Key Points
-
•
Aspacytarabine enables delivery of high-dose cytarabine to unfit patients without the prohibitive cerebellar and gastrointestinal toxicities.
-
•
Aspacytarabine treatment resulted in a CR rate of 36.9% with no prolonged neutropenia or thrombocytopenia during postremission therapy.
Visual Abstract
Abstract
High-dose cytarabine is associated with gastrointestinal and cerebellar toxicity, precluding its use for older or unfit patients with acute myeloid leukemia (AML). Aspacytarabine, an inactive prodrug of cytarabine, was evaluated as monotherapy in a phase 2b study of patients unfit for intensive chemotherapy (NCT03435848). Sixty-five patients with AML were treated with aspacytarabine 4.5 g/m2 per day (equimolar to 3 g/m2 per day cytarabine) for 6 doses per treatment. The median age was 75 years; 60.6% of patients had de novo AML, 28.8% had AML secondary to myelodysplastic syndrome, and 10.6% had therapy-related AML. Overall, 36.9% achieved complete remission (CR) with full count recovery. CR rates in patients with secondary AML, patients with prior treatment with hypomethylating agents, and patients with TP53 mutation were 26.7%, 25%, and 36%, respectively. Median overall survival was 9 months (range, 6-15.9) and was not reached among responders. Hematologic recovery was observed in all responding patients by day 26 without prolonged cytopenias. Adverse events typically precluding the use of high-dose cytarabine in older or unfit patients were not observed. These data suggest that aspacytarabine may be an effective regimen with a reduction in the attendant toxicities associated with high-dose cytarabine, an important consideration when treating AML and other hematologic disorders that use high-dose cytarabine. This trial was registered at www.clinicaltrials.gov as #NCT03435848.
Introduction
Intensive chemotherapy (IC) remains the standard of care for younger and fit patients with newly diagnosed acute myeloid leukemia (AML). However, IC is unsuitable for many older patients with AML who have consistently demonstrated a lower response rate and higher treatment-related mortality and morbidity, leaving this population with few effective treatments.1
Aspacytarabine (BST-236) is a novel cytarabine prodrug composed of cytarabine covalently bound to asparagine via its cytosine residue. This formulation is designed to deliver high doses of cytarabine with lower systemic exposure to peak levels of free cytarabine, thereby reducing systemic toxicity. In a phase 1/2 study conducted in patients with AML and acute lymphoblastic leukemia, aspacytarabine was found to be well-tolerated, with 6 g/m2 per day determined to be the maximal tolerated dose.2
Given these data, an open-label, multicenter, phase 2b study was conducted to assess the efficacy and safety of aspacytarabine in patients with previously untreated de novo or secondary AML unfit for IC.
Methods
This study was conducted at 20 sites in the United States and Israel and was approved by the local institutional review boards. Informed consent was obtained from all subjects in accordance with the Declaration of Helsinki. Newly diagnosed patients with AML (de novo, secondary to myelodysplastic syndrome, or therapy-related, with or without prior treatment with hypomethylating agents [HMAs] for myelodysplastic syndrome) not eligible for IC because of advanced age (≥75 years) or significant comorbidities were enrolled.
Treatment consisted of 1 or 2 induction courses followed by up to 3 consolidation courses. Patients who did not reach at least a complete remission (CR) with incomplete count recovery (CRi) after 2 induction courses received no additional treatment in the study.
Induction and consolidation courses consisted of aspacytarabine monotherapy at 4.5 g/m2 per day, administered IV over 1 hour for 6 consecutive days. The third course of consolidation was permitted for those with evidence of measurable residual disease (MRD) after the second course. The induction course was administered at the hospital, and subsequent treatment was permitted in an outpatient setting. The dose of aspacytarabine was reduced to 2.3 g/m2 per day in any course beyond the first induction for any grade ≥3 nonhematologic, treatment-related adverse event (AE) and during the second and third consolidation courses regardless of toxicity at the discretion of the treating physician. During treatment courses, patients received supportive care as per institutional standards. Only 1 dose reduction was allowed in the study. Response was assessed at the time of count recovery (absolute neutrophil count ≥ 1000/μL and/or platelet count ≥ 100 × 103/μL) or by day 42 (±3) in the absence of recovery, whichever came first. Consolidation was offered when CR, CRi, or CR with partial count recovery (CRh) was achieved.
Responses were assessed based on the revised International Working Group Response Criteria.3 The overall response rate included patients who achieved CR, CRh, CRi, or morphologic leukemia–free state. MRD was assessed in each bone marrow aspirate using multicolor flow cytometry performed centrally at the University of Washington (sensitivity, 10-3).
AEs were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.03.
This study was designed to evaluate the efficacy and safety of aspacytarabine compared with an age-matched control, which was the best standard of care used at protocol initiation. Specifically, the primary end point of CR was compared with an age-matched, random effects meta-analysis of the historical CR rate of 1530 patients from 10 multicenter studies of low-dose cytarabine (LDAC) and HMA in an older and unfit AML population.
The assumptions were that administration of aspacytarabine may 1) enable delivery of safer high-dose cytarabine to a population otherwise unfit for IC and 2) result in a better response compared with standard LDAC/HMA. The trial was designed to have 80% power to demonstrate a 1-sided P value < .025, in comparison with a meta-analysis of historical studies if the true CR rate was between 26% and 29%.
Results
Sixty-six patients were enrolled, and 65 were evaluable for response. One patient was excluded when re-evaluation of the bone marrow did not reveal AML. Baseline demographics and disease characteristics are summarized in Table 1. Most of the patients were ineligible to receive standard IC because of their age (51.5%). Clinically significant cardiac or pulmonary comorbidities accounted for an additional 24% of the study population, and the remaining patients had either a comorbidity that the investigator judged incompatible with intensive induction chemotherapy or a contraindication to anthracycline therapy.
Table 1.
Baseline characteristics (safety set)
| Demographics |
|---|
| Overall age (y), N = 66 |
| Median, 75 |
| Max-min, 47-88 |
| Parameter | Number of patients (N = 66) | Proportion (%) |
|---|---|---|
| Age categories (y) | ||
| ≥75 | 34 | 51.5 |
| 65-75 | 26 | 39.4 |
| 50-65 | 5 | 7.6 |
| <50 | 1 | 1.5 |
| Sex | ||
| Female | 28 | 42.4 |
| Male | 38 | 57.6 |
| Race | ||
| White | 54 | 81.8 |
| Black or African American | 5 | 7.6 |
| Unknown | 4 | 6.1 |
| Asian | 2 | 3 |
| Multiple | 1 | 1.5 |
| ECOG performance status | ||
| 0-1 | 40 | 60.6 |
| 2-3 | 26 | 39.4 |
| AML type | ||
| De novo | 40 | 60.6 |
| Secondary | 26 | 39.4 |
| Previous HMA therapy | ||
| Yes | 12 | 18.2 |
| No | 54 | 81.8 |
| Number of HMA courses | ||
| 0 | 54 | 81.8 |
| 1-2 | 1 | 1.5 |
| 6-10 | 4 | 6.1 |
| >10 | 7 | 10.6 |
| WBC counts | ||
| 10.0-49.9 × 109/L | 15 | 22.7 |
| <10.0 × 109/L | 51 | 77.3 |
| ELN 2017 risk group | ||
| Favorable | 9 | 13.9 |
| Intermediate | 15 | 22.7 |
| Adverse | 33 | 50 |
| Unknown | 9 | 13.6 |
| NPM1 mutation | ||
| Missing | 14 | 21.2 |
| No | 44 | 66.7 |
| Yes | 8 | 12.1 |
| FLT3 mutation | ||
| Missing | 10 | 15.2 |
| No | 50 | 75.8 |
| Yes | 6 (3 with low ITD, 1 high, and 2 IDT unknown) | 9.1 |
| TP53 mutation | ||
| Missing | 18 | 27.3 |
| No | 36 | 54.5 |
| Yes | 11 | 18.2 |
AML, acute myeloid leukemia; ECOG, Eastern Cooperative Oncology Group; ELN, European LeukemiaNet; FLT3, FMS-like receptor tyrosine kinase-3; HMA, hypomethylating agents; ITD, intertandem duplication; max, maximum; min, minimum; NPM1, nucleophosmin 1; TP53, tumor protein 53.
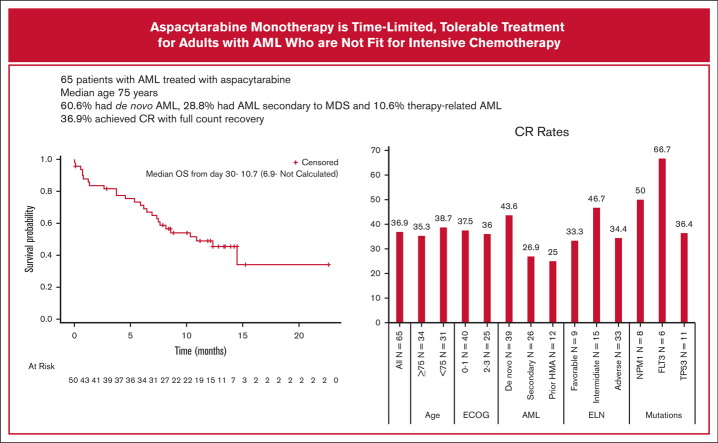
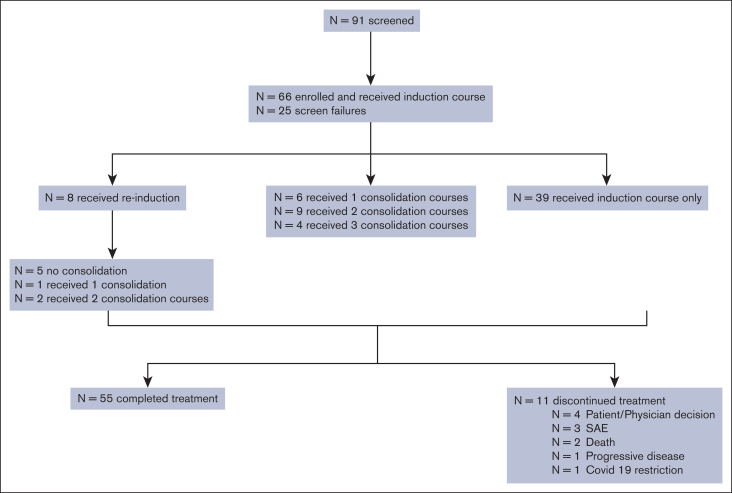
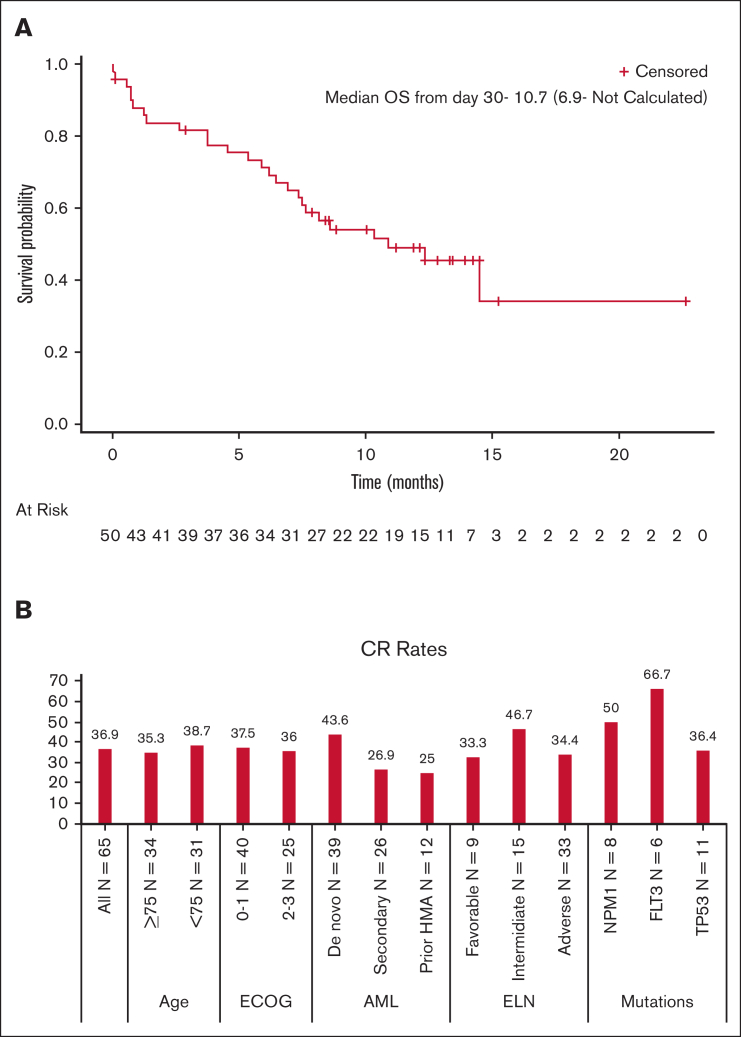
Twenty-four patients (36.9% of the intention-to-treat [ITT] population) achieved CR (with full count recovery); 16 reached CR after the first induction and 8 after the second induction. This CR rate is significantly higher than that achieved in an age-matched meta-analysis of historical studies of LDAC and HMA, in which the CR rate for a similarly aged population was 14.9% (P < .0001). The CR rates among patients with de novo and secondary AML were 43.6% and 26.9%, respectively. Of 12 patients with prior HMA therapy, 3 (25%) achieved CR, whereas 21 of 53 patients (39.6%) with HMA-naïve AML achieved CR. Patients with FLT3, NPM1, FLT3 + NPM1, or TP53 mutations had CR rates of 66.7%, 50.0%, 66%, and 36.4%, respectively. MRD negativity was achieved in 12 patients (18.5% of the ITT) and in 50% of the responders (12 of 24). One patient with a complex karyotype achieved morphologic leukemia–free state. Patient disposition is described in Figure 1. The majority (59.1%) received only 1 induction course because of disease progression or study termination, and 33.3% received at least 1 consolidation course. Treatment was completed as planned for 55 of the 65 patients with AML, but 11 discontinued treatment before the completion of all intended courses, including the 1 patient who did not have AML. Reasons for treatment discontinuation included death (n = 2), withdrawal of consent (n = 2), physician preference (n = 2), disease progression (n = 1), and inability to travel because of COVID restrictions (n = 1). Three patients discontinued because of AEs; 1 patient discontinued the second treatment 6 days course because of symptomatic acute or chronic left subdural hemorrhage.
Figure 1.
CONSORT diagram. SAE, serious adverse event.
Of 66 patients, 56 (84.8%) received dose intensity as planned (4.5g/m2 per day), and the others had reduced dose intensity either because of AEs or the physician's decision, as allowed per protocol in the second consolidation.
The median number of courses to CR was 1, with a median CR duration of 6.5 months (95% CI, 3.9-7.5). The median time for absolute neutrophil count to reach ≥500 cells per μL after induction and consolidation was 24 and 21 days, respectively, and to reach >1000 cells per μL was 26 and 21 days, respectively (Table 2). The median time for platelets to reach ≥50 000 cells per μL after induction and consolidation was 24 and 23.5 days, respectively, and to reach >100 000 cells per μL was 24 and 25.5 days, respectively (Table 2). The time to count recovery in the consolidation courses compared with that of the induction courses was not prolonged.
Table 2.
ANC and platelet count recovery per course in patients who reached CR/CRi/CRh
| Course | Course day | ANC |
Platelets |
||
|---|---|---|---|---|---|
| Mean cells per μL | ±SE cells per μL | Mean cells per μL | ±SE cells per μL | ||
| Induction | 4 | 1174 | 616-1731 | 44 412 | 35 570-53 255 |
| 10 | 302 | 106-499 | 21 846 | 18 874-24 818 | |
| 17 | 227 | 64-390 | 21 349 | 17 256-25 442 | |
| 24 | 1224 | 805-1642 | 136 166 | 109 964-162 368 | |
| 31 | 3342 | 2392-4291 | 219 239 | 185 154-253 324 | |
| 38 | 5038 | 3271-6805 | 250 873 | 210 604-291 143 | |
| 45 | 4565 | 3380-5749 | 151 500 | 128 026-174 973 | |
| Reinduction | 4 | 2399 | 1493-3305 | 124 833 | 76 248-182 418 |
| 10 | 954 | 604-1304 | 46 357 | 29 896-62 828 | |
| 17 | 87 | 35-139 | 21 000 | 9 255-32 744 | |
| 24 | 1087 | 293-1882 | 171 805 | 105 242-238 368 | |
| 31 | 2746 | 1459-4033 | 490 666 | 436 622-544 710 | |
| 38 | 5565 | 5330-5800 | 475 000 | 464 000-486 000 | |
| 45 | 10 020 | NA | 404 000 | NA | |
| First consolidation | 4 | 4791 | 4173-5408 | 202 401 | 174 579-230 224 |
| 10 | 3056 | 2453-3659 | 46 062 | 35 856-56 268 | |
| 17 | 1605 | 963-2247 | 31 666 | 18 211-45 121 | |
| 24 | 3405 | 2426-4383 | 183 218 | 145 852-220 585 | |
| 31 | 8395 | 5505-11 284 | 354 145 | 300 442-407 849 | |
| 38 | 3380 | 2909-3850 | 227 500 | 198 591-256 408 | |
| Second consolidation | 4 | 4146 | 2916-5375 | 245 479 | 212 279-278 678 |
| 10 | 5385 | 2019-8751 | 66 937 | 53 911-79 953 | |
| 17 | 735 | 452-1018 | 21 791 | 15 155-28 427 | |
| 24 | 2460 | 1686-3235 | 186 645 | 111 170-262 120 | |
| 31 | 2850 | 2429-3270 | 279 125 | 199 853-358 396 | |
| 38 | 2810 | NA | 145 000 | NA | |
| Third consolidation | 4 | 4700 | NA | 19 516 667 | 92 333-298 000 |
| 10 | 2856 | NA | 57 000 | 34 000-80 000 | |
| 17 | 190 | NA | 1250 | 500-2000 | |
| 24 | 2390 | NA | 118 000 | 30 000-206 000 | |
| 31 | 3776 | NA | 236 500 | 142 000-331 000 | |
| 38 | 3503 | NA | 131 666 | NA | |
NA, not applicable.
The median follow-up for all patients was 13.7 months (range, 12.5-15.3). Thirty-six patients (55%) died, including 28 nonresponders and 8 who achieved CR. Causes of death among patients who achieved CR were AML recurrence (n = 6), sepsis (n = 1), and acute respiratory failure (n = 1). The median relapse-free survival was 8.7 months, with a 1-year rate of 40.6%. The median overall survival (OS) was 9.0 (95% CI, 6.0-15.9) months in the ITT population and not reached among patients who achieved CR (n = 24) compared with 4 months (95% CI, 2.0-7.9 months) (n = 41) in nonresponding patients. The median OS calculated according to landmark analysis from day 30 (N = 50) was 10.7 months (Figure 2A). The 1-year OS for responders was 79.2%. CR rates by subgroups are presented in Figure 2B.
Figure 2.
Efficacy results. (A) OS for all patients using landmark analysis. Efficacy results: OS for all patients using a landmark analysis. (B) Efficacy results: CR rates according to subgroups. CR rates based on subgroups.ECOG, Eastern Cooperative Oncology Group; ELN, European LeukemiaNet.
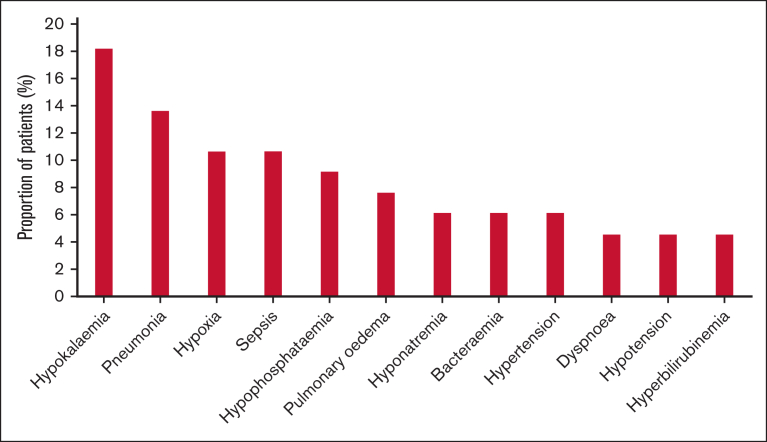
All patients were evaluable for safety. All experienced at least 1 treatment-emergent AE (TEAE), with 62 patients (94%) experiencing a grade ≥3 TEAE. The most common grade ≥3 nonhematologic TEAEs (>10%) were hypokalemia (18.2%), pneumonia (13.6%), hypoxia (10.6%), and sepsis (10.6%; Figure 3). Grade ≥3 gastrointestinal AEs were reported by 13.6% of patients; diarrhea grade >2 by 3% of patients, and stomatitis grade >1 by 4.5% of patients. There were no TEAEs of cerebellar toxicity grade ≥3.
Figure 3.
Nonhematologic grade >2 AEs in >2 patients.
Ten patients died after TEAEs. The most common causes were sepsis (n = 4) and pulmonary edema (n = 2). One patient each died of multiorgan failure, COVID-19 pneumonia, cerebrovascular accident, and unknown cause. The 30-day all-cause mortality rate was 12.6%.
AEs leading to dose modification occurred in 1 patient (1.5%). Discontinuation of aspacytarabine because of an AE occurred in 1 patient because of cognitive disorder, dysarthria, and confusional state in the context of acute on chronic subdural hemorrhage. Two patients in CR discontinued the study after induction and did not receive any consolidation course; 1 because of fluctuations in creatinine levels and the other because of fluid overload due to transfusions. No gastrointestinal toxicities above grade 3 and no cases of grade ≥3 cerebellar toxicity were reported. Thus, AEs precluding the use of high-dose cytarabine in unfit patients, including severe gastrointestinal and cerebellar toxicity, were minimal in the study population, even though the median age was 75 and patients with moderate renal failure (lower limit of creatinine clearance = 45 mL/min) were eligible to enroll.
Discussion
Recently, venetoclax in combination with HMA or LDAC and targeted therapies, such as FLT3 and isocitrate dehydrogenase inhibitors, have transformed the treatment landscape in AML, including for patients unfit for IC. However, 34% to 39% of reported newly diagnosed older patients may not respond to venetoclax-based lower-intensity regimens,4,5 and patients without specific mutations do not benefit from targeted therapies. In addition, responses to venetoclax-based regimens are often short-lived, accompanied by prolonged cytopenias,6 and associated with an extremely poor prognosis upon relapse with a median OS of 2.9 months.7 Thus, there is still an unmet need for effective treatment for unfit patients with AML.
Collectively, given the response achieved in this study with limited treatment courses, without prolonged cytopenias after repeated administration, and a relatively favorable toxicity profile, these data suggest that aspacytarabine may provide an alternative firstline treatment option for patients with AML who are either old or otherwise unfit for IC. Studies of aspacytarabine in other hematologic diseases that commonly incorporate high-dose cytarabine, such as acute lymphoblastic leukemia or lymphoma, have been proposed, and a phase 1 study combining aspacytarabine with venetoclax as induction treatment for newly diagnosed unfit patients with AML is currently ongoing (NCT05503355).
Conflict-of-interest disclosure: J.K.A. is on the data monitoring committee at GlycoMimetics; received consulting or advisory fees from AbbVie, Astellas Pharma, BioSight, bluebird bio, Curio, Daiichi Sankyo, Gilead, Kura Oncology, Kymera, Rigel Pharmaceuticals, Stemline Therapeutics, and Syros; received personal fees from the American Society of Hematology, Aptitude Health, HMP Education, MD Education, PeerView, and VJ HemOnc; received travel support from BioSight, Insights in Hematology, and National Comprehensive Cancer Network; received research funding (all to institution) from AbbVie, Agios, ALX Oncology, Amgen, Amphivena, Aprea AB, Aptose Biosciences, Astellas Pharma, BioSight, Boehringer Ingelheim, Bristol Myers Squibb (BMS), Celgene, Cyclacel, Fujifilm, ImmunoGen, Kartos Therapeutics, Kura Oncology, Loxo, Pfizer, and Telios; and received travel, accommodations, expenses from Astellas Pharma, BioSight, and Dava Oncology. T.Z. is a paid consultant for BioSight and received honorarium from AbbVie, Gilead Sciences, Janssen, Novartis, BMS, Sanofi, and Takeda. J.K. is on the advisory board/consultant for Apellis, Novartis, GlaxoSmithKline, and Alexion, and on the speaker's bureau for Alexion, Apellis, Amgen, Jazz, CTI, BMS, and AbbVie. V.K. is on the advisory board for Pfizer and Novartis. Y.A. received research funding (to institution) from ALX Oncology, BioSight, Curis, Biomea, and Novartis, and is on the advisory board/received honoraria from BMS, Kite, Pfizer, Servier, Astellas, and Rigel. B.B. received research funding from Karyopharm Pharmaceuticals, Cell Therapeutics, and Sumitomo Pharma Oncology, and received advisory board honoraria from BMS, Novartis, Celgene, Karyopharm, Daiichi Sankyo, and Cell Therapeutics. S.M.L. received research funding from Onconova, Celgene, BioSight, Hoffman-La Roche, and Kura (travel included); is on data safety monitoring board at Marker Therapeutics; and is on the advisory board of AbbVie, Amgen, BMS, Pluristem, Syros, and Agios. M.-E.P. received clinical trial support to the institution from Pfizer, Glycomimetics, BioSight, BMS, AbbVie, Ascentage, Telios, and Astex. O.W. received research support from AbbVie; received speaker honoraria from AbbVie, Astellas, and Novartis; and has an advisory role at AbbVie, Astellas, Novartis, Pfizer, Medison, and Teva. L.F., S.T., and C.B. are BioSight employees. S.G. is a paid consultant at BioSight. R.B.Y. is a BioSight employee and shareholder. J.M.R. is a paid consultant at BioSight. The remaining authors declare no competing financial interests.
Acknowledgments
Authorship
Contribution: J.K.A. and J.M.R. performed and designed research, and wrote the manuscript; T.Z. and A.G. designed and performed research; J.K., J.M., V.K., M.K., O.F., D.L.B., A.E., M.B., B.B., S.M.L., M.-E.P., O.W., M.C., C.G., G.R., I.L. performed research; Y.A. wrote the manuscript; L.F. and R.B.Y. designed research, analyzed and interpreted data, and wrote the manuscript; S.T. designed research and analyzed and interpreted data; C.B. collected data and analyzed and interpreted data; and S.G. designed research.
Footnotes
The protocol is available on request from the corresponding author, Jessica K. Altman (j-altman@northwestern.edu).
References
- 1.Kantarjian H, Ravandi F, O’Brien S, et al. Intensive chemotherapy does not benefit most older patients (age 70 years or older) with acute myeloid leukemia. Blood. 2010;116(22):4422–4429. doi: 10.1182/blood-2010-03-276485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zuckerman T, Ram R, Akria L, et al. BST-236, a novel cytarabine prodrug for patients with acute leukemia unfit for standard induction: a phase 1/2a study. Blood Adv. 2019;3(22):3740–3749. doi: 10.1182/bloodadvances.2019000468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheson BD, Bennett JM, Kopecky KJ, et al. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol. 2003;21(24):4642–4649. doi: 10.1200/JCO.2003.04.036. [DOI] [PubMed] [Google Scholar]
- 4.DiNardo CD, Jonas BA, Pullarkat V, et al. Azacitidine and venetoclax in previously untreated acute myeloid leukemia. N Engl J Med. 2020;383(7):617–629. doi: 10.1056/NEJMoa2012971. [DOI] [PubMed] [Google Scholar]
- 5.Fleischmann M, Scholl S, Frietsch JJ, et al. Clinical experience with venetoclax in patients with newly diagnosed, relapsed, or refractory acute myeloid leukemia. J Cancer Res Clin Oncol. 2022;148(11):3191–3202. doi: 10.1007/s00432-022-03930-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rausch CR, DiNardo CD, Maiti A, et al. Duration of cytopenias with concomitant venetoclax and azole antifungals in acute myeloid leukemia. Cancer. 2021;127(14):2489–2499. doi: 10.1002/cncr.33508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maiti A, Rausch CR, Cortes JE, et al. Outcomes of relapsed or refractory acute myeloid leukemia after frontline hypomethylating agent and venetoclax regimens. Haematologica. 2021;106(3):894–898. doi: 10.3324/haematol.2020.252569. [DOI] [PMC free article] [PubMed] [Google Scholar]