Abstract
A strong and specific comprehensive physiological association has been documented between mandibular jaw movements and related periods of normal or disturbed breathing across different sleep stages. The mandibular jaw movement biosignal can be incorporated in the polysomnography, displayed on the screen as a function of time like any standard polysomnography signal (eg, airflow, oxygen saturation, respiratory inductance plethysmography bands) and interpreted in the context of the target period of breathing and its associated respiratory effort level. Overall, the mandibular jaw movement biosignal that depicts the muscular trigeminal respiratory drive is a highly effective tool for differentiating between central and obstructive sleep episodes including hypopneas and for providing clinicians with valuable insights into wake/sleep states, arousals, and sleep stages. These fundamental characteristics of the mandibular jaw movement biosignal contrast with photoplethysmography, airflow, or oxygen saturation signals that provide information more about the consequence of the disturbed breathing episode than about the event itself.
Citation:
Malhotra A, Martinot J-B, Pépin J-L. Insights on mandibular jaw movements during polysomnography in obstructive sleep apnea. J Clin Sleep Med. 2024;20(1):151–163.
Keywords: mandibular jaw movements, obstructive sleep apnea, sleep staging, sleep-disordered breathing
BRIEF SUMMARY
Current Knowledge/Study Rationale: The aim of this review is to show that mandibular jaw movement monitoring, utilizing a single point of contact sensor positioned on the patient’s chin, enables accurate measurement of changes in respiratory effort level across different sleep stages and types of respiratory events. The article seeks to provide sleep practitioners with a comprehensive understanding of the significance of mandibular jaw movements as a reliable and informative tool for characterizing breathing disturbances during sleep, as compared to conventional polysomnography signals.
Study Impact: By establishing a correlation between mandibular jaw movements and respiratory effort, and making therefore possible the differentiation between obstructive and central events, mandibular jaw movements have the potential to significantly improve diagnostic accuracy in individuals suspected of having obstructive sleep apnea. Equipping practitioners with this knowledge will enable them to make well-informed decisions and implement personalized interventions, leading to more effective management of obstructive sleep apnea.
INTRODUCTION
The crucial role of sleep mandibular jaw movements (MJM) in stabilizing the pharynx and maintaining the openness of the upper airway is widely recognized.1–5 These movements exhibit specific amplitude and frequency patterns across different sleep states and stages.6 Studies have demonstrated that MJM provide valuable insights into the respiratory drive, variations in respiratory effort, and the cumulative burden of respiratory effort during abnormal respiratory events.6–9 Additionally, the accurate measurement of MJM allows for precise differentiation between obstructive and central events.7–9 Through the development of a robust technology positioned on the chin,10 overnight monitoring of MJM has become possible, providing a comprehensive biosignal that can be interpreted like conventional polysomnography channels (Figure 1). This technology proves instrumental in facilitating the diagnosis of sleep-disordered breathing.10–13
Figure 1. Interpretation of mandibular jaw movement (MJM) biosignal.
This 30-second fragment (zoomed part) displays six respiratory cycles. The ventilatory displacement is measured using thoracic and abdominal respiratory inductance plethysmography (RIP) belts, nasal pressure airflow, and thermal sensors. Additionally, mandibular jaw’s rotational movement and position are respectively acquired by the gyroscope and accelerometer embedded in the inertial unit of the capturing technology. When MJM biosignals are displayed in parallel with simultaneously recorded standard polysomnography channels, they oscillate at the breathing frequency, precisely matching the airflow and respiratory effort signals. One inspiratory phase is marked by a green vertical line, demonstrating changes in rotational movement (positive in channels GyrX and GyrY and negative in channel GyrZ). Simultaneously, the mandible undergoes a displacement of a few tenths of a millimeter, as indicated by channels AccX and AccZ. In addition, an arrow highlights a notch in the rotational movement waveform during the expiratory phase of the indicated cycle. This observation suggests that the physiological displacement velocity may transiently vary based on the stability of the central drive or local anatomical conditions.
Signals from the top to the bottom: pulsed oxygen saturation (SpO2), thoracic and abdominal respiratory inductance plethysmography (RIP Thx, RIP Abd), nasal airflow pressure (FlowPr), oronasal thermal flow (FlowTh), mandibular jaw movements captured by the gyroscope (MJM GyrX, GyrZ, GyrY) and the accelerometer (MJM AccX, AccZ).
REVIEW
Background and modes of signal collection
Specific waveforms, frequencies, and amplitudes of MJM are seen across a variety of breathing disturbances that are recommended to be scored by the American Academy of Sleep Medicine. In particular, recent studies have shown that monitoring stereotypical MJM during sleep-disordered breathing can accurately predict the hourly indices of obstructive respiratory disturbances and more specifically of apneas/hypopneas.10–12 MJM also differ in amplitude and frequency depending on the sleep stage.13
The automated algorithmic MJM analysis has made possible the interpretation of these movements at home over multiple nights,10–13 which is becoming a recommended practice for diagnosing sleep apnea. However, to understand better this new stand-alone MJM biosignal, we have incorporated this new biosignal into polysomnography (PSG) fragments after clocks synchronizing. This approach enables visualization of PSG fragments with MJM biosignals recorded simultaneously, allowing for comparison between conventional PSG signals such as airflow or oxygen saturation and MJM during sleep normal and disturbed breathing.6
The episodes illustrated in this manuscript were gathered from patients undergoing type 1 PSG in a laboratory setting, utilizing a Somnoscreen Plus acquisition system. Various parameters were monitored, including electroencephalogram recordings, electrooculogram, submental electromyography (EMG), respiratory motion measurements via respiratory inductance plethysmography (RIP) (SleepSense; SLP, Elgin, IL, USA), and measurements of nasal and oral airflow using a pressure transducer and thermistor, respectively. Oxygen saturation levels were also recorded using a digital oximeter (Nonin; Nonin Medical, Plymouth, MN, USA), and MJM were captured through a lightweight, single point of contact sensor placed on the patient’s chin and controlled externally via a smartphone application (Sunrise, Namur, Belgium). The embedded inertial measurement unit of the sensor includes a gyroscope and an accelerometer that measure along their three axes (X, Y, Z) the rotational movement and position of the mandible, respectively, providing a total of six derived MJM channels (GyrX, GyrY, GyrZ, AccX, AccY, AccZ). The rotational movement captured by the gyroscope is produced by rotation of the mandibular condyle in the temporo-mandibular joint. We have chosen to present on each figure the most relevant derived MJM channel(s) for the gyroscope and for the accelerometer in comparison to the other PSG signals. It is important to note that all six derived MJM channels may not be equally informative simultaneously due to variations in head and jaw positions. The informativeness of the derived MJM channels will indeed depend on how an individual is moving or positioning their head and jaw, with these factors influencing the amplitude of the vectors along the X, Y, and Z axes of the gyroscope and accelerometer that is used to compute the derived MJM signals.
Detailed information about the validity of automated MJM analysis is provided in the supplementary material (455.5KB, pdf) .
Physiology of sleep mandibular jaw movements
The upper airway muscles are involved in complex interactions that prioritize or combine breathing, swallowing, speech, and masticatory activities. The mandibular jaw plays a central role in all these oro-facial functions. Consequently, awake MJM exhibit high variability and speed and are unpredictable in amplitude and frequency.
Under normal sleep circumstances, the mandibular jaw undergoes slight movements around a fixed position, and the mouth remains nearly closed. Only a minor physiological displacement of a few tenths of millimeters, linked to the respiratory cycle and regulated by the respiratory centers, takes place during regular sleep (Figure 1). This rotational movement is generated by the rotation of the mandibular condyle within the temporomandibular joint.
During sleep, the patency of the upper airway relies on a delicate dynamic balance, with the mandibular jaw acting as a lever to stabilize and strengthen the pharyngeal walls. It is indeed crucial for the pharynx to stiffen before diaphragmatic contraction, as the accompanying decrease in airway pressure can destabilize pharyngeal caliber. This negative pressure reflex likely contributes to the recruitment of trigeminal motoneurons and the resulting MJM. In particular, MJM during sleep occur at frequencies between 0.15 to 0.60 Hz, suggesting that they are primarily driven by the brainstem, specifically by the motor nucleus of the motor branch of the trigeminal nerve located in the midpons.2,3 This branch of the fifth cranial nerve provides motor nerves to the mandible’s agonist and antagonist muscles. The so-called cyclical breathing activity of the mandible4,5 begins when non-rapid eye movement stage N1 light sleep is detected on the electroencephalogram, based on a specific ratio of theta/alpha waves. The observed tight connectivity between the motor trigeminal nerves and the respiratory centers in the brainstem explains these phenomena. Similar connections are well known between the hypoglossal nucleus, responsible for tongue movements during sleep, and the spinal nuclei controlling the diaphragm and other accessory respiratory muscles.
The innovative sleep monitoring technology illustrated here utilizes MJM to capture the physiological displacement linked to breathing cycles, measuring changes in respiratory effort across various sleep disorders but also identifying sleep/wake states, arousals, and sleep stages.13
In-depth studies have explored the association between MJM biosignal properties and both physiological and pathological breathing patterns during sleep. These studies have demonstrated a close relationship between MJM biosignal amplitudes and changes in respiratory effort, correlating with endoesophageal diaphragmatic EMG activity signal estimates. It has been shown that MJM amplitudes approximately increase by 0.28 mm for each 10 µV of increase in EMG activity and that variations in MJM amplitudes reflect patterns observed with EMG activity, differentiating normal breathing, obstructive, mixed, and central apnea periods.7 In addition, a recent study validated the MJM biosignal captured by Sunrise as a highly reliable and noninvasive surrogate of the esophageal pressure gold-standard signal to measure respiratory effort during sleep.8
MJM amplitudes provide efficient differentiation between central and obstructive events and clearly identify mixed episodes with obstructive events characterized by higher MJM amplitudes across all quartiles and a wider range of amplitudes than central events. The high level of agreement between the esophageal pressure and the MJM biosignal sequences emphasizes the reliability of the MJM capturing technology with median similarity scores of 0.82–0.99.8 The MJM respiratory biosignal can be read and interpreted like the esophageal pressure during the prearousal period. The respective changes of their amplitudes at the breathing frequency across the normal or different disturbed respiratory cycles of obstructive or central nature are very similar (Figure 2 and inset). By contrast, the esophageal pressure signal on arousal normalizes and does not reflect the intensity of the muscular respiratory drive compared to brisk and abrupt MJM indicating the closure of the mouth on arousal (see the arrow in Figure 2).
Figure 2. Typical obstructive breathing cycles highlighted with mandibular jaw movement (MJM) changes.
The MJM and esophageal pressure signals clearly illustrate changes in breathing patterns, indicating increased respiratory effort during breathing cycles coinciding with inspiratory flow limitation, respiratory inductance plethysmography band phase shift, and snoring. The nasal airflow pressure is marked by vibrations in relation with snoring. Despite arousal (see the arrow), inspiratory flow limitation persists in this fragment, strongly suggesting that the pharynx is not fully reopened at this moment. The MJM signal mimics the changes observed in esophageal pressure during the respiratory cycle (see the inset). These changes are characterized by increasing peak-to-peak amplitudes of the MJM signal while the esophageal pressure becomes more and more negative. This trend continues until an arousal event takes place.
Signals from the top to the bottom: pulsed oxygen saturation (SpO2), thoracic and abdominal respiratory inductance plethysmography (RIP Thx, RIP Abd), nasal airflow pressure (FlowPr), oronasal thermal flow (FlowTh), snore, mandibular jaw movements captured by the gyroscope (MJM Gyr) and the accelerometer (MJM Acc), esophageal pressure signal (POES), electroencephalography derivation C4A1. Arousal is marked with the red arrow. Indications about sleep/wake state or sleep stage is reported as well as the hour and the duration of the polysomnography fragment.
Obstructive compared to central sleep-disturbed breathing episodes in PSG supplemented with mandibular jaw movements
During obstructive events, the intensity or magnitude of MJM can offer valuable insights into the level of respiratory effort exerted by an individual to overcome airway obstruction. The stereotypical sequence of an obstructive apnea/hypopnea event or respiratory effort related arousals consists of a period of increasing respiratory drive reflected by an increasing peak to peak amplitude of the rotational MJM that oscillates at the breathing frequency followed by a prominent displacement of the jaw (up and down movement closing the mouth) often linked to arousal depicted by the accelerometer MJM biosignal (Figure 3 and Video 1 (8MB, wmv) in the supplemental material).
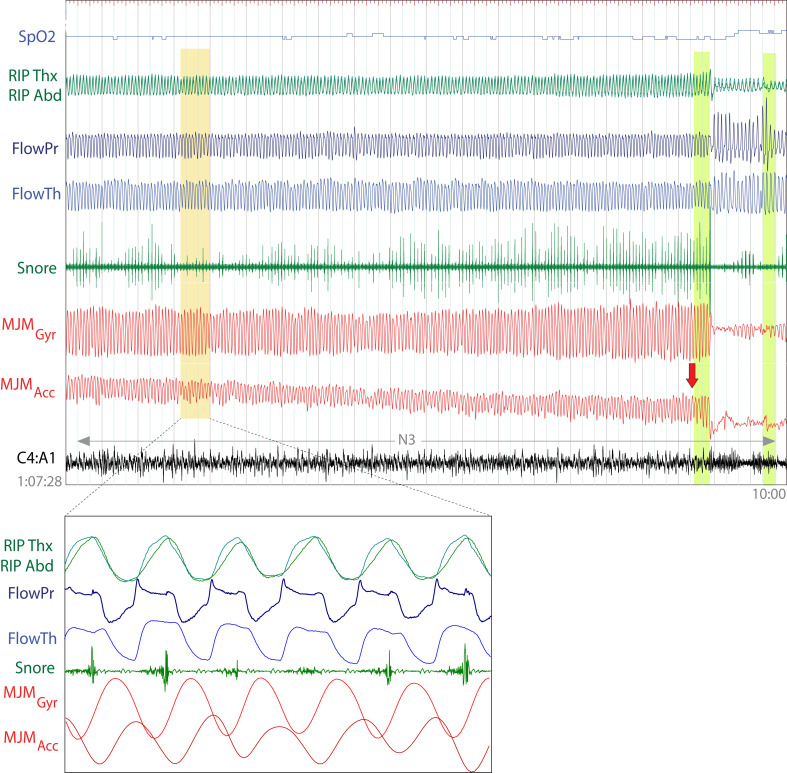
Figure 3. Mandibular jaw movement (MJM) patterns during successive obstructive hypopneas.
Successive episodes of obstructive hypopneas that end in an awakening are evident, followed by a central apnea. The awakening (yellow rectangle) is clearly indicated by large, irregular MJM that are no longer repeated at the prearousal respiratory frequency. It is worth noting that the abdominal respiratory inductance plethysmography in this obese patient is occasionally altered.
Signals from the top to the bottom: pulsed oxygen saturation (SpO2), thoracic and abdominal respiratory inductance plethysmography (RIP Thx, RIP Abd), nasal airflow pressure (FlowPr), oronasal thermal flow (FlowTh), snore, mandibular jaw movements captured by the gyroscope (MJM Gyr) and the accelerometer (MJM Acc), electroencephalography derivation C4A1. Vertical green bars indicate an arousal. Indications about sleep/wake state or sleep stage is reported as well as the hour and the duration of the polysomnography fragment.
During wake periods, there are irregularities in the respiratory frequency and large changes in MJM amplitude are observed (Figure 3). When an individual is asleep, the MJM amplitude is a function of the respiratory drive amplitude rather than the upper airway resistance. The MJM amplitude decreases during central events and increases during obstructive episodes, regardless of the degree of upper airway resistance (Figure 3 and Figure 4). The airflow amplitude decreases during central events as a function of the residual respiratory drive, while it decreases during classic obstructive sleep apnea or increases during the hyperventilation phase of a period of Cheyne-Stokes breathing as a function of the degree of upper airway resistance, although the muscular respiratory drive highlighted by MJM augments (Figure 5 and Figure 6).
Figure 4. Differentiating mandibular jaw movements (MJM) between obstructive and central sleep-disturbed breathing.
Left segment (1 minute): The MJM amplitude (MJM Gyr) increases during a period of intraevent negative effort dependence, which indicates a decrease in flow signal during the second part of inspiration, followed by a peak in termination (see the vertical blue). This signal association clearly demonstrates how much the respiratory effort, as shown by MJM, increases while the intraluminal pressure decreases and suctions the pharyngeal walls. The more negative the accelerometric signal, the wider the mouth opens until an arousal occurs, closing the mouth with a mandibular jaw prominent displacement and peaking the flow (see the dotted line and the horizontal arrow respectively). It is noteworthy that during this period of increased effort, the respiratory inductance plethysmography bands remain in phase.
Right segment (2 minutes, 20 seconds): Central apneas are characterized by a pronounced decrease in MJM amplitude. By contrast, arousals are well depicted by the MJMAcc channel. The remaining small and irregular displacements can be attributed to the signal-to-noise ratio (see red arrows).
Signals from the top to the bottom: pulsed oxygen saturation (SpO2), thoracic and abdominal respiratory inductance plethysmography (RIP Thx, RIP Abd), nasal airflow pressure (FlowPr), oronasal thermal flow (FlowTh), snore, mandibular jaw movements captured by the gyroscope (MJM Gyr) and the accelerometer (MJM Acc), electroencephalography derivation C4A1. Vertical green bars indicate an arousal. Indications about sleep/wake state or sleep stage is reported as well as the hour and the duration of the polysomnography fragment.
Figure 5. Mandibular jaw movements (MJM) during a period of Cheyne-Stokes periodic breathing.
This fragment illustrates a typical period of Cheyne-Stokes breathing, as evidenced by the MJM signal and the intermixed central apnea and hypopnea (see A and H vertical arrows, respectively). The change in the trigeminal muscular drive amplitude is well reflected in corresponding changes in airflow and ventilation. A cortical arousal is indicated during the hyperventilation period and is accompanied by a change in the MJM morphology (see the horizontal arrow).
Signals from the top to the bottom: pulsed oxygen saturation (SpO2), thoracic and abdominal respiratory inductance plethysmography (RIP Thx, RIP Abd), nasal airflow pressure (FlowPr), oronasal thermal flow (FlowTh), snore, mandibular jaw movements captured by the gyroscope (MJM Gyr) and the accelerometer (MJM Acc), electroencephalography derivation C4A1. The vertical green bar indicates an arousal. Indications about sleep/wake state or sleep stage is reported as well as the hour and the duration of the polysomnography fragment.
Figure 6. Changes in respiratory drive amplitudes and arousal impact on mandibular jaw movements (MJM).
This 5-minute fragment demonstrates the effect of a cortical arousal on respiratory flow, which peaks with a high respiratory drive, as seen in the MJMGyr channel, while the mouth closes, as seen in the MJMAcc channel (see the arrow). Subsequently, respiratory effort gradually increases without normalizing inspiratory flow limitation. In the middle part of the fragment, upper airway resistances remain elevated and trigeminal respiratory drive is increased, but to a lesser extent than during the first arousal. The changes in MJM amplitudes across these successive obstructive episodes are clearly delineated.
Signals from the top to the bottom: pulsed oxygen saturation (SpO2), thoracic and abdominal respiratory inductance plethysmography (RIP Thx, RIP Abd), nasal airflow pressure (FlowPr), oronasal thermal flow (FlowTh), snore, mandibular jaw movements captured by the gyroscope (MJM Gyr) and the accelerometer (MJM Acc), electroencephalography derivation C4A1. The vertical green bar indicates an arousal. Indications about sleep/wake state or sleep stage is reported as well as the hour and the duration of the polysomnography fragment.
Differentiating hypopneas
The MJM analysis is highly effective in differentiating between central and obstructive hypopneas during routine PSG, highlighting the power of this biosignal.9
A hypopnea is a common respiratory event during sleep, and correctly characterizing its subtype as either obstructive or central can provide valuable information about its origin and aid in personalized therapeutic decision-making.14–17
While previous efforts have focused on using routine PSG signals to characterize hypopnea better, the MJM analysis reproducibly and reliably identifies respiratory effort in patients being evaluated for suspected obstructive sleep apnea. By considering an ensemble of readable and comprehensible features with regard to routine signals used during PSG for assessing respiratory effort, hypopneas can be accurately characterized as either obstructive or central (Figure 7 and Figure 8).
Figure 7. Respiratory effort across different obstructive episodes well depicted by the mandibular jaw movement biosignal.
The recording shows a four-minute period of increased respiratory effort terminated by a cortical arousal, followed by two successive obstructive apneas. The increased respiratory effort is well demonstrated by a shift in phase in the thoraco-abdominal movements recorded by respiratory inductance plethysmography, inspiratory flow limitation at the nasal cannula, and inspiratory snoring.
Signals from the top to the bottom: pulsed oxygen saturation (SpO2), thoracic and abdominal respiratory inductance plethysmography (RIP Thx, RIP Abd), nasal airflow pressure (FlowPr), oronasal thermal flow (FlowTh), snore, mandibular jaw movements captured by the gyroscope (MJM Gyr) and the accelerometer (MJM Acc), electroencephalography derivation C4A1. Vertical green bars indicate an arousal. Indications about sleep/wake state or sleep stage is reported as well as the hour and the duration of the polysomnography fragment.
Figure 8. Mandibular jaw movements during non-rapid eye movement (NREM) and rapid eye movement (REM) central hypopneas.
Left segment: Typical central hypopneas during a two-minute period of N2 stage with a mild mouth opening while the motor trigeminal drive addressed to the elevators of the mandible is decreased (see vertical arrows).
Right segment: Typical central hypopneas during a period of REM sleep: the arrow A indicates the trigeminal drive tendency to decrease from one cycle to the other strongly suggesting that the episode is of central nature; the breathing cycles B and C are marked with a mild rebound in the drive, which is well depicted with an intraevent negative effort dependence pattern at the nasal pressure signal.
Signals from the top to the bottom: pulsed oxygen saturation (SpO2), thoracic and abdominal respiratory inductance plethysmography (RIP Thx, RIP Abd), nasal airflow pressure (FlowPr), oronasal thermal flow (FlowTh), snore, mandibular jaw movements captured by the gyroscope (MJM Gyr) and the accelerometer (MJM Acc), electroencephalography derivation C4A1. Vertical green bars indicate an arousal. Indications about sleep/wake state or sleep stage is reported as well as the hour and the duration of the polysomnography fragment.
A comparative analysis of the surface EMG activities and MJM biosignals recorded concomitantly is shown in Figure 9. This fragment highlights the intricate contributions of masticatory muscles responsible for jaw closure and opening. It helps connect the dots between how the muscles work and how the mandibular jaw moves. This connection is significant and can give us a better understanding of the respiratory disturbance dynamics. MJM recordings provide clinicians with valuable insights into the patient’s work of breathing, including patterns of negative effort dependence (Figure 6), long sustained periods of respiratory effort (Figure 10), and periodic breathing (Figure 5 and Figure 11).18,19 Events during classic or more drive-dependent obstructive sleep apnea can also be well illustrated with MJM recordings (Figure 2 and Figure 7). Mixed episodes are well delineated with MJM (Figure 12). Additionally, the MJM analysis is capable of detecting central hypopneas during rapid eye movement sleep as well as obstructive hypopneas of short duration scored in the absence of cortical arousal or only in the presence of a minimal 3% pulsed oxygen saturation desaturation (Figure 8 and Figure 13).
Figure 9. Mandibular jaw movement patterns in comparison with electromyography (EMG) activities during obstructive hypopnea.
The figure provides a clear representation of the changes in respiratory effort during an episode of obstructive hypopnea, along with the corresponding changes in surface EMG activities of the masseter and submental muscles. The episode terminates with an increase in the EMG activity of elevator muscles when an arousal occurs and the flow peaks.
Signals from the top to the bottom: pulsed oxygen saturation (SpO2), thoracic and abdominal respiratory inductance plethysmography (RIP Thx, RIP Abd), nasal airflow pressure (FlowPr), oronasal thermal flow (FlowTh), snore, mandibular jaw movements captured by the gyroscope (MJM Gyr) and the accelerometer (MJM Acc), electroencephalography derivation C4A1, surface EMG activities of the left (L) and right (R) submental and masseter muscles. Indications about sleep/wake state or sleep stage is reported as well as the hour and the duration of the polysomnography fragment.
Figure 10. A 10-minute period of sustained respiratory effort illustrated with mandibular jaw movements.
The recording shows a prolonged period of sustained respiratory effort lasting 10 minutes, during which the respiratory inductance plethysmography belts remain synchronous. A pattern of negative effort dependence is indicated at the nasal cannula with snoring. An increase in respiratory drive is well demonstrated by an increase in the rotational movement of the mandibular jaw with a mild mouth opening over a distance of 1.6 mm at the level of the arrow. This period ends with a cortical arousal.
Signals from the top to the bottom: pulsed oxygen saturation (SpO2), thoracic and abdominal respiratory inductance plethysmography (RIP Thx, RIP Abd), nasal airflow pressure (FlowPr), oronasal thermal flow (FlowTh), snore, mandibular jaw movements captured by the gyroscope (MJM Gyr) and the accelerometer (MJM Acc), electroencephalography derivation C4A1. Vertical green bars indicate an arousal. Indications about sleep/wake state or sleep stage is reported as well as the hour and the duration of the polysomnography fragment.
Figure 11. Periodic respiration associated with opioids outlined with mandibular jaw movements.
Biot’s respiration, characterized by an unpredictable breathing pattern, differs from Cheyne-Stokes breathing and involves central hypopneas or apneas of varying durations highlighted by yellow or pink squares, respectively. Additionally, in Biot’s respiration, ventilatory recoveries occur with variable tidal volumes.
Signals from the top to the bottom: pulsed oxygen saturation (SpO2), thoracic and abdominal respiratory inductance plethysmography (RIP Thx, RIP Abd), nasal airflow pressure (FlowPr), oronasal thermal flow (FlowTh), snore, mandibular jaw movements captured by the gyroscope (MJM Gyr) and the accelerometer (MJM Acc), electroencephalography derivation C4A1. Indications about sleep/wake state or sleep stage is reported as well as the hour and the duration of the polysomnography fragment.
Figure 12. Mandibular jaw movements during mixed apneas.
During the initial phase of apnea, an augmented respiratory effort is absent, leading to a period of reduced or no breathing. Subsequently, both the spinal (diaphragm) and trigeminal drives intensify, contributing to an increase in respiratory effort. This intensification continues until an arousal event takes place, leading eventually to a full awakening.
Signals from the top to the bottom: pulsed oxygen saturation (SpO2), thoracic and abdominal respiratory inductance plethysmography (RIP Thx, RIP Abd), nasal airflow pressure (FlowPr), oronasal thermal flow (FlowTh), snore, mandibular jaw movements captured by the gyroscope (MJM Gyr) and the accelerometer (MJM Acc), electroencephalography derivation C4A1. Indications about sleep/wake state or sleep stage is reported as well as the hour and the duration of the polysomnography fragment.
Figure 13. Rules for hypopnea scoring illustrated with mandibular jaw movements (MJM).
This fragment highlights the occurrence of short episodes of obstructive hypopneas that are scored in the absence of arousal but with a drop of 3% or 4% in SpO2 (see arrows A).
In contrast, another obstructive hypopnea is scored at arrow B only in the presence of an arousal. These hypopneas are characterized by inspiratory flow limitations, with or without inspiratory time prolongation. These flow limitations are clearly observed, accompanied by an increase in the MJM amplitude. Arrows C indicate hypopneas scored with both SpO2 drop and cortical arousal. While the shift phase of the respiratory inductance plethysmography bands remains minimal, the indicated hypopneas are of obstructive nature, suggesting partial or temporary blockages in the upper airway well depicted by the changes in the trigeminal drive.
Signals from the top to the bottom: pulsed oxygen saturation (SpO2), thoracic and abdominal respiratory inductance plethysmography (RIP Thx, RIP Abd), nasal airflow pressure (FlowPr), oronasal thermal flow (FlowTh), snore, mandibular jaw movements captured by the gyroscope (MJM Gyr) and the accelerometer (MJM Acc), electroencephalography derivation C4A1. Vertical green bars indicate an arousal. Indications about sleep/wake state or sleep stage is reported as well as the hour and the duration of the polysomnography fragment.
Nonrespiratory sleep mandibular jaw movements
The occurrence of rhythmic masticatory muscles activities in sleep bruxism does not alter the interpretation of MJM, as the typical sequence linking sleep-disordered breathing and rhythmic masticatory muscles activities can be characterized by stereotypical MJM that can be visually and algorithmically detected (Figure 14).20,21 Other displacements of the mandible during sleep such as swallowing (Figure 15) or suckling (Figure 16) are clearly distinguishable and more pronounced in comparison to its respiratory movements.
Figure 14. Mandibular jaw movement patterns of rhythmic masticatory muscle activity (RMMA).
The respiratory activity of the mandibular jaw is interrupted by a period of RMMA depicted by mandibular jaw movements around 1 Hz and of high amplitude (see blue arrows) accompanying an arousal, which terminates a period of obstructive event.
Signals from the top to the bottom: pulsed oxygen saturation (SpO2), thoracic and abdominal respiratory inductance plethysmography (RIP Thx, RIP Abd), nasal airflow pressure (FlowPr), oronasal thermal flow (FlowTh), snore, mandibular jaw movements captured by the gyroscope (MJM Gyr) and the accelerometer (MJM Acc), electroencephalography derivation C4A1. Indications about sleep/wake state or sleep stage is reported as well as the hour and the duration of the polysomnography fragment.
Figure 15. Mandibular jaw movement biosignal during swallowing.
The figure shows successive and brief episodes of swallowing (see blue arrows) that can be easily identified based on the background respiratory activity of the mandibular jaw.
Signals from the top to the bottom: pulsed oxygen saturation (SpO2), thoracic and abdominal respiratory inductance plethysmography (RIP Thx, RIP Abd), nasal airflow pressure (FlowPr), oronasal thermal flow (FlowTh), snore, mandibular jaw movements captured by the gyroscope (MJM Gyr) and the accelerometer (MJM Acc), chin electromyography signal (EMG1), electroencephalography derivation C4A1. These signals were recorded during N2 sleep stage. Hour and the duration of the polysomnography fragment are reported.
Figure 16. Mandibular jaw movement biosignal depicting suckling movements.
The figure illustrates how breastfeeding a 20-month-old child while they are asleep and suckling their mother’s breast can disrupt the background respiratory activity of the child’s mandibular jaw.
Signals from the top to the bottom: pulsed oxygen saturation (SpO2), thoracic and abdominal respiratory inductance plethysmography (RIP Thx, RIP Abd), nasal airflow pressure (FlowPr), oronasal thermal flow (FlowTh), snore, mandibular jaw movements captured by the gyroscope (MJM Gyr) and the accelerometer (MJM Acc), electroencephalography derivation C4A1. The entire fragment was in N1 sleep stage. The hour and the duration of the polysomnography fragment are reported.
CONCLUSIONS
A strong and specific comprehensive physiological association has been highlighted between MJM and concomitant periods of normal or disturbed breathing, arousals, and different sleep stages. In one notable instance, the MJM biosignal supports clearly the differentiation between central and obstructive hypopneas.
DISCLOSURE STATEMENT
J.B.M. reports being a scientific advisor to Sunrise and being an investigator in pharmacy trials for Jazz Pharmaceuticals, Theranexus, and Desitin. A.M. is funded by the US National Institutes of Health. He reports income related to medical education from Jazz, Zoll, Eli Lilly, Sunrise, and Livanova. ResMed provided a philanthropic donation to the University of California San Diego. J.L.P. reports being a scientific advisor to Sunrise; receiving grants and/or personal fees from ResMed, Philips, Fisher & Paykel, Sefam, AstraZeneca, AGIR à dom, Elevie, VitalAire, Boehringer Ingelheim, Jazz Pharmaceuticals, and Itamar Medical; and receiving research support for clinical studies from Mutualia and Air Liquide Foundation.
EDITOR'S NOTE
The Emerging Technologies section focuses on new tools and techniques of potential utility in the diagnosis and management of any and all sleep disorders. The technologies may not yet be marketed, and indeed may only exist in prototype form. Some preliminary evidence of efficacy must be available, which can consist of small pilot studies or even data from animal studies, but definitive evidence of efficacy will not be required, and the submissions will be reviewed according to this standard. The intent is to alert readers of Journal of Clinical Sleep Medicine of promising technology that is in early stages of development. With this information, the reader may wish to (1) contact the author(s) in order to offer assistance in more definitive studies of the technology; (2) use the ideas underlying the technology to develop novel approaches of their own (with due respect for any patent issues); and (3) focus on subsequent publications involving the technology in order to determine when and if it is suitable for application to their own clinical practice. The Journal of Clinical Sleep Medicine and the American Academy of Sleep Medicine expressly do not endorse or represent that any of the technology described in the Emerging Technologies section has proven efficacy or effectiveness in the treatment of human disease, nor that any required regulatory approval has been obtained.
ACKNOWLEDGMENTS
The authors are indebted to Professor Michael I Polkey (Royal Brompton Hospital London) for constructive discussion of the data discussed in this manuscript.
ABBREVIATIONS
- Acc
accelerometer
- EMG
electromyography
- FlowPr
nasal airflow pressure
- FlowTh
oronasal thermal flow
- Gyr
gyroscope
- MJM
mandibular jaw movements
- PSG
polysomnography
- REM
rapid eye movement
- RIP
respiratory inductance plethysmography
- RIP
Abd abdominal respiratory inductive plethysmography
- RIP
Thx thoracic respiratory inductive plethysmography
- RMMA
rhythmic masticatory muscles activity
- SpO2
pulsed oxygen saturation
REFERENCES
- 1. Martinot JB, Le-Dong NN, Cuthbert V, Denison S, Silkoff P, Borel JC, Pépin JL . The key role of the mandible in modulating airflow amplitude during sleep . Respir Physiol Neurobiol. 2020. ; 279 : 103447 . [DOI] [PubMed] [Google Scholar]
- 2. Kubin L . Neural control of the upper airway: respiratory and state-dependent mechanisms . Compr Physiol. 2016. ; 6 ( 4 ): 1801 – 1850 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Moore JD, Kleinfeld D, Wang F . How the brainstem controls orofacial behaviors comprised of rhythmic actions . Trends Neurosci. 2014. ; 37 ( 7 ): 370 – 380 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hollowell DE, Suratt PM . Mandible position and activation of submental and masseter muscles during sleep . J Appl Physiol 1985. 1991. ; 71 ( 6 ): 2267 – 2273 . [DOI] [PubMed] [Google Scholar]
- 5. Hollowell DE, Suratt PM . Activation of masseter muscles with inspiratory resistance loading . J Appl Physiol 1985. 1989. ; 67 ( 1 ): 270 – 275 . [DOI] [PubMed] [Google Scholar]
- 6. Martinot JB, Pépin JL . Mandibular jaw movements as a non-invasive measure of respiratory effort during sleep: application in clinical practice . Front Sleep. 2023. ; 2 : 1145620 . [Google Scholar]
- 7. Martinot JB, Le-Dong NN, Cuthbert V, et al . Mandibular movements as accurate reporters of respiratory effort during sleep: validation against diaphragmatic electromyography . Front Neurol. 2017. ; 8 : 353 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pépin JL, Le-Dong NN, Cuthbert V, Coumans N, Tamisier R, Malhotra A, Martinot JB . Mandibular movements are a reliable noninvasive alternative to esophageal pressure for measuring respiratory effort in patients with sleep apnea syndrome . Nat Sci Sleep. 2022. ; 14 : 635 – 644 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Martinot JB, Le-Dong NN, Cuthbert V, Denison S, Borel JC, Gozal D, Pépin JL . Respiratory mandibular movement signals reliably identify obstructive hypopnea events during sleep . Front Neurol. 2019. ; 10 : 828 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pépin JL, Letesson C, Le-Dong NN, et al . Assessment of mandibular movement monitoring with machine learning analysis for the diagnosis of obstructive sleep apnea . JAMA Netw Open. 2020. ; 3 ( 1 ): e1919657 – e1919657 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kelly JL, Ben Messaoud R, Joyeux-Faure M, et al . Diagnosis of sleep apnoea using a mandibular monitor and machine learning analysis: one-night agreement compared to in-home polysomnography . Front Neurosci. 2022. ; 16 : 726880 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Martinot JB, Pépin JL, Malhotra A, et al . Near-boundary double-labelling based classification: the new standard when evaluating performances of new sleep apnea diagnosis solution against polysomnography? Sleep. 2022. ; 45 ( 10 ): zcac188 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Le-Dong NN, Martinot JB, Coumans N, Cuthbert V, Tamisier R, Bailly S, Pépin JL . Machine learning-based sleep staging in patients with sleep apnea using a single mandibular movement signal . Am J Respir Crit Care Med. 2021. ; 204 ( 10 ): 1227 – 1231 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bradley TD, Logan AG, Kimoff RJ, et al. CANPAP Investigators . Continuous positive airway pressure for central sleep apnea and heart failure . N Engl J Med. 2005. ; 353 ( 19 ): 2025 – 2033 . [DOI] [PubMed] [Google Scholar]
- 15. Cowie MR, Woehrle H, Wegscheider K, et al . Adaptive servo-ventilation for central sleep apnea in systolic heart failure . N Engl J Med. 2015. ; 373 ( 12 ): 1095 – 1105 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. National Institutes of Health Clinical Center . The Impact of Low-Flow Oxygen Therapy on Hospital Admissions and Mortality in Patients with Heart Failure and Central Sleep Apnea (LOFT-HF). NCT03745898. ClinicalTrials.gov. Bethesda, MD: National Institutes of Health; 2019. . https://clinicaltrials.gov/ct2/show/NCT03745898 ; Accessed on January 26, 2020.
- 17. Javaheri S . Effects of continuous positive airway pressure on sleep apnea and ventricular irritability in patients with heart failure . Circulation. 2000. ; 101 ( 4 ): 392 – 397 . [DOI] [PubMed] [Google Scholar]
- 18. Martinot JB, Le-Dong NN, Malhotra A, Pépin JL . Respiratory effort during sleep and prevalent hypertension in obstructive sleep apnoea . Eur Respir J. 2023. ; 61 ( 3 ): 2201486 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Martinot JB, Borel JC, Le-Dong NN, et al . Monitoring mandibular movements to detect Cheyne-Stokes breathing . Respir Res. 2017. ; 18 ( 1 ): 66 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Martinot JB, Le-Dong NN, Cuthbert V, Denison S, Gozal D, Lavigne G, Pépin JL . Artificial intelligence analysis of mandibular movements enables accurate detection of phasic sleep bruxism in OSA patients: a pilot study . Nat Sci Sleep. 2021. ; 13 : 1449 – 1459 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Martinot JB, Borel JC, Le-Dong NN, Silkoff PE, Denison S, Gozal D, Pépin JL . Bruxism relieved under CPAP treatment in a patient with OSA syndrome . Chest. 2020. ; 157 ( 3 ): e59 – e62 . [DOI] [PubMed] [Google Scholar]