Abstract
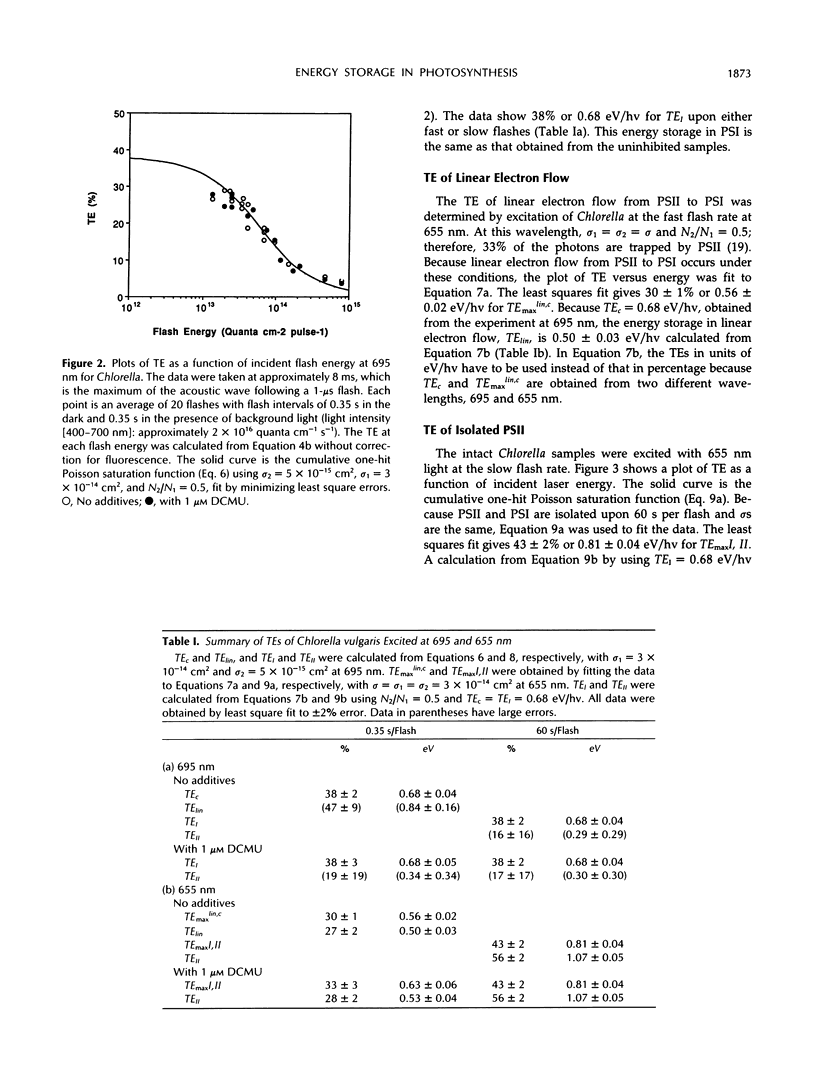
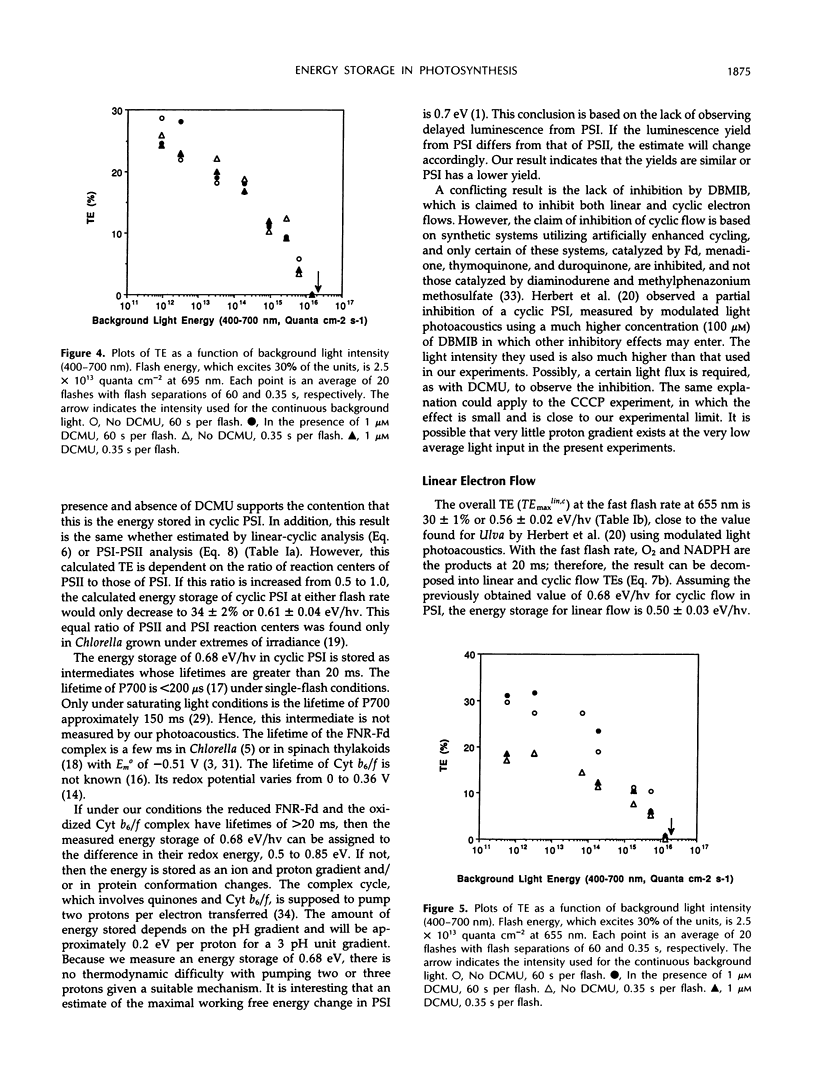
The energy storage of photosynthesis in the green alga Chlorella vulgaris was determined by pulsed, time-resolved photoacoustics. The energy storage of the linear electron transfer process in photosynthesis, of cyclic photosystem (PS) I, and possibly of PSII was determined by selection of excitation wavelength and of flash interval. At 695 nm excitation, a rather large cyclic PSI energy storage of 0.68 ± 0.04 eV/quantum of energy at 8 ms after a 1-μs flash was obtained. This energy remained the same at flash intervals of 0.35 to 60 s and was independent of the presence of 3-(3,4-dichlorophenyl)-1,1-dimethylurea. We tentatively assign this energy to the ferredoxin-NADP-reductase-ferredoxin and oxidized cytochrome b6/f complexes. An efficient distribution of energy between cyclic and linear systems is obtained with the simple assumption that the turnover time of the cyclic system is slower than that of the linear system. The energy storage of linear electron flow was determined by 655 nm excitation of Chlorella with a short flash interval of 0.35 s per flash. It was calculated to be 0.50 ± 0.03 eV/hv, close to that expected for oxygen and NADPH formation. The energy storage of PSII is determined by excitation of Chlorella at 655 nm with a long flash interval of 60 s per flash. It was calculated to be 1.07 ± 0.05 eV/hv, consistent with the energy storage being in S-states and the secondary electron acceptor of PSII with a calculated redox energy of 1.03 eV/hv. In the presence of 1 μm 3-(3,4-dichlorophenyl)-1,1-dimethylurea, the calculated energy storage in PSII is still significant, 0.53 ± 0.04 eV/hv. This probably indicates a significant cyclic electron flow around PSII. These cyclic flows may contribute considerably to energy storage in photosynthesis.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. Role of ferredoxin in photosynthesis. Naturwissenschaften. 1969 Jun;56(6):295–305. doi: 10.1007/BF00602160. [DOI] [PubMed] [Google Scholar]
- Batie C. J., Kamin H. The relation of pH and oxidation-reduction potential to the association state of the ferredoxin . ferredoxin:NADP+ reductase complex. J Biol Chem. 1981 Aug 10;256(15):7756–7763. [PubMed] [Google Scholar]
- Bennoun P. Réoxydation du quencher de fluorescence "Q" en présence de 3-(3,4-dichlorophényl)-1,1-diméthylurée. Biochim Biophys Acta. 1970 Sep 1;216(2):357–363. doi: 10.1016/0005-2728(70)90227-6. [DOI] [PubMed] [Google Scholar]
- Bouges-Bocquet B. Absorption changes from 437 nm to 530 nm in Chlorella pyrenoidosa under flash excitation. Probable detection of ferredoxin--NADP-reductase. FEBS Lett. 1978 Jan 15;85(2):340–344. doi: 10.1016/0014-5793(78)80487-6. [DOI] [PubMed] [Google Scholar]
- Bouges-Bocquet B. Kinetic models for the electron donors of photosystem II of photosynthesis. Biochim Biophys Acta. 1980 Dec;594(2-3):85–103. doi: 10.1016/0304-4173(80)90006-3. [DOI] [PubMed] [Google Scholar]
- Bowes J. M., Crofts A. R. Binary oscillations in the rate of reoxidation of the primary acceptor of photosystem II. Biochim Biophys Acta. 1980 May 9;590(3):373–384. doi: 10.1016/0005-2728(80)90208-x. [DOI] [PubMed] [Google Scholar]
- Canaani O., Malkin S., Mauzerall D. Pulsed photoacoustic detection of flash-induced oxygen evolution from intact leaves and its oscillations. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4725–4729. doi: 10.1073/pnas.85.13.4725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowther D., Hind G. Partial characterization of cyclic electron transport in intact chloroplasts. Arch Biochem Biophys. 1980 Oct 15;204(2):568–577. doi: 10.1016/0003-9861(80)90069-7. [DOI] [PubMed] [Google Scholar]
- Custović I., Veljković M., Popović Z., Konstantinovi0c P. O proteinima kolostruma i zrelog zeninog mlijeka. Med Arh. 1968 Sep-Dec;22(5):27–32. [PubMed] [Google Scholar]
- Delosme R., Zickler A., Joliot P. Turnover kinetics of photosystem I measured by the electrochromic effect in Chlorella. Biochim Biophys Acta. 1978 Oct 11;504(1):165–174. doi: 10.1016/0005-2728(78)90015-4. [DOI] [PubMed] [Google Scholar]
- Herbert S. K., Fork D. C., Malkin S. Photoacoustic measurements in vivo of energy storage by cyclic electron flow in algae and higher plants. Plant Physiol. 1990 Nov;94(3):926–934. doi: 10.1104/pp.94.3.926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauzerall D. C. Determination of oxygen emission and uptake in leaves by pulsed, time resolved photoacoustics. Plant Physiol. 1990 Sep;94(1):278–283. doi: 10.1104/pp.94.1.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauzerall D. Fluorescence and photosynthesis: gated detection and analysis of nanosecond pulse excitation. Adv Biol Med Phys. 1980;17:173–198. doi: 10.1016/b978-0-12-005217-2.50012-9. [DOI] [PubMed] [Google Scholar]
- Maxwell P. C., Biggins J. Role of cyclic electron transport in photosynthesis as measured by the photoinduced turnover of P700 in vivo. Biochemistry. 1976 Sep 7;15(18):3975–3981. doi: 10.1021/bi00663a011. [DOI] [PubMed] [Google Scholar]
- Pulles M. P., Van Gorkom H. J., Willemsen J. G. Absorbance changes due to the charge-accumulating species in system 2 of photosynthesis. Biochim Biophys Acta. 1976 Dec 6;449(3):536–540. doi: 10.1016/0005-2728(76)90162-6. [DOI] [PubMed] [Google Scholar]
- Trumpower B. L. The protonmotive Q cycle. Energy transduction by coupling of proton translocation to electron transfer by the cytochrome bc1 complex. J Biol Chem. 1990 Jul 15;265(20):11409–11412. [PubMed] [Google Scholar]
- Whitmarsh J., Cramer W. A. Kinetics of the photoreduction of cytochrome b-559 by photosystem II in chloroplasts. Biochim Biophys Acta. 1977 May 11;460(2):280–289. doi: 10.1016/0005-2728(77)90214-6. [DOI] [PubMed] [Google Scholar]


