Abstract
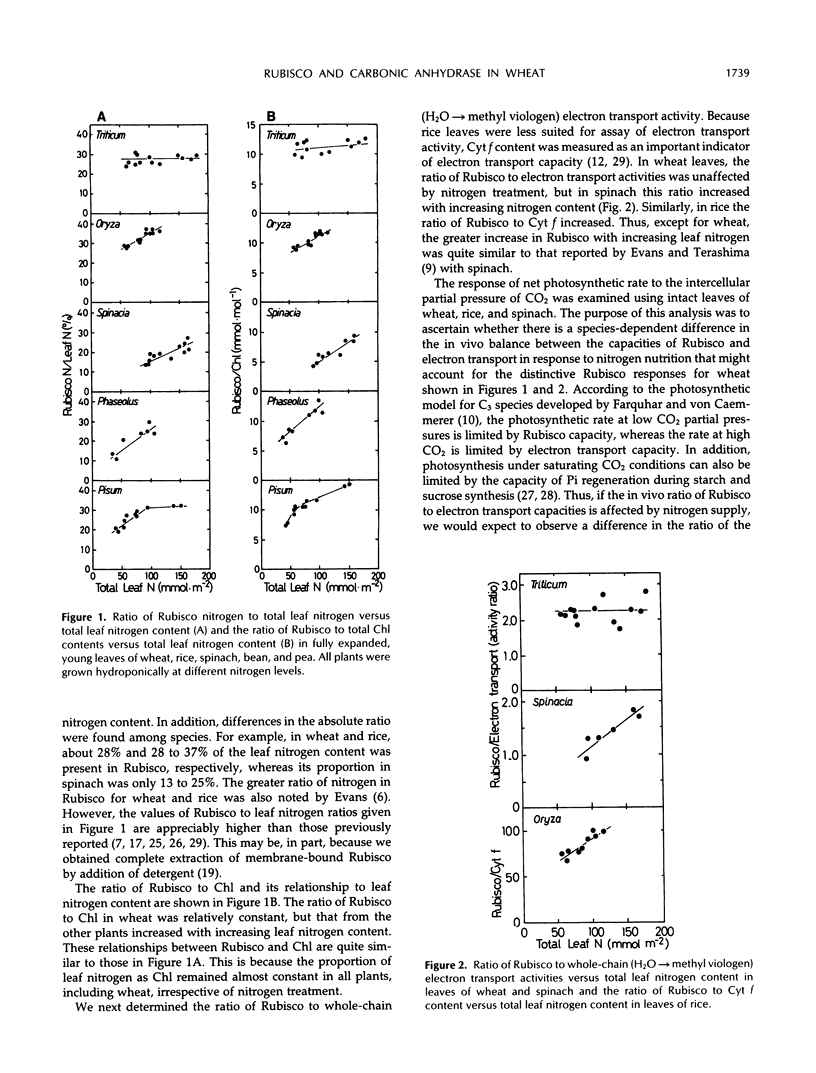
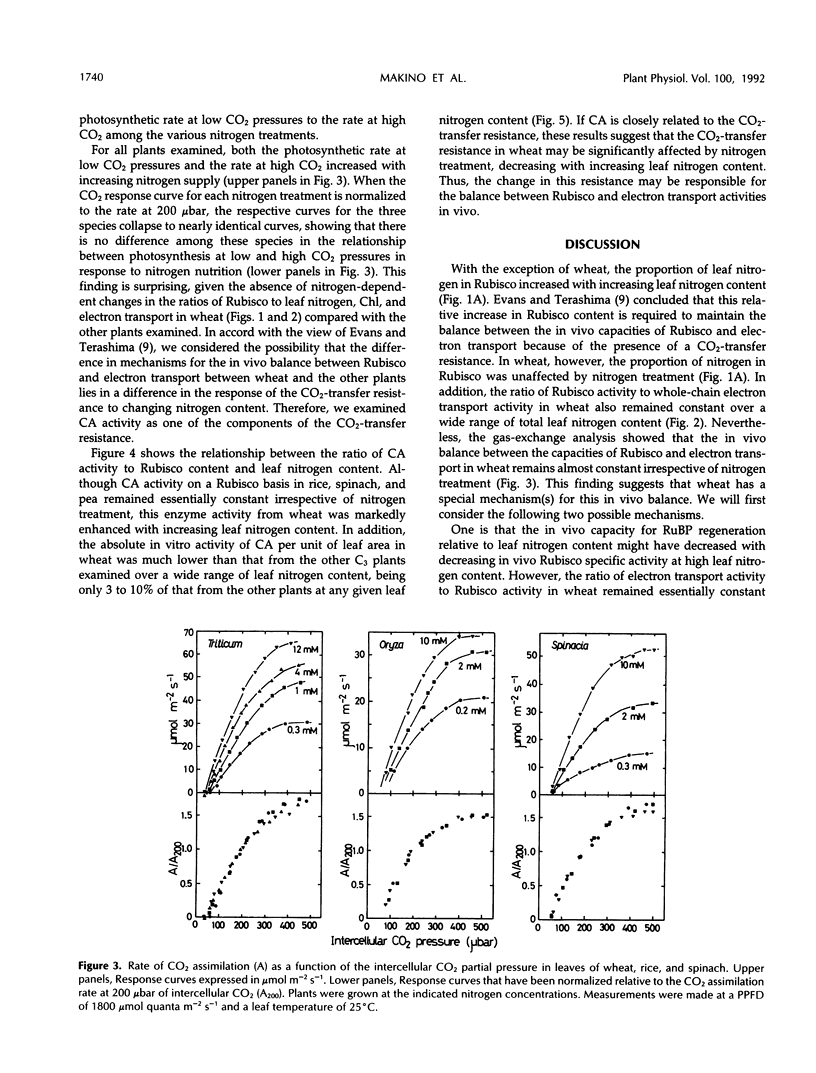
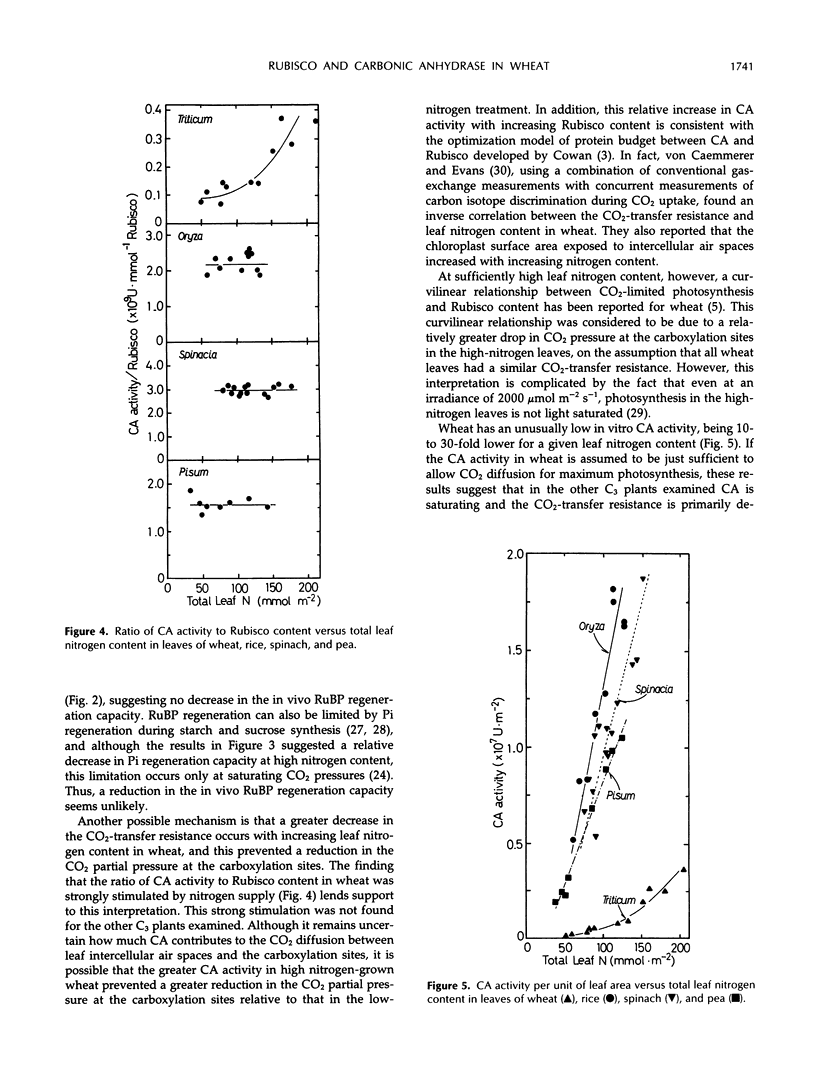
The amounts of ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco), total chlorophyll (Chl), and total leaf nitrogen were measured in fully expanded, young leaves of wheat (Triticum aestivum L.), rice (Oryza sativa L.), spinach (Spinacia oleracea L.), bean (Phaseolus vulgaris L.), and pea (Pisum sativum L.). In addition, the activities of whole-chain electron transport and carbonic anhydrase were measured. All plants were grown hydroponically at different nitrogen concentrations. Although a greater than proportional increase in Rubisco content relative to leaf nitrogen content and Chl was found with increasing nitrogen supply for rice, spinach, bean, and pea, the ratio of Rubisco to total leaf nitrogen or Chl in wheat was essentially independent of nitrogen treatment. In addition, the ratio of Rubisco to electron transport activities remained constant only in wheat. Nevertheless, gas-exchange analysis showed that the in vivo balance between the capacities of Rubisco and electron transport in wheat, rice, and spinach remained almost constant, irrespective of nitrogen treatment. The in vitro carbonic anhydrase activity in wheat was very low and strongly responsive to increasing nitrogen content. Such a response was not found for the other C3 plants examined, which had 10- to 30-fold higher carbonic anhydrase activity than wheat at any leaf-nitrogen content. These distinctive responses of carbonic anhydrase activity in wheat were discussed in relation to CO2-transfer resistance and the in vivo balance between the capacities of Rubisco and electron transport.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Evans J. R. Nitrogen and Photosynthesis in the Flag Leaf of Wheat (Triticum aestivum L.). Plant Physiol. 1983 Jun;72(2):297–302. doi: 10.1104/pp.72.2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidema J., Makino A., Mae T., Ojima K. Photosynthetic Characteristics of Rice Leaves Aged under Different Irradiances from Full Expansion through Senescence. Plant Physiol. 1991 Dec;97(4):1287–1293. doi: 10.1104/pp.97.4.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson B. S., Fong F., Heath R. L. Carbonic anhydrase of spinach: studies on its location, inhibition, and physiological function. Plant Physiol. 1975 Mar;55(3):468–474. doi: 10.1104/pp.55.3.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longstreth D. J., Nobel P. S. Nutrient Influences on Leaf Photosynthesis: EFFECTS OF NITROGEN, PHOSPHORUS, AND POTASSIUM FOR GOSSYPIUM HIRSUTUM L. Plant Physiol. 1980 Mar;65(3):541–543. doi: 10.1104/pp.65.3.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loreto F., Harley P. C., Di Marco G., Sharkey T. D. Estimation of Mesophyll Conductance to CO(2) Flux by Three Different Methods. Plant Physiol. 1992 Apr;98(4):1437–1443. doi: 10.1104/pp.98.4.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino A., Osmond B. Effects of Nitrogen Nutrition on Nitrogen Partitioning between Chloroplasts and Mitochondria in Pea and Wheat. Plant Physiol. 1991 Jun;96(2):355–362. doi: 10.1104/pp.96.2.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall P. J., Bouma D. Zinc deficiency, carbonic anhydrase, and photosynthesis in leaves of spinach. Plant Physiol. 1973 Sep;52(3):229–232. doi: 10.1104/pp.52.3.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage R. F., Sharkey T. D., Seemann J. R. Acclimation of Photosynthesis to Elevated CO(2) in Five C(3) Species. Plant Physiol. 1989 Feb;89(2):590–596. doi: 10.1104/pp.89.2.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seemann J. R., Sharkey T. D., Wang J., Osmond C. B. Environmental effects on photosynthesis, nitrogen-use efficiency, and metabolite pools in leaves of sun and shade plants. Plant Physiol. 1987 Jul;84(3):796–802. doi: 10.1104/pp.84.3.796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharkey T. D. O(2)-insensitive photosynthesis in c(3) plants : its occurrence and a possible explanation. Plant Physiol. 1985 May;78(1):71–75. doi: 10.1104/pp.78.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitt M. Limitation of Photosynthesis by Carbon Metabolism : I. Evidence for Excess Electron Transport Capacity in Leaves Carrying Out Photosynthesis in Saturating Light and CO(2). Plant Physiol. 1986 Aug;81(4):1115–1122. doi: 10.1104/pp.81.4.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]


