Abstract
Pectinolytic enzymes are among the important group of industrial enzymes that have wide applications in different food industries. In this study, pectinase-based silica nanocarriers were synthesized using co-precipitation and cross-linking techniques. The resulting silica nanoparticles were investigated using scanning electron microscopy (SEM), energy-dispersive electron microscopy (EDEX), and X-ray diffraction (XRD) for determination of its morphology, elemental composition, and crystalline pattern. Under the optimal immobilization conditions like 1.5 % glutaraldehyde, 3000 IU/mg pectinase concentration, 90 min immobilization time and 40 °C immobilization temperature, pectinase showed maximum immobilization yield. The immobilization of pectinase onto the silica nanocarriers led to enhanced catalytic characteristics, displaying higher enzymatic activity across various temperature and pH levels compared to soluble pectinase. Moreover, the immobilization substantially improved the temperature stability of pectinase, exhibiting 100 % of its initial activity even after 120 h of pre-incubation at 50 °C. Additionally, the silica nanocarrier pectinase retained 100 % of its original activity even after being reused 10 times in a single batch of reactions. These findings indicate that the immobilization of silica nanocarriers effectively enhances pectinase's industrial capabilities, making it economically feasible for industrial use and an efficient system for various biotechnological applications.
Keywords: Co-precipitation, Crosslinking, Pectinase, Silica nanocarriers, Immobilization, Industrial capabilities, Biotechnological applications
1. Introduction
Enzymes have found widespread application as biocatalysts in various industrial processes, such as food production, textiles, papermaking, wastewater treatment, and pharmaceuticals, due to their advantageous attributes like high substrate selectivity, catalytic efficiency, mild reaction conditions, and environmental friendliness [1]. Among these enzymes, pectinolytic enzymes are a group of enzymes responsible for hydrolysis of pectinolytic substances. Pectinolytic enzymes have utilization in diverse industrial applications, including extraction of fruit juices, bioscouring of cotton fibers, coffee and tea fermentation and many more [2,3]. However, the unfavourable conditions prevalent in industrial processes can adversely affect the catalytic properties of enzymes, limiting their effectiveness in industrial preparations.
To address this challenge, immobilization and protein engineering techniques have been explored to enhance the operational stability of enzymes under harsh industrial conditions. Immobilization not only improves the stability of enzymes but also allows for their reuse in continuous industrial processes, making them cost-effective for commercial use [4,5]. Various methods have been developed for enzyme immobilization, with enzyme crosslinking onto solid supports being considered particularly effective, as it enhances the catalytic activities and reusability of the enzymes. This involves the reaction of the free amine groups of enzyme protein chains with the aldehyde group of glutaraldehyde, forming Schiff bases that link the enzymes to solid supports [[6], [7], [8], [9]].
Several support materials, such as calcium alginate, agar-agar, polyacrylamide, diethylaminoethyl (DEAE) cellulose and silica have been investigated for enzyme immobilization. Among these, nanocarriers have emerged as highly suitable support materials because of their unique properties, including large surface area, shape retention, availability in different sizes and arrangements, and the ability to withstand harsh chemical modifications while maintaining their surface characteristics [[10], [11], [12], [13], [14], [15]].
In this study, functionally activated SiO2 nanoparticles were synthesized for the immobilization of pectinase, aiming to achieve enhanced stability and reusability. Silica-based nanoparticles exhibit environmental benignness, high biocompatibility, and resistance to organic solvents and microbial attacks, making them ideal candidates for enzyme immobilization [[16], [17], [18]]. Silica nanoparticles improve the activity and durability of enzymes by providing precise spatiotemporal control as well as by maintaining systemic biodistributions for boosting the catalytic outcome. Therefore, the main objectives of this research are to optimize the immobilization process parameters and kinetic parameters of both free and immobilized enzymes, as well as to evaluate the multiple reuse of the immobilized pectinase for continuous product formation. These investigations provide valuable insights into enhancing the industrial applicability of pectinase and contribute to the development of efficient and stable biocatalysts for continuous industrial use. Moreover, the findings will be used to overcome the issues of soluble enzymes.
2. Material and methods
2.1. Production of pectinase
Pectinase was produced through submerged fermentation using a bacterial strain (Bacillus subtilis) as reported previously [8]. To partially purify the pectinase from the culture medium, centrifugation (10,000 g for 15 min at 4 °C) and ammonium sulfate precipitation with 50 % saturation were employed. The resulting precipitates were redissolved in glycine-NaOH buffer and subsequently dialyzed for 03 h against the aforesaid buffer. These dialyzed precipitates were then used for the immobilization of enzyme and kinetic experiments [8].
2.2. Synthesis of silica nanoparticles
Silica nanoparticles were synthesized using the sol-gel method with some modifications [10,19]. In this process, 5 ml of tetraethylorthosilicate (99.5 %) was mixed deionized water (10 ml) and 99.9 % ethanol (20 ml). The mixture was then sonicated for 15 min. Additionally, 2 g of CTAB (cetyltrimethylammonium bromide) surfactant was added to the mixture. After 15 min, a dropwise addition of 30 % ammonia solution resulted in the formation of a white-turbid solution, indicating hydrolysis and condensation. The reaction mixture was sonicated for 1 h, and the resulting powder was dried overnight at 100 °C. The dried product was then calcined at 650 °C for 1 h and was placed in a vacuum desiccator for further analysis.
2.3. Scanning electron microscopy (SEM) and energy dispersive electron microscopy (EDX)
The surface morphology and size of the silica nanoparticles were examined using a JSM Jeol 6380A scanning electron microscope (City, Japan). The elemental composition of the silica nanoparticles was determined using energy dispersive electron microscopy (EDX) with the standard addition method, employing an accelerating voltage of 20 keV.
2.4. X-ray diffraction analysis
X-ray diffraction analyses were performed to characterize the crystalline phase of the silica nanoparticles. A Bruker D8 Advance X-Ray Diffractometer equipped with a CuK radiation source (k = 1.5405 A) was used for the analysis. The samples were analyzed at a rate of 10° per minute, and the 2 theta range was set between 10° and 80°.
2.5. Immobilization of pectinase on silica nanoparticles
To immobilize pectinase on silica nanoparticles, a solution was prepared containing silica nanoparticles (0.1 gm) and 5 % glutaraldehyde (10 ml). This solution was kept at room temperature for 03 h. To remove unbound glutaraldehyde the nanoparticles were washed 03 times with 50 mM sodium phosphate buffer of pH 7.5. Subsequently, the prepared nanoparticles were then mixed with pectinase (1 ml) at room temperature for around 03 h.
The immobilization yield of pectinase immobilized silica nanoparticles was calculated at using the formula mentioned in equation (1):
| (Equation 1) |
2.6. Pectinase assay
Pectinase activity was determined by measuring the amount of reducing sugar using the DNS (3,5-dinitrosalicylic acid) method with citrus pectin as the substrate [[8], [20]]. One unit (IU) of enzyme activity was defined as the amount of pectinase required to produce 1 μmol of product (galacturonic acid) under optimum reaction conditions (pH 10 at 45 °C for 15 min). Pectinase assay was carried out in triplicate in order to calculate mean and standard deviation.
2.7. Influence of immobilization conditions
2.7.1. Influence of glutaraldehyde as crosslinking agent
Different concentrations of glutaraldehyde (0.5 %–5.0 %) were investigated by treating the silica nanoparticles with pectinase in order to determine the optimum concentration while all other parameters were kept constant.
2.7.2. Influence of pectinase concentration, crosslinking time, and temperature
The influence of pectinase concentration on the immobilization of silica nanoparticles was determined by varying the units of pectinase from 500 to 5000 IU/mg during the immobilization procedure. The effect of cross-linking time and temperature was also determined by incubating pectinase with silica nanoparticles for different time intervals (15–120 min) and at various temperatures (5 °C–40 °C).
2.8. Analysis of catalytic properties of pectinase-immobilized silica nanocarriers
The catalytic properties of pectinase immobilized on silica nanocarriers were analyzed and compared with soluble pectinase.
2.8.1. Reaction time and temperature
The influence of reaction time on the catalytic activity of pectinase immobilized on silica nanocarriers was investigated by evaluating the enzyme assay for various time periods (ranging from 5 to 60 min) while keeping the other parameters constant, and the results were compared with soluble enzyme activity. The effect of reaction temperature on the enzymatic activity of pectinase immobilized on silica nanoparticles was analyzed by measuring the enzyme assay at different reaction temperatures (ranging from 20 to 50 °C) while keeping the other parameters constant.
2.8.2. Reaction pH, thermal stability, and kinetic parameters
The influence of reaction pH on the activity of pectinase immobilized on silica nanoparticles was evaluated by determining the enzyme activity at pH 5 to 10 while keeping the other parameters constant and comparing it with the soluble enzyme. The effect of different temperatures on the stability of pectinase immobilized on silica nanoparticles was investigated by pre-incubating the enzymes at various temperatures (ranging from 25 °C to 60 °C) for different time periods. The enzyme activity was measured at regular intervals of 24 h. The kinetic parameters (Km and Vmax) of pectinase immobilized on silica nanocarriers were determined by measuring the enzyme assay using different substrate concentrations. The values of Km and Vmax were determined by Lineweaver Burk plot [21].
2.9. Reusability
To evaluate the reusability of the immobilized enzymes, pectinase immobilized on silica nanocarriers was employed in ten consecutive batches of reactions. The immobilized pectinase was washed with deionized water before starting each new reaction cycle.
3. Results and discussion
The foremost significance of enzyme immobilization is to enhance the kinetic properties of that enzyme along with increase in its stability and reusability. This study was also designed with the aim to achieve aforementioned purposes. Hence, pectinase was immobilized using silica nanoparticles to improve the commercial applications of this enzyme.
3.1. Characterization of SiO2 nanoparticles
Silica has been the most explored material in research world, due to its versatile properties. Nanosized silica has some unique characteristics as compared to micro size due its enhanced surface area. Synthesized silica nanoparticles were characterized by scanning electron microscopy to access the particle size and morphology of the nanoparticles. Analysis was carried out KV and X10000 zoom level. SEM image of synthesize silica nanoparticles is given in Fig. 1A. It is quite obvious from the figure that the particles are spherical in size and show symmetrical geometry. Particle size is estimated to be around 80–200 nm. Particles seems to be agglomerated, it is due to the presence of surface hydroxyl groups which contribute to interparticle hydrogen bonding which can be reduced by proper dispersion in ethanol. The elemental composition analysis (Fig. 1B) showed that the sample consisted primarily of silicon (52.92 %) and oxygen (43.99 %), confirming the formation of silica particles. The X-ray diffraction (XRD) analysis (Fig. 1C) indicated that the silica nanoparticles were amorphous, as evidenced by the absence of distinct diffraction peaks, except for a broad band centered around 22°, characteristic of amorphous silica [22].
Fig. 1.
Characterization of silica nanoparticles. A) Scanning electron microscopy B) Energy dispersive X-ray spectroscopy. C) X-ray Diffractometer.
3.2. Effect of glutaraldehydre concentration
The concentration of glutaraldehyde was optimized for maximum crosslinking of pectinase to SiO2 nanoparticles. The results indicated that 2.5 % glutaraldehyde concentration achieved the highest crosslinking and immobilization yield of pectinase onto SiO2 nanoparticles (Fig. 2A). Lower glutaraldehyde concentrations were insufficient for maximum crosslinking, while higher concentrations had the potential to affect the enzyme's structure and enzymatic activity. Excessive crosslinking agent could also block the enzyme's active site and hinder the formation of enzyme-substrate complexes. So the concentration of glutaraldehyde plays an important role in the immobilization of pectinase.
Fig. 2.
Optimization of parameters for immobilization of pectinase on silica nanoparticles. A) Effect of glutaraldehyde concentration B) Effect of pectinase concentration C) Effect of cross-linking time D) Effect of cross-linking temperature.
3.3. Influence of pectinase concentration
The effect of pectinase concentration on the immobilization efficiency and enzymatic activity was evaluated by incubating different concentrations of partially purified pectinase (ranging from 500 to 5000 IU/mg) for 3 h. The results showed that the immobilization yield of the silica nanocatalyst increased with increasing pectinase concentration, reaching maximum yield at 3000 IU/mg pectinase (Fig. 2B). However, further increases in pectinase concentration did not significantly affect the catalytic activity of the silica nanocatalyst, indicating saturation of the pectinase-nanoparticle system at 3000 IU/mg.
3.4. Influence of enzyme-nanoparticles incubation time on pectinase immobilization
The influence of incubation time on pectinase immobilization onto silica nanoparticles was investigated by varying the incubation period from 30 to 180 min using a fixed pectinase concentration of 3000 IU/mg. The results demonstrated that the immobilization yield of pectinase increased with longer incubation times, reaching maximum yield after 90 min (Fig. 2C). However, extending the incubation period beyond 90 min did not significantly affect the immobilization yield or the catalytic activity of the nanocatalyst. Less than 90 min of incubation time was insufficient for effective crosslinking between the enzyme units and silica nanoparticles, whereas 90 min provided adequate time for maximum enzyme binding.
3.5. Influence of incubation temperature on pectinase immobilization onto silica nanoparticles
The influence of incubation temperature on pectinase immobilization onto silica nanoparticles was investigated by incubating the enzyme solution with the nanoparticles at different temperatures. The results showed that increasing the incubation temperature enhanced the crosslinking of pectinase with nanoparticles, with maximum enzyme crosslinking observed at 40 °C, as evidenced by the highest catalytic activity of the pectinase nanocarrier (Fig. 2D). However, beyond 40 °C, further increases in the incubation temperature led to a decrease in catalytic activity. This decrease may be attributed to conformational changes in the enzyme at higher temperatures, which affect the crosslinking efficiency. Similar observations have been reported for the immobilization of pectinase using glutaraldehyde as a crosslinking agent with other support materials.
3.6. Catalytic properties of pectinase-based silica nanocarriers
The catalytic properties of pectinase immobilized on silica nanocarriers were compared to those of soluble pectinase.
3.6.1. Effect of reaction time
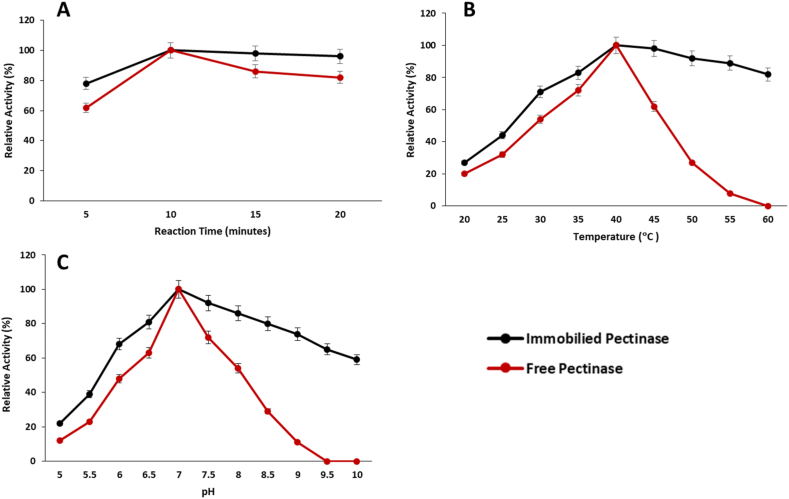
The effect of reaction time on the catalytic activity of immobilized pectinase was evaluated by comparing the enzymatic assay of the immobilized enzyme at different time periods with the free enzyme. Both immobilized and free pectinase showed maximum enzymatic activities after 10 min of reaction time. The immobilization of pectinase onto silica nanocarriers did not affect the reaction time for pectin degradation, indicating that the crosslinking of pectinase with nanoparticles did not induce significant structural changes that would alter the enzyme's reaction time (Fig. 3A).
Fig. 3.
Catalytic parameters optimization of immobilized and free pectinase. A) Effect of reaction time B) Effect of reaction temperatures C) Effect of reaction pH.
3.6.2. Effect of reaction temperature
The effect of reaction temperature on the catalytic performance of pectinase-based silica nanocarriers was assessed by measuring the enzyme assay at different temperatures and comparing it with free pectinase. The results demonstrated that the crosslinking of pectinase with silica nanoparticles did not affect the catalytic performance of the enzyme. Both immobilized and free pectinases exhibited maximum activity at 40 °C (Fig. 3B). However, the immobilized pectinase showed higher relative activity at various temperatures compared to the free enzyme. Even at 60 °C, the immobilized pectinase retained more than 80 % relative activity, while the free pectinase lost its complete activity. This increased relative activity of the immobilized pectinase at different temperatures can be attributed to improved conformational rigidity of the non-active site of the enzyme after binding with glutaraldehyde.
3.6.3. Effect of reaction pH
The effect of pH was determined by measuring the enzyme activity at different pH levels and comparing it with the free enzyme. Both immobilized and free pectinases exhibited maximum relative activity at pH 7.0. However, the immobilized pectinase showed higher relative activity (60 %) across a range of pH values as compared to the free enzyme, which exhibit no product formation at pH 9.5 (Fig. 3C). These results suggest that the crosslinking of pectinase onto silica nanocarriers using glutaraldehyde induced positive conformational changes and improved the enzyme's resistance to variations in ionic strength. Similar results were previously reported by Nauri and Khodaiyan [12,13].
After enzyme immobilization there is generally a shift of characterization conditions though in this study the optimum conditions of bothy free and immobilized enzymes are same. However the stability beyond optimum conditions were observed in case of immobilized enzyme which showed its better performance as compared to free enzyme.
3.6.4. Thermal stability of silica nanoparticles-immobilized pectinase
The thermal stability of silica nanoparticle-immobilized pectinase was evaluated by subjecting the enzymes to different temperatures and comparing their stability to free pectinase. The results showed that the immobilized pectinase exhibited enhanced stability at elevated temperatures compared to the free enzyme (Fig. 4). After 120 h of preincubation at 60 °C, the immobilized pectinase retained more than 70 % of its initial activity, while the free pectinase lost its complete activity after only 24 h of preincubation at the same temperature. The improved thermal stability of the immobilized pectinase can be attributed to the structural support provided by the silica nanoparticles, which helps the enzyme withstand thermal denaturation at higher temperatures. Similar results were previously reported for pectinase immobilization on chitosan nanoparticles and laccase immobilization on silica nanoparticles [10,[23], [24], [25], [26]].
Fig. 4.
Thermal stability of silica nanoparticles immobilized pectinase against different temperatures. A) 30 °C B) 40 °C C) 50 °C D) 60 °C.
3.6.5. Kinetic parameters
The kinetic parameters, Km and Vmax, of pectinase immobilized on silica nanocarriers were determined and compared to those of the soluble enzyme. The results showed that the immobilization of pectinase did not significantly alter the affinity of the enzyme for the substrate, as evidenced by similar Km and Vmax values between the immobilized and soluble enzymes (Table 1). This indicates that the conformational structure of the enzyme's active site remained intact after immobilization, allowing for efficient substrate binding. Previous studies also showed that immobilization of laccase on silica nanoparticles does not significantly affect enzyme substrate binding efficiency [[10], [23], [24]].
Table 1.
Kinetic parameters of silica nanoparticles immobilized pectinase with the comparison of soluble pectinase.
| Enzyme | Km Value (mg ml−1) | Vmax Value (μM min−1) |
|---|---|---|
| Free Pectinase | 1.017 | 23,800 |
| Silica Nanoparticles Immobilized Pectinase | 1.019 | 23,500 |
3.7. Reusability
The reusability of the silica nanoparticle-immobilized pectinase was evaluated by consecutively reusing the immobilized enzyme in ten reaction cycles. The results demonstrated that the immobilized pectinase retained nearly 100 % of its initial activity after ten cycles of reuse (Fig. 5). This indicates that the crosslinking of enzymes with silica nanoparticles using glutaraldehyde facilitated their reusability in continuous industrial applications. The improved operational stability and reusability can be attributed to the strong and stable crosslinking between the enzyme and the support material. Previously pectinase immobilized on chitosan nanoparticles lost their 20 % enzyme activity even after 4 cycles [26]. Thus this study design showed that pectinase immobilized silica nanoparticles can be economically and effectively used for various industrial processes.
Fig. 5.
Reusability analysis of silica nanoparticles immobilized pectinase.
4. Conclusion
In this study, pectinase was successfully produced from Bacillus subtilis and immobilized on silica nanoparticles, aiming to enhance its industrial applicability and stability. The silica nanoparticles immobilized pectinase exhibited more relative activity under wide range of conditions, showcasing its enhanced versatility. Furthermore, the thermal stability of the pectinase was also significantly improved after immobilizing enzyme with this method. Additionally, the silica nanocarrier-immobilized pectinase demonstrated remarkable reusability which increases product formation and decreases time and efforts. This approach holds great potential for various industrial applications, offering an economically viable and efficient system for biotechnological processes.
5. Funding
This research was funded by the researchers supporting project no RSP - 2023R339) at king saud university Riyadh, Saudi Arabia.
CRediT authorship contribution statement
Tayyaba Behram: Writing – original draft. Sidra Pervez: Methodology. Muhammad Asif Nawaz: Supervision. Rahim Ullah: Data curation. Azmat Ali Khan: Funding acquisition. Bushra Ahmad: Writing – review & editing. Amer M. Alanzai: Investigation. Asrar Ahmad: Formal analysis. Abdul Khaliq Jan: Methodology. Haneef Ur Rahman: Writing – review & editing. Muhsin Jamal: Data curation. Tour jan: Conceptualization. Abrar Mohyuddin: Resources. Nasir Mehmood Khan: Formal analysis. Shujaat Ahmad: Writing – review & editing.
Declaration of competing interest
The authors declare no conflict of interest.
Acknowledgments
This work was funded by the researchers supporting project no RSP - 2023R339) at king saud university Riyadh, Saudi Arabia. Authors also gratefully acknowledge SBBU, Sheringal for providing resources for completion of this research work.
Contributor Information
Sidra Pervez, Email: drsidrapervez@sbbwu.edu.pk.
Muhammad Asif Nawaz, Email: asif_biotech33@sbbu.edu.pk.
References
- 1.Haile S., Ayele A. Pectinase from microorganisms and its industrial applications. Sci. World J. 2022;2022 doi: 10.1155/2022/1881305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oumer O.J., Abate D. Screening and molecular identification of pectinase producing microbes from coffee pulp. BioMed Res. Int. 2018;2018 doi: 10.1155/2018/2961767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prathyusha K., Suneetha V. Bacterial pectinases and their potent biotechnological application in fruit processing/juice production industry: a review. J. Phytol. 2011;3:16–19. [Google Scholar]
- 4.Muller S., Concha D., Vasquez P., Rodriguez-Nuñez K., Martinez R., Bernal C. Effect of the immobilization of pectinase on the molecular weight distribution of pectin oligosaccharides obtained from citrus pectin. Biocatal. Agric. Biotechnol. 2022;43 [Google Scholar]
- 5.Lee Y.E., Kang Y.R., Chang Y.H. Effect of pectic oligosaccharide on probiotic survival and physicochemical properties of hydrogel beads for synbiotic encapsulation of Lactobacillus bulgaricus. Food Biosci. 2023;51 [Google Scholar]
- 6.Ozyilmaz G., & Gunay E. Clarification of apple, grape and pear juices by co-immobilized amylase, pectinase and cellulase. Food Chem. 2023;398 doi: 10.1016/j.foodchem.2022.133900. [DOI] [PubMed] [Google Scholar]
- 7.Veljković M., Stepanović R., Banjanac K., Ćorović M., Milivojević A., Simović M., Bezbradica D. Continuous production of fructo-oligosaccharides using selectively immobilized fructosyltransferase from Aspergillus aculeatus onto Purolite® A109. J. Ind. and Eng. Chem. 2023;117:149–156. [Google Scholar]
- 8.Rehman H.U., Nawaz M.A., Pervez S., Jamal M., Attaullah M., Aman A., Qader S.A.U. Encapsulation of pectinase within polyacrylamide gel: characterization of its catalytic properties for continuous industrial uses. Heliyon. 2020;6(8) doi: 10.1016/j.heliyon.2020.e04578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kharazmi S., Taheri-Kafrani A., Soozanipour A. Efficient immobilization of pectinase on trichlorotriazine-functionalized polyethylene glycol-grafted magnetic nanoparticles: a stable and robust nanobiocatalyst for fruit juice clarification. Food Chem. 2020;325 doi: 10.1016/j.foodchem.2020.126890. [DOI] [PubMed] [Google Scholar]
- 10.Patel S.K., Kalia V.C., Choi J.H., Haw J.R., Kim I.W., Lee J.K. Immobilization of laccase on $ SiO_2 $ nanocarriers improves its stability and reusability. J. Microbiol. Biotechnol. 2014;24(5):639–647. doi: 10.4014/jmb.1401.01025. [DOI] [PubMed] [Google Scholar]
- 11.Nouri M. Handbook of Magnetic Hybrid Nanoalloys and Their Nanocomposites. Springer; Cham: 2022. Oxidation Behavior of magnetic Hybrid nanoalloys. [DOI] [Google Scholar]
- 12.Nouri M., Khodaiyan F. Green synthesis of chitosan magnetic nanoparticles and their application with poly-aldehyde kefiran cross-linker to immobilize pectinase enzyme. Biocatal. Agric. Biotechnol. 2020;29 [Google Scholar]
- 13.Nouri M., Khodaiyan F. Magnetic biocatalysts of pectinase: synthesis by macromolecular cross-linker for application in apple juice clarification. Food Technol. Biotechnol. 2020;58:391–401. doi: 10.17113/ftb.58.04.20.6737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lu J., Nie M., Li Y., Zhu H., Shi G. Design of composite nanosupports and applications thereof in enzyme immobilization: a review. Colloids Surf. B Biointerfaces. 2022;217 doi: 10.1016/j.colsurfb.2022.112602. [DOI] [PubMed] [Google Scholar]
- 15.Gkantzou E., Chatzikonstantinou A.V., Fotiadou R., Giannakopoulou A., Patila M., Stamatis H. Trends in the development of innovative nanobiocatalysts and their application in biocatalytic transformations. Biotechnol. Adv. 2021;51 doi: 10.1016/j.biotechadv.2021.107738. [DOI] [PubMed] [Google Scholar]
- 16.Behram T., Pervez S., Nawaz M.A., Ahmad S., Jan A.U., Rehman H.U., Khan F.A. Development of pectinase based nanocatalyst by immobilization of pectinase on magnetic iron oxide nanoparticles using glutaraldehyde as crosslinking agent. Molecules. 2023;28(1):404. doi: 10.3390/molecules28010404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arruebo M., Galan M., Navascues N., Tellez C., Marquina C., Ibarra M.R., Santamaria J. Development of magnetic nanostructured silica-based materials as potential vectors for drug-delivery applications. Chem. Mater. 2006;18:1911–1919. [Google Scholar]
- 18.Zhao W., Gu J., Zhang L., Chen H., Shi J. Fabrication of uniform magnetic nanocomposite spheres with a magnetic core/mesoporous silica shell structure. J. Amer. Chem. Soc. 2005;127:8916–8917. doi: 10.1021/ja051113r. [DOI] [PubMed] [Google Scholar]
- 19.Lin J., Siddiqui J.A., Ottenbrite R.M. Surface modification of inorganic oxide particles with silane coupling agent and organic dyes. Polym. Adv. Technol. 2001;12:285–292. [Google Scholar]
- 20.Miller G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugars. Analyt. Chem. 1959;31:426–428. [Google Scholar]
- 21.Lineweaver H., Burk D. The determination of enzyme dissociation constants. J. Am. Chem. Soc. 1934;56:658–666. [Google Scholar]
- 22.Mohamad N.R., Marzuki N.H.C., Buang N.A., Huyop F., Wahab R.A. An overview of technologies for immobilization of enzymes and surface analysis techniques for immobilized enzymes. Biotechnol. & Biotechnolog. Equip. 2015;2:205–220. doi: 10.1080/13102818.2015.1008192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lei Z., Bi S. The silica-coated chitosan particle from a layer-by-layer approach for pectinase immobilization. Enzyme Microbial. Technol. 2007;40:1442–1447. [Google Scholar]
- 24.Kalsoom U., Ahsan Z., Bhatti H.N., Amin F., Nadeem R., Aftab K., Bilal M. Iron oxide nanoparticles immobilized Aspergillus flavus manganese peroxidase with improved biocatalytic, kinetic, thermodynamic, and dye degradation potentialities Process. Biochemistry. 2022;117:117–133. [Google Scholar]
- 25.Rehman H.U., Aman A., Silipo A., Qader S.A.U., Molinaro A., Ansari A. Degradation of complex carbohydrate: immobilization of pectinase from Bacillus licheniformis KIBGE-IB21 using calcium alginate as a support. Food Chem. 2013;139:1081–1086. doi: 10.1016/j.foodchem.2013.01.069. [DOI] [PubMed] [Google Scholar]
- 26.Navarro-Lopez D.E., Bautista-Ayala, la Cruz A.R., Efr Martínez-Beltran S., Rojas-Torres D.E., Sanchez-Martinez A., Ceballos-Sanchez O., Jauregui J.J.A., Lozano L.M., Sepúlveda-Villegas M., Tiwari N., Lopez-Mena E.R. Nanocatalytic performance of pectinase immobilized over in situ prepared magnetic nanoparticles. Heliyon. 2023;9 doi: 10.1016/j.heliyon.2023.e19021. [DOI] [PMC free article] [PubMed] [Google Scholar]