Abstract
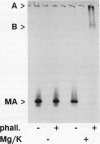
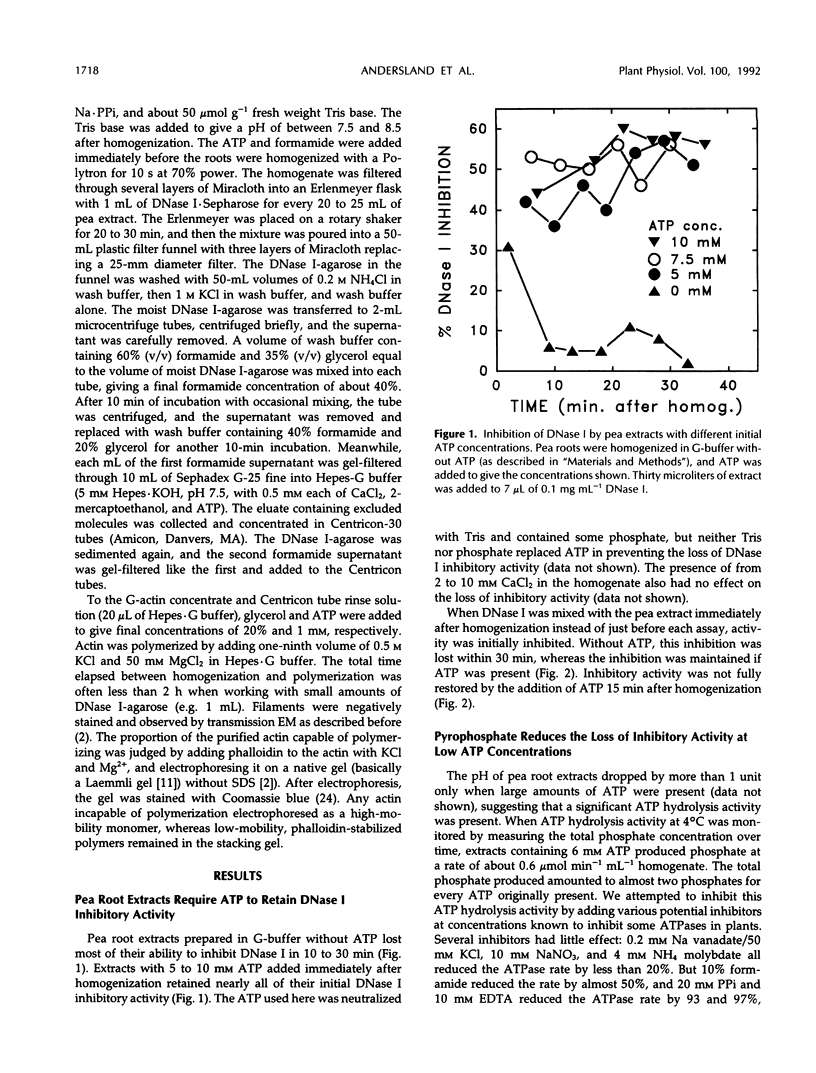
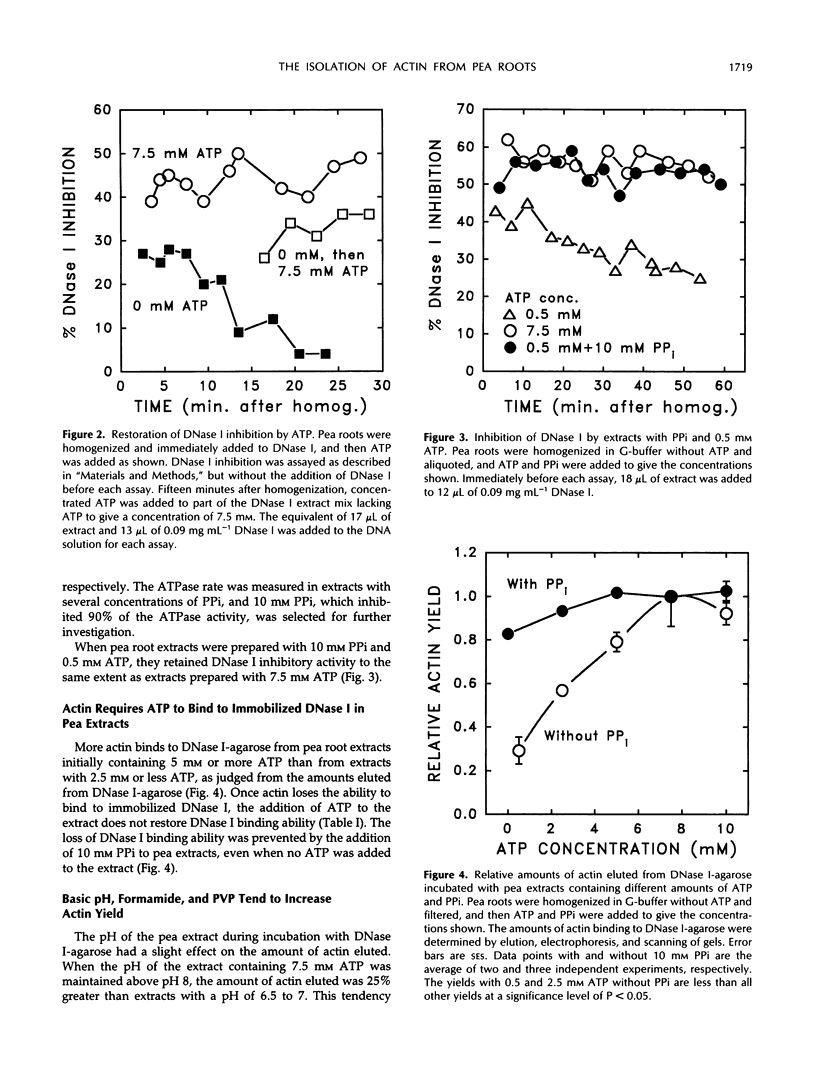
Native actin can be isolated from pea (Pisum sativum L.) roots by DNase I affinity chromatography, but the resulting yields and quality of actin are variable. By use of two assays for actin, a DNase I inhibition assay and a gel scanning assay, we identified several factors that increased actin yield. ATP is required for the actin in crude pea root extracts to bind to immobilized DNase I. Low amounts of ATP are hydrolyzed rapidly by an endogenous ATPase in the extract, and the actin then irreversibly loses the ability to bind to DNase I. High ATP concentrations (5-10 mm) or inhibition of the ATPase (with 10 mm pyrophosphate) are required for pea actin to retain DNase I binding ability. When adequate amounts of ATP are present, actin binding from the extract is further enhanced by basic pH, formamide, and soluble polyvinyl-pyrrolidone. Once actin is bound to the DNase I-agarose and washed free of extract, high ATP concentrations are not required to keep actin bound. Actin eluted from the DNase I-agarose with formamide retained its ability to polymerize into filaments with the addition of KCl and Mg2+. The advantages and disadvantages of this procedure and its application to other plant materials are discussed.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bedinger P. A., Edgerton M. D. Developmental staging of maize microspores reveals a transition in developing microspore proteins. Plant Physiol. 1990 Feb;92(2):474–479. doi: 10.1104/pp.92.2.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blikstad I., Markey F., Carlsson L., Persson T., Lindberg U. Selective assay of monomeric and filamentous actin in cell extracts, using inhibition of deoxyribonuclease I. Cell. 1978 Nov;15(3):935–943. doi: 10.1016/0092-8674(78)90277-5. [DOI] [PubMed] [Google Scholar]
- Gallagher S. R., Leonard R. T. Effect of vanadate, molybdate, and azide on membrane-associated ATPase and soluble phosphatase activities of corn roots. Plant Physiol. 1982 Nov;70(5):1335–1340. doi: 10.1104/pp.70.5.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirono M., Kumagai Y., Numata O., Watanabe Y. Purification of Tetrahymena actin reveals some unusual properties. Proc Natl Acad Sci U S A. 1989 Jan;86(1):75–79. doi: 10.1073/pnas.86.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabsch W., Mannherz H. G., Suck D., Pai E. F., Holmes K. C. Atomic structure of the actin:DNase I complex. Nature. 1990 Sep 6;347(6288):37–44. doi: 10.1038/347037a0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Larson E., Howlett B., Jagendorf A. Artificial reductant enhancement of the Lowry method for protein determination. Anal Biochem. 1986 Jun;155(2):243–248. doi: 10.1016/0003-2697(86)90432-x. [DOI] [PubMed] [Google Scholar]
- Laub F., Kaplan M., Gitler C. Actin polymerization accompanies Thy-1-capping on mouse thymocytes. FEBS Lett. 1981 Feb 9;124(1):35–38. doi: 10.1016/0014-5793(81)80048-8. [DOI] [PubMed] [Google Scholar]
- Lazarides E., Lindberg U. Actin is the naturally occurring inhibitor of deoxyribonuclease I. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4742–4746. doi: 10.1073/pnas.71.12.4742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBel D., Poirier G. G., Beaudoin A. R. A convenient method for the ATPase assay. Anal Biochem. 1978 Mar;85(1):86–89. doi: 10.1016/0003-2697(78)90277-4. [DOI] [PubMed] [Google Scholar]
- Loomis W. D. Overcoming problems of phenolics and quinones in the isolation of plant enzymes and organelles. Methods Enzymol. 1974;31:528–544. doi: 10.1016/0076-6879(74)31057-9. [DOI] [PubMed] [Google Scholar]
- Ma Y. Z., Yen L. F. Actin and Myosin in pea tendrils. Plant Physiol. 1989 Feb;89(2):586–589. doi: 10.1104/pp.89.2.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannherz H. G., Rohr G. 5'-Nucleotidase reverses the inhibitory action of actin on pancreatic deoxyribonuclease I. FEBS Lett. 1978 Nov 15;95(2):284–289. doi: 10.1016/0014-5793(78)81012-6. [DOI] [PubMed] [Google Scholar]
- McLean B. G., Eubanks S., Meagher R. B. Tissue-specific expression of divergent actins in soybean root. Plant Cell. 1990 Apr;2(4):335–344. doi: 10.1105/tpc.2.4.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meagher R. B. Divergence and differential expression of actin gene families in higher plants. Int Rev Cytol. 1991;125:139–163. doi: 10.1016/s0074-7696(08)61218-8. [DOI] [PubMed] [Google Scholar]
- Meza I., Sabanero M., Cazares F., Bryan J. Isolation and characterization of actin from Entamoeba histolytica. J Biol Chem. 1983 Mar 25;258(6):3936–3941. [PubMed] [Google Scholar]
- Neuhoff V., Arold N., Taube D., Ehrhardt W. Improved staining of proteins in polyacrylamide gels including isoelectric focusing gels with clear background at nanogram sensitivity using Coomassie Brilliant Blue G-250 and R-250. Electrophoresis. 1988 Jun;9(6):255–262. doi: 10.1002/elps.1150090603. [DOI] [PubMed] [Google Scholar]
- Pardee J. D., Spudich J. A. Purification of muscle actin. Methods Enzymol. 1982;85(Pt B):164–181. doi: 10.1016/0076-6879(82)85020-9. [DOI] [PubMed] [Google Scholar]
- Polzar B., Nowak E., Goody R. S., Mannherz H. G. The complex of actin and deoxyribonuclease I as a model system to study the interactions of nucleotides, cations and cytochalasin D with monomeric actin. Eur J Biochem. 1989 Jun 15;182(2):267–275. doi: 10.1111/j.1432-1033.1989.tb14826.x. [DOI] [PubMed] [Google Scholar]
- Villanueva M. A., Ho S. C., Wang J. L. Isolation and characterization of one isoform of actin from cultured soybean cells. Arch Biochem Biophys. 1990 Feb 15;277(1):35–41. doi: 10.1016/0003-9861(90)90546-b. [DOI] [PubMed] [Google Scholar]
- Zechel K. Isolation of polymerization-competent cytoplasmic actin by affinity chromatography on immobilized DNAse I using formamide as eluant. Eur J Biochem. 1980 Sep;110(2):343–348. doi: 10.1111/j.1432-1033.1980.tb04873.x. [DOI] [PubMed] [Google Scholar]
- Zimmerle C. T., Frieden C. Effect of pH on the mechanism of actin polymerization. Biochemistry. 1988 Oct 4;27(20):7766–7772. doi: 10.1021/bi00420a027. [DOI] [PubMed] [Google Scholar]