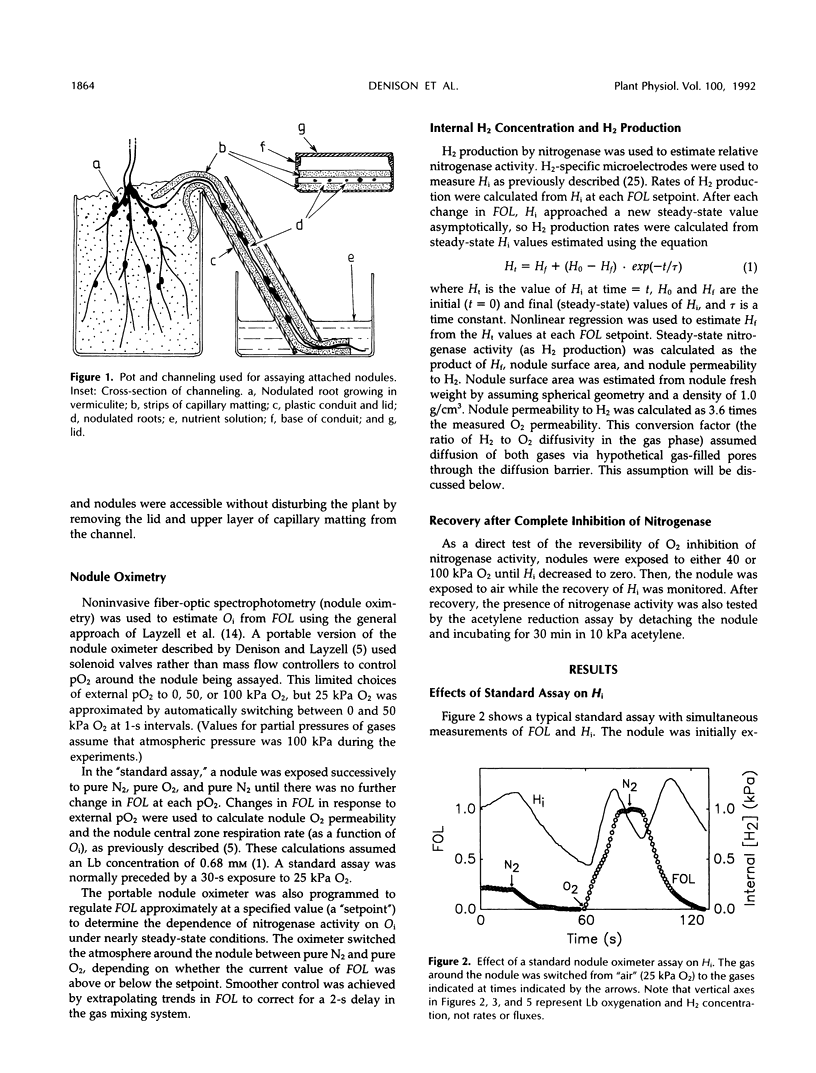
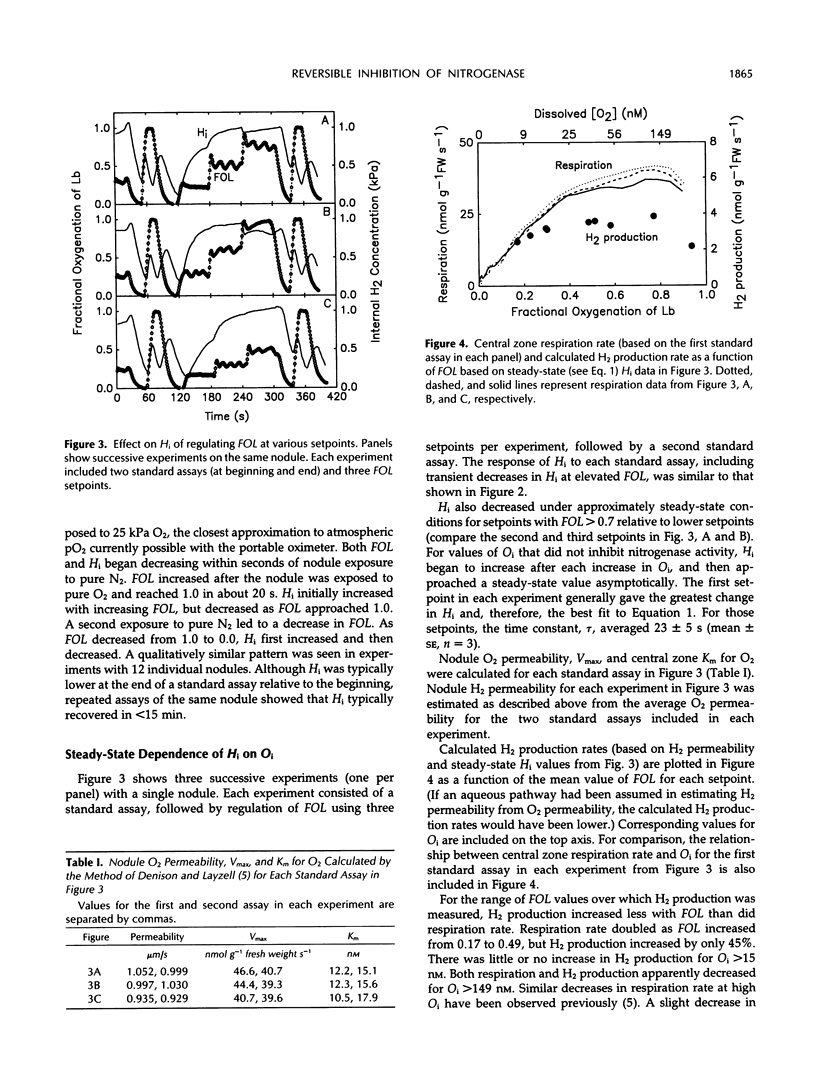
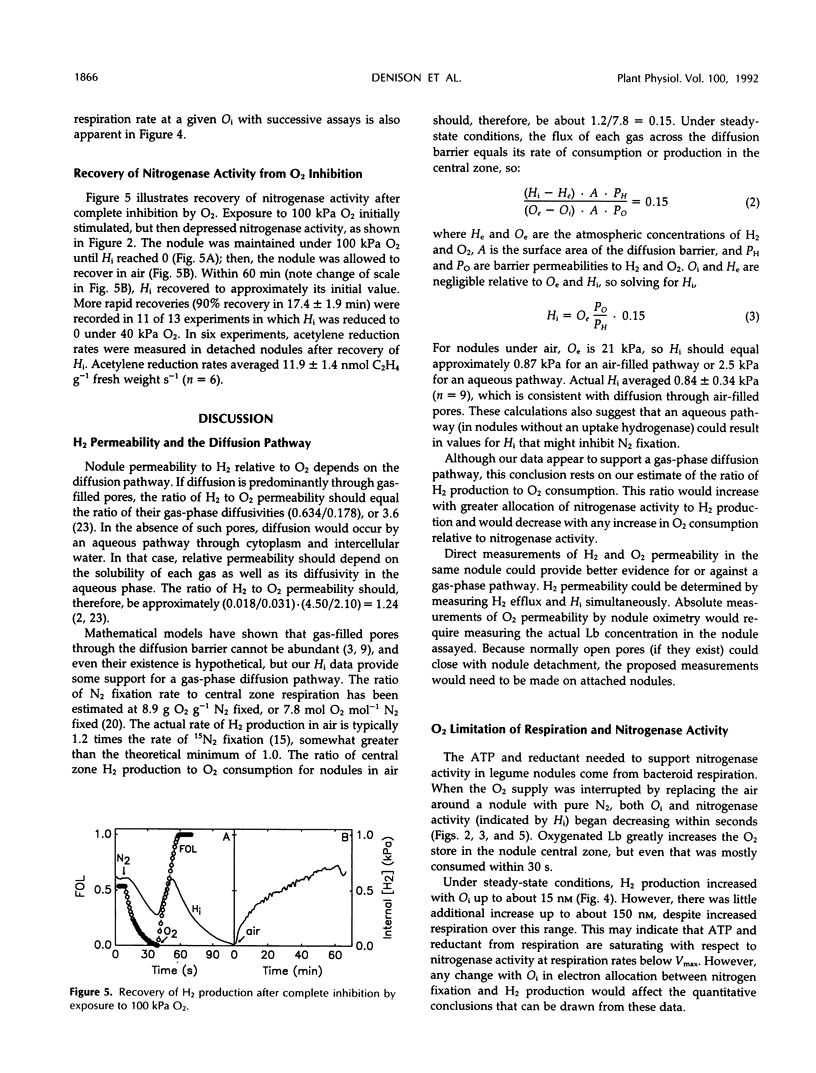
Abstract
Various forms of stress result in decreased O2 permeability or decreased capacity to consume O2 in legume root nodules. These changes alter the nodule interior O2 concentration (Oi). To determine the relationship between Oi and nitrogenase activity in attached soybean (Glycine max) nodules, we controlled Oi by varying external pO2 while monitoring internal H2 concentration (Hi) with microelectrodes. Oi was monitored by noninvasive leghemoglobin spectrophotometry (nodule oximetry). After each step-change in Oi, Hi approached a new steady state, with a time constant averaging 23 s. The rate of H2 production by nitrogenase was calculated as the product of Hi, nodule surface area, and nodule H2 permeability. H2 permeability was estimated from O2 permeability (measured by nodule oximetry) by assuming diffusion through air-filled pores; support for this assumption is presented. Oi was nearly optimal for nitrogenase activity (H2 production) between 15 and 150 nm. A 1- to 2-min exposure to elevated external pO2 (40-100 kPa) reduced Hi to zero, but nitrogenase activity recovered quickly under air, often in <20 min. This rapid recovery contrasts with previous reports of much slower recovery with longer exposures to elevated pO2. The mechanism of nitrogenase inhibition may differ between brief and prolonged O2 exposures.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Denison R. F., Hunt S., Layzell D. B. Nitrogenase activity, nodule respiration, and o(2) permeability following detopping of alfalfa and birdsfoot trefoil. Plant Physiol. 1992 Mar;98(3):894–900. doi: 10.1104/pp.98.3.894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denison R. F., Layzell D. B. Measurement of legume nodule respiration and o(2) permeability by noninvasive spectrophotometry of leghemoglobin. Plant Physiol. 1991 May;96(1):137–143. doi: 10.1104/pp.96.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denison R. F. Mathematical modeling of oxygen diffusion and respiration in legume root nodules. Plant Physiol. 1992 Mar;98(3):901–907. doi: 10.1104/pp.98.3.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denison R. F., Weisz P. R., Sinclair T. R. Analysis of acetylene reduction rates of soybean nodules at low acetylene concentrations. Plant Physiol. 1983 Nov;73(3):648–651. doi: 10.1104/pp.73.3.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt S., King B. J., Canvin D. T., Layzell D. B. Steady and nonsteady state gas exchange characteristics of soybean nodules in relation to the oxygen diffusion barrier. Plant Physiol. 1987 May;84(1):164–172. doi: 10.1104/pp.84.1.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt S., King B. J., Layzell D. B. Effects of gradual increases in o(2) concentration on nodule activity in soybean. Plant Physiol. 1989 Sep;91(1):315–321. doi: 10.1104/pp.91.1.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King B. J., Hunt S., Weagle G. E., Walsh K. B., Pottier R. H., Canvin D. T., Layzell D. B. Regulation of o(2) concentration in soybean nodules observed by in situ spectroscopic measurement of leghemoglobin oxygenation. Plant Physiol. 1988 Jun;87(2):296–299. doi: 10.1104/pp.87.2.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King B. J., Layzell D. B. Effect of Increases in Oxygen Concentration during the Argon-Induced Decline in Nitrogenase Activity in Root Nodules of Soybean. Plant Physiol. 1991 Jun;96(2):376–381. doi: 10.1104/pp.96.2.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layzell D. B., Hunt S., Palmer G. R. Mechanism of Nitrogenase Inhibition in Soybean Nodules : Pulse-Modulated Spectroscopy Indicates that Nitrogenase Activity Is Limited by O(2). Plant Physiol. 1990 Apr;92(4):1101–1107. doi: 10.1104/pp.92.4.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robson R. L., Postgate J. R. Oxygen and hydrogen in biological nitrogen fixation. Annu Rev Microbiol. 1980;34:183–207. doi: 10.1146/annurev.mi.34.100180.001151. [DOI] [PubMed] [Google Scholar]
- Silvester W. B., Winship L. J. Transient responses of nitrogenase to acetylene and oxygen in actinorhizal nodules and cultured frankia. Plant Physiol. 1990 Feb;92(2):480–486. doi: 10.1104/pp.92.2.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair T. R., Goudriaan J. Physical and morphological constraints on transport in nodules. Plant Physiol. 1981 Jan;67(1):143–145. doi: 10.1104/pp.67.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisz P. R., Sinclair T. R. Regulation of Soybean Nitrogen Fixation in Response to Rhizosphere Oxygen: II. Quantification of Nodule Gas Permeability. Plant Physiol. 1987 Jul;84(3):906–910. doi: 10.1104/pp.84.3.906. [DOI] [PMC free article] [PubMed] [Google Scholar]


