Abstract
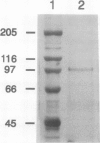
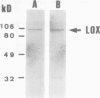
Membrane-associated lipoxygenase from green tomato (Lycopersicon esculentum L. cv Caruso) fruit has been purified 49-fold to a specific activity of 8.3 μmol·min−1·mg−1 of protein by solubilization of microsomal membranes with Triton X-100, followed by anion- exchange and size-exclusion chromatography. The apparent molecular mass of the enzyme was estimated to be 97 and 102 kD by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and size-exclusion chromatography, respectively. The purified membrane lipoxygenase preparation consisted of a single major band following sodium dodecyl sulfate-polyacrylamide gel electrophoresis, which cross-reacts with immunoserum raised against soluble soybean lipoxygenase 1. It has a pH optimum of 6.5, an apparent Km of 6.2 μm, and Vmax of 103. μmol·min−1·mg−1 of protein with linoleic acid as substrate. Corresponding values for the partially purified soluble lipoxygenase from tomato are 3.8 μm and 1.3 μmol·min−1·mg−1 of protein, respectively. Thus, the membrane-associated enzyme is kinetically distinguishable from its soluble counterpart. Sucrose density gradient fractionation of the isolated membranes indicated that the membrane-associated lipoxygenase sediments with thylakoids. A lipoxygenase band with a corresponding apparent mol wt of 97,000 was identified immunologically in sodium dodecyl sulfate-polyacrylamide gel electrophoresis-resolved proteins of purified thylakoids prepared from intact chloroplasts isolated from tomato leaves and fruit.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Britton G. General carotenoid methods. Methods Enzymol. 1985;111:113–149. doi: 10.1016/s0076-6879(85)11007-4. [DOI] [PubMed] [Google Scholar]
- Ghosh S., Gepstein S., Heikkila J. J., Dumbroff E. B. Use of a scanning densitometer or an ELISA plate reader for measurement of nanogram amounts of protein in crude extracts from biological tissues. Anal Biochem. 1988 Mar;169(2):227–233. doi: 10.1016/0003-2697(88)90278-3. [DOI] [PubMed] [Google Scholar]
- Mack A. J., Peterman T. K., Siedow J. N. Lipoxygenase isozymes in higher plants: biochemical properties and physiological role. Isozymes Curr Top Biol Med Res. 1987;13:127–154. [PubMed] [Google Scholar]
- Paliyath G., Thompson J. E. Senescence-Related Changes in ATP-Dependent Uptake of Calcium into Microsomal Vesicles from Carnation Petals. Plant Physiol. 1988 Oct;88(2):295–302. doi: 10.1104/pp.88.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo A. D., Chereskin B. M., Castelfranco P. A., Franceschi V. R., Wezelman B. E. ATP requirement for mg chelatase in developing chloroplasts. Plant Physiol. 1980 May;65(5):956–960. doi: 10.1104/pp.65.5.956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddanna P., Whelan J., Reddy P. S., Reddy C. C. Isolation and characterization of 5-lipoxygenase from tulip bulbs. Biochem Biophys Res Commun. 1988 Dec 30;157(3):1348–1351. doi: 10.1016/s0006-291x(88)81023-4. [DOI] [PubMed] [Google Scholar]
- Rouet-Mayer M. A., Bureau J. M., Laurière C. Identification and characterization of lipoxygenase isoforms in senescing carnation petals. Plant Physiol. 1992 Mar;98(3):971–978. doi: 10.1104/pp.98.3.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouzer C. A., Ford-Hutchinson A. W., Morton H. E., Gillard J. W. MK886, a potent and specific leukotriene biosynthesis inhibitor blocks and reverses the membrane association of 5-lipoxygenase in ionophore-challenged leukocytes. J Biol Chem. 1990 Jan 25;265(3):1436–1442. [PubMed] [Google Scholar]
- Rouzer C. A., Kargman S. Translocation of 5-lipoxygenase to the membrane in human leukocytes challenged with ionophore A23187. J Biol Chem. 1988 Aug 5;263(22):10980–10988. [PubMed] [Google Scholar]
- Todd J. F., Paliyath G., Thompson J. E. Characteristics of a membrane-associated lipoxygenase in tomato fruit. Plant Physiol. 1990 Nov;94(3):1225–1232. doi: 10.1104/pp.94.3.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tranbarger T. J., Franceschi V. R., Hildebrand D. F., Grimes H. D. The soybean 94-kilodalton vegetative storage protein is a lipoxygenase that is localized in paraveinal mesophyll cell vacuoles. Plant Cell. 1991 Sep;3(9):973–987. doi: 10.1105/tpc.3.9.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace J. M. Spot test for lipoxygenase activity. J Lipid Res. 1972 Mar;13(2):282–284. [PubMed] [Google Scholar]
- Wong A., Cook M. N., Foley J. J., Sarau H. M., Marshall P., Hwang S. M. Influx of extracellular calcium is required for the membrane translocation of 5-lipoxygenase and leukotriene synthesis. Biochemistry. 1991 Sep 24;30(38):9346–9354. doi: 10.1021/bi00102a030. [DOI] [PubMed] [Google Scholar]
- Wong A., Hwang S. M., Cook M. N., Hogaboom G. K., Crooke S. T. Interactions of 5-lipoxygenase with membranes: studies on the association of soluble enzyme with membranes and alterations in enzyme activity. Biochemistry. 1988 Sep 6;27(18):6763–6769. doi: 10.1021/bi00418a018. [DOI] [PubMed] [Google Scholar]
- Wray W., Boulikas T., Wray V. P., Hancock R. Silver staining of proteins in polyacrylamide gels. Anal Biochem. 1981 Nov 15;118(1):197–203. doi: 10.1016/0003-2697(81)90179-2. [DOI] [PubMed] [Google Scholar]