Abstract
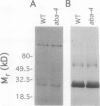
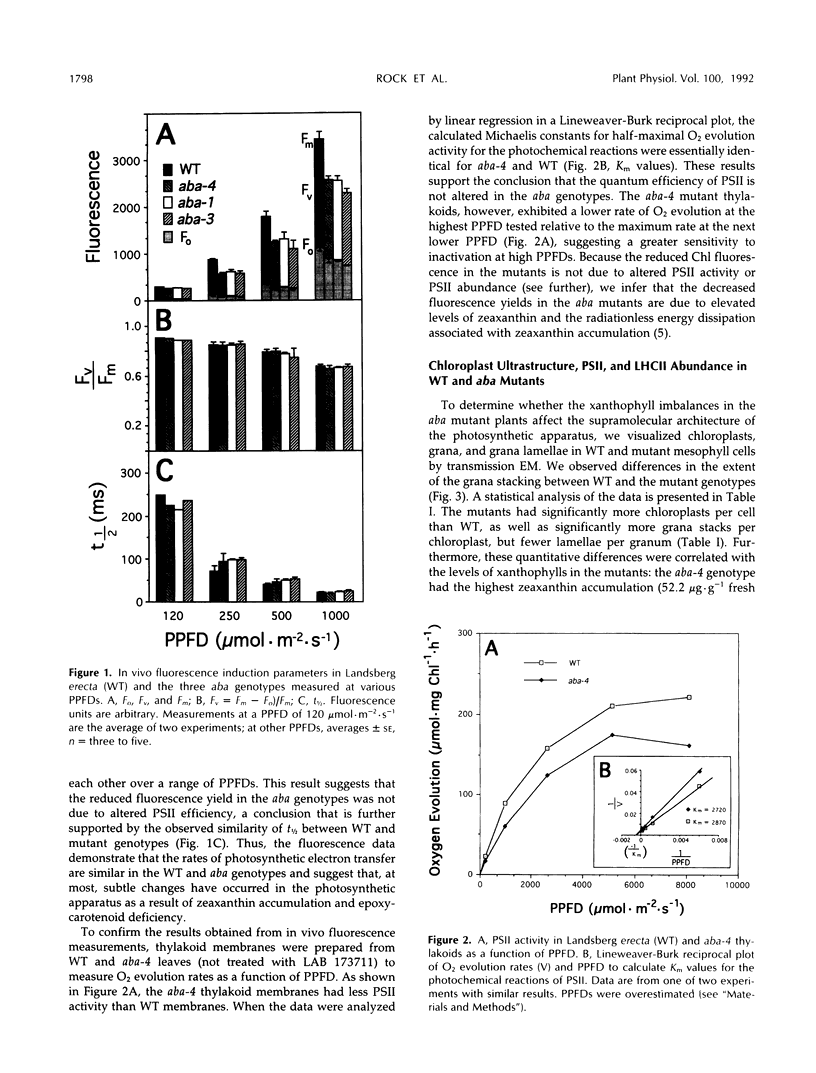
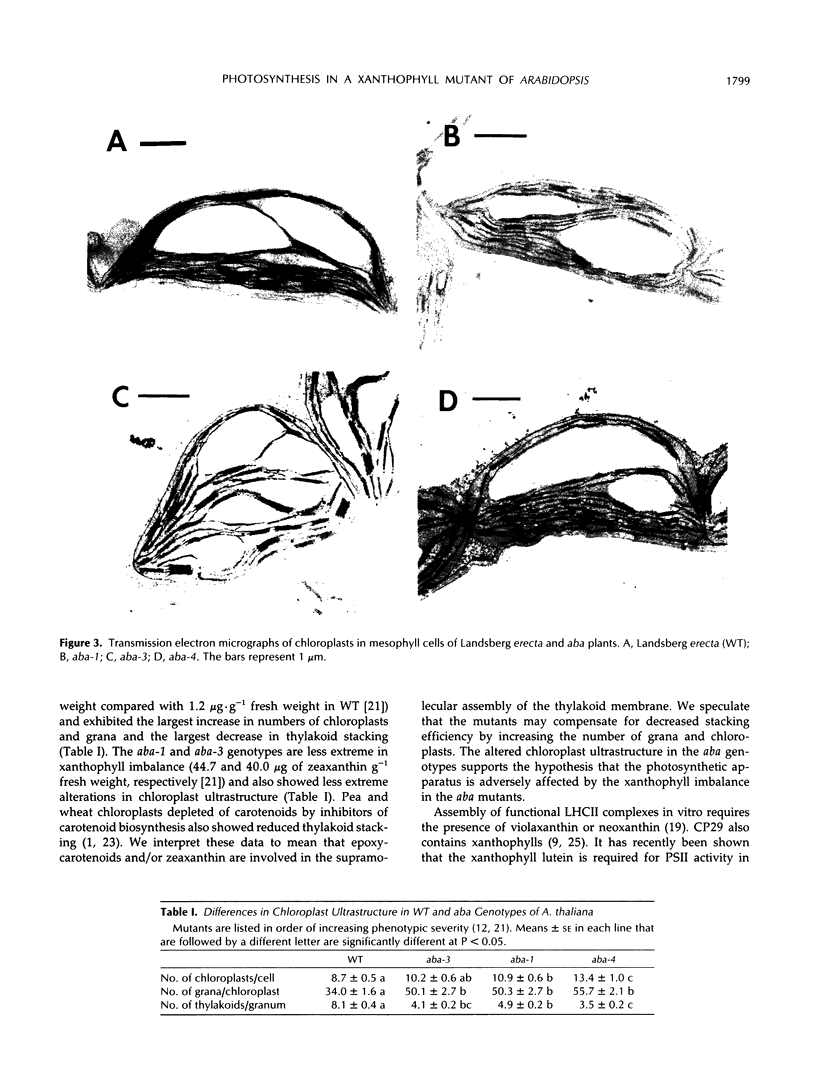
It has been shown that the aba mutant of Arabidopsis thaliana (L.) Heynh. is impaired in epoxy-carotenoid biosynthesis and accumulates the epoxy-carotenoid precursor, zeaxanthin (C.D. Rock, J.A.D. Zeevaart [1991] Proc Natl Acad Sci USA 88: 7496-7499). In addition to providing conclusive evidence for the indirect pathway of abscisic acid biosynthesis from epoxy-carotenoids, the aba mutation offers a powerful means to study the function of xanthophylls (oxygenated carotenoids) in photosynthesis. We measured in vivo the chlorophyll (Chl) fluorescence parameters Fo (initial), Fm (maximum), Fv (variable = Fm − Fo), and t½ (half-rise time of fluorescence induction) of wild-type (WT) and three allelic aba mutants. The mutant genotypes had significantly lower Fo and Fm values relative to those of WT. The Fv/Fm ratio and t½, which are parameters affected by photochemical efficiency, photosystem II (PSII), and plastoquinone pool sizes, were similar in the aba alleles and WT. Because the aba genotypes accumulate high levels of zeaxanthin, which is involved in nonphotochemical quenching of Chl fluorescence, we propose that the reduced fluorescence yields in the aba genotypes are a consequence of the accumulated zeaxanthin. Measurement of PSII oxygen evolution rates in isolated thylakoid membranes of WT and aba-4 confirmed that quantum efficiency was not altered in aba-4 but indicated that the mutant had reduced PSII activity in vitro. Electron microscopy revealed an abnormal chloroplast ultrastructure in the aba plants: the mutants had significantly fewer thylakoid lamellae per granum stack but significantly more grana per chloroplast, as well as more chloroplasts per cell than WT. Immunoblot analysis established that aba-4 had normal levels of the Chl a/b-binding core polypeptide of PSII (CP29) and the PSII light-harvesting Chl a/b-binding complex. These results provide evidence for the role of zeaxanthin in nonphotochemical fluorescence quenching and suggest involvement of epoxy-carotenoids and/or zeaxanthin in thylakoid stacking and PSII activity.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartels P. G., Hyde A. Chloroplast Development in 4-Chloro-5-(dimethylamino)-2-(alpha,alpha,alpha-trifluoro-m-tolyl)-3 (2H)-pyridazinone (Sandoz 6706)-treated Wheat Seedlings: A Pigment, Ultrastructural, and Ultracentrifugal Study. Plant Physiol. 1970 Jun;45(6):807–810. doi: 10.1104/pp.45.6.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua N. H., Bennoun P. Thylakoid membrane polypeptides of Chlamydomonas reinhardtii: wild-type and mutant strains deficient in photosystem II reaction center. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2175–2179. doi: 10.1073/pnas.72.6.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darr S. C., Somerville S. C., Arntzen C. J. Monoclonal antibodies to the light-harvesting chlorophyll a/b protein complex of photosystem II. J Cell Biol. 1986 Sep;103(3):733–740. doi: 10.1083/jcb.103.3.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demmig-Adams B., Adams W. W., Heber U., Neimanis S., Winter K., Krüger A., Czygan F. C., Bilger W., Björkman O. Inhibition of zeaxanthin formation and of rapid changes in radiationless energy dissipation by dithiothreitol in spinach leaves and chloroplasts. Plant Physiol. 1990 Feb;92(2):293–301. doi: 10.1104/pp.92.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demmig B., Winter K., Krüger A., Czygan F. C. Photoinhibition and zeaxanthin formation in intact leaves : a possible role of the xanthophyll cycle in the dissipation of excess light energy. Plant Physiol. 1987 Jun;84(2):218–224. doi: 10.1104/pp.84.2.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore A. M., Yamamoto H. Y. Dark induction of zeaxanthin-dependent nonphotochemical fluorescence quenching mediated by ATP. Proc Natl Acad Sci U S A. 1992 Mar 1;89(5):1899–1903. doi: 10.1073/pnas.89.5.1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene B. A., Allred D. R., Morishige D. T., Staehelin L. A. Hierarchical Response of Light Harvesting Chlorophyll-Proteins in a Light-Sensitive Chlorophyll b-Deficient Mutant of Maize. Plant Physiol. 1988 Jun;87(2):357–364. doi: 10.1104/pp.87.2.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markwell J. P., Webber A. N., Lake B. Mutants of Sweetclover (Melilotus alba) Lacking Chlorophyll b: Studies on Pigment-Protein Complexes and Thylakoid Protein Phosphorylation. Plant Physiol. 1985 Apr;77(4):948–951. doi: 10.1104/pp.77.4.948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray D. L., Kohorn B. D. Chloroplasts of Arabidopsis thaliana homozygous for the ch-1 locus lack chlorophyll b, lack stable LHCPII and have stacked thylakoids. Plant Mol Biol. 1991 Jan;16(1):71–79. doi: 10.1007/BF00017918. [DOI] [PubMed] [Google Scholar]
- Ohad I., Adir N., Koike H., Kyle D. J., Inoue Y. Mechanism of photoinhibition in vivo. A reversible light-induced conformational change of reaction center II is related to an irreversible modification of the D1 protein. J Biol Chem. 1990 Feb 5;265(4):1972–1979. [PubMed] [Google Scholar]
- Plumley F. G., Schmidt G. W. Reconstitution of chlorophyll a/b light-harvesting complexes: Xanthophyll-dependent assembly and energy transfer. Proc Natl Acad Sci U S A. 1987 Jan;84(1):146–150. doi: 10.1073/pnas.84.1.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock C. D., Zeevaart J. A. The aba mutant of Arabidopsis thaliana is impaired in epoxy-carotenoid biosynthesis. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7496–7499. doi: 10.1073/pnas.88.17.7496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryrie I. J. Immunological evidence for apoproteins of the light-harvesting chlorophyll-protein complex in a mutant of barley lacking chlorophyll b. Eur J Biochem. 1983 Mar 1;131(1):149–155. doi: 10.1111/j.1432-1033.1983.tb07242.x. [DOI] [PubMed] [Google Scholar]
- Sagar A. D., Briggs W. R. Effects of High Light Stress on Carotenoid-Deficient Chloroplasts in Pisum sativum. Plant Physiol. 1990 Dec;94(4):1663–1670. doi: 10.1104/pp.94.4.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith B. M., Morrissey P. J., Guenther J. E., Nemson J. A., Harrison M. A., Allen J. F., Melis A. Response of the Photosynthetic Apparatus in Dunaliella salina (Green Algae) to Irradiance Stress. Plant Physiol. 1990 Aug;93(4):1433–1440. doi: 10.1104/pp.93.4.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White M. J., Green B. R. Polypeptides belonging to each of the three major chlorophyll a + b protein complexes are present in a chlorophyll-b-less barley mutant. Eur J Biochem. 1987 Jun 15;165(3):531–535. doi: 10.1111/j.1432-1033.1987.tb11471.x. [DOI] [PubMed] [Google Scholar]