Abstract
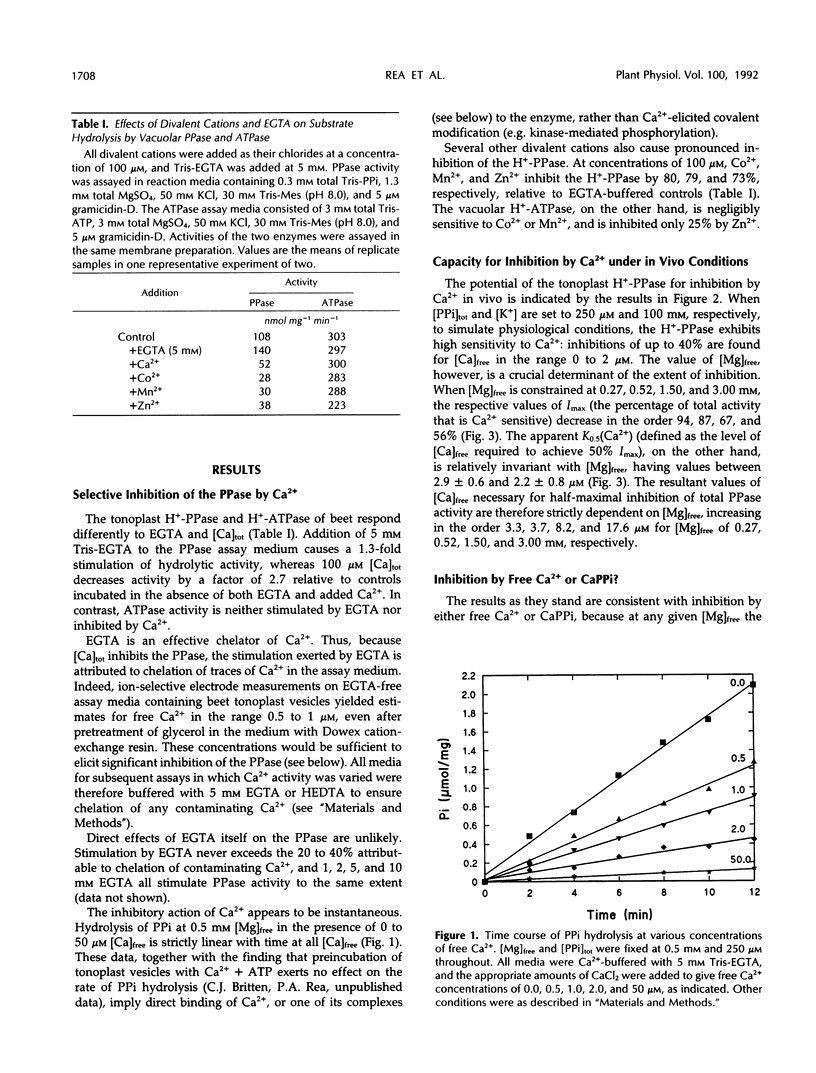
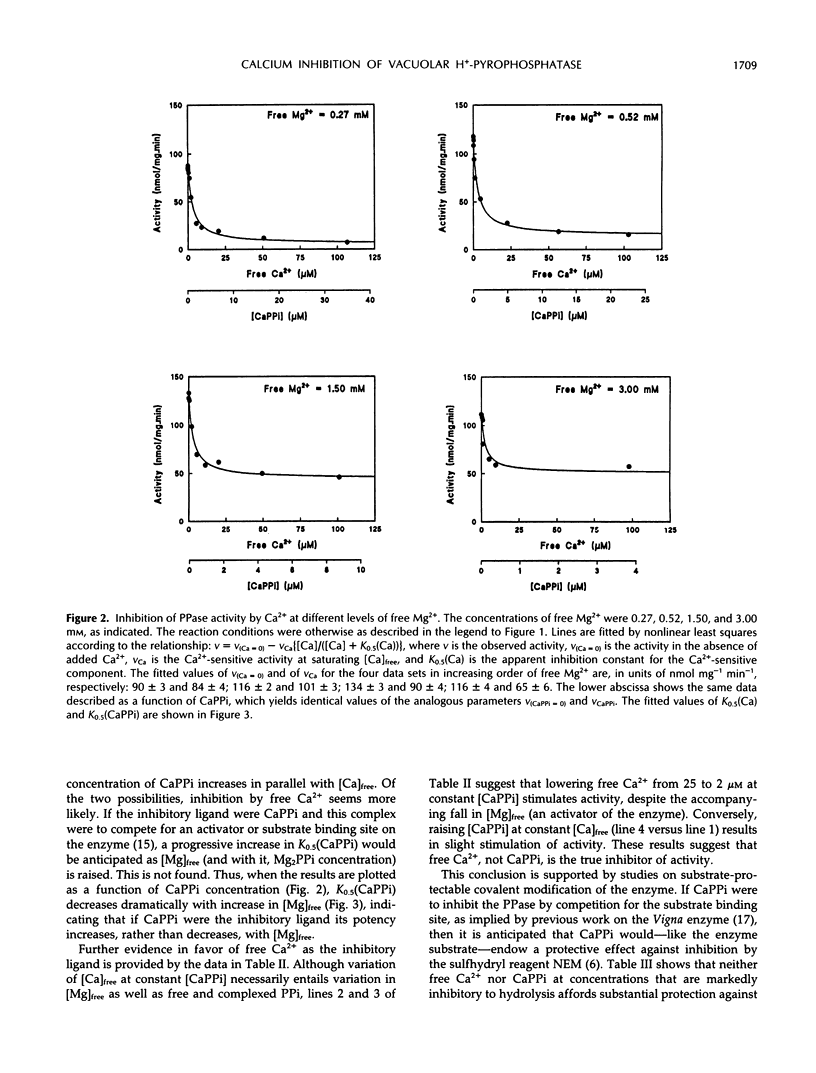
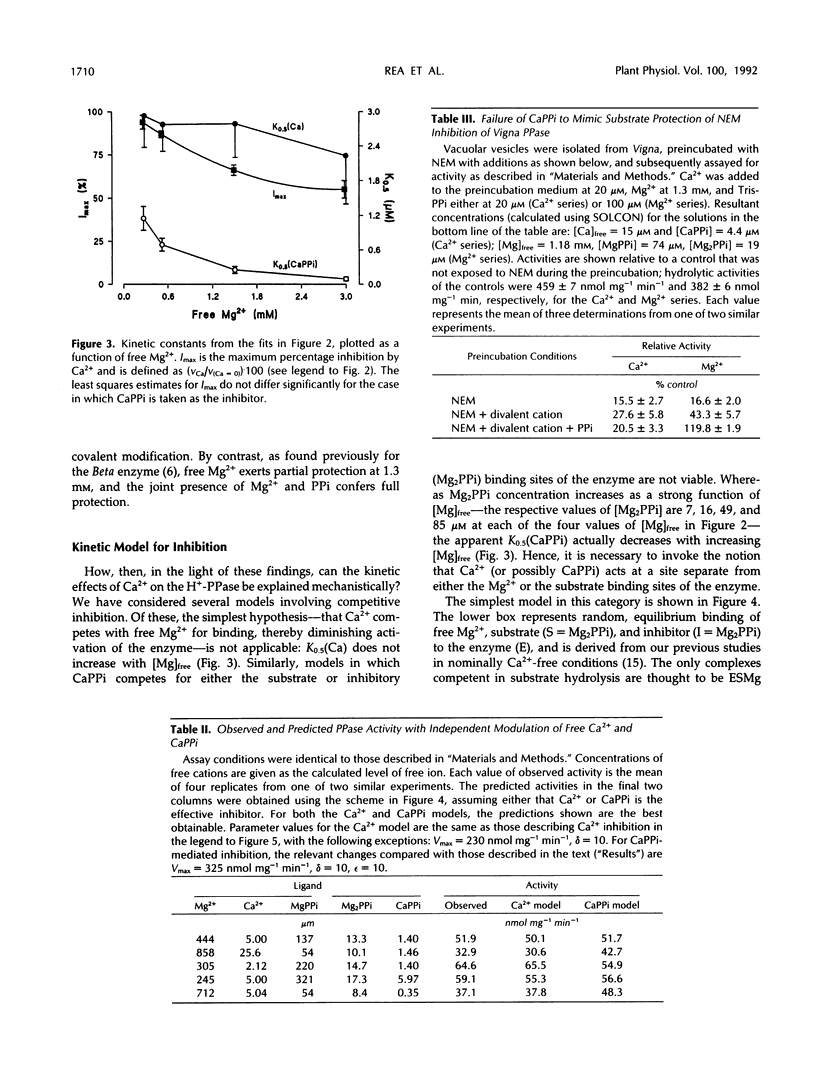
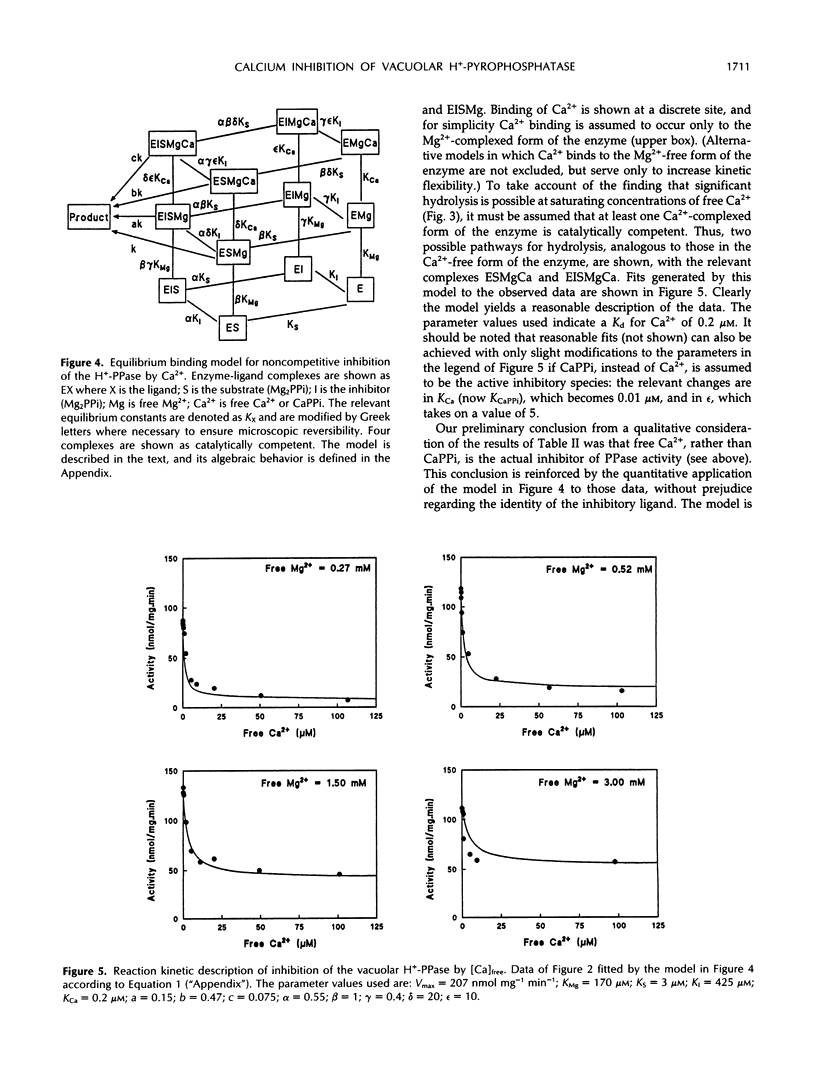
The H+-translocating inorganic pyrophosphatase (H+-PPase) associated with vesicles of the vacuolar membrane (tonoplast) isolated from beet (Beta vulgaris L.) is subject to direct inhibition by Ca2+ and a number of other divalent cations (Co2+, Mn2+, Zn2+). By contrast, the H+-translocating ATPase (H+-ATPase) located on the same membrane is insensitive to Ca2+. Here we examine the mechanism and feasibility of regulation of the vacuolar H+-PPase by cytosolic free Ca2+ under the conditions thought to prevail in vivo with respect to Mg2+, inorganic pyrophosphate (PPi), and pH. The minimal reaction scheme that satisfactorily describes the effects of elevated Ca2+ or CaPPi on the enzyme is one that invokes equilibrium binding of substrate (Mg2PPi) at one site, inhibitory binding of Mg2PPi to a lower-affinity second site, binding of activator (Mg2+) at a third site, and direct binding of Ca2+ or CaPPi to a fourth site. Changes in enzyme activity in response to selective manipulation of either Ca2+ or CaPPi are explicable only if Ca2+, rather than CaPPi, is the inhibitory ligand. This conclusion is supported by the finding that CaPPi fails to mimic substrate in protection of the enzyme from inhibition by N-ethylmaleimide. Furthermore, the reaction scheme quantitatively and independently predicts the observed noncompetitive effects of free Ca2+ on the substrate concentration dependence of H+-PPase activity. The results are discussed in relation to the previous proposal that CaPPi is the principal inhibitory ligand of the vacuolar H+-PPase (M. Maeshima [1991] Eur J Biochem 196: 11-17) and the possibility that in vivo modulation of cytosolic free Ca2+ might constitute a specific mechanism for selective regulation of this enzyme, and consequently for stabilization of PPi levels in the cytoplasm of plant cells.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baykov A. A., Volk S. E., Unguryte A. Inhibition of inorganic pyrophosphatase of animal mitochondria by calcium. Arch Biochem Biophys. 1989 Sep;273(2):287–291. doi: 10.1016/0003-9861(89)90486-4. [DOI] [PubMed] [Google Scholar]
- Bencini D. A., Wild J. R., O'Donovan G. A. Linear one-step assay for the determination of orthophosphate. Anal Biochem. 1983 Jul 15;132(2):254–258. doi: 10.1016/0003-2697(83)90004-0. [DOI] [PubMed] [Google Scholar]
- Blackford S., Rea P. A., Sanders D. Voltage sensitivity of H+/Ca2+ antiport in higher plant tonoplast suggests a role in vacuolar calcium accumulation. J Biol Chem. 1990 Jun 15;265(17):9617–9620. [PubMed] [Google Scholar]
- Davidson A. M., Halestrap A. P. Inhibition of mitochondrial-matrix inorganic pyrophosphatase by physiological [Ca2+], and its role in the hormonal regulation of mitochondrial matrix volume. Biochem J. 1989 Mar 15;258(3):817–821. doi: 10.1042/bj2580817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies J. M., Rea P. A., Sanders D. Vacuolar proton-pumping pyrophosphatase in Beta vulgaris shows vectorial activation by potassium. FEBS Lett. 1991 Jan 14;278(1):66–68. doi: 10.1016/0014-5793(91)80085-h. [DOI] [PubMed] [Google Scholar]
- Gilroy S., Fricker M. D., Read N. D., Trewavas A. J. Role of Calcium in Signal Transduction of Commelina Guard Cells. Plant Cell. 1991 Apr;3(4):333–344. doi: 10.1105/tpc.3.4.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedrich R., Kurkdjian A. Characterization of an anion-permeable channel from sugar beet vacuoles: effect of inhibitors. EMBO J. 1988 Dec 1;7(12):3661–3666. doi: 10.1002/j.1460-2075.1988.tb03247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaestner K. H., Sze H. Potential-dependent anion transport in tonoplast vesicles from oat roots. Plant Physiol. 1987 Mar;83(3):483–489. doi: 10.1104/pp.83.3.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leigh R. A., Pope A. J., Jennings I. R., Sanders D. Kinetics of the Vacuolar H-Pyrophosphatase : The Roles of Magnesium, Pyrophosphate, and their Complexes as Substrates, Activators, and Inhibitors. Plant Physiol. 1992 Dec;100(4):1698–1705. doi: 10.1104/pp.100.4.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeshima M. H(+)-translocating inorganic pyrophosphatase of plant vacuoles. Inhibition by Ca2+, stabilization by Mg2+ and immunological comparison with other inorganic pyrophosphatases. Eur J Biochem. 1991 Feb 26;196(1):11–17. doi: 10.1111/j.1432-1033.1991.tb15779.x. [DOI] [PubMed] [Google Scholar]
- Maeshima M., Yoshida S. Purification and properties of vacuolar membrane proton-translocating inorganic pyrophosphatase from mung bean. J Biol Chem. 1989 Nov 25;264(33):20068–20073. [PubMed] [Google Scholar]
- Moe O. A., Butler L. G. Yeast inorganic pyrophosphatase. 3. Kinetics of Ca 2+ inhibition. J Biol Chem. 1972 Nov 25;247(22):7315–7319. [PubMed] [Google Scholar]
- Parry R. V., Turner J. C., Rea P. A. High purity preparations of higher plant vacuolar H+-ATPase reveal additional subunits. Revised subunit composition. J Biol Chem. 1989 Nov 25;264(33):20025–20032. [PubMed] [Google Scholar]
- Rea P. A., Poole R. J. Proton-Translocating Inorganic Pyrophosphatase in Red Beet (Beta vulgaris L.) Tonoplast Vesicles. Plant Physiol. 1985 Jan;77(1):46–52. doi: 10.1104/pp.77.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarafian V., Poole R. J. Purification of an h-translocating inorganic pyrophosphatase from vacuole membranes of red beet. Plant Physiol. 1989 Sep;91(1):34–38. doi: 10.1104/pp.91.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeshige K., Tazawa M. Determination of the inorganic pyrophosphate level and its subcellular localization in Chara corallina. J Biol Chem. 1989 Feb 25;264(6):3262–3266. [PubMed] [Google Scholar]
- Volk S. E., Baykov A. A., Duzhenko V. S., Avaeva S. M. Kinetic studies on the interactions of two forms of inorganic pyrophosphatase of heart mitochondria with physiological ligands. Eur J Biochem. 1982 Jun 15;125(1):215–220. doi: 10.1111/j.1432-1033.1982.tb06671.x. [DOI] [PubMed] [Google Scholar]


