Abstract
Background
Severe refractory hypotension and cardiogenic shock are the main contributors to death in acute aluminum phosphide (ALP) poisoning. Shock index (SI) and modified shock index (MSI) are easily obtained parameters that reflect shock at an early stage.
Aim
This study aimed to evaluate the role of SI and MSI in the prediction of the severity and outcomes of acute ALP poisoned patients.
Patients and methods
This cross sectional study was conducted on patients admitted to Tanta University Poison Control Centre with acute ALP poisoning from April 2022 to March 2023. Socio-demographics and toxicological data were taken, findings of clinical examination and laboratory investigations were recoded, SI was calculated by dividing heart rate over systolic blood pressure, and MSI was obtained by dividing heart rate over mean arterial pressure. Poisoning severity was assessed using poisoning severity score (PSS). Patients were divided into groups according to intensive care unit (ICU) admission and mortality.
Results
The study enrolled 94 patients. The median values of SI and MSI were significantly higher in ICU-admitted patients and non-survivors rather than their comparable groups. Significant positive correlations were observed between each of SI and MSI and PSS. At cut-off >1.14, SI conveyed fair performance to predict ICU admission and mortality (AUC = 0.710 and 0.739, respectively). Similarly, MSI had fair performance to predict ICU admission (AUC = 0.731) and mortality (AUC = 0.744) at cut-off >1.47 and >1.5, respectively.
Conclusion
Both SI and MSI could be considered simple bedside adjuncts to predict ICU admission and mortality in acute ALP poisoning.
Keywords: aluminum phosphide poisoning, intensive care unit, mortality, prediction, shock index, modified shock index
Introduction
Aluminum phosphide (ALP) is a well-known pesticide that is used as a cheap fumigant in many countries to protect crops during transportation or storage.1 Regrettably, ALP poisoning is associated with high mortality and has been identified as the most lethal pesticide poisoning.2
Toxicity is mediated through the release of phosphine gas when ALP tablets come in contact with the gastric acidity or exposed to the moisture in the surrounding environment. Phosphine is cytotoxic and causes oxidative stress. In addition, it inhibits cytochrome c oxidase enzyme and oxidative phosphorylation with adenosine triphosphate depletion and subsequently cellular death occurs.3,4
Among different body organs, heart is highly susceptible to mitochondrial dysfunction and oxidative stress caused by ALP toxicity because it is rich in mitochondria and has low antioxidant capacity. Cardiotoxicity is a critical event related to acute ALP poisoning that nearly 70% of fatalities were attributable to cardiovascular disorders with severe and refractory hypotension is the dominant clinical manifestation.5,6 Accordingly, previous studies investigated the role of cardiac markers like troponin, electrocardiographic changes, and echocardiographic findings to predict the outcome of acute ALP poisoning.7–9 However, to the best of the authors’ knowledge, shock index (SI) and modified shock index (MSI) were not previously investigated with regard to ALP poisoning.
Shock index is a simple bedside assessment parameter determined easily by dividing the heart rate (HR) over the systolic blood pressure (SBP). Modified shock index is defined as the ratio between HR and mean arterial pressure (MAP).10 Shock index can evaluate acute hypovolemia and bleeding, as well as, acute circulatory failure and was found as an independent predictor of microvascular damage and extent of myocardial injury. Moreover, it may reflect the worsening of stroke volume, cardiac index, and left ventricular stroke work.11 Remarkably, MAP is thought to be the best evaluator for organs perfusion compared with SBP and diastolic blood pressure (DBP) alone and is considered the recommended indicator for deciding fluid resuscitation and vasopressors titration.10
Both SI and MSI were previously investigated in patients with different clinical conditions including trauma,12 sepsis,13,14 COVID-19 infection,15 and acute myocardial infarction.16 However, after meticulous search through the literature, only one study has investigated SI in the field of clinical toxicology. Lau and Wong17 conducted a study on calcium channel blockers poisoned patients and found that, using univariate and multivariate regression analysis, SI was significantly associated with life-threatening manifestations, mortality, and ICU admission. They also concluded that higher SI is associated with more serious patient outcome.
Hence, the current study aimed to evaluate the role of SI and MSI in predicting the poisoning severity and outcomes (intensive care unit [ICU] admission and mortality) in patients with acute ALP poisoning.
Patients and methods
Study design and setting
The current cross-sectional study was conducted on patients with acute ALP poisoning who were admitted to Tanta University Poison Control Centre, Tanta Emergency Hospitals, Egypt during the period from April 2022 to March 2023.
Ethical consideration
The study followed the World Medical Association Declaration of Helsinki and was conducted after the agreement of our institution ethics committee (Approval code: 35329/2/22). A written informed consent was obtained from each patients or his/her guardian in case of incompetency. To maintain patients’ confidentiality, a code number was assigned for each patient for anonymous analysis of the collected data.
Inclusion criteria
All male and female patients with the age of 18 years or more and presented with symptomatic acute ALP poisoning were enrolled in the study. Diagnosis depended upon the history of exposure, requesting the container if available, presence of suggestive clinical manifestations (such as: metabolic acidosis, hypotension, and typical garlic odor of breath), and performing silver nitrate test on the gastric aspirate to detect phosphine gas.18,19
Exclusion criteria
The current study excluded patients with co-exposure of other pharmaceutical or non-pharmaceutical preparations and patients with comorbidities such as cardiovascular, hepatic, or renal disorders. Patients who gave a history of acute ALP exposure, but remained asymptomatic during the in-hospital follow-up and those who received any medical intervention before admission to our center were also excluded.
Data collection
Patients who fulfilled the eligibility criteria were subjected to history taking including socio-demographics (age, sex, and residence), toxicological history (route of exposure, alleged mode of poisoning, and time interval between exposure and hospital admission). Findings of initial clinical assessment including HR, SBP, DBP, MAP, and respiratory rate (RR) were recorded, as well as, the level of consciousness as assessed by Glasgow Coma Scale (GCS), and oxygen saturation (O2 saturation).
The following laboratory investigations were performed at admission and before the start of the treatment. Arterial blood gas (ABG) analysis (pH, HCO3; serum bicarbonate, and PaCO2; partial arterial carbon dioxide pressure) using pHOx plus L Stat profile calibrator cartridge C from Nova Biomedical GmbH, Germany (catalog number: 34086). Serum sodium (Na) and potassium (K) levels done using diestro electrolyte analyzer using ISE calibrating pack from Diestro, Argentina (catalog number: IN0100). Kidney function tests (urea and creatinine) and liver enzymes (AST; aspartate aminotransferase and ALT; alanine aminotransferase) were measured by Konelab Prime 60i apparatus using kits obtained from Thermo Fisher Scientifc-Finland (catalog numbers:, 981,820, 981,811, 981,769, and 981,771, respectively).
Grading the severity of the poisoning
To grade the severity of the poisoning condition of each patients, poisoning severity score (PSS) was applied according to the International Programme on Chemical Safety (IPCS). The grading was based on the patient’s most severe symptoms or signs including five grades: grade 0 (no symptoms), grade 1 (mild transient with spontaneously resolving symptoms), grade 2 (pronounced or prolonged symptoms), grade 3 (severe or life-threatening symptoms), and grade 4 (death).20
Calculation of shock index and modified shock index
The following formulae were used to calculate SI and MSI14:
SI = HR/SBP
MSI = HR/MAP
MAP = [(DBP × 2) + SBP]/3
Treatment
The included patients received the standard and routine treatment in the form of emergency treatment care to maintain patent airway, breathing, and circulation. Intravenous fluids and vasopressors (norepinephrine) were given for hypotensive patients. Gastric lavage was done using paraffin oil. Additionally, treatment of metabolic acidosis and any other expected manifestations was considered.
Outcome
According to the requirement of ICU admission, the enrolled patients were divided into ICU-admitted and non-ICU-admitted groups. Patients were also grouped according to mortality into survivors and non-survivors.
Criteria for ICU admission included hemodynamic instability, severe metabolic acidosis, respiratory distress, severe central nervous system depression, and the requirement for mechanical ventilation or vasopressor administration.21
Statistical analysis
Statistical analysis was carried out using the Statistical Package for Social Sciences (IBM SPSS Statistics), version 26 for Windows (IBM Corp., Armonk, N.Y., USA). Numerical variables were assessed for normality of distribution using the Shapiro-Wilk test. Variables following the normal distribution were summarized as the mean and standard deviation (SD), and the comparisons between the groups were done using the independent samples T-test. Variables not following the normal distribution were summarized as the median and interquartile range (IQR; expressed as the 25th–75th percentiles), and the comparisons were done using the Mann-Whitney test. Correlations between numerical variables were tested using Spearman’s rank-order correlation. Categorical data were summarized as frequencies (count and percentage), and associations between categorical variables were done using Pearson’s Chi-square test or Fisher’s exact test. To assess the diagnostic performance of the studied scores, receiver operating characteristics (ROC) curve analysis was performed with identification of the optimal cut-off point and its associated sensitivity, specificity, positive and negative predictive values (PPV, NPV), and overall accuracy. A P-value <0.05 was chosen to define the significance of the statistical test results.
Results
The current study included 94 acute ALP poisoned patients; of them 84 patients (89.4%) required ICU admission and non-survivors were 68 patients (72.3%). The age of the enrolled patients ranged from 18–57 years (mean ± SD: 26.9 ± 10.7 years) with female predominance (56.4%). Patients from rural areas constituted the major counterpart 88.3%. All patients alleged suicidal ingestion of ALP tablets. The delay time ranged from 0.5 to 6 h with median and [IQR] of 2.0 [1.5–3.5] hours. Table 1 shows socio-demographics and toxicological characteristics of the studied patients.
Table 1.
Socio-demographics and toxicological data of patients with acute aluminum phosphide poisoning (n = 94).
| ICU admitted (n = 84) |
Non-ICU admitted (n = 10) | P-value | Non-survivors (n = 68) |
Survivors (n = 26) |
P-value | Total (n = 94) |
|
|---|---|---|---|---|---|---|---|
|
Age
Mean ± SD (Min–Max) |
27.5 ± 11.0 (18.0–57.0) |
22.6 ± 6.9 (18.0–35.0) |
0.178 t | 28.8 ± 11.4 (18.0–57.0) |
22.1 ± 6.7 (18.0–35.0) |
<0.001* t | 26.9 ± 10.7 (18.0–57.0) |
|
Gender
Male n (%) Female n (%) |
36 (42.9%) 48 (57.1%) |
5 (50.0%) 5 (50.0%) |
0.743 X2 | 33 (48.5%) 35 (51.5%) |
8 (30.8%) 18 (69.2%) |
<0.001* Z | 41 (43.6%) 53 (56.4%) |
|
Residence
Rural n (%) Urban |
77 (91.7%) 7 (8.3%) |
6 (60.0%) 4 (40.0%) |
0.015* X2 | 61 (89.7%) 7 (10.3%) |
22 (84.6%) 4 (15.4%) |
0.490 X2 | 83 (88.3%) 11 (11.7%) |
|
Mode
Suicidal n (%) Accidental n (%) |
84 (100.0%) 0 (0.0%) |
10 (100.0%) 0 (0.0%) |
NA | 68 (100.0%) 0 (0.0%) |
26 (100.0%) 0 (0.0%) |
NA | 94 (100.0%) 0 (0.0%) |
|
Route
Oral Inhalation |
84 (100.0%) 0 (0.0%) |
10 (100.0%) 0 (0.0%) |
NA | 68 (100.0%) 0 (0.0%) |
26 (100.0%) 0 (0.0%) |
NA | 94 (100.0%) 0 (0.0%) |
|
Delay (hours)
Median [IQR] (Min–Max) |
2.0 [1.5–4.0] (0.5–6.0) |
2.0 [1.0–2.5] (1.0–4.0) |
0.195 Z | 2.0 [1.3–4.0] (0.5–6.0) |
2.0 [2.0–3.0] (1.0–6.0) |
0.952 Z | 2.0 [1.5–3.5] (0.5–6.0) |
n: number, ICU: intensive care unit, SD: standard deviation, Min: minimum, Max: maximum, IQR: interquartile range (25th–75th percentiles), t: Independent samples T-test, Z: Mann–Whitney test, X2: Pearson’s Chi-square test/ Fisher’s exact test, *significant at P < 0.05
The initial clinical assessment and laboratory investigations results of the studied patients are illustrated in (Table 2). Pulse rate did not show significant difference between ICU-admitted and non-ICU-admitted patients, while it was significantly lower in non-survivors rather than the survivors (P = 0.012). The adverse outcome groups had significantly lower SBP, DBP, and MAP rather than their comparable groups. Conversely, ICU-admitted patients and non-survivors had significantly higher RR in comparison with non-ICU admitted and survived patients (P < 0.001 for each). Regarding GCS, a significant difference was observed between survivors and non-survivors (P = 0.023), while it did not show significant difference between ICU-admitted and non-ICU admitted groups. Oxygen saturation and HCO3 were significantly lower in ICU-admitted patients compared with non-ICU admitted patients (P < 0.001 and 0.033, respectively). However, pH and PaCO2 did not exhibit significant difference between ICU admitted and non-ICU admitted patients. Non-survivors had significantly lower O2 saturation, pH, and HCO3 rather than survivors (P < 0.001 for each) with no significant difference as regard to PaCO2. The mean value of serum K level was significantly lower and median value of serum creatinine was significantly higher in non-survivors in comparison with the survivors (P = 0.034 and 0.006, respectively), however they did not show significant difference between ICU-admitted and non-ICU admitted patients. No significant difference was observed regarding serum Na level, blood urea, and liver enzymes (AST and ALT) between adverse outcome groups and their respective groups.
Table 2.
Comparison between acute aluminum phosphide poisoned groups regarding initial clinical assessment and laboratory investigations (n = 94).
| ICU admitted (n = 84) |
Non-ICU admitted (n = 10) | P-value | Non-survivors (n = 68) |
Survivors (n = 26) |
P-value | Total (n = 94) |
|
|---|---|---|---|---|---|---|---|
|
Pulse rate (b/min)
Mean ± SD (Min–Max) |
90.1 ± 22.0 (36.0–148.0) |
93.4 ± 18.3 (63.0–120.0) |
0.648 t | 87.0 ± 21.2 (36.0–136.0) |
99.4 ± 20.1 (63.0–148.0) |
0.012* t | 90.4 ± 21.5 (36.0–148.0) |
|
SBP (mmHg)
Median [IQR] (Min–Max) |
70.0 [40.0–90.0] (40.0–150.0) |
85.0 [80.0–90.0] (70.0–160.0) |
0.021* Z | 70.0 [40.0–80.0] (40.0–150.0) |
90.0 [80.0–110.0] (40.0–160.0) |
<0.001* Z | 80.0 [40.0–90.0] (40.0–160.0) |
|
DBP (mmHg)
Median [IQR] (Min–Max) |
40.0 [20.0–50.0] (20.0–100.0) |
55.0 [50.0–60.0] (30.0–90.0) |
0.014* Z | 35.0 [20.0–50.0] (20.0–100.0) |
55.0 [50.0–60.0] (20.0–100.0) |
<0.001* Z | 40.0 [20.0–50.0] (20.0–100.0) |
|
MAP (mmHg)
Median [IQR] (Min–Max) |
53.3 [26.7–63.3] (26.7–116.7) |
65.0 [60.0–70.0] (46.7–113.3) |
0.019* Z | 48.3 [26.7–56.7] (26.7–116.7) |
70.0 [60.0–76.7] (26.7–116.7) |
<0.001* Z | 53.3 [26.7–70.0] (26.7–116.7) |
|
RR (cycle/minute)
Mean ± SD (Min–Max) |
27.1 ± 6.7 (16.0–48.0) |
19.2 ± 4.4 (15.0–30.0) |
<0.001* t | 28.5 ± 6.1 (16.0–48.0) |
20.4 ± 5.3 (15.0–40.0) |
<0.001* t | 26.3 ± 6.9 (15.0–48.0) |
|
GCS
Median [IQR] (Min–Max) |
15 [15–15] (3–15) |
15 [15–15] (15–15) |
0.204 Z | 15 [15–15] (3–15) |
15 [15–15] (15–15) |
0.023* Z | 15 [15–15] (3–15) |
|
O
2
saturation (%)
Mean ± SD (Min–Max) |
88.6 ± 11.3 (35.0–100.0) |
97.2 ± 2.0 (93.0–99.0) |
<0.001* t | 87.0 ± 11.8 (35.0–100.0) |
96.2 ± 3.4 (86.0–100.0) |
<0.001* t | 89.6 ± 11.0 (35.0–100.0) |
|
pH
Median [IQR] (Min–Max) |
7.34 [7.27–7.40] (7.03–7.55) |
7.38 [7.33–7.45] (7.30–7.48) |
0.100 Z |
7.31 [7.25–7.37] (7.03–7.55) |
7.40 [7.35–7.45] (7.12–7.52) |
<0.001 * Z | 7.35 [7.28–7.40] (7.03–7.55) |
|
HCO
3
(mmol/L)
Median [IQR] (Min–Max) |
13.1 [10.5–16.6] (6.5–26.0) |
16.6 [14.2–18.0] (12.0–19.9) |
0.033* Z | 12.8 [10.3–15.5] (6.5–26.0) |
17.5 [14.2–19.9] (9.2–23.0) |
<0.001* Z | 13.8 [10.6–17.4] (6.5–26.0) |
|
PaCO
2
(mmHg)
Median [IQR] (Min–Max) |
24.3 [19.1–32.9] (11.0–49.0) |
23.9 [21.5–38.0] (14.3–42.1) |
0.516 Z |
23.6 [18.7–31.9] (11.0–49.0) |
27.0 [22.0–34.6] (14.2–42.1) |
0.147 Z |
24.3 [19.4–33.7] (11.0–49.0) |
|
K level (mmol/L)
Mean ± SD (Min–Max) |
3.57 ± 0.50 (2.10–5.20) |
3.82 ± 0.48 (3.08–4.59) |
0.130 t |
3.53 ± 0.44 (2.80–4.40) |
3.77 ± 0.61 (2.10–5.20) |
0.034* t | 3.60 ± 0.50 (2.10–5.20) |
|
Na level (mmol/L)
Mean ± SD (Min–Max) |
141.2 ± 5.4 (130.0–165.5) |
141.6 ± 4.2 (135.6–150.0) |
0.814 t | 141.0 ± 5.3 (130.0–165.5) |
141.7 ± 5.5 (133.0–155.5) |
0.610 t | 141.2 ± 5.3 (130.0–165.5) |
|
Serum creatinine (mg/dL)
Median [IQR] (Min–Max) |
1.10 [1.00–1.30] (0.60–2.10) |
1.00 [1.00–1.20] (0.90–1.20) |
0.303 Z |
1.15 [1.00–1.35] (0.60–2.10) |
1.00 [0.90–1.20] (0.60–1.60) |
0.006* Z | 1.10 [1.00–1.30] (0.60–2.10) |
|
Blood urea (mg/dL)
Mean ± SD (Min–Max) |
36.9 ± 11.5 (16.0–85.0) |
33.6 ± 7.3 (20.0–47.0) |
0.376 t |
37.6 ± 11.8 (16.0–85.0) |
34.0 ± 9.2 (20.0–51.0) |
0.162 t |
36.6 ± 11.2 (16.0–85.0) |
|
AST (U/L)
Median [IQR] (Min–Max) |
29.0 [15.0–35.5] (7.0–150.0) |
25.0 [16.0–36.0] (15.0–74.0) |
0.801 Z | 30.0 [15.0–39.5] (7.0–150.0) |
25.0 [16.0–32.0] (10.0–74.0) |
0.337 Z |
29.0 [15.0–36.0] (7.0–150.0) |
|
ALT (U/L)
Median [IQR] (Min–Max) |
24.0 [15.5–33.0] (7.0–161.0) |
19.5 [16.0–35.0] (12.0–54.0) |
0.717 Z |
26.5 [15.5–34.5] (8.0–161.0) |
18.0 [16.0–27.0] (7.0–54.0) |
0.196 Z |
23.0 [16.0–33.0] (7.0–161.0) |
n: number, IQR: interquartile range (25th–75th percentiles), Min: minimum, Max: maximum, SD: standard deviation, t: Independent samples T-test, Z: Mann–Whitney test, ICU: Intensive Care Unit, b/min: beats/minute, SBP: systolic blood pressure, DBP: diastolic blood pressure, MAP: mean arterial pressure, RR: respiratory rate, GCS: Glasgow Coma Scale, O2 saturation: oxygen saturation, HCO3: serum bicarbonate, PaCO2: partial arterial carbon dioxide pressure, K: potassium, Na: Sodium, AST: aspartate aminotransferase, ALT: alanine aminotransferase, *significant at P < 0.05.
As demonstrated in (Table 3), ICU-admitted patients and non-survivors had significantly higher median values of PSS (P < 0.001 for each). The median values of SI were significantly higher in ICU-admitted patients rather than non-ICU admitted patients (1.33 versus 0.97, P = 0.031) and in non-survivors compared with survivors (1.41 versus 0.95, P < 0.001). Similarly, MSI median value in ICU-admitted patients was significantly higher than non-ICU admitted patients (1.86 versus 1.27, P = 0.017) and it was also significantly higher in non-survivors (1.99) compared with the survivors (1.33) (P < 0.001).
Table 3.
Comparison between acute aluminum phosphide poisoned groups regarding poisoning severity score, shock index, and modified shock index (n = 94).
| Variables | ICU admitted (n = 84) |
Non-ICU admitted (n = 10) | P-value | Non-survivors (n = 68) |
Survivors (n = 26) |
P-value | Total (n = 94) |
|---|---|---|---|---|---|---|---|
|
PSS
Median [IQR] (Min–Max) |
3 [3–3] (1–3) |
2 [1–2] (1–2) |
<0.001* Z | 3 [3–3] (1–3) |
2 [2–2] (1–3) |
<0.001* Z | 3 [2–3] (1–3) |
|
SI
Median [IQR] (Min–Max) |
1.33 [0.94–1.84] (0.58–3.70) |
0.97 [0.86–1.14] (0.61–1.50) |
0.031* Z | 1.41 [1.07–1.90] (0.58–3.40) |
0.95 [0.86–1.20] (0.61–3.70) |
<0.001* Z | 1.32 [0.93–1.75] (0.58–3.70) |
|
MSI
Median [IQR] (Min–Max) |
1.86 [1.35–2.72] (0.70–5.55) |
1.27 [1.16–1.47] (0.86–2.57) |
0.017* Z | 1.99 [1.50–2.85] (0.70–4.76) |
1.33 [1.16–1.64] (0.86–5.55) |
<0.001* Z | 1.83 [1.31–2.60] (0.70–5.55) |
n: number, IQR: interquartile range (25th–75th percentiles), Min: minimum, Max: maximum, Z: Mann-Whitney test, PSS: poisoning severity score, SI: shock index, MSI: modified shock index, *significant at P < 0.05.
A significant positive correlation was observed between SI and PSS (r = 0.454, P < 0.001). Similarly, MSI had significant positive correlation with PSS (r = 0.482, P < 0.001) (Figs 1 and 2).
Fig. 1.
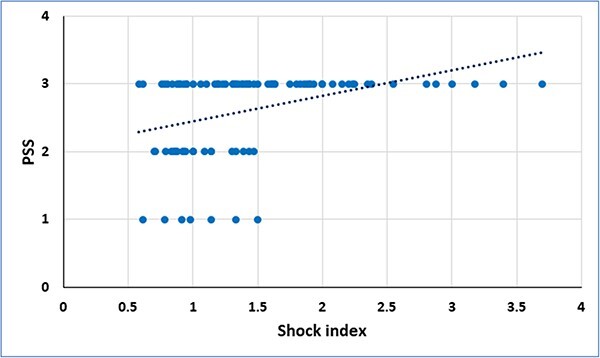
Correlation between shock index and poisoning severity score (PSS) in acute aluminum phosphide poisoned patients.
Fig. 2.
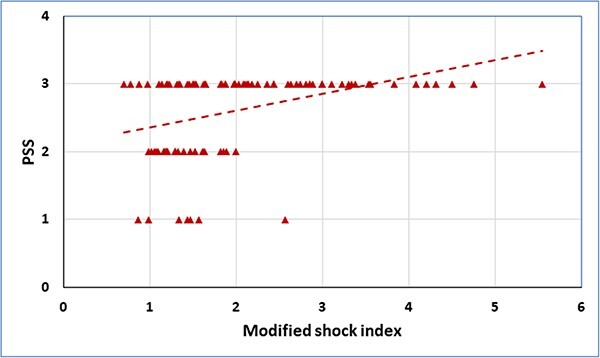
Correlation between modified shock index and poisoning severity score (PSS) in acute aluminum phosphide poisoned patients.
Table 4 depicts the results of ROC curve analysis. Both SI and MSI were significantly valid to predict the need for ICU admission and mortality among ALP poisoned patients. At cut-off >1.14, SI conveyed fair performance to predict ICU admission (AUC = 0.710) and mortality (AUC = 0.739). Similarly, MSI showed fair performance to predict ICU admission and mortality (AUC = 0.731 and 0.744, respectively) at cut-off >1.47 and >1.5, respectively.
Table 4.
The receiver operating characteristic (ROC) curve analysis for shock index and modified shock index to predict the risk of ICU admission and mortality in acute aluminum phosphide poisoning.
| Variables | AUC | 95% CI | P-value | Cut-off value | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | Accuracy (%) |
|---|---|---|---|---|---|---|---|---|---|
| ICU admission | |||||||||
| SI | 0.710 | 0.565 to 0.854 | 0.004* | >1.14 | 64.29 | 80.00 | 96.4 | 21.1 | 66.0 |
| MSI | 0.731 | 0.580 to 0.882 | 0.003* | >1.47 | 69.05 | 80.00 | 96.7 | 23.5 | 70.2 |
| Mortality | |||||||||
| SI | 0.739 | 0.629 to 0.849 | <0.001 | >1.14 | 72.06 | 73.08 | 87.5 | 50.0 | 72.3 |
| MSI | 0.744 | 0.634 to 0.854 | <0.001* | >1.5 | 76.47 | 69.23 | 86.7 | 52.9 | 74.5 |
AUC: area under the curve, CI: confidence interval, PPP: positive predictive value, NPP: negative predictive value, PSS: poisoning severity score, SI: shock index, MSI: modified shock index, *significant at P < 0.05
Discussion
Over the last years, ALP has become a great challenge in many developing countries because of its wide availability and substantial toxicity that is associated with high morbidity and mortality.22 In absence of specific antidote, intensive supportive care remains the backbone of ALP poisoning treatment. Therefore, early prognostic stratification of patients is critical to initiate appropriate interventions, including proactive ICU transfers and better allocation of limited resources.19 Therefore, the current study aimed to evaluate the role of SI and MSI as simple parameters to predict the severity and outcomes of acute ALP poisoned patients.
In the current study, ICU admission and mortality were reported in 89.4 and 72.3% of the enrolled patients, respectively. According to Ahmed et al.,23 ICU admission was reported in 69.3% of ALP poisoned patients. Furthermore, non-survivors represented 77.4 and 89% of acute ALP poisoned patients according to Dorooshi et al.24 and Sakr et al.,25 respectively. High incidence of ICU admission and mortality among ALP poisoned patients can be justified by its potent toxic effects and progressive deteriorating clinical manifestations, as well as, the absence of specific antidote.21
In the present study, the value of the mean age and SD was 26.9 ± 10.7 years and suicidal ingestion was alleged by all patients. Stressful life conditions, associated anxiety, unemployment, and failure in education or love are common triggers for suicidal attempts especially in young adults.26 Additionally, females represented 56.4% of the studied patients. Female predominance was previously indicated by Abdel Wahab et al.7 and Bogale et al.27 who reported that females constituted 57.6% and 63.3% of their enrolled patients, respectively. In contrast, males outnumbered females according to Navabi et al.28 They contributed their results that men may access this poison easier than women through their occupational activities.
The risk of ALP exposure in rural areas is substantial because of its extensive use for different agricultural purposes without sufficient legal regulations.29 Consequently, patients from rural areas constituted the major counterpart (88.3%) in this study. Near results (82%) were also reported by ELabdeen et al.30
Because of rapid onset of clinical manifestations and easy transportation, the median value of the duration between the exposure to the poison and the arrival to our treating center was 2 h. In agreement with our results, El-Sarnagawy et al.31 and Elsharkawy et al.32 reported that the median values of the delay time were 2 and 1 h, respectively.
In the current study, SBP, DBP, and MAP were significantly lower in all adverse outcome groups. These results are comparable to findings of Ghonem et al.,4 Pannu et al.,19 Ahmed et al.23 Hypotension is one of the significant factors that contribute to poor outcome in ALP poisoning.19 Myocardial and adrenal gland damage together with volume depletion are the main underlying causes of shock in ALP poisoning.33
It was detected that, pulse was significantly lower in non-survivors compared to the survivors while it did not show significant difference between ICU-admitted and non-ICU admitted groups. This was in line with Elhosary and Hodeib26 and Erfantalab et al.34 However, reference-wise, pulse rate had no significant difference between survivors and non-survivors according to Sheta et al.,29 El-Sarnagawy,31 and Anbalagan et al.35 While Elgazzar et al.9 observed that pulse was significantly higher in non-survived acute ALP poisoned patient who were admitted to the ICU. Furthermore, pulse rate was significantly lower in patients who were admitted to the ICU according to Ahmed et al.23 Different types of dysrhythmias are expected with ALP poisoning including both tachycardia and bradycardia.24
Respiratory system is vulnerable to acute ALP poisoning with different manifestations like tachypnea and dyspnea, as well as, the development of crepitation and pulmonary edema may occur.36 In this regard, ICU-admitted patients and non-survivors had significantly higher RR and significantly lower O2 saturation. These finding agreed with the results of Sheta et al.29 and Abd Elghany et al.37
In the current study, GCS was significantly different between survivors and non-survivors. This was in agreement with Sheta et al.29 and Sharma et al.38 Brain cells hypoxia may occur with ALP poisoning as a result of oxygen free radicals formation and hypotension.24 Moreover, with low GCS and lack of protective airway reflexes, patients are susceptible to some complications as aspiration pneumonia.39
In the current study, pH was significantly lower in non-survivors and HCO3 was significantly lower in both ICU-admitted and non-survivors groups. Parallel with these results, Sagah and Elhawary40 found that pH and HCO3 were significantly lower in non-survivors. Moreover, they observed that pH was the best predictor of mortality among different ABG parameters. Low median values of pH and HCO3 refers to metabolic acidosis which is known to be a significant contributor for death in acute ALP poisoning.41 Inhibition of cytochrome c oxidase and massive hypoperfusion usually account for metabolic acidosis.42
Potassium level was significantly lower and serum creatinine was significantly higher in non-survivors. However, Na, blood urea, and liver enzymes did not have significant difference between the comparable groups. Hypokalaemia in acute ALP poisoning may occur due to vomiting. Renal affection may be precipitated by either hypoperfusion or hypoxia. However, in this regard, literature revealed controversial results.23,39,42,43
Statistical analysis of the current study revealed that ICU-admitted patients and non-survivors had significantly higher median values of PSS rather than their comparable groups. In the same line, El-Sarnagawy et al.21 documented that the median value of PSS in ALP poisoned patients who were admitted to the ICU was significantly higher than those who did not require ICU admission (3 versus 1, respectively).
Phosphine gas inhibits mitochondrial cytochrome c oxidase enzyme causing disruption of the cellular respiration and impairment of the energy production. Heart is the most vulnerable organ to ALP toxicity because of its high mitochondrial content, oxygen consumption, and metabolic activity. Moreover, heart is highly sensitive to oxidative stress caused by ALP. Consequently, most of fatalities are related to cardiovascular complications including refractory hypotension, serious dysrhythmias, and cardiogenic shock.32,44,45 This definitely emphasizes the importance of HR and blood pressure to evaluate patients with acute ALP poisoning and predict their outcome.
However, vital signs are often initially within the normal ranges during the compensatory phase of shock. Accordingly, SI and MSI come to the fore; the elevation of SI has been correlated with reduced left ventricular end-diastolic pressure and circulatory volume, even when the values of HR and SBP are within the normal limits.46 While SI includes only SBP, MSI incorporates also DBP owing to its undeniable importance in determining the clinical severity.47
In the current study, SI and MSI were calculated for each patient and statistical analysis showed that the median values of SI and MSI were significantly higher in ICU-admitted patients and non-survivors. Despite normal ranges were defined for both SI (0.5–0.7) and MSI (0.7–1.3),14 it is appropriate to consider a risk value specific for each medical condition. The study herein demonstrated a value >1.14 as the best cut-off of SI to predict ICU admission and mortality. For MSI, the best cut-off to predict ICU admission was >1.47 and the best cut-off to predict mortality was >1.5.
According to the results of ROC analysis, SI, and MSI had fair performance to predict ICU admission and mortality among ALP poisoned patients. Furthermore, both SI and MSI showed significant positive correlations with PSS. In this regard, both SI and MSI could be introduced as simple, rapid, and applicable predictive and risk stratification adjuncts for patients presented with acute ALP poisoning. Additionally, MSI considers valuable information related to cardiovascular and hemodynamic stability by integrating HR, SBP, and DBP, which makes it a reasonable tool for assessment.48
Conclusion
In conclusion, acute ALP poisoning is a serious toxicological challenge with high rates of morbidity and mortality. Both SI and MSI have significant positive correlations with the severity of acute ALP poisoning. They have fair performance to predict the need for ICU admission and mortality. However, the simplicity of SI and MSI could support their utility as rapid bedside assessment tools for early evaluation of the patients with acute ALP poisoning and predicting their outcomes.
Strength and limitations
To the best of the authors’ knowledge, this is the first study to investigate the role of SI and MSI in ALP poisoning. Although these parameters conveyed fair performance to predict the outcome, they are simple and easily obtained from vital signs that are initially measured at hospital admission. The main limitations of this study were small sample size and uni-centered nature of the study. Therefore, further studies are recommended for evaluation of the findings of our study.
Author contribution
Mona M. Ghonem and Aliaa A. Hodeib contributed to the study’s conception and design. Material preparation and data collection were performed by Mona M. Ghonem, Aliaa A. Hodeib, and Amira A. Abdelnoor. The first draft of the manuscript was written by Mona M. Ghonem and Aliaa A. Hodeib. All authors read and approved the final manuscript.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Conflict of interest statement
The authors have no relevant financial or non-financial interests to disclose.
Data availability
The datasets used and/or analyzed during this study are available from the corresponding author on reasonable request.
Ethical approval
The study followed the World Medical Association Declaration of Helsinki and was conducted after the agreement of Faculty of Medicine – Tanta University ethics committee (Approval code: 35329/2/22).
Consent to participate
A written informed consent was obtained from each patients or his/her guardian in case of incompetency. To maintain patients’ confidentiality, a code number was assigned for each patient for anonymous analysis of the collected data.
Contributor Information
Mona M Ghonem, Department of Forensic Medicine and Clinical Toxicology, Faculty of Medicine, Tanta University, Tanta City 31527, Egypt.
Amira A Abdelnoor, Department of Forensic Medicine and Clinical Toxicology, Faculty of Medicine, Tanta University, Tanta City 31527, Egypt.
Aliaa A Hodeib, Department of Forensic Medicine and Clinical Toxicology, Faculty of Medicine, Tanta University, Tanta City 31527, Egypt.
References
- 1. Oghabian Z, Ahmadi J, Pakravan S, Dabaghzadeh F, Heidari MR, Tajaddini S, Karami-Mohajeri S. Successful treatment of aluminium phosphide poisoning by dihydroxyacetone: a two-case report study. Clin Pharmacol Ther. 2020:45(5):1194–1198. [DOI] [PubMed] [Google Scholar]
- 2. Manouchehri A, Ghareghani S, Shamaei S, Nilechi M, Bossaghzadeh F. A review on Aluminum phosphide (Rice Tablets) poisoning; from exposure to the applicable and new strategies of clinical management. Adv Life Sci. 2021:8(4):326–332. [Google Scholar]
- 3. Eshraghi A, Rajaei N, Balali Mood M, Vakili V, Ramezani J. Changes of QT dispersion in patients suffering from aluminium phosphide poisoning (Rice Pill). Open Access Maced J Med Sci. 2019:7(14):2251–2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ghonem M, El Sharkawy S, Lashin H. Predictive variables of acute aluminum phosphide poisoning outcome: a new proposed model. Egypt J Forensic Appl Toxicol. 2020:20(2):45–60. [Google Scholar]
- 5. Bansal P, Giri S, Bansal R, Tomar LR. Survival in a case of aluminum phosphide poisoning with severe myocardial toxicity. Indian J Health Sci Biomed Res. 2017:10(3):343–346. [Google Scholar]
- 6. Salimi A, Jamali Z, Shabani M. Antioxidant potential and inhibition of mitochondrial permeability transition pore by myricetin reduces aluminium phosphide-induced cytotoxicity and mitochondrial impairments. Front Pharmacol. 2021:12:719081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Abdel Wahab M, Shalaby S, El Awady E, Hussien R, Salah Eldin W. Assessment of the role of total antioxidant capacity and troponin I as possible predictors for phosphides -induced cardiotoxicity. Ain-Shams J Forensic Med Clin Toxicol. 2020:34(1):82–94. [Google Scholar]
- 8. Wahdan A, Khalifa H. Clinical data, laboratory investigations and electrocardiographic changes as predictors of mortality in acute aluminum phosphide poisoning. MJFMCT. 2020:28(1):111–123. [Google Scholar]
- 9. Elgazzar FM, Shama MA, Shoeib O, Hafez ASAF. The role of echocardiographic findings in estimating survival probability of intensive care unit admitted aluminum phosphide poisoned patients. J Med Toxicol. 2022:18(2):128–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Althunayyan SM, Alsofayan YM, Khan AA. Shock index and modified shock index as triage screening tools for sepsis. J Infect Public Health. 2019:12(6):822–826. [DOI] [PubMed] [Google Scholar]
- 11. el-Menyar A, al Habib KF, Zubaid M, Alsheikh-Ali AA, Sulaiman K, Almahmeed W, Amin H, AlMotarreb A, Ullah A, Al Suwaidi J. Utility of shock index in 24,636 patients presenting with acute coronary syndrome. Eur Heart J Acute Cardiovasc Care. 2020:9(6):546–556. [DOI] [PubMed] [Google Scholar]
- 12. Singh A, Ali S, Agarwal A, Srivastava RN. Correlation of shock index and modified shock index with the outcome of adult trauma patients: a prospective study of 9860 patients. N Am J Med Sci. 2014:6(9):450–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sotello D, Yang S, Nugent K. Comparison of the shock index, modified shock index, and age shock index in adult admissions to a tertiary hospital. Southwest Respir Crit Care Chron. 2019:7(28):18–23. [Google Scholar]
- 14. Ojeda E, Diaz M, Hidalgo K. Modified shock index as a predictor of mortality in septic patients. MOJ Surg. 2023:11(1):51–53. [Google Scholar]
- 15. Kurt E, Bahadirli S. The usefulness of shock index and modified shock index in predicting the outcome of COVID-19 patients. Disaster Med Public Health Prep. 2022:16(4):1558–1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schmitz T, Harmel E, Linseisen J, Kirchberger I, Heier M, Peters A, Meisinger C. Shock index and modified shock index are predictors of long-term mortality not only in STEMI but also in NSTEMI patients. Ann Med. 2022:54(1):900–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lau MT, Wong CLW. Utility of triage shock index in predicting patient outcome in calcium channel blocker poisoning. Hong Kong J Emerg Med. 2020:30(2):1–6. [Google Scholar]
- 18. Chugh SN, Ram S, Chugh K, Malhotra KC. Spot diagnosis of aluminium phosphide ingestion: an application of a simple test. J Assoc Physicians India. 1989:37(3):219–220. [PubMed] [Google Scholar]
- 19. Pannu AK, Jhuria L, Bhalla A, Sharma N. PGI score: prospective validation and correlation with SOFA, SAPS-II, and APACHE-II scores for predicting outcomes in acute aluminum phosphide poisoning. Toxicol Res (Camb). 2022:11(2):361–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Persson HE, Sjöberg GK, Haines JA, De Garbino JP. Poisoning severity score. Grading of acute poisoning. J Toxicol Clin Toxicol. 1998:36(3):205–213. [DOI] [PubMed] [Google Scholar]
- 21. el-Sarnagawy GN, Abdelnoor AA, Abuelfadl AA, el-Mehallawi IH. Comparison between various scoring systems in predicting the need for intensive care unit admission of acute pesticide-poisoned patients. Environ Sci Pollut Res. 2022:29(23):33999–34009. [DOI] [PubMed] [Google Scholar]
- 22. Elshama SS. Aluminum phosphide poisoning in Egypt. Biomed J Sci & Tech Res. 2022:46(3):37428–37432. [Google Scholar]
- 23. Ahmed N, El-Mehallawi I, Abo Elnoor M, Hodeib A. Potential clinical and laboratory prognostic factors for prediction of need for ICU admission in acute aluminum phosphide poisoning. Ain-Shams J Forensic Med Clin Toxicol. 2021:37(2):98–106. [Google Scholar]
- 24. Dorooshi G, Mirzae M, Fard NT, Zoofaghari S, Mood NE. Investigating the outcomes of aluminum phosphide poisoning in Khorshid referral hospital, Isfahan, Iran: a retrospective study. J Res Pharm Pract. 2022:10(4):166–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sakr S, Atef M, Shalaby NMM. PGI score as a predictor of cardiotoxicity and mortality in patients with acute aluminum phosphide poisoning. Zagazig J Forensic Med & Toxicol. 2023:21(1):32–48. [Google Scholar]
- 26. Elhosary N, Hodeib A. Blood lactate and lactate pyruvate ratio as prognostic biomarkers of outcome in acute aluminium phosphide poisoning. Egypt J Forensic Sci Appli Toxicol. 2020:20(2):15–30. [Google Scholar]
- 27. Bogale DE, Ejigu BD, Muche TA. Clinical profile and treatment outcome of aluminum phosphide poisoning in Felege Hiwot referral hospital, Northwest Ethiopia: a retrospective study. Open Access Emerg Med. 2021:13:239–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Navabi SM, Navabi J, Aghaei A, Shaahmadi Z, Heydari R. Mortality from aluminum phosphide poisoning in Kermanshah Province, Iran: characteristics and predictive factors. Epidemiol Health. 2018:40:e2018022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sheta AA, El-Banna AS, Elmeguid RA, Mohamed HE, Gad NH. A study of the predictive factors of mortality in acute poisoning with aluminum phosphide with special reference to echocardiography and SOFA score. Environ Sci Pollut Res. 2019:26(32):33135–33145. [DOI] [PubMed] [Google Scholar]
- 30. ELabdeen S, Saad K, Oreby M, Elgazzar F. Assessment of intravenous lipid emulsion as an adjuvant therapy in acute aluminum phosphide poisoning: a randomized controlled trial. Ain-Shams J Forensic Med Clin Toxicol. 2020:34(1):51–68. [Google Scholar]
- 31. El-Sarnagawy G. Predictive factors of mortality in acute aluminum phosphide poisoning: 5 years retrospective study in Tanta poison control unit. Ain-Shams J Forensic Med Clin Toxicol. 2017:29(2):70–79. [Google Scholar]
- 32. Elsharkawy RE, Ghonem MM, El-Sarnagawy GN, Nagy AA, Heshmat MM. Cardioprotective role of the coenzyme Q10 and coconut oil in acute aluminum phosphide poisoning: a randomized controlled clinical trial. Toxicol Res (Camb). 2023:12(3):507–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Proudfoot AT. Aluminium and zinc phosphide poisoning. Clin Toxicol (Phila). 2009:47(2):89–100. [DOI] [PubMed] [Google Scholar]
- 34. Erfantalab P, Soltaninejad K, Shadnia S, Zamani N, Hassanian-Moghaddam H, Mahdavinejad A, Damaneh BH. Trend of blood lactate level in acute aluminum phosphide poisoning. World J Emerg Med. 2017:8(2):116–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Anbalagan LC, Pannu AK, Bhalla A, Dhibar DP, Sharma N. Prognostic significance of poison-related factors and consumption patterns in acute aluminum phosphide poisoning. Turk J Emerg Med. 2023:23(2):88–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Demir U, Hekimoglu Y, Asirdizer M, Etli Y, Kartal E, Gumus O. A case who died due to the suicidal intake of aluminum phosphide. Cumhuriyet Med J. 2017:39(1):458–465. [Google Scholar]
- 37. Abd Elghany SA, Heshmat MM, Oreby M, Elsarnagawy GN. Evaluation of various scoring systems in prediction of acute aluminum phosphide (ALP) poisoning outcome. Ain-Shams J Forensic Med Clin Toxicol. 2018:30(1):117–127. [Google Scholar]
- 38. Sharma A, Balasubramanian P, Gill KD, Bhalla A. Prognostic significance of blood glucose levels and alterations among patients with aluminium phosphide poisoning. Sultan Qaboos Univ Med J. 2018:18(3):e299–e303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Farzaneh E, Ghobadi H, Akbarifard M, Nakhaee S, Amirabadizadeh A, Akhavanakbari G, Keyler DE, Mehrpour O. Prognostic factors in acute aluminium phosphide poisoning: a risk-prediction nomogram approach. Basic Clin Pharmacol Toxicol. 2018:123(3):347–355. [DOI] [PubMed] [Google Scholar]
- 40. Sagah G, Elhawary A. Prognostic significance of acid base disturbances among patients with acute aluminum phosphide poisoning. Egypt J Forensic Sci Appli Toxicol. 2022:22(2):113–125. [Google Scholar]
- 41. Hosseini SF, Forouzesh M, Maleknia M, Valiyari S, Maniati M, Samimi A. The molecular mechanism of aluminum phosphide poisoning in cardiovascular disease: pathophysiology and diagnostic approach. Cardiovasc Toxicol. 2020:20(5):454–461. [DOI] [PubMed] [Google Scholar]
- 42. Abd-Allah MA, Abdalla AA, Mohamed NA, Rady MM, Farrag AA, Abd el Nasser G, Salama K, Elfakharany YM. Updates on toxicology of aluminum phosphide and different management protocols. Zagazig Univ Med J. 2022:28(6):1176–1183. [Google Scholar]
- 43. Hosseinian A, Pakravan N, Rafiei A, Feyzbakhsh SM. Aluminum phosphide poisoning known as rice tablet: a common toxicity in North Iran. Indian J Med Sci. 2011:65(4):143–150. [PubMed] [Google Scholar]
- 44. Karimani A, Mohammadpour AH, Zirak MR, Rezaee R, Megarbane B, Tsatsakis A, Karimi G. Antidotes for aluminum phosphide poisoning—an update. Toxicol Rep. 2018:5:1053–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. El Shehaby DM, Sayed SA, Abd El-Kareem DM, Elsherif R, Almaz D. Trimetazedine with hyperinsulinimea-euoglycemia, N-acetyl cysteine, and vitamin C: a new approach concept for management of aluminum phosphide poisoning. J Biochem Mol Toxicol. 2021:36(1):e22931. [DOI] [PubMed] [Google Scholar]
- 46. Koch E, Lovett S, Nghiem T, Riggs RA, Rech MA. Shock index in the emergency department: utility and limitations. Open Access Emerg Med. 2019:11:179–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Gouda M, Saad AM, Al-Daydamony MM. Modified shock index as a predictor of in-hospital outcome in cases of ST-segment elevation myocardial infarction treated with primary percutaneous coronary intervention. J Cardiol Curr Res. 2016:7(4):00255. [Google Scholar]
- 48. Devendra Prasad K, Abhinov T, Himabindu K, Rajesh K, Krishna Moorthy DGSR. Modified shock index as an indicator for prognosis among sepsis patients with and without comorbidities presenting to the emergency department. Cureus. 2021:13(12):e20283. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during this study are available from the corresponding author on reasonable request.


