Abstract
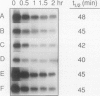
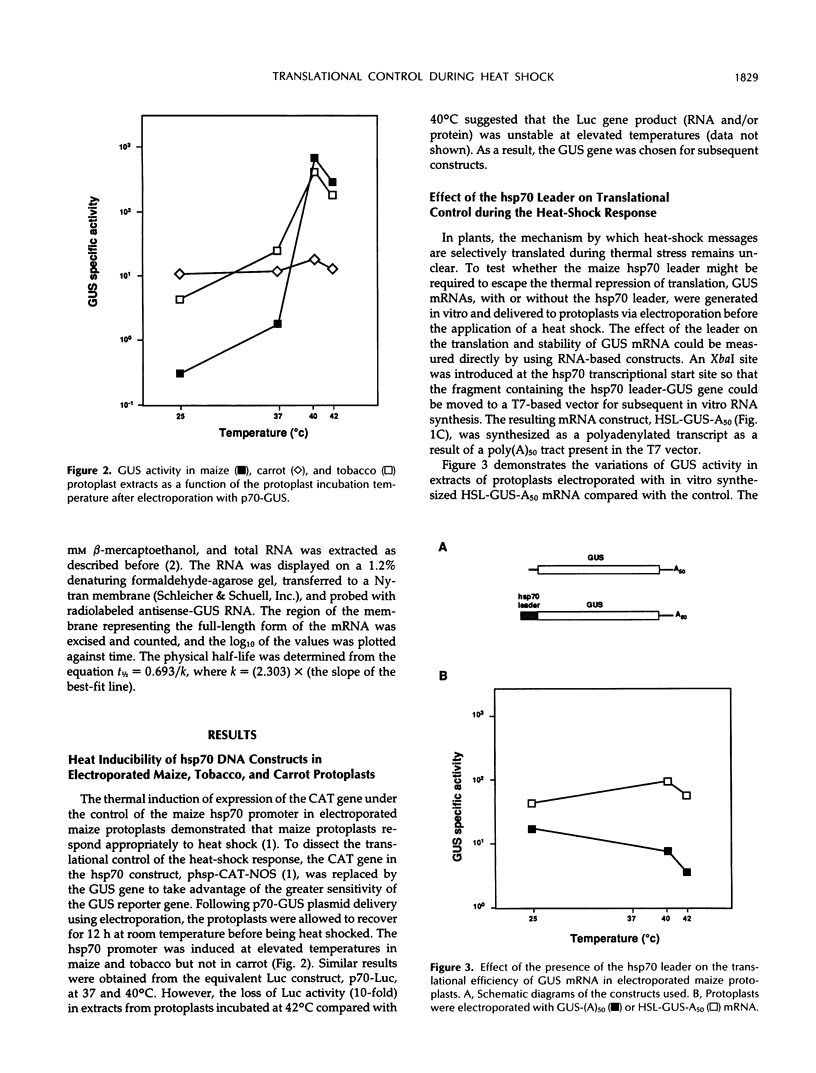
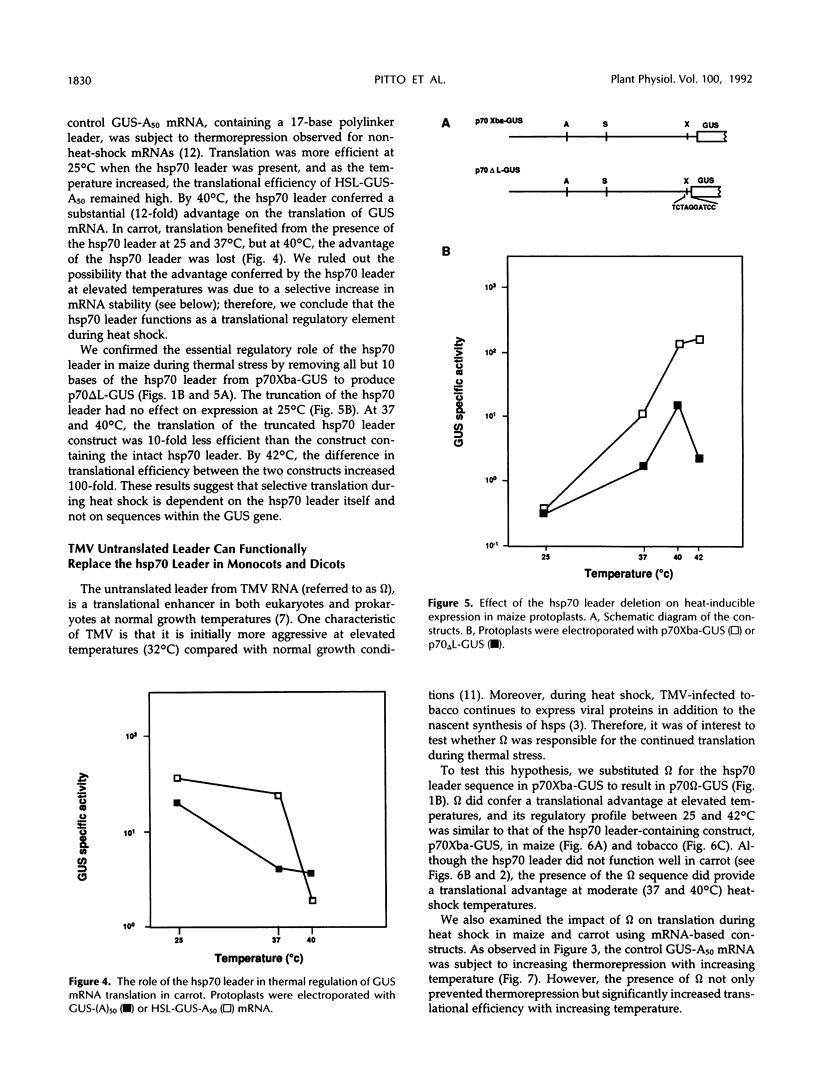
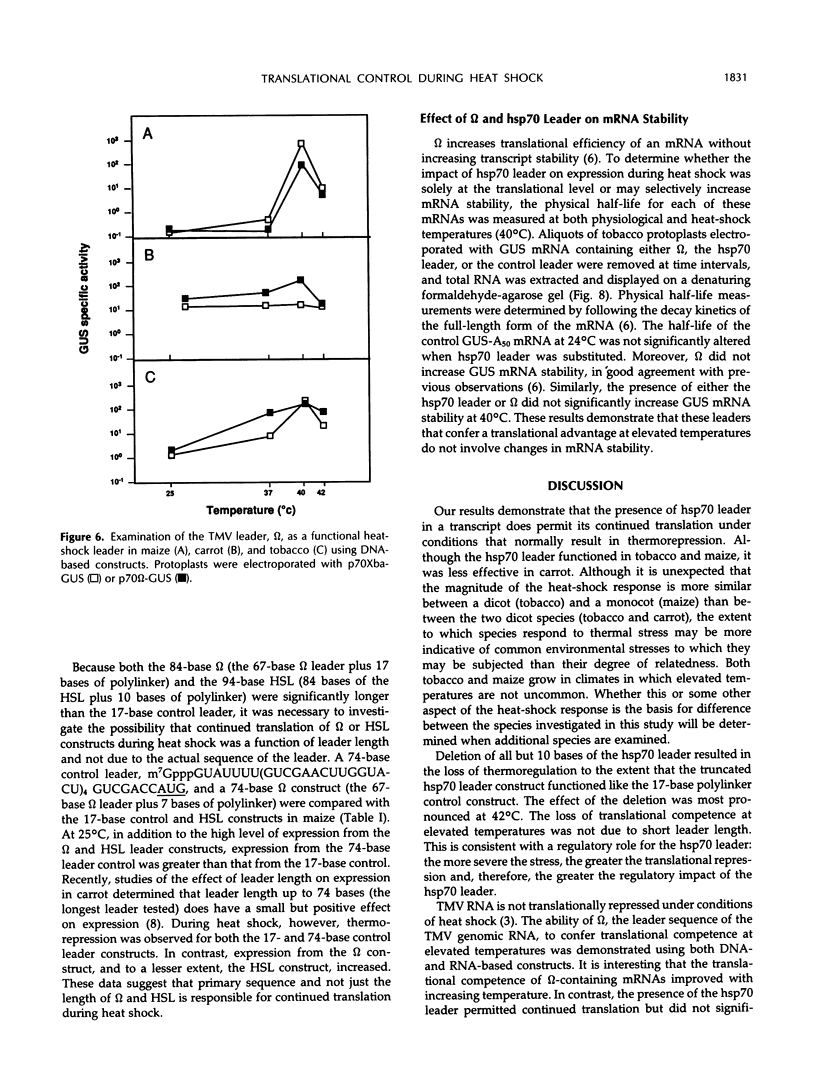
The 5′-untranslated leader of maize (Zea mays) heat-shock protein (hsp) 70 mRNA is required for translational competence during heat shock in protoplasts. When the β-glucuronidase gene was used as a reporter mRNA, expression at elevated temperatures increased more than 10-fold when the hsp70 leader constituted the 5′-untranslated region. The hsp70 leader did not affect the physical half-life of the mRNA and, therefore, does not function at the level of transcript stability. The maize hsp70 leader was required to escape thermal repression in both maize and tobacco (Nicotiana tabacum) but was less effective in carrot. In addition, mRNAs containing the tobacco mosaic virus untranslated leader (ω) were also efficiently translated during heat shock, data suggesting that the presence of the ω sequence enables the transcript to escape the translational repression that occurs during thermal stress.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Callis J., Fromm M., Walbot V. Heat Inducible Expression of a Chimeric Maize hsp70CAT Gene in Maize Protoplasts. Plant Physiol. 1988 Dec;88(4):965–968. doi: 10.1104/pp.88.4.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Duncan R., Milburn S. C., Hershey J. W. Regulated phosphorylation and low abundance of HeLa cell initiation factor eIF-4F suggest a role in translational control. Heat shock effects on eIF-4F. J Biol Chem. 1987 Jan 5;262(1):380–388. [PubMed] [Google Scholar]
- Gallie D. R., Lucas W. J., Walbot V. Visualizing mRNA expression in plant protoplasts: factors influencing efficient mRNA uptake and translation. Plant Cell. 1989 Mar;1(3):301–311. doi: 10.1105/tpc.1.3.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallie D. R., Sleat D. E., Watts J. W., Turner P. C., Wilson T. M. A comparison of eukaryotic viral 5'-leader sequences as enhancers of mRNA expression in vivo. Nucleic Acids Res. 1987 Nov 11;15(21):8693–8711. doi: 10.1093/nar/15.21.8693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallie D. R., Walbot V. Identification of the motifs within the tobacco mosaic virus 5'-leader responsible for enhancing translation. Nucleic Acids Res. 1992 Sep 11;20(17):4631–4638. doi: 10.1093/nar/20.17.4631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurley W. B., Czarnecka E., Nagao R. T., Key J. L. Upstream sequences required for efficient expression of a soybean heat shock gene. Mol Cell Biol. 1986 Feb;6(2):559–565. doi: 10.1128/mcb.6.2.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultmark D., Klemenz R., Gehring W. J. Translational and transcriptional control elements in the untranslated leader of the heat-shock gene hsp22. Cell. 1986 Feb 14;44(3):429–438. doi: 10.1016/0092-8674(86)90464-2. [DOI] [PubMed] [Google Scholar]
- Lebeurier G., Hirth L. Effect of elevated temperatures on the development of two strains of tobacco mosaic virus. Virology. 1966 Jul;29(3):385–395. doi: 10.1016/0042-6822(66)90214-5. [DOI] [PubMed] [Google Scholar]
- Lindquist S. The heat-shock response. Annu Rev Biochem. 1986;55:1151–1191. doi: 10.1146/annurev.bi.55.070186.005443. [DOI] [PubMed] [Google Scholar]
- McGarry T. J., Lindquist S. The preferential translation of Drosophila hsp70 mRNA requires sequences in the untranslated leader. Cell. 1985 Oct;42(3):903–911. doi: 10.1016/0092-8674(85)90286-7. [DOI] [PubMed] [Google Scholar]
- Pelham H. R. A regulatory upstream promoter element in the Drosophila hsp 70 heat-shock gene. Cell. 1982 Sep;30(2):517–528. doi: 10.1016/0092-8674(82)90249-5. [DOI] [PubMed] [Google Scholar]
- Rochester D. E., Winer J. A., Shah D. M. The structure and expression of maize genes encoding the major heat shock protein, hsp70. EMBO J. 1986 Mar;5(3):451–458. doi: 10.1002/j.1460-2075.1986.tb04233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozen F., Edery I., Meerovitch K., Dever T. E., Merrick W. C., Sonenberg N. Bidirectional RNA helicase activity of eucaryotic translation initiation factors 4A and 4F. Mol Cell Biol. 1990 Mar;10(3):1134–1144. doi: 10.1128/mcb.10.3.1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schöffl F., Rieping M., Baumann G., Bevan M., Angermüller S. The function of plant heat shock promoter elements in the regulated expression of chimaeric genes in transgenic tobacco. Mol Gen Genet. 1989 Jun;217(2-3):246–253. doi: 10.1007/BF02464888. [DOI] [PubMed] [Google Scholar]
- Vancanneyt G., Rosahl S., Willmitzer L. Translatability of a plant-mRNA strongly influences its accumulation in transgenic plants. Nucleic Acids Res. 1990 May 25;18(10):2917–2921. doi: 10.1093/nar/18.10.2917. [DOI] [PMC free article] [PubMed] [Google Scholar]