Abstract
Study Objectives:
Polycystic ovary syndrome (PCOS) confers a high risk of obstructive sleep apnea (OSA). Here we investigated the effect of OSA on first in vitro fertilization (IVF) cycle metrics and outcomes in patients with PCOS.
Methods:
This was a prospective cohort study of patients with PCOS undergoing their first IVF at a single tertiary center between October 1, 2021, and September 30, 2022. Patients were screened for OSA before IVF and grouped accordingly. Clinical and IVF cycle data were compared between groups.
Results:
OSA was found in 37.2% of 156 patients with PCOS, with longer infertility duration (4.3 ± 2.5 vs. 3.4 ± 2.0 years) and lower levels of anti-Müllerian and luteinizing hormones than patients without OSA (6.44 ± 2.96 vs 8.69 ± 4.03 µg/L and 6.30 ± 5.02 vs 8.46 ± 6.09 U/L). Antral follicle count was lower in patients with OSA (28.9 ± 12.4 vs 33.2 ± 12.9). During ovarian stimulation, patients with OSA required significantly higher doses of gonadotropin (2080.8 ± 1008.7 vs 1682.8 ± 619.9 U) and had lower peak estradiol level (4473.5 ± 2693.0 vs 5455.7 ± 2955.1 pmol/L) and fewer retrieved oocytes, high-quality, and available embryos (17.8 ± 7.2 vs 21.9 ± 10.5, 4.5 ± 4.4 vs 6.2 ± 4.6, 5.2 ± 4.3 vs 7.4 ± 5.0). Eleven patients were excluded for having no embryos or missing transfer. Therefore, we analyzed the outcome of the first embryo transfer in 145 patients. The biochemical and clinical pregnancy rates were lower in patients with OSA than patients without OSA (51.9% vs 66.7% and 42.3% vs 60.2%). OSA was independently associated with clinical pregnancy rate after controlling for several confounders (P = .043).
Conclusions:
OSA impairs female fertility in patients with polycystic ovary syndrome, suggesting an adverse effect on in vitro fertilization cycle stimulation characteristics and clinical outcomes.
Citation:
Zhang Q, Wang Z, Ding J, et al. Effect of obstructive sleep apnea on in vitro fertilization outcomes in women with polycystic ovary syndrome. J Clin Sleep Med. 2024;20(1):31–38.
Keywords: obstructive sleep apnea, polycystic ovarian syndrome, in vitro fertilization, female fertility, pregnancy
BRIEF SUMMARY
Current Knowledge/Study Rationale: There are currently no clinical data on female fertility in patients with obstructive sleep apnea and in vitro fertilization cycle outcomes. This study explored associations between obstructive sleep apnea and fertility and in vitro fertilization cycle outcomes in patients with polycystic ovary syndrome of reproductive age.
Study Impact: The analysis shows that obstructive sleep apnea has a negative impact on several metrics of female fertility that could translate into poor pregnancy outcomes. If confirmed in more patients, as a reversible risk factor affecting female fertility, recognition of the impact of obstructive sleep apnea could improve fertility outcomes.
INTRODUCTION
Obstructive sleep apnea (OSA) is characterized by repeated upper airway collapse during sleep leading to chronic intermittent hypoxia and sleep fragmentation. It is conservatively estimated that 14% of men and 5% of women in the general adult population are affected by OSA, with the incidence increasing with age and obesity. Patients with OSA have a poorer quality of life and a higher risk of developing cardiovascular, cerebrovascular, and metabolic diseases.1
Polycystic ovary syndrome (PCOS) is characterized by sporadic menstruation, anovulation, insulin resistance, centripetal obesity, and infertility, and it is the most common endocrine disorder of reproductive age, affecting about 8%–13% of women. Patients with PCOS have poorer sleep quality and a higher incidence of OSA.2,3 A meta-analysis of 17 studies and 648 patients with PCOS calculated a 35% prevalence of OSA,4 approximately 2–3 times higher than reported by women in the general population. Endocrine Society guidelines in Europe and the United States have long recognized the association between PCOS and OSA.5,6 In vitro fertilization (IVF) is clinically recommended for patients with PCOS who have failed lifestyle and ovulation induction therapy or who have other infertility factors,7 so the IVF cycle characteristics and outcomes in PCOS women continue to be of interest.8
Sleep disturbance has been shown to be associated with decreased female fertility.9 Objective sleep duration is associated with the number of oocytes retrieved during IVF10 and the completion of embryo transfer.11 Self-reported perceived sleep quality is linearly correlated with pregnancy rate in IVF.12 However, there are no clinical data on female fertility in patients with OSA and IVF cycle outcomes. We therefore explored the associations between OSA and fertility and first IVF cycle outcomes in patients with PCOS of reproductive age.
METHODS
Participants
Patients with PCOS who underwent IVF for the first time at the Reproductive Medicine Center of Renmin Hospital of Wuhan University between October 1, 2021, and September 30, 2022, were consecutively recruited. Inclusion criteria were: patients ages 20–35 years weighing > 40 kg13 with indications for IVF and a diagnosis of PCOS according to the modified Rotterdam criteria.14 Patients with any of the following conditions were excluded: previously diagnosed or treated for OSA; organic disease such as unilateral oophorectomy or previously diagnosed with uterine abnormalities, such as malformed uterus (unicornate uterus, septate uterus, double uterus), adenomyosis, submucosal myoma, or uterine adhesiosis; chromosomal karyotype abnormalities in either spouse; abnormal semen examination of the male; a medical condition contraindicating assisted reproductive technology or pregnancy, eg, poorly controlled diabetes, undiagnosed liver or kidney disease, severe anemia, history of pulmonary embolism or cerebrovascular accident, uncontrolled hypertension, or known symptomatic heart disease; a history (or suspicion) of cervical, endometrial, or breast cancer; and abnormal uterine bleeding of unknown cause. The Ethics Committee of Renmin Hospital of Wuhan University approved the study. All enrolled patients signed written informed consent.
Study design
This was a prospective cohort study in which all patients with PCOS meeting the inclusion criteria and without exclusion criteria were monitored for sleep breathing prior to IVF. Patients were divided into OSA and non-OSA groups according to the apnea-hypopnea index to compare IVF cycle characteristics and outcomes. The OSA group was divided into mild or moderate-severe OSA, with intergroup comparisons conducted to evaluate associations between fertility/pregnancy outcomes metrics and OSA severity. Linear and logistic regression models of OSA and outcomes were developed. Demographic information and years of infertility were obtained through a short questionnaire. Follicle-stimulating hormone (FSH), luteinizing hormone (LH), estradiol (E2), testosterone, and progesterone were measured by Access 2 chemiluminescence immunoassays (Beckman, Chaska, MN, USA) following the manufacturer’s protocols. Free triiodothyronine, free thyroxine, and thyroid-stimulating hormone were measured by Centaur XP autoanalyzer (Siemens). Glucose was measured by ADVIA 2400 Clinical Chemistry System (Siemens) following the manufacturer’s instructions. Anti-Müllerian hormone was analyzed by chemiluminescence immunoassay using Cobas e601 (Roche Diagnostics, Germany). Patients underwent transvaginal pelvic ultrasound (Philips EnVisor C HD Ultrasound) for antral follicle counting and endometrial thickness measurement.
IVF procedure
A gonadotropin (Gn)-releasing hormone antagonist regimen was followed, and all patients were treated with this regimen. Briefly, exogenous Gn was administered on the second day of menstruation after ultrasonography showed that there was no large follicle in the ovary and blood reproductive hormones were at their basal state. Ovulation was induced with recombinant follicle-stimulating hormone (FSH) (Merck Serono, Geneva, Switzerland) and urinary human menopausal Gn (Livzon Pharm, Zhuhai, Guangdong, China). The Gn dose was adjusted according to follicle growth and estradiol level; 250 µg antagonist (Merck Serono) was added when the dominant follicle diameter reached 14 mm or when luteinizing hormone (LH) was ≥ 10U/L. When there were three dominant follicles with diameters ≥ 18 mm, 250 µg recombinant human chorionic gonadotropin (hCG, Livzon) was injected intramuscularly and oocytes retrieved 36 hours later. Oocytes were fertilized 4–6 hours after retrieval. Transfer or embryo cryopreservation was selected according to the indications and the patient’s wishes.
Sleep studies
We used a portable, noncontact sleep apnea monitor to detect each participant’s sleep for at least 7 hours during the night. The Zhaoguan ultra-wideband biological radar sleep screening device (China) consisted of a radar transmitter (OrbSense, ZG-S01D) that tracked respiratory and body movements and a finger plethysmograph (ZG-P11F) that measured oxygen saturation. Both components were supplied by Megasens Technology Co. Ltd. (HangZhou, China). The apnea-hypopnea index, mean oxygen saturation, lowest oxygen saturation, oxygen desaturation index (number of times per hour of sleep that the blood oxygen level dropped by ≥ 4% from baseline), and the percentage of time with oxygen saturation < 90% were measured. The apnea-hypopnea index represents the number of occurrences of apnea and hypopnea per hour during sleep and was used to classify the severity of OSA as mild (5–15 events/h), moderate (15–30 events/h), and severe (≥ 30 events/h).15 The radar sleep apnea monitor has previously been shown to be a reliable device for screening OSA.16 Patients’ sleep monitoring results were blind to the attending physician.
Outcomes
The primary outcome was IVF first embryo transfer pregnancy rate. Biochemical pregnancy was defined as a serum hCG > 10 mIU/mL. Clinical pregnancy was defined as the observation of a gestational sac on ultrasonography. Secondary outcomes were basal antral follicle count (AFC), serum anti-Müllerian hormone (AMH), duration and dosage of Gn used, total number of oocytes retrieved, two pronuclei (2PN) fertilization, cleavage, 2PN cleavage, and high-quality and available embryos. Normal fertilization results in a fertilized oocyte with 2PN zygote. 2PN cleavage denotes the embryos that originate from a 2PN zygote, which are indicative of normal fertilization. 2PN is the basis for screening and grading embryos, which can reflect the quality of oocytes and fertilized embryos of women undergoing IVF.
Statistical analysis
SPSS v26 software (IBM Statistics, Armonk, NY, USA) was used for data analysis. Continuous data are expressed as mean ± standard deviation, with Student’s t test or analysis of variance used to compare two or more than two groups, respectively. Categorical data are represented by the number of cases (%) for comparison of differences between groups using the nonparametric chi-squared test. Multivariate linear and logistic regression was used to adjust for the effect of baseline characteristics and groups. A two-sided alpha level of 0.05 was considered statistically significant.
RESULTS
Demographic and clinical characteristics
A total of 156 patients with PCOS were included, and 58 (37.2%) patients were diagnosed with OSA: 34 mild, 19 moderate, and 5 severe. The mean age of the patients was 30.1 ± 3.5 years, and the mean body mass index (BMI) was 23.9 ± 3.6 kg/m2. The OSA group had a significantly higher BMI and longer period of infertility than those in the non-OSA group (P < .05) (Table 1). AFC, AMH, LH, and LH/FSH were significantly lower in the OSA group (P < .05). There were no significant differences in age, fasting blood glucose, thyroid function, E2, testosterone, and endometrial thickness between the two groups. On the hCG injection day, the OSA group had significantly lower peak E2 levels, and there was no significant difference in progesterone and endometrial thickness between the two groups.
Table 1.
Comparison of demographic and clinical data of the OSA and non-OSA groups at first IVF-assisted pregnancy.
| Variables | Total (n = 156) | Non-OSA Group (n = 98) | OSA Group (n = 58) | t | P |
|---|---|---|---|---|---|
| Age, years | 30.1 ± 3.5 | 29.7 ± 3.5 | 30.8 ± 3.5 | 1.935 | .055 |
| Height, cm | 160.1 ± 4.5 | 160.5 ± 4.5 | 159.6 ± 4.4 | −1.191 | .236 |
| Weight, kg | 61.3 ± 10.3 | 59.3 ± 9.7 | 64.8 ± 10.3 | 3.368 | .001 |
| BMI, kg/m2 | 23.9 ± 3.6 | 23.0 ± 3.3 | 25.4 ± 3.5 | 4.310 | <.001 |
| AHI, events/h | 6.8 ± 8.9 | 1.7 ± 1.3 | 15.3 ± 9.8 | 10.581 | <.001 |
| Mean oxygen saturation, % | 96.1 ± 2.1 | 97.1 ± 1.4 | 94.4 ± 1.8 | −10.044 | <.001 |
| Lowest oxygen saturation, % | 87.3 ± 6.1 | 90.4 ± 3.7 | 82.2 ± 5.9 | −9.567 | <.001 |
| ODI, events/h | 7.4 ± 7.7 | 2.8 ± 2.2 | 14.9 ± 7.7 | 11.664 | <.001 |
| T90, % | 3.17 ± 8.97 | 0.52 ± 2.06 | 7.55 ± 13.32 | 3.988 | <.001 |
| Fasting glucose, mmol/L | 5.0 ± 0.6 | 5.0 ± 0.6 | 5.1 ± 0.6 | 0.698 | .486 |
| FT3, pg/mL | 3.44 ± 0.36 | 3.41 ± 0.32 | 3.50 ± 0.40 | 1.406 | .162 |
| FT4, ng/dL | 1.22 ± 0.14 | 1.21 ± 0.14 | 1.24 ± 0.15 | 1.272 | .206 |
| TSH, µIU/mL | 2.33 ± 1.16 | 2.35 ± 1.22 | 2.30 ± 1.06 | −0.244 | .807 |
| Infertility duration, years | 3.7 ± 2.2 | 3.4 ± 2.0 | 4.3 ± 2.5 | 2.467 | .015 |
| AFC, n | 31.6 ± 12.9 | 33.2 ± 12.9 | 28.9 ± 12.4 | −1.993 | .048 |
| FSH, U/L | 6.86 ± 1.61 | 6.89 ± 1.41 | 6.82 ± 1.90 | −0.260 | .795 |
| LH, U/L | 7.66 ± 5.80 | 8.46 ± 6.09 | 6.30 ± 5.02 | −2.259 | .025 |
| LH/FSH | 1.12 ± 0.82 | 1.24 ± 0.90 | 0.91 ± 0.60 | −2.727 | .007 |
| E2, pmol/L | 44.58 ± 20.87 | 45.34 ± 22.58 | 43.27 ± 17.71 | −0.594 | .553 |
| P, U/L | 0.68 ± 0.56 | 0.72 ± 0.63 | 0.62 ± 0.45 | −0.991 | .323 |
| T, µg/L | 35.92 ± 11.58 | 34.80 ± 11.59 | 37.62 ± 11.55 | 1.056 | .295 |
| AMH, µg/L | 7.85 ± 3.82 | 8.69 ± 4.03 | 6.44 ± 2.96 | −4.003 | <.001 |
| Endometrial thickness, mm | 5.6 ± 2.0 | 5.5 ± 2.0 | 5.8 ± 2.0 | 0.987 | .325 |
| hCG injection day | |||||
| E2, pmol/L | 5089.8 ± 2890.8 | 5455.7 ± 2955.1 | 4473.5 ± 2693.0 | −2.053 | .042 |
| P, U/L | 1.10 ± 0.48 | 1.10 ± 0.47 | 1.10 ± 0.51 | −0.058 | .954 |
| Endometrial thickness, mm | 11.2 ± 2.3 | 11.1 ± 2.4 | 11.4 ± 2.3 | 0.861 | .391 |
Data are presented as mean ± standard deviation. AFC = antral follicle count, AHI = apnea-hypopnea index, AMH = anti-Müllerian hormone, BMI = body mass index, E2 = estradiol, FSH = follicle-stimulating hormone, FT3 = free triiodothyronine, FT4 = free thyroxine, hCG = human chorionic gonadotropin, IVF, in vitro fertilization, LH = luteinizing hormone, ODI = oxygen desaturation index, OSA = obstructive sleep apnea, P = progesterone, T = testosterone, T90 = the percentage of time with oxygen saturation less than 90%, TSH = thyroid-stimulating hormone.
Cycle stimulation characteristics
The total dosage of Gn used was higher in OSA group (Table 2). The number of oocytes retrieved, 2PN fertilization, cleavage, 2PN cleavage, and high-quality and available embryos were significantly lower in the OSA group than in the non-OSA group (P < .05).
Table 2.
Analysis of initial IVF cycle data between the two groups.
| Variables | Total (n = 156) | Non-OSA Group (n = 98) | OSA Group (n = 58) | t | P |
|---|---|---|---|---|---|
| Duration of Gn used, d | 10.6 ± 2.4 | 10.6 ± 2.4 | 10.7 ± 2.5 | 0.258 | .797 |
| Dosage of Gn used, U | 1830.8 ± 807.4 | 1682.8 ± 619.9 | 2080.8 ± 1008.7 | 2.717 | .008 |
| Total number of oocytes retrieved, n | 20.4 ± 9.6 | 21.9 ± 10.5 | 17.8 ± 7.2 | −2.613 | .010 |
| No. of 2PN zygotes, n | 11.6 ± 6.2 | 12.2 ± 6.8 | 10.5 ± 4.9 | −1.849 | .066 |
| No. of cleavage, n | 15.8 ± 7.8 | 16.9 ± 8.3 | 14.0 ± 6.4 | −2.260 | .025 |
| No. of 2PN cleavage, n | 11.3 ± 6.1 | 12.0 ± 6.6 | 10.1 ± 4.9 | −2.041 | .043 |
| No. of high-quality embryos, n | 5.6 ± 4.6 | 6.2 ± 4.6 | 4.5 ± 4.4 | −2.185 | .030 |
| No. of available embryos, n | 6.6 ± 4.9 | 7.4 ± 5.0 | 5.2 ± 4.3 | −2.728 | .007 |
Data are presented as means ± standard deviation. 2PN = two pronuclei, Gn = gonadotropin, IVF = in vitro fertilization, OSA = obstructive sleep apnea.
Clinical and cycle stimulation characteristics patients with OSA of different severity
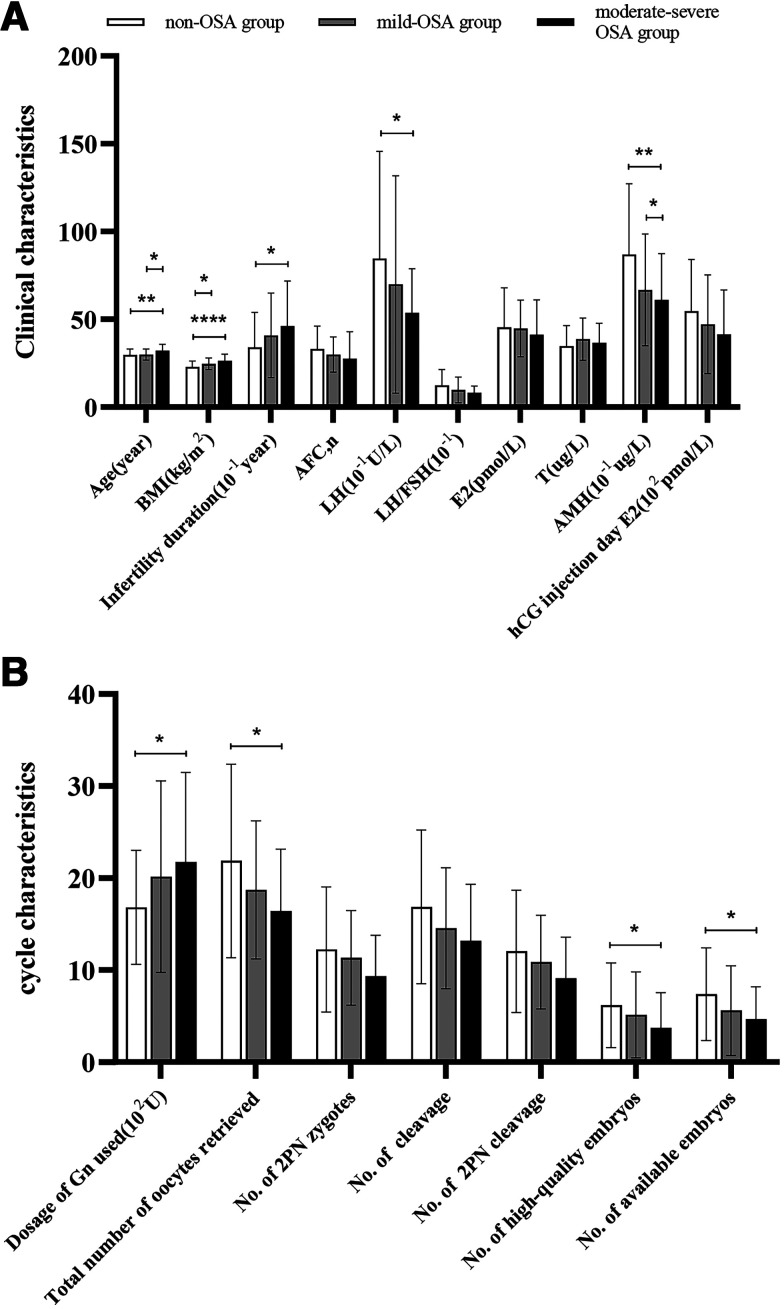
Since there were only five patients with severe OSA and the pathological impairment was similar to that of moderate OSA, we pooled moderate and severe patients into one group and performed intergroup comparisons to evaluate trends in fertility according to OSA severity. Patients with more severe OSA were older and had a higher BMI, longer infertility duration, and lower levels of AFC, AMH, LH, LH/FSH, basal E2, and E2 on the hCG injection day (Table S1 (173.5KB, pdf) in the supplemental material and Figure 1A). The total doses of Gn increased and the number of oocytes retrieved, 2PN fertilization, cleavage, 2PN cleavage, and high-quality and available embryos decreased with OSA severity (Table S2 (173.5KB, pdf) and Figure 1B).
Figure 1. Clinical and biochemical characteristics and cycle stimulation characteristics.
(A) Clinical and biochemical characteristics at baseline and on hCG day. (B) Cycle stimulation characteristics. Asterisks represent significant differences: *P < .05, **P < .01, ****P < .0001. 2PN = two pronuclei, AFC = antral follicle count, AMH = anti-Müllerian hormone, BMI = body mass index, E2 = estradiol, FSH = follicle-stimulating hormone, Gn = gonadotropin, hCG = human chorionic gonadotropin, LH = luteinizing hormone, OSA = obstructive sleep apnea, T = testosterone.
IVF first embryo transfer outcomes
Thirty-five patients received fresh embryo transfer and 110 received embryo cryopreservation followed by frozen embryo transfer for their first IVF cycle, resulting in 35 fresh cycles and 133 frozen cycles. In total, nine patients had no available embryos and two patients did not attend for transfer. The posttransfer biochemical and clinical pregnancy rates were analyzed for the two groups. As shown in Table 3, the first embryo transfer biochemical pregnancy rate in PCOS was 61.4% and the clinical pregnancy rate was 53.8%. The first fresh embryo transfer biochemical pregnancy rate in PCOS was 57.1% and the clinical pregnancy rate was 45.7%. The first frozen embryo transfer biochemical pregnancy rate in PCOS was 62.4%, and the clinical pregnancy rate was 56.4%. The biochemical and clinical pregnancy rates of the first IVF transfer were lower in the OSA group than the non-OSA group (51.9% vs 66.7%, P = .080; 42.3% vs 60.2%, P = .038).
Table 3.
Biochemical and clinical pregnancy rates after embryo transfer.
| Variables | Total (n = 145) | Non-OSA Group (n = 93) | OSA Group (n = 52) | χ2 | P |
|---|---|---|---|---|---|
| First fresh embryo transfer (n = 35), % | |||||
| Biochemical pregnancy rate | 57.1 (20/35) | 60 (15/25) | 50 (5/10) | 0.292 | .589 |
| Clinical pregnancy rate, % | 45.7 (16/35) | 48 (12/25) | 40 (4/10) | 0.184 | .668 |
| First frozen embryo transfer (n = 133), % | |||||
| Biochemical pregnancy rate | 62.4 (83/133) | 65.9 (54/82) | 56.9 (29/51) | 1.083 | .298 |
| Clinical pregnancy rate | 56.4 (75/133) | 62.2 (51/82) | 57.1 (24/51) | 2.929 | .087 |
| First embryo transfer (n = 145), % | |||||
| Biochemical pregnancy rate | 61.4 (89/145) | 66.7 (62/93) | 51.9 (27/52) | 3.058 | .08 |
| Clinical pregnancy rate | 53.8 (78/145) | 60.2 (56/93) | 42.3 (22/52) | 4.303 | .038 |
Data are presented as % (n). OSA = obstructive sleep apnea.
Transfer outcomes in OSA of different severity
As shown in Table S3 (173.5KB, pdf) , the biochemical and clinical pregnancy rates of the first frozen embryo transfer and first embryo transfer for IVF decreased with OSA severity (first frozen embryo transfer clinical pregnancy rate 62.2% in the non-OSA group vs 51.7% in the mild-OSA group vs 40.9% in the moderate-severe OSA group; P = .172).
Clinical characteristics of nonpregnant patients
Sixty-seven (46.2%) patients failed their first clinical pregnancy with IVF. Their clinical characteristics are shown in Table S4 (173.5KB, pdf) . Patients with clinical pregnancy failure had longer infertility duration (4.2 ± 2.1 years vs 3.4 ± 2.3, P = .035), fewer oocytes retrieved (18.9 ± 8.8 vs 22.1 ± 10.5), and lower 2PN cleavage (10.2 ± 6.0 vs 12.2 ± 6.1). In addition, these patients tended to have higher BMI, apnea-hypopnea index, and percentage of time with oxygen saturation < 90% and lower 2PN zygotes and cleavage (percentage of time with oxygen saturation < 90%; 4.56 ± 12.48 vs 1.71 ± 3.97; P = .078).
Association between OSA and clinical outcomes in IVF
Finally, we constructed a binary logistic regression model of clinical pregnancy rate of first transfer (Table 4). In univariate logistic regression analysis, OSA and infertility duration were negatively correlated with clinical pregnancy rate. After adjusting for fresh embryo transfer, age, BMI, infertility duration, fasting blood glucose, and endometrial thickness, OSA was independently and negatively associated with clinical pregnancy rate (odds ratio = 0.379, 95% confidence interval: 0.148–0.969; P = .043). In addition, multiple linear regression analysis showed that E2 on the hCG injection day, total number of oocytes retrieved, high-quality embryos, and available embryos in patients with OSA were lower than those in patients without OSA, but the differences were no longer statistically significant after adjusting for age, BMI, infertility duration, fasting blood glucose, and endometrial thickness (Table 5).
Table 4.
Binary logistic regression model of clinical pregnancy rate.
| Variables | Univariable | Multivariable | ||||
|---|---|---|---|---|---|---|
| P | OR | 95% CI | P | OR | 95% CI | |
| OSA | 0.039 | 0.485 | 0.243–0.965 | 0.043 | 0.379 | 0.148–0.969 |
| Fresh embryo transfer | 0.273 | 0.652 | 0.304–1.400 | 0.132 | 0.491 | 0.194–1.238 |
| Age | 0.614 | 0.976 | 0.889–1.072 | 0.861 | 1.011 | 0.897–1.138 |
| BMI | 0.098 | 0.924 | 0.841–1.015 | 0.854 | 1.011 | 0.896–1.141 |
| Fasting glucose | 0.408 | 0.752 | 0.383–1.477 | 0.448 | 0.755 | 0.365–1.561 |
| Infertility duration | 0.040 | 0.848 | 0.725–0.992 | 0.126 | 0.850 | 0.691–1.047 |
| Endometrial thickness | 0.541 | 1.053 | 0.893–1.241 | 0.210 | 1.133 | 0.932–1.379 |
BMI = body mass index, CI = confidence interval, OR = odds ratio, OSA = obstructive sleep apnea.
Table 5.
Multiple linear regression model of OSA and potential mediators.
| Parameters | Unadjusted | Adjusted* | ||||
|---|---|---|---|---|---|---|
| B (95% CI) | Adj. R2 | P | B (95% CI) | Adj. R2 | P | |
| hCG injection day E2 | −982.1 (−1927.3 to −37.0) | 0.021 | 0.042 | −387.7 (−1619.7 to 844.3) | 0.004 | .534 |
| Total number of oocytes retrieved | −4.113 (−7.223 to −1.003) | 0.036 | 0.010 | −3.013 (−7.022 to 0.996) | 0.058 | .139 |
| No. of high-quality embryos | −1.640 (−3.122 to −0.157) | 0.024 | 0.030 | −0.609 (−2.505 to 1.288) | 0.070 | .526 |
| No. of available embryos | −2.177 (−3.754 to −0.600) | 0.040 | 0.007 | −1.495 (−3.480 to 0.491) | 0.076 | .139 |
Adjusted for age, body mass index, fasting glucose, infertility duration, endometrial thickness. Adj. = adjusted, CI = confidence interval, E2 = estradiol, hCG = human chorionic gonadotropin.
DISCUSSION
Here, the overall prevalence of OSA was 37.2% in consecutive patients with PCOS enrolled in the study, similar to a recent study from China reporting an overall prevalence of OSA in PCOS of 40%, most of whom were mild (70%).17 It is well known that sleep affects the secretion cycle of several female hormones including E2, progesterone, prolactin, LH, and FSH. Sleep disorders may have adverse effects on female health including fecundity.18 A small prospective cohort study found that female sleep-disordered breathing of any severity was associated with adverse reproductive events.19 This study further confirmed that OSA adversely affects female fertility and reduces the effectiveness of infertility interventions. The more severe the OSA, the more significant the decline in female fertility.
Our study showed that patients with OSA and PCOS had a lower LH, AMH, and AFC and also required a higher dosage of Gn and exhibited a lower E2 peak and fewer retrieved oocytes. These results indicate that OSA may impair the fertility potential of patients with PCOS by affecting the functional ovarian reserve, ovarian reactivity, and IVF cycle stimulation characteristics. However, we did not find a significant difference in endometrial thickness between the OSA and non-OSA groups, which may be due to sample size or the confounding effect of PCOS itself. Serum AMH is expressed by granulosa cells of growing follicles and is relatively stable throughout the menstrual cycle, reflecting a woman’s functional ovarian reserve.20 PCOS is characterized by a pathological increase in the number of follicles at all growth stages, and serum AMH levels in patients with PCOS are 2–4 times higher than in healthy women.21 Recent studies on PCOS showed that after controlling for confounders such as age, AMH was not related to clinical pregnancy rate but was related to the cycle stimulation characteristics. Patients with PCOS with higher AMH used less Gn, had a tendency to increase the peak of E2 on hCG day, and had more oocytes retrieved and higher-quality embryos.22 There was a positive correlation between LH and AMH levels in PCOS.23 LH and AMH levels were also significantly reduced in female rats exposed to intermittent hypobaric hypoxia, with significant pathological changes observed in the ovaries and uterus that could affect oogenesis and embryo implantation.24 The endometrial thickness on the hCG day is positively correlated with the level of E2, which could predict the clinical pregnancy rate. Indeed, when the endometrial thickness was less than 8 mm, there were fewer successful pregnancies.25 However, experimental and clinical data suggest that the endometrium differs in women with PCOS when compared to healthy controls.26 It is possible that OSA has no additional effect on endometrial thickness or that its effect is masked by the effect of PCOS.
We found that patients with OSA had a higher BMI, but there was no significant difference in fasting glucose levels between the two groups, perhaps due to the improved health of these patients with metformin prior to receiving IVF. Obesity, glucose dysregulation, and an imbalance in insulin homeostasis may be the pathological mechanisms underlying unsatisfactory reproductive outcomes in PCOS.27 IVF is the standard assisted reproduction protocol for patients with PCOS-related infertility after ovulation induction therapy has failed. However, even if ovulation disorder is circumvented through IVF, the embryo quality in PCOS is still worrying, especially for patients with a high BMI, where it is more difficult to achieve clinical pregnancy.27 A large prospective cohort study observed a clinical pregnancy rate of 58.7% in PCOS infertility patients undergoing frozen embryo transfer.28 Here, 75.3% (110/146) of patients underwent embryo cryopreservation followed by first-time frozen embryo transfer, and the clinical pregnancy rate was 56.4%.
The analysis indicated that the clinical pregnancy rate was significantly lower in the OSA group than in the non-OSA group, and this decreased with OSA severity. Moreover, OSA was independently and negatively associated with clinical pregnancy rate after adjusting for confounders such as age, BMI, and infertility duration (P = .043). Previous studies have shown a negative correlation between AFC and the clinical pregnancy rate following IVF. Patients with PCOS have a higher AFC but a lower clinical pregnancy rate than women with other causes of infertility.29 A previous large-scale retrospective study found that women with OSA were twice as likely to develop infertility than healthy women.30 Here, patients with OSA and PCOS had a lower AFC, and OSA further increased infertility in PCOS. In addition, PCOS oocytes with normal morphology have been shown to harbor genetic defects in meiosis and early embryo development, and the embryo quality of patients with PCOS is worse than that of normal women.31 In rat experiments, intermittent hypobaric hypoxia caused systemic oxidative stress and induced apoptosis of uterine and ovarian epithelial and granulosa cells.24 Furthermore, increased granulosa cell reactive oxygen species expression in PCOS induced apoptosis, further affecting oocyte quality and reducing pregnancy rates.32 We also found that patients who failed their first embryo transfer in IVF tended to have lower mean and minimum oxygen saturation at night and increased percentage of time with oxygen saturation < 90%, thus experiencing more severe nighttime hypoxia than patients who had a successful transfer. Given that oxidative stress is an important pathological feature of OSA,33 it is hypothesized that this may also be the cause of decreased female fertility. Further larger sample sizes must be studied to confirm these observations since, as a reversible risk factor affecting female fertility, recognition of the impact of OSA could improve fertility outcomes.
There were several limitations in this study. The sample size was relatively small, especially patients with severe OSA. The sample was from only one clinical site in one region of China, which limits generalizability, and only one night of OSA screening was performed. Another limitation was that we did not screen for OSA with Epworth Sleepiness Score or STOP-BANG score, which are commonly used tools to assess the risk of OSA. Also, it is possible that these findings do not apply to women with PCOS who are not undergoing IVF therapy who may get pregnant with other methodologies. Our study lacked detailed data on the patients’ medications before IVF, so we could not adjust for them as confounding factors.
CONCLUSIONS
In conclusion, OSA appears to have a negative impact on fertility in PCOS, highlighting the need to screen for OSA as a reversible risk factor affecting female fertility in infertile women with PCOS. The effectiveness of targeted treatments still requires further exploration.
DISCLOSURE STATEMENT
All authors have seen and approved the manuscript. All studies were conducted and completed at Renmin Hospital of Wuhan University. This research was supported by a grant from the National Natural Science Foundation of China (No. 82270101), chaired by Ke Hu; the other authors of the study declare no financial support. The authors report no conflicts of interest.
ACKNOWLEDGMENTS
The authors thank all the patients for participating in this study. The authors also thank all the research assistants at the Reproductive Medicine Center and Department of Respiratory and Critical Care Medicine, Renmin Hospital of Wuhan University for their assistance in data collection.
Author contributions: Z.W. and Q.Z. contributed to study design, data collection, data analysis, and drafting the manuscript. J.D. and S.Y. contributed to expertise and data collection. Y.H. and H.C. contributed to study design and data collection. J.Y. and K.H. contributed to correcting the manuscript, final approval, and obtaining funding.
ABBREVIATIONS
- 2PN
two pronuclei
- AFC
antral follicle count
- AMH
anti-Müllerian hormone
- BMI
body mass index
- E2
estradiol
- FSH
follicle-stimulating hormone
- Gn
gonadotropin
- hCG
human chorionic gonadotropin
- IVF
in vitro fertilization
- LH
luteinizing hormone
- OSA
obstructive sleep apnea
- PCOS
polycystic ovarian syndrome
REFERENCES
- 1. Patel SR . Obstructive sleep apnea . Ann Intern Med. 2019. ; 171 ( 11 ): ITC81 – ITC96 . [DOI] [PubMed] [Google Scholar]
- 2. Moran LJ, March WA, Whitrow MJ, Giles LC, Davies MJ, Moore VM . Sleep disturbances in a community-based sample of women with polycystic ovary syndrome . Hum Reprod. 2015. ; 30 ( 2 ): 466 – 472 . [DOI] [PubMed] [Google Scholar]
- 3. Kumarendran B, Sumilo D, O’Reilly MW, et al . Increased risk of obstructive sleep apnoea in women with polycystic ovary syndrome: a population-based cohort study . Eur J Endocrinol. 2019. ; 180 ( 4 ): 265 – 272 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kahal H, Kyrou I, Uthman OA, et al . The prevalence of obstructive sleep apnoea in women with polycystic ovary syndrome: a systematic review and meta-analysis . Sleep Breath. 2020. ; 24 ( 1 ): 339 – 350 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Legro RS, Arslanian SA, Ehrmann DA, Hoeger KM, Murad MH, Pasquali R, Welt CK ; Endocrine Society . Diagnosis and treatment of polycystic ovary syndrome: an Endocrine Society clinical practice guideline . J Clin Endocrinol Metab. 2013. ; 98 ( 12 ): 4565 – 4592 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Conway G, Dewailly D, Diamanti-Kandarakis E, et al. ESE PCOS Special Interest Group . The polycystic ovary syndrome: a position statement from the European Society of Endocrinology . Eur J Endocrinol. 2014. ; 171 ( 4 ): 1 – 29 . [DOI] [PubMed] [Google Scholar]
- 7. Balen AH, Morley LC, Misso M, et al . The management of anovulatory infertility in women with polycystic ovary syndrome: an analysis of the evidence to support the development of global WHO guidance . Hum Reprod Update. 2016. ; 22 ( 6 ): 687 – 708 . [DOI] [PubMed] [Google Scholar]
- 8. Sha T, Wang X, Cheng W, Yan Y . A meta-analysis of pregnancy-related outcomes and complications in women with polycystic ovary syndrome undergoing IVF . Reprod Biomed Online. 2019. ; 39 ( 2 ): 281 – 293 . [DOI] [PubMed] [Google Scholar]
- 9. Kloss JD, Perlis ML, Zamzow JA, Culnan EJ, Gracia CR . Sleep, sleep disturbance, and fertility in women . Sleep Med Rev. 2015. ; 22 : 78 – 87 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Goldstein CA, Lanham MS, Smith YR, O’Brien LM . Sleep in women undergoing in vitro fertilization: a pilot study . Sleep Med. 2017. ; 32 : 105 – 113 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pimolsri C, Lyu X, Goldstein C, et al . Objective sleep duration and timing predicts completion of in vitro fertilization cycle . J Assist Reprod Genet. 2021. ; 38 ( 10 ): 2687 – 2696 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Philipsen MT, Knudsen UB, Zachariae R, Ingerslev HJ, Hvidt JEM, Frederiksen Y . Sleep, psychological distress, and clinical pregnancy outcome in women and their partners undergoing in vitro or intracytoplasmic sperm injection fertility treatment . Sleep Health. 2022. ; 8 ( 2 ): 242 – 248 . [DOI] [PubMed] [Google Scholar]
- 13. He Y, Lu Y, Zhu Q, et al . Influence of metabolic syndrome on female fertility and in vitro fertilization outcomes in PCOS women . Am J Obstet Gynecol. 2019. ; 221 ( 2 ): 138.e1 – 138.e12 . [DOI] [PubMed] [Google Scholar]
- 14. Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group . Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome . Fertil Steril. 2004. ; 81 ( 1 ): 19 – 25 . [DOI] [PubMed] [Google Scholar]
- 15. Berry RB, Budhiraja R, Gottlieb DJ, et al. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine . Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events . J Clin Sleep Med. 2012. ; 8 ( 5 ): 597 – 619 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wei Z, Xu J, Li W, Wang X, Qin Z, Zhou J, Wang W . Evaluation of a non-contact ultra-wideband bio-radar sleep monitoring device for screening of sleep breathing disease . Sleep Breath. 2022. ; 26 ( 2 ): 689 – 696 . [DOI] [PubMed] [Google Scholar]
- 17. Yang R, Gao C, Yan Y, et al . Analysis of the proportion and clinical characteristics of obstructive sleep apnea in women with polycystic ovary syndrome . Sleep Breath. 2022. ; 26 ( 1 ): 497 – 503 . [DOI] [PubMed] [Google Scholar]
- 18. Lee EK, Gutcher ST, Douglass AB . Is sleep-disordered breathing associated with miscarriages? An emerging hypothesis . Med Hypotheses. 2014. ; 82 ( 4 ): 481 – 485 . [DOI] [PubMed] [Google Scholar]
- 19. Walter JR, Lee JY, Snoll B, Park JB, Kim DH, Xu S, Barnhart K . Pregnancy outcomes in infertility patients diagnosed with sleep disordered breathing with wireless wearable sensors . Sleep Med. 2022. ; 100 : 511 – 517 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Moolhuijsen LME, Visser JA . Anti-Müllerian hormone and ovarian reserve: update on assessing ovarian function . J Clin Endocrinol Metab. 2020. ; 105 ( 11 ): 3361 – 3373 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dumont A, Robin G, Dewailly D . Anti-Müllerian hormone in the pathophysiology and diagnosis of polycystic ovarian syndrome . Curr Opin Endocrinol Diabetes Obes. 2018. ; 25 ( 6 ): 377 – 384 . [DOI] [PubMed] [Google Scholar]
- 22. Liu S, Hong L, Mo M, et al . Association of anti-Müllerian hormone with polycystic ovarian syndrome phenotypes and pregnancy outcomes of in vitro fertilization cycles with fresh embryo transfer . BMC Pregnancy Childbirth. 2022. ; 22 ( 1 ): 171 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Piouka A, Farmakiotis D, Katsikis I, Macut D, Gerou S, Panidis D . Anti-Mullerian hormone levels reflect severity of PCOS but are negatively influenced by obesity: relationship with increased luteinizing hormone levels . Am J Physiol Endocrinol Metab. 2009. ; 296 ( 2 ): E238 – E243 . [DOI] [PubMed] [Google Scholar]
- 24. Liu W, Pu L, Deng B, et al . Intermittent hypobaric hypoxia causes deleterious effects on the reproductive system in female rats . Biomed Pharmacother. 2020. ; 130 : 110511 . [DOI] [PubMed] [Google Scholar]
- 25. Liu KE, Hartman M, Hartman A, Luo ZC, Mahutte N . The impact of a thin endometrial lining on fresh and frozen-thaw IVF outcomes: an analysis of over 40 000 embryo transfers . Hum Reprod. 2018. ; 33 ( 10 ): 1883 – 1888 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Palomba S, Piltonen TT, Giudice LC . Endometrial function in women with polycystic ovary syndrome: a comprehensive review . Hum Reprod Update. 2021. ; 27 ( 3 ): 584 – 618 . [DOI] [PubMed] [Google Scholar]
- 27. Gupta S, Sinha A . Is in vitro fertilization the answer in polycystic ovary syndrome? Fertil Steril. 2021. ; 115 ( 2 ): 330 – 331 . [DOI] [PubMed] [Google Scholar]
- 28. Chen ZJ, Shi Y, Sun Y, et al . Fresh versus frozen embryos for infertility in the polycystic ovary syndrome . N Engl J Med. 2016. ; 375 ( 6 ): 523 – 533 . [DOI] [PubMed] [Google Scholar]
- 29. Liao S, Xiong J, Tu H, et al . Prediction of in vitro fertilization outcome at different antral follicle count thresholds combined with female age, female cause of infertility, and ovarian response in a prospective cohort of 8269 women . Medicine (Baltimore). 2019. ; 98 ( 41 ): e17470 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lim ZW, Wang ID, Wang P, et al . Obstructive sleep apnea increases risk of female infertility: a 14-year nationwide population-based study . PLoS One. 2021. ; 16 ( 12 ): e0260842 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wood JR, Dumesic DA, Abbott DH, Strauss JF 3rd . Molecular abnormalities in oocytes from women with polycystic ovary syndrome revealed by microarray analysis . J Clin Endocrinol Metab. 2007. ; 92 ( 2 ): 705 – 713 . [DOI] [PubMed] [Google Scholar]
- 32. Lai Q, Xiang W, Li Q, et al . Oxidative stress in granulosa cells contributes to poor oocyte quality and IVF-ET outcomes in women with polycystic ovary syndrome . Front Med. 2018. ; 12 ( 5 ): 518 – 524 . [DOI] [PubMed] [Google Scholar]
- 33. Lavie L . Oxidative stress—a unifying paradigm in obstructive sleep apnea and comorbidities . Prog Cardiovasc Dis. 2009. ; 51 ( 4 ): 303 – 312 . [DOI] [PubMed] [Google Scholar]