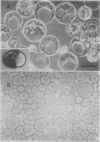
Abstract
Bulk vacuole isolation, gas chromatography-mass spectrometry,, and high-performance liquid chromatography have been used to investigate the accumulation and partitioning of assimilated nitrogen supplied as 15NH4Cl between vacuolar and extravacuolar (cytoplasmic) fractions of protoplasts from suspension cultures of carrot (Daucus carota L. cv Chantenay). Glutamine was the most abundant amino acid in the vacuole of protoplasts from late-exponential phase cells, whereas alanine, glutamate, and γ-aminobutyric acid were located primarily in the cytoplasmic fraction. In 15N-feeding studies, newly synthesized glutamine partitioned strongly to the vacuole, whereas glutamate partitioned strongly to the cytoplasm, γ-aminobutyric acid was totally excluded from the vacuole, and alanine was distributed in both compartments. Comparison of the 15N-enrichment patterns suggests that initial assimilation to glutamine occurs within a subcompartment of the cytoplasmic fraction. The protoplast-feeding technique may be extended to investigate cytoplasmic compartmentation further.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boudet A. M., Canut H., Alibert G. Isolation and Characterization of Vacuoles from Melilotus alba Mesophyll. Plant Physiol. 1981 Dec;68(6):1354–1358. doi: 10.1104/pp.68.6.1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Bush D. R., Sze H. Calcium transport in tonoplast and endoplasmic reticulum vesicles isolated from cultured carrot cells. Plant Physiol. 1986 Feb;80(2):549–555. doi: 10.1104/pp.80.2.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke R. J., Roberts K., Davies D. D. Model for Stress-induced Protein Degradation in Lemna minor. Plant Physiol. 1980 Dec;66(6):1119–1122. doi: 10.1104/pp.66.6.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz K. J., Jäger R., Kaiser G., Martinoia E. Amino Acid Transport across the Tonoplast of Vacuoles Isolated from Barley Mesophyll Protoplasts : Uptake of Alanine, Leucine, and Glutamine. Plant Physiol. 1990 Jan;92(1):123–129. doi: 10.1104/pp.92.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fentem P. A., Lea P. J., Stewart G. R. Ammonia Assimilation in the Roots of Nitrate- and Ammonia-Grown Hordeum Vulgare (cv Golden Promise). Plant Physiol. 1983 Mar;71(3):496–501. doi: 10.1104/pp.71.3.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Robinson S. A., Slade A. P., Fox G. G., Phillips R., Ratcliffe R. G., Stewart G. R. The role of glutamate dehydrogenase in plant nitrogen metabolism. Plant Physiol. 1991 Feb;95(2):509–516. doi: 10.1104/pp.95.2.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streeter J. G., Thompson J. F. In Vivo and In Vitro Studies on gamma-Aminobutyric Acid Metabolism with the Radish Plant (Raphanus sativus, L.). Plant Physiol. 1972 Apr;49(4):579–584. doi: 10.1104/pp.49.4.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thom M., Maretzki A., Komor E. Vacuoles from Sugarcane Suspension Cultures : I. ISOLATION AND PARTIAL CHARACTERIZATION. Plant Physiol. 1982 Jun;69(6):1315–1319. doi: 10.1104/pp.69.6.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner G. J. Content and vacuole/extravacuole distribution of neutral sugars, free amino acids, and anthocyanin in protoplasts. Plant Physiol. 1979 Jul;64(1):88–93. doi: 10.1104/pp.64.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]