Abstract
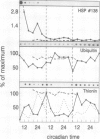
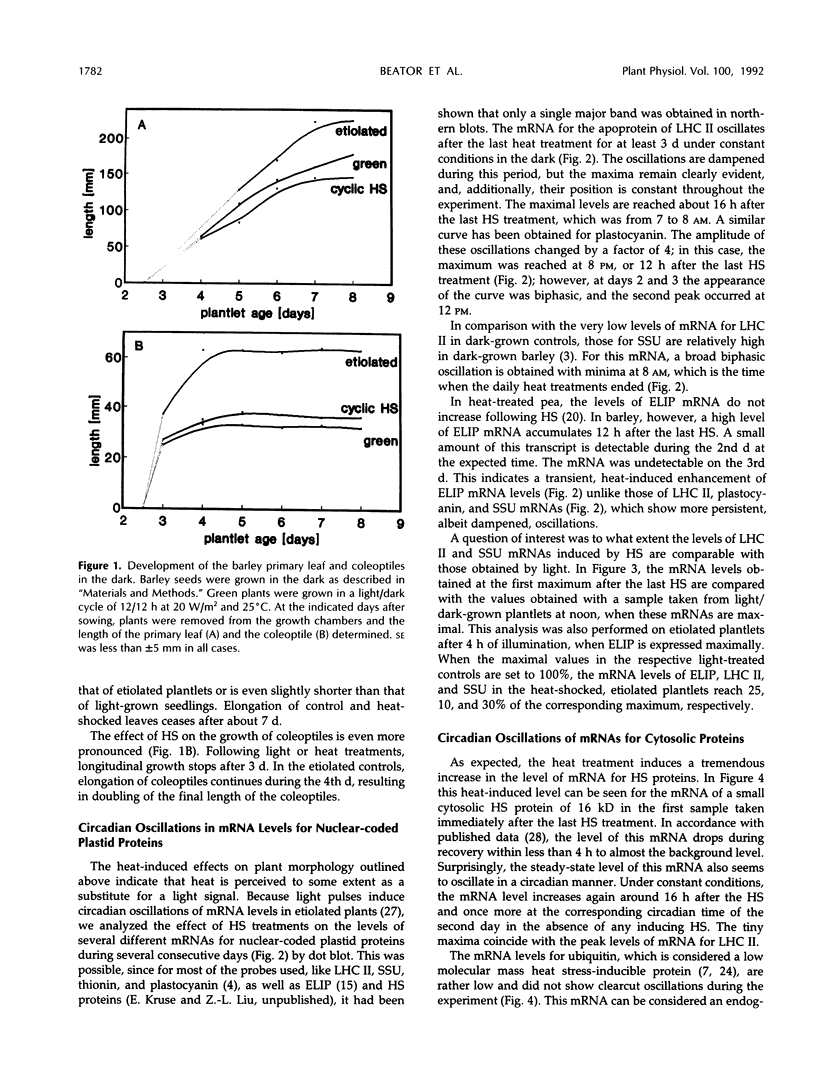
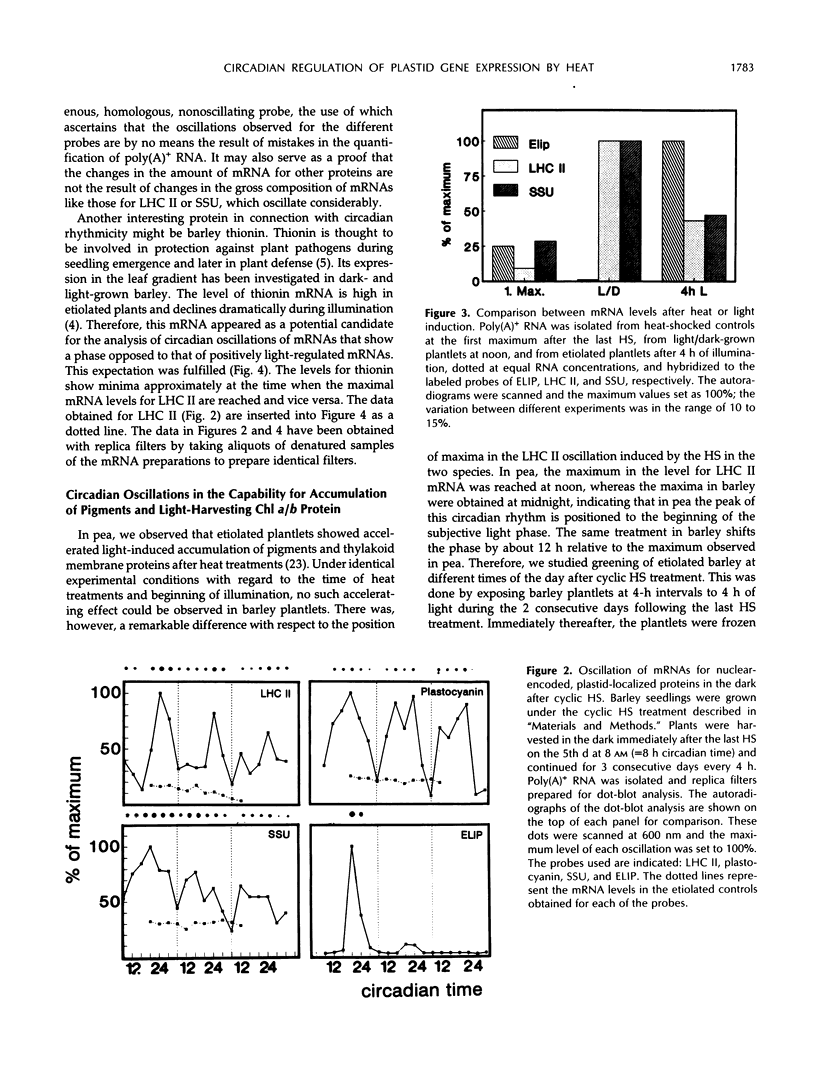
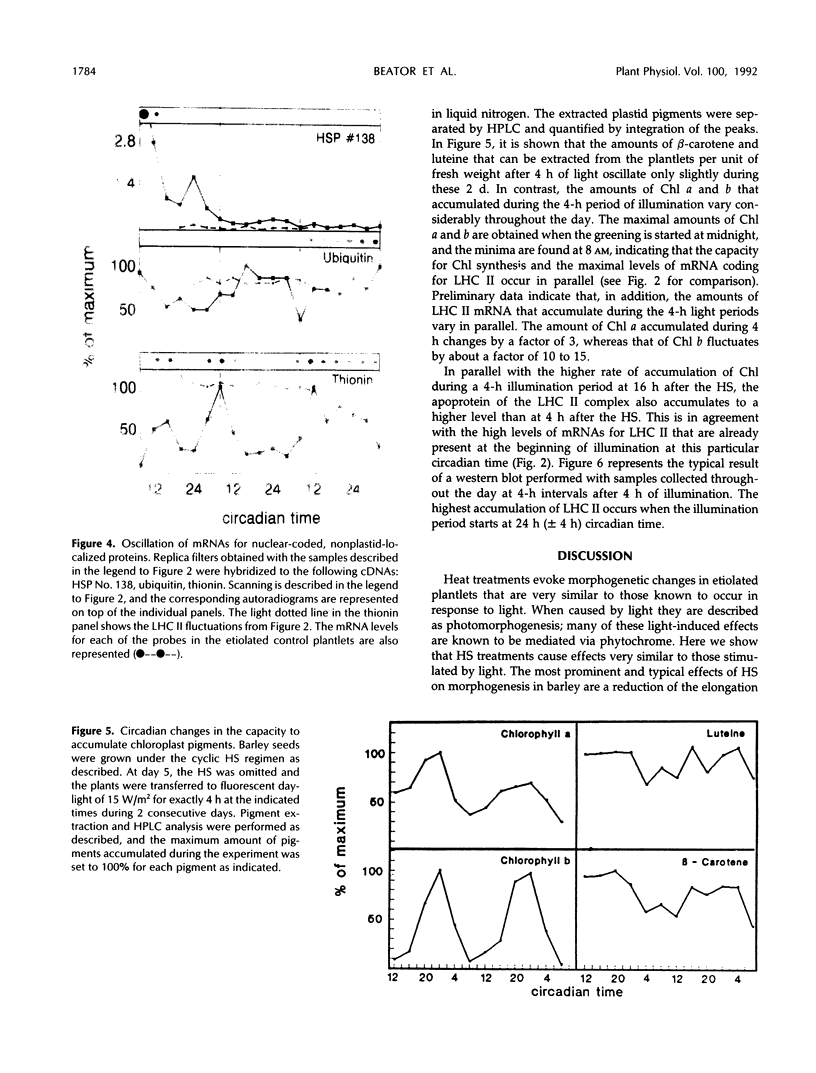
The effect of daily heat-shock treatments on gene expression and morphogenesis of etiolated barley (Hordeum vulgare) was investigated. Heat-shock treatments in the dark induced shortening of the primary leaves and the coleoptiles to the length of those in light-grown plantlets. In addition, the mRNA levels of the light-induced genes that were investigated were raised under these conditions and showed distinct oscillations over a period of at least 3 d. While the mRNA levels for chlorophyll a/b binding protein (LHC II), plastocyanin, and the small subunit of ribulose-1,5-bisphosphate carboxylase had maxima between 8 and 12 pm (12-16h after the last heat-shock treatment), the mRNA levels for thionin oscillated with a phase opposed to that of LHC II. Etiolated barley, the circadian oscillator of which was synchronized by cyclic heatshock treatments, was illuminated for a constant interval at different times of the day; this led to the finding that greening was fastest at the time when the maximal levels of mRNA for LHC II were also observed. Whereas accumulation of chlorophyll a during a 4-h period of illumination oscillated by a factor of 3, chlorophyll b accumulation changed 10- to 15-fold. Similarly, accumulation of LHC II was highest when pigments accumulated maximally. Hence, greening or, in other words, thylakoid membrane assembly is under control of the circadian oscillator.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamska I., Scheel B., Kloppstech K. Circadian oscillations of nuclear-encoded chloroplast proteins in pea (Pisum sativum). Plant Mol Biol. 1991 Nov;17(5):1055–1065. doi: 10.1007/BF00037144. [DOI] [PubMed] [Google Scholar]
- Apel K., Kloppstech K. The plastid membranes of barley (Hordeum vulgare). Light-induced appearance of mRNA coding for the apoprotein of the light-harvesting chlorophyll a/b protein. Eur J Biochem. 1978 Apr 17;85(2):581–588. doi: 10.1111/j.1432-1033.1978.tb12273.x. [DOI] [PubMed] [Google Scholar]
- Apel K. Phytochrome-induced appearance of mRNA activity for the apoprotein of the light-harvesting chlorophyll a/b protein of barley (Hordeum vulgare). Eur J Biochem. 1979 Jun;97(1):183–188. doi: 10.1111/j.1432-1033.1979.tb13101.x. [DOI] [PubMed] [Google Scholar]
- Bohlmann H., Clausen S., Behnke S., Giese H., Hiller C., Reimann-Philipp U., Schrader G., Barkholt V., Apel K. Leaf-specific thionins of barley-a novel class of cell wall proteins toxic to plant-pathogenic fungi and possibly involved in the defence mechanism of plants. EMBO J. 1988 Jun;7(6):1559–1565. doi: 10.1002/j.1460-2075.1988.tb02980.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke T. J., Callis J., Vierstra R. D. Characterization of a polyubiquitin gene from Arabidopsis thaliana. Mol Gen Genet. 1988 Aug;213(2-3):435–443. doi: 10.1007/BF00339613. [DOI] [PubMed] [Google Scholar]
- Chory J., Peto C. A. Mutations in the DET1 gene affect cell-type-specific expression of light-regulated genes and chloroplast development in Arabidopsis. Proc Natl Acad Sci U S A. 1990 Nov;87(22):8776–8780. doi: 10.1073/pnas.87.22.8776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichacker L. A., Soll J., Lauterbach P., Rüdiger W., Klein R. R., Mullet J. E. In vitro synthesis of chlorophyll a in the dark triggers accumulation of chlorophyll a apoproteins in barley etioplasts. J Biol Chem. 1990 Aug 15;265(23):13566–13571. [PubMed] [Google Scholar]
- Fejes E., Pay A., Kanevsky I., Szell M., Adam E., Kay S., Nagy F. A 268 bp upstream sequence mediates the circadian clock-regulated transcription of the wheat Cab-1 gene in transgenic plants. Plant Mol Biol. 1990 Dec;15(6):921–932. doi: 10.1007/BF00039431. [DOI] [PubMed] [Google Scholar]
- Gallagher T. F., Ellis R. J. Light-stimulated transcription of genes for two chloroplast polypeptides in isolated pea leaf nuclei. EMBO J. 1982;1(12):1493–1498. doi: 10.1002/j.1460-2075.1982.tb01345.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuliano G., Hoffman N. E., Ko K., Scolnik P. A., Cashmore A. R. A light-entrained circadian clock controls transcription of several plant genes. EMBO J. 1988 Dec 1;7(12):3635–3642. doi: 10.1002/j.1460-2075.1988.tb03244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm B., Kruse E., Kloppstech K. Transiently expressed early light-inducible thylakoid proteins share transmembrane domains with light-harvesting chlorophyll binding proteins. Plant Mol Biol. 1989 Nov;13(5):583–593. doi: 10.1007/BF00027318. [DOI] [PubMed] [Google Scholar]
- Kloppstech K., Otto B., Sierralta W. Cyclic temperature treatments of dark-grown pea seedlings induce a rise in specific transcript levels of light-regulated genes related to photomorphogenesis. Mol Gen Genet. 1991 Mar;225(3):468–473. doi: 10.1007/BF00261689. [DOI] [PubMed] [Google Scholar]
- Kreuzaler F., Ragg H., Fautz E., Kuhn D. N., Hahlbrock K. UV-induction of chalcone synthase mRNA in cell suspension cultures of Petroselinum hortense. Proc Natl Acad Sci U S A. 1983 May;80(9):2591–2593. doi: 10.1073/pnas.80.9.2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto B., Ohad I., Kloppstech K. Temperature treatments of dark-grown pea seedlings cause an accelerated greening in the light at different levels of gene expression. Plant Mol Biol. 1992 Mar;18(5):887–896. doi: 10.1007/BF00019203. [DOI] [PubMed] [Google Scholar]
- Robertson D., Laetsch W. M. Structure and function of developing barley plastids. Plant Physiol. 1974 Aug;54(2):148–159. doi: 10.1104/pp.54.2.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavladoraki P., Kloppstech K., Argyroudi-Akoyunoglou J. Circadian Rhythm in the Expression of the mRNA Coding for the Apoprotein of the Light-Harvesting Complex of Photosystem II : Phytochrome Control and Persistent Far Red Reversibility. Plant Physiol. 1989 Jun;90(2):665–672. doi: 10.1104/pp.90.2.665. [DOI] [PMC free article] [PubMed] [Google Scholar]