Abstract
The TraR and TraI proteins of Agrobacterium tumefaciens mediate cell-density-dependent expression of the Ti plasmid tra regulon. TraI synthesizes the autoinducer pheromone N-(3-oxooctanoyl)-l-homoserine lactone (3-oxo-C8-HSL), while TraR is an 3-oxo-C8-HSL-responsive transcriptional activator. We have compared the abilities of 3-oxo-C8-HSL and 32 related compounds to activate expression of a TraR-regulated promoter. In a strain that expresses wild-type levels of TraR, only 3-oxo-C8-HSL was strongly stimulatory, four compounds were detectably active only at high concentrations, and the remaining 28 compounds were inactive. Furthermore, many of these compounds were potent antagonists. In contrast, almost all of these compounds were stimulatory in a congenic strain that overexpresses TraR and no compound was a potent antagonist. We propose a model in which autoinducers enhance the affinity of TraR either for other TraR monomers or for DNA binding sites and that overexpression of TraR potentiates this interaction by mass action. Wild-type A. tumefaciens released a rather broad spectrum of autoinducers, including several that antagonize induction of a wild-type strain. However, under all conditions tested, 3-oxo-C8-HSL was more abundant than any other analog, indicating that other released autoinducers do not interfere with tra gene induction. We conclude that (i) in wild-type strains, only 3-oxo-C8-HSL significantly stimulates tra gene expression, while many autoinducer analogs are potent antagonists; (ii) TraR overexpression increases agonistic activity of autoinducer analogs, allowing sensitive biodetection of many autoinducers; and (iii) autoinducer stimulatory activity is potentiated by TraR overproduction, suggesting that autoinducers may shift an equilibrium between TraR monomers and dimers or oligomers. When autoinducer specificities of other quorum-sensing proteins are tested, care should be taken not to overexpress those proteins.
Many genera of bacteria use diffusible chemicals to exchange information (3, 5, 12, 18, 26, 36, 41, 45). An important class of chemical signal molecules is the family of N-acyl-homoserine lactones (N-acyl-HSLs), which are generally synthesized by an enzyme related to the LuxI protein of Vibrio fischeri. These compounds, called autoinducers, passively diffuse across the bacterial envelope and therefore accumulate intracellularly only at high bacterial densities (25). These chemicals are thought to bind to a protein related to the LuxR protein of V. fischeri, whose amino terminus contains an autoinducer binding site and whose carboxyl terminus binds to a DNA site directly upstream of the lux promoter (1, 7, 21, 42). Cell density-dependent gene expression is denoted quorum sensing, and this sort of regulation is used by a wide spectrum of bacteria to regulate diverse genes, including the pathogenicity genes of several plant and animal pathogens.
The Ti plasmids of several Agrobacterium tumefaciens strains bear genes that encode a LuxI-type protein called TraI, which synthesizes N-(3-oxooctanoyl)-l-HSL (3-oxo-C8-HSL) and a LuxR-type protein called TraR, which presumably binds 3-oxo-C8-HSL (17, 23, 30, 34, 46). Putative TraR-autoinducer complexes activate transcription of several genes required for Ti plasmid conjugal transfer as well as other Ti plasmid-borne genes. As expected, Ti plasmid conjugation occurs only at high donor population densities (16). The expression of TraR is regulated by particular opines (17). Octopine induces expression of the traR genes of plasmids related to pTi15955, pTiA6, and pTiR10, while agrocinopines A and B induce the traR gene of pTiC58 (4, 17). These observations explain older findings that Ti plasmid conjugation occurs only within crown gall tumors or in the presence of particular opines (9). The traR and traI genes are positively autoregulated (17).
All autoinducers described to date contain an HSL moiety and a fatty acyl group whose members have various lengths, saturation levels, and oxidation states. Two of these compounds have been shown to derive their fatty acids from acyl-ACP (22, 30, 38), which indicates that these fatty acids are drawn from pools of fatty acid biosynthetic intermediates. The fatty acids of acyl-ACPs always have even numbers of carbon atoms. They also have either 3-oxo, 3-hydroxyl, or fully reduced methylene groups at the C-3 position or a have a 2,3 unsaturated bond (8). Accordingly, the acyl groups of all natural autoinducers have even numbers of carbon atoms and have 3-oxo, 3-hydroxyl, or fully reduced acyl groups. Curiously, no autoinducer has been reported to contain a 2,3 unsaturated bond in its acyl moiety, although two autoinducers have 7,8 unsaturated bonds (20, 35, 39). The lengths of these side chains contain between 4 and 14 carbon atoms.
In several studies, the ability of autoinducer analogs to induce expression of quorum-sensing systems has been tested (6, 13, 19, 32, 37, 46). In general, compounds closely related to the cognate autoinducer caused weak-to-moderate gene expression while less similar compounds were less active. In some cases, autoinducer analogs acted as antagonists of the native autoinducer and thereby inhibited the induction of target genes. In two studies, autoinducer analogs were demonstrated to inhibit binding of radiolabeled autoinducers (32, 37).
There are a few reports of a single LuxI-type protein synthesizing more than one autoinducer. LuxI synthesizes primarily 3-oxo-C6-HSL (see Fig. 1, compound C) but also synthesizes small amounts of C6-HSL (compound K; see reference 27). Similarly, an Escherichia coli strain expressing the LasI protein of Pseudomonas aeruginosa synthesizes primarily 3-oxo-C12-HSL but also synthesizes trace amounts of additional compounds, including two that coelute by reversed-phase high-performance liquid chromatography (HPLC) with 3-oxo-C6-HSL and 3-oxo-C8-HSL (33, 44). The SwrI protein of Serratia liquefaciens synthesizes both C4-HSL and C6-HSL in a 10:1 ratio (14). HPLC fractionation of Agrobacterium autoinducers revealed two bioactive fractions (46). One fraction contained 3-oxo-C8-HSL, while a second, less homogeneous, fraction contained compounds having molecular masses of 274, 214, and 200 Da. While the 274-Da compound was thought to be due to a purification artifact, the other two compounds were not since they had molecular masses identical to those of 3-oxo-C6-HSL and C6-HSL, respectively. That study did not determine whether all three compounds were synthesized by TraI.
FIG. 1.
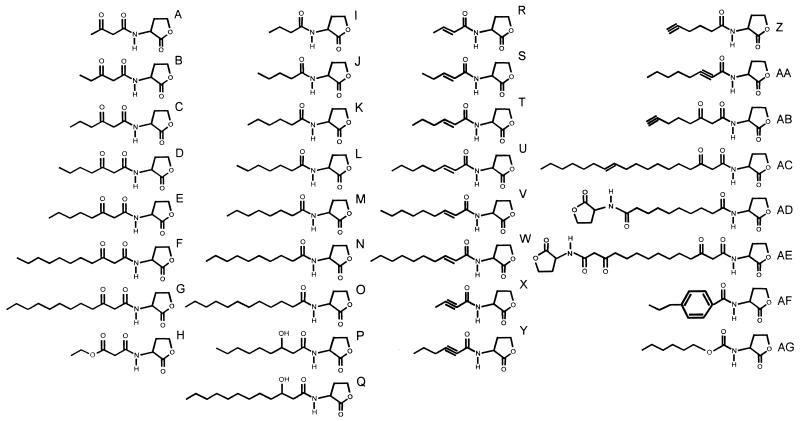
Chemical structures of N-acyl-HSL compounds used in this study. Non-IUPAC descriptions of compounds are as follows: A, 3-oxobutanoyl-HSL; B, 3-oxopentanoyl-HSL; C, 3-oxohexanoyl-HSL; D, 3-oxoheptanoyl-HSL; E, 3-oxooctanoyl-HSL; F, 3-oxoundecanoyl-HSL; G, 3-oxododecanoyl-HSL; H, 4-oxa-3-oxohexanoyl-HSL; I, butanoyl-HSL; J, pentanoyl-HSL; K, hexanoyl-HSL; L, heptanoyl-HSL; M, octanoyl-HSL; N, decanoyl-HSL; O, dodecanoyl-HSL; P, 3-hydroxynonanoyl-HSL; Q, 3-hydroxydodecanoyl-HSL; R, 2-butenoyl-HSL; S, 2-pentenoyl-HSL; T, 2-hexenoyl-HSL; U, 2-octenoyl-HSL; V, 2-nonenoyl-HSL; W, 2-decenoyl-HSL; X, 2-butynoyl-HSL; Y, 2-hexynoyl-HSL; Z, 5-hexynoyl-HSL; AA, 2-octynoyl-HSL; AB, 3-oxo-7-octynoyl-HSL; AC, 3-oxo-11-octadecenoyl-HSL; AD, diHSL-decandioate; AE, diHSL-3,12-dioxotetradecandioate; AF, p-propylbenzoyl-HSL; and AG, O-hexyl-N-HSL carbamate.
Shaw and colleagues recently described an elegant method for using an A. tumefaciens strain to detect a broad range of autoinducers (40). The strain used in these studies was able to recognize no fewer than nine autoinducers having a wide variety of acyl side groups. However, in an earlier report, an A. tumefaciens strain discriminated between different autoinducers far more strongly (46). One difference between these two studies is the genotypes of the bioassay strains. In the present study, we evaluated whether differences in the traR genotype that affect its expression level might alter the ability of A. tumefaciens to detect analogs of 3-oxo-C8-HSL. We report that TraR overproduction allows A. tumefaciens to detect a broad range of autoinducer analogs and abolishes the ability of autoinducer analogs to act as antagonists.
MATERIALS AND METHODS
Strains and plasmids.
WCF47 is a derivative of R10 (10) containing a nonpolar internal deletion of traI. The traI deletion was constructed with two fragments generated by PCR, one containing the 5′ end of traI and upstream sequences and the other containing the 3′ end of traI and downstream sequences. These fragments were ligated together, and the resulting traI deletion was introduced into the suicide plasmid pKNG101 (24). The resulting plasmid, pCF393, was introduced into strain R10 by conjugation and selected with streptomycin. Streptomycin-resistant transconjugants were plated on solid medium lacking streptomycin and containing 5% sucrose. The sacB gene of pCF393 confers sucrose sensitivity, and sucrose therefore selects for Campbell-type excision. Sucrose-resistant derivatives were screened for the inability to produce 3-oxo-C8-HSL. pCF218 is an IncP plasmid that expresses TraR from a vector promoter (17), while pCF372 contains a PtraI-lacZ fusion (16).
Synthesis of autoinducer analogs.
The chemical structures of autoinducer analogs used in this study are shown in Fig. 1. Synthesis of compounds A, E, I, J, K, L, M, and N was previously described (13). Synthesis of compounds B, T, and Y was as described in reference 37, while synthesis of compounds C and G was as described in references 11 and 33, respectively.
Compounds D, F, AB, AC, and AE were synthesized by the same procedures as those used to make compound B but with the corresponding acyl acid chlorides. Compounds H, O, R, S, U, V, W, X, Z, AA, and AF were synthesized by the same procedures as those used to make compound I but with the sodium salt of the corresponding fatty acid. Monoethyl malonate was used to make compound H. Compound AD was synthesized by reaction of the dicarboxylic acid chlorides with two equivalents of HSL hydrobromide in pyridine followed by adsorption to a BondElut column and purification by preparative HPLC. Compound AG was made similarly from hexyl chloroformate. Compounds P and Q were synthesized by a Reformatsky reaction followed by coupling of the resulting 3-hydroxycarboxylates with HSL hydrobromide by the same technique used to make compound I. The purity of all compounds was carefully tested by reversed-phase HPLC.
The formal International Union of Pure and Applied Chemistry (IUPAC) chemical designations for the compounds used in this study are as follows: A, 3-oxo-N-(tetrahydro-2-oxo-3-furanyl)butanamide; B, 3-oxo-N-(tetrahydro-2-oxo-3-furanyl)pentanamide; C, 3-oxo-N-(tetrahydro-2-oxo-3-furanyl)hexanamide; D, 3-oxo-N-(tetrahydro-2-oxo-3-furanyl)heptanamide; E, 3-oxo-N-(tetrahydro-2-oxo-3-furanyl)octanamide; F, 3-oxo-N-(tetrahydro-2-oxo-3-furanyl)undecanamide; G, 3-oxo-N-(tetrahydro-2-oxo-3-furanyl)dodecanamide; H, 2-ethoxycarbonyl-N-(tetrahydro-2-oxo-3-furanyl)ethanamide; I, N-(tetrahydro-2-oxo-3-furanyl)butanamide; J, N-(tetrahydro-2-oxo-3-furanyl)pentanamide; K, N-(tetrahydro-2-oxo-3-furanyl)hexanamide; L, N-(tetrahydro-2-oxo-3-furanyl)heptanamide; M, N-(tetrahydro-2-oxo-3-furanyl)octanamide; N, N-(tetrahydro-2-oxo-3-furanyl)decanamide; O, N-(tetrahydro-2-oxo-3-furanyl)dodecanamide; P, 3-hydroxy-N-(tetrahydro-2-oxo-3-furanyl)nonanamide; Q, 3-hydroxy-N-(tetrahydro-2-oxo-3-furanyl)dodecanamide;R, N-(tetrahydro-2-oxo-3-furanyl)-2-butenamide; S, N-(tetrahydro-2-oxo-3-furanyl)-2-pentenamide; T, N-(tetrahydro-2-oxo-3-furanyl)-2-hexenamide; U, N-(tetrahydro-2-oxo-3-furanyl)-2-octenamide; V, N-(tetrahydro-2-oxo-3-furanyl)-2-nonenamide; W, N-(tetrahydro-2-oxo-3-furanyl)-2-decenamide; X, N-(tetrahydro-2-oxo-3-furanyl)-2-butynamide; Y, N-(tetrahydro-2-oxo-3-furanyl)-2-hexynamide; Z, N-(tetrahydro-2-oxo-3-furanyl)-5-hexynamide; AA, N-(tetrahydro-2-oxo-3-furanyl)-2-octynamide; AB, 3-oxo-N-(tetrahydro-2-oxo-3-furanyl)-7-octynamide; AC, 3-oxo-N-(tetrahydro-2-oxo-3-furanyl)-11-octadecenamide; AD, N,N′-bis(tetrahydro-2-oxo-3-furanyl)decandiamide; AE, 3,12-dioxo-N,N′-bis(tetrahydro-2-oxo-3-furanyl)tetradecandiamide; AF, 4-propyl-N-(tetrahydro-2-oxo-3-furanyl)benzamide; and AG, 1-hexoxy-N-(tetrahydro-2-oxo-3-furanyl)methanamide. See the legend to Fig. 1 for non-IUPAC descriptions of these compounds.
Bioassays of autoinducer activity.
Autoinducer analogs were stored in ethyl acetate at −80°C. Prior to use, autoinducers were warmed to room temperature and 0.017 μmol was added in 34 μl of ethyl acetate to empty culture tubes. After the solvent was allowed to evaporate, 1.75 ml of AT medium (43) supplemented with 400 μg of octopine per ml and approximately 107 bacterial cells [strain WCF47(pCF372) or WCF47(pCF372)(pCF218)] was added. After the autoinducer analog was allowed to dissolve, 0.55 ml was removed and added to 1.2 ml of the same culture (a 3.16-fold dilution). This dilution was repeated serially nine times, resulting in 10 culture tubes having analogs at concentrations ranging from 10−1 to 104 to nM. These cultures were incubated with aeration for 12 h and assayed for β-galactosidase specific activity (29). The bacterial cultures remained in exponential growth phase during this interval. To assay the abilities of analogs to prevent induction, the assays described above were repeated, except that 3-oxo-C8-HSL (final concentration, 102 nM) was added in addition to the indicated amounts of other compounds.
Western immunoblots of TraR.
To compare intracellular concentrations of TraR in the two bioassay strains described above, we cultured each strain in 100 ml of AT broth containing 100 nM 3-oxo-C8-HSL to an optical density at 600 nm of 0.6, concentrated the cultures by centrifugation, resuspended the cultures in 1 ml of TEDG (50 mM Tris-HCL [pH 7.9], 0.5 mM EDTA, 1 mM dithiothreitol, 5% glycerol), disrupted the cells using a French pressure cell, and ultracentrifuged the lysates at 150,000 × g for 30 min. The resulting cleared lysates were size fractionated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred to a nitrocellulose membrane, and detected with affinity-purified polyclonal anti-TraR rabbit antiserum.
TLC analysis of autoinducers released by A. tumefaciens.
The isolation of autoinducers by strains expressing TraI was done by culturing strains to stationary phase in 20 ml of broth, removing bacteria by centrifugation, and extracting the cell-free spent broth three times with 3 volumes of ethyl acetate. In control experiments, this procedure was shown to recover virtually all of the 3-oxo-C8-HSL and 3-oxo-C6-HSL, although it may not result in quantitative recovery of more polar compounds. The ethyl acetate fractions were pooled and evaporated to dryness, and each residue was resuspended in 1 ml of ethyl acetate, transferred to a 1-ml glass vial, and reevaporated. The residue was resuspended in 200 μl of ethyl acetate and stored at −80°C. Aliquots (1 μl) were applied to C18 reversed-phase thin-layer chromatography (TLC) plates (Whatman) and chromatographed with 60% methanol–40% water as described previously (40). After chromatography, the plates were dried and overlaid with 200 ml of agar containing AT medium, 40 μg of X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside), and approximately 107 bacteria per ml [strain WCF47(pCF372)(pCF218)]. TLC plates were incubated overnight at 28°C and examined for X-Gal hydrolysis.
In experiments to identify products of alkaline hydrolysis of autoinducers, 0.2 nmol of 3-oxo-C8-HSL or 2 nmol of 3-oxo-C6-HSL that had been dissolved in ethyl acetate was transferred to empty microcentrifuge tubes and the ethyl acetate was removed by evaporation. The dried autoinducers were resuspended in 20 μl of water, 20 μl of 0.01 M NaOH (pH 12), or 20 μl of 0.1 M NaOH (pH 13) and then incubated at room temperature for 1 h. One microliter of each was spotted directly onto a reversed-phase TLC plate, which was developed as described above.
RESULTS
Recognition of autoinducer analogs by a strain expressing wild-type levels of TraR.
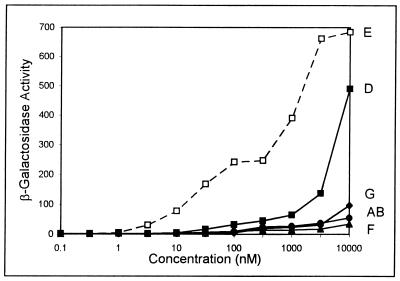
We assayed 33 autoinducers and chemically related compounds (Fig. 1) for the ability to induce β-galactosidase activity in strain WCF47(pCF372), which lacks its own traI gene and which has a plasmid-borne PtraI-lacZ fusion. Synthetic 3-oxo-C8-HSL (compound E) detectably activated this fusion at a concentration of 3.0 nM (Fig. 2). A concentration of 104 nM caused production of approximately 700 U of enzyme activity. The dose-response curve shown in Fig. 2 suggests that this regulatory system was not saturated by a 104 nM concentration of this autoinducer. In marked contrast to these results, of the 32 other compounds tested, only four caused production of significant levels of β-galactosidase activity (Fig. 2 and Table 1). These compounds are 3-oxo-C7-HSL (compound D), 3-oxo-C11-HSL (F), 3-oxo-C12-HSL (G), and 3-oxo-7-octynoyl-HSL (AB). All of these compounds closely resemble 3-oxo-C8-HSL, differing only in the length or level of desaturation of the acyl moeity. All compounds except 3-oxo-C8-HSL were weakly active or inactive at concentrations less than 103 nM (Fig. 2).
FIG. 2.
Dose-response curves with A. tumfaciens WCF47(pCF372). Results with compounds E (□), D (■), F (▴), G (⧫), and AB (•) are shown. All other compounds were inactive.
TABLE 1.
Abilities of A. tumefaciens strains to respond to autoinducer analogs
| Compound | β-Galactosidase activity ina:
|
|||
|---|---|---|---|---|
| WCF47(pCF372) with analog at concn (nM):
|
WCF47(pCF372) (pCF218) with analog at concn (nM):
|
|||
| 10 | 104 | 10 | 104 | |
| 3-Oxobutanoyl-HSL (A) | 1.8 | 0.4 | 1.6 | 24 |
| 3-Oxopentanoyl-HSL (B) | 2.1 | 1.6 | 1.2 | 710 |
| 3-Oxohexanoyl-HSL (C) | 1.6 | 2.5 | 180 | 1,200 |
| 3-Oxoheptanoyl-HSL (D) | 10 | 470 | 1,200 | 1,400 |
| 3-Oxooctanoyl-HSL (E) | 70 | 700 | 1,300 | 1,500 |
| 3-Oxoundecanoyl-HSL (F) | 4.9 | 45 | 440 | 1,100 |
| 3-Oxododecanoyl-HSL (G) | 4.3 | 73 | 91 | 1,300 |
| 4-Oxa-3-oxohexanoyl-HSL (H) | 2.2 | 0.3 | 1.9 | 850 |
| Butanoyl-HSL (I) | 1.2 | 0.8 | 1.8 | 1.7 |
| Pentanoyl-HSL (J) | 1.6 | 1.2 | 2.2 | 210 |
| Hexanoyl-HSL (K) | 2.5 | 2.1 | 0.9 | 1,500 |
| Heptanoyl-HSL (L) | 1.4 | 2.5 | 300 | 680 |
| Octanoyl-HSL (M) | 3.2 | 6.1 | 650 | 1,100 |
| Decanoyl-HSL (N) | 2.6 | 1.9 | 1.7 | 1,000 |
| Dodecanoyl-HSL (O) | 2.2 | 1.6 | 2.3 | 72 |
| 3-Hydroxynonanoyl-HSL (P) | 2.7 | 0.7 | 290 | 1,400 |
| 3-Hydroxydodecanoyl-HSL (Q) | 1.8 | 2.0 | 1.1 | 1,000 |
| 2-Butenoyl-HSL (R) | 2.1 | 2.3 | 2.0 | 2.4 |
| 2-Pentenoyl-HSL (S) | 1.9 | 1.1 | 1.7 | 640 |
| 2-Hexenoyl-HSL (T) | 2.5 | 1.8 | 1.1 | 280 |
| 2-Octenoyl-HSL (U) | 1.3 | 2.2 | 3.1 | 940 |
| 2-Nonenoyl-HSL (V) | 1.8 | 1.8 | 1.7 | 800 |
| 2-Decenoyl-HSL (W) | 2.0 | 1.3 | 0.8 | 300 |
| 2-Butynoyl-HSL (X) | 3.0 | 1.0 | 1.3 | 1.6 |
| 2-Hexynoyl-HSL (Y) | 2.3 | 0.7 | 1.3 | 120 |
| 5-Hexynoyl-HSL (Z) | 1.9 | 0.5 | 2.1 | 380 |
| 2-Octynoyl-HSL (AA) | 1.9 | 1.1 | 1.3 | 710 |
| 3-Oxo-7-octynoyl-HSL (AB) | 1.3 | 45 | 710 | 1,500 |
| 3-Oxo-11-octadecenoyl-HSL (AC) | 2.8 | 1.7 | 1.3 | 27 |
| diHSL decandioate (AD) | 1.7 | 1.3 | 1.8 | 17 |
| diHSL-3,12-dioxotetradecandioate (AE) | 1.6 | 1.0 | 19 | 460 |
| p-Propylbenzoyl-HSL (AF) | ND | 1.5 | ND | 1.8 |
| O-Hexyl-N-HSL carbamate (AG) | 2.9 | 1.2 | 7.1 | 1,000 |
The indicated analogs were provided at the noted concentrations to cultures of WCF47(pCF372) containing or lacking pCF218 in AT broth supplemented with 2 mg of opine per ml. Bacteria were cultured for 12 h and assayed for β-galactosidase specific activity (29). β-Galactosidase expression in the absence of autoinducers was less than 2 Miller units. ND, not determined.
We also tested whether any of these compounds could antagonize induction by 3-oxo-C8-HSL. We assayed cultures of strain WCF47(pCF372) in the presence of 102 nM 3-oxo-C8-HSL and a 102, 103, or 104 nM concentration of each of the other analogs. Approximately half of these analogs inhibited induction at least fourfold when they were provided at a 100:1 molar ratio (Table 2). In some cases, activity was reduced 100-fold or more. Six compounds inhibited induction at least fourfold when they were supplied at a 10:1 molar ratio, and one compound (C8-HSL, compound M) inhibited induction when it was provided at a 1:1 molar ratio (Table 2). The most effective antagonists included 3-oxo-C6-HSL (compound C), C7-HSL (L), C8-HSL (M), C10-HSL (N), and 3-hydroxy-C9-HSL (P), all of which closely resemble 3-oxo-C8-HSL in structure.
TABLE 2.
Measure of competitive inhibition in A. tumefaciens strains with 100 nM 3-oxo-C8-HSL
| Inhibitor | β-Galactosidase activity ina:
|
|||||
|---|---|---|---|---|---|---|
| WCF47(pCF372) with analog at concn (nM):
|
WCF47(pCF372)(pCF218) with analog at concn (nM):
|
|||||
| 102 | 103 | 104 | 102 | 103 | 104 | |
| None | 340 | NA | NA | 1,400 | NA | NA |
| A | 360 | 250 | 150 | 1,600 | 1,300 | 1,200 |
| B | 350 | 240 | 110 | 1,500 | 1,500 | 1,300 |
| C | 340 | 11 | 0.9 | 1,600 | 1,500 | 1,200 |
| D | 210 | 120 | 470 | 1,500 | 1,500 | 1,100 |
| F | 280 | 38 | 74 | 1,300 | 1,200 | 1,000 |
| G | 250 | 200 | 180 | 1,400 | 1,400 | 1,000 |
| H | 360 | 320 | 17 | 1,800 | 1,500 | 850 |
| I | 400 | 330 | 380 | 1,400 | 840 | 1,300 |
| J | 350 | 350 | 97 | 1,600 | 1,600 | 1,200 |
| K | 380 | 340 | 46 | 1,500 | 1,600 | 940 |
| L | 180 | 19 | 2.4 | 1,400 | 1,200 | 950 |
| M | 95 | 6.0 | 5.7 | 1,600 | 1,500 | 1,200 |
| N | 370 | 93 | 2.6 | 1,400 | 1,300 | 1,100 |
| O | 410 | 330 | 17 | 1,600 | 1,400 | 1,500 |
| P | 220 | 9.5 | 3.6 | 1,500 | 1,500 | 1,300 |
| Q | 350 | 99 | 1.9 | 1,400 | 1,400 | 1,000 |
| R | 320 | 330 | 330 | 1,600 | 1,600 | 1,500 |
| S | 380 | 320 | 260 | 1,600 | 1,600 | 1,400 |
| T | 330 | 320 | 76 | 1,400 | 1,600 | 890 |
| U | 340 | 170 | 3.9 | 1,500 | 1,300 | 1,100 |
| V | 370 | 250 | 8.6 | 1,500 | 1,500 | 780 |
| W | 360 | 360 | 140 | 1,400 | 1,300 | 1,100 |
| X | 340 | 330 | 310 | 1,500 | 990 | 1,300 |
| Y | 360 | 350 | 340 | 1,600 | 930 | 1,700 |
| Z | 370 | 390 | 300 | 1,600 | 1,100 | 1,500 |
| AA | 320 | 370 | 210 | 1,300 | 1,500 | 1,100 |
| AB | 230 | 48 | 33 | 1,300 | 1,400 | 940 |
| AC | 380 | 370 | 300 | 1,400 | 1,500 | 950 |
| AD | 340 | 310 | 270 | 1,600 | 1,200 | 1,500 |
| AE | 340 | 220 | 13 | 1,600 | 1,500 | 970 |
| AF | 360 | 350 | 320 | 1,600 | 1,500 | 970 |
| AG | 370 | 350 | 64 | 1,400 | 1,100 | 1,000 |
The indicated analogs were provided at the noted concentrations to cultures of WCF47(pCF372) containing or lacking pCF218 in AT broth supplemented with 2 mg of opine per ml. Bacteria were cultured for 12 h and assayed for β-galactosidase specific activity (29). β-Galactosidase expression in the absence of autoinducers was less than 2 Miller units. NA, not applicable.
Autoinducer recognition by a strain that overexpresses TraR.
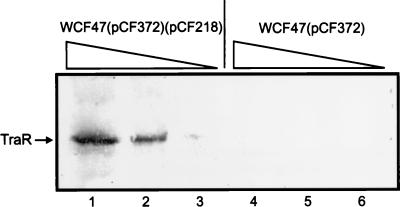
To determine whether TraR overexpression altered the detection of autoinducers, we constructed a derivative of WCF47(pCF372) that contains plasmid pCF218, which bears DNA that overexpresses TraR from a vector promoter (17). While WCF47(pCF372) synthesizes extremely low levels of TraR, WCF47(pCF372)(pCF218) synthesizes amounts that are readily detectable by Western immunoblotting (Fig. 3). WCF47(pCF372)(pCF218) was far more sensitive to low concentrations of 3-oxo-C8-HSL than its parent, since it was detectably induced at concentrations as low as 0.1 nM and the response was saturated by an approximately 10 nM concentration of this autoinducer (Fig. 4A). The reporter fusion in this strain, when fully induced, expressed about twofold more β-galactosidase than the same reporter fusion in the strain that expresses wild-type levels of TraR.
FIG. 3.
Western immunoblot of serially diluted extracts of WCF47(pCF372)(pCF218) (left) and WCF47(pCF372) (right). Lanes 1 and 4, 10 μl of lysate; lanes 2 and 5, 2.5 μl of lysate; lanes 3 and 6, 0.625 μl of lysate.
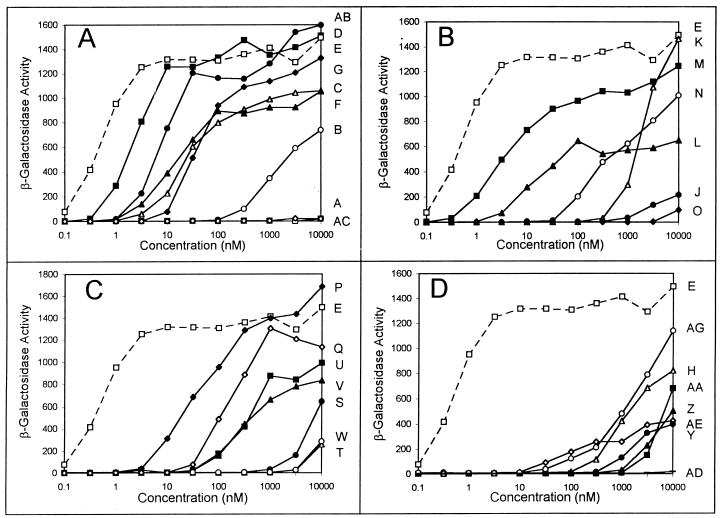
FIG. 4.
Dose-response curves with an A. tumefaciens strain that overexpresses TraR. (A) Results with compounds A (□), B (○), C (▵), D (■), E (□), F (▴), G (⧫), AB (•), and AC (◊); (B) results with compounds E (□), J (•), K (▵), L (▴), M (■), N (○), and O (⧫); (C) results with compounds E (□), P (⧫), Q (◊), S (•), T (▵), U (■), V (▴), and W (○); (D) results with compounds E (□), H (▵), Y (•), Z (▴), AA (■), AD (–), AE (◊), and AG (○).
We also tested the ability of the 32 autoinducer analogs to induce the reporter fusion of WCF47(pCF372)(pCF218). The responses to two concentrations of these compounds are shown in Table 1, and dose-response curves for most compounds are shown in Fig. 4. Surprisingly, this strain was detectably stimulated by no fewer than 29 of the 33 tested compounds. Among the most active compounds were 3-oxo-C7-HSL (compound D), 3-oxo-7-octynoyl-HSL (AB), and C8-HSL (M), all of which can half-maximally induce the fusion when they are provided at 3 to 10 nM. All three compounds are closely related to 3-oxo-C8-HSL. Slightly less active compounds include 3-oxo-C6-HSL (C), C7-HSL (L), 3-oxo-C11-HSL (F), and 3-hydroxy-C9-HSL (P). Almost all other compounds were stimulatory when they were supplied at higher concentrations. The four inactive compounds were C4-HSL (I), 2-butenoyl-HSL (R), 2-butynoyl-HSL (X), and p-propylbenzoyl-HSL (AF). 3-Oxo-C4-HSL (A) was only weakly active, indicating that TraR is stimulated very poorly or not at all by autoinducers having four-carbon acyl moieties. Two other compounds, 3-oxo-11-octadecenoyl-HSL (AC) and diHSL-decandioate (AD), were also very weak inducers.
We tested these 32 compounds for the ability to antagonize 3-oxo-C8-HSL. Strikingly, none of these compounds was able to inhibit induction more than twofold (Table 2). Several compounds appeared to cause very modest inhibition, although these differences may lie within experimental error.
Production of multiple autoinducers by TraI.
We have shown that several naturally occurring autoinducers are potent antagonists of 3-oxo-C8-HSL in a strain that expresses wild-type levels of TraR. In an earlier report, Zhang and colleagues reported that an A. tumefaciens strain containing an octopine-type Ti plasmid synthesized compounds having molecular masses identical to those of 3-oxo-C8-HSL, 3-oxo-C6-HSL, and C6-HSL (46). We have found that the latter two compounds can antagonize 3-oxo-C8-HSL. To determine whether A. tumefaciens synthesizes inhibitory concentrations of these compounds, we used TLC (40) to visualize all bioactive autoinducers made by A. tumefaciens and to estimate the concentrations of some of these compounds.
Strain R10(pCF372) was cultured in broth (AT salts supplemented with octopine) to stationary phase and assayed for β-galactosidase activity. The culture expressed only 36 U of activity, indicating that its tra regulon was only weakly induced and suggesting that the culture medium contained only limited amounts of autoinducers. These data agree with earlier observations that the high cell densities required for induction of this system are more readily achieved on semisolid medium than in broth culture (17). We therefore diluted this culture fivefold into fresh broth (AT salts and octopine) and incubated it once again to stationary phase. This second culture expressed 282 U of β-galactosidase activity, indicating that its tra regulon was far more strongly induced than that of the first culture, presumably due to the autoinducers released during the first growth interval. Bacteria were removed by centrifugation from 20 ml of this culture, and the cell-free supernatant was extracted and resuspended in 200 μl of ethyl acetate as described in Materials and Methods. The concentrated extract was serially diluted in fivefold steps, and 1 μl of each dilution was applied to a reversed-phase TLC plate.
In the most concentrated sample, no fewer than six bioactive compounds were detected (Fig. 5A). The two most prominent spots had Rfs identical to those of 3-oxo-C8-HSL and 3-oxo-C6-HSL, and smaller amounts of a compound with an Rf identical to that of C6-HSL was also detected, consistent with the data of Zhang and colleagues (46). In addition, trace amounts of several more hydrophobic compounds were detected. Two very polar compounds were also detected, as described more fully below. WCF47(pCF372), which lacks traI, did not synthesize any compounds active in this assay (data not shown), suggesting that all detected compounds were synthesized by TraI.
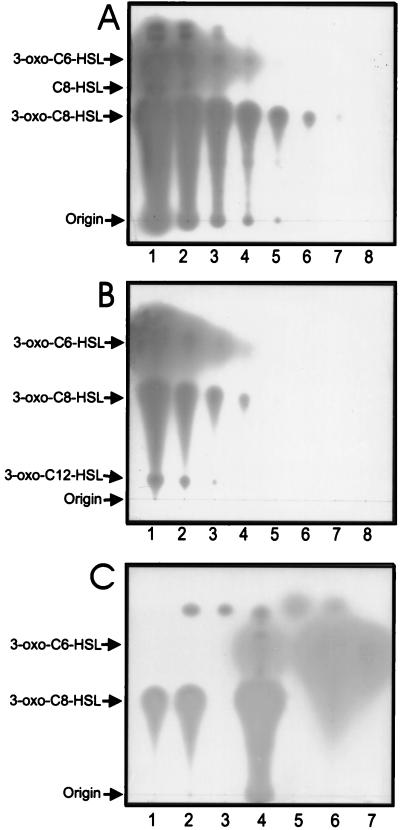
FIG. 5.
TLC of autoinducers synthesized by a wild-type A. tumefaciens strain. (A) Lane 1 represents the activities of autoinducers obtained from 100 μl of culture supernatant. Lanes 2 to 8 show fivefold serial dilutions of the sample chromatographed in lane 1. (B) Synthetic racemic autoinducers. Lane 1 contains 50 pmol of 3-oxo-C6-HSL, 50 pmol of 3-oxo-C8-HSL, and 1,500 pmol of 3-oxo-C12-HSL. Lanes 2 to 8 show fivefold serial dilutions of the sample chromatographed in lane 1. (C) Conversion of 3-oxo-C8-HSL and 3-oxo-C6-HSL to a more polar active form by treatment with alkali. Lane 1, 3-oxo-C8-HSL incubated at pH 7.0; lane 2, 3-oxo-C8-HSL incubated at pH 12 for 1 h; lane 3, 3-oxo-C8-HSL incubated at pH 13 for 1 h; lane 4, autoinducers produced by strain R10(pCF372); lane 5, 3-oxo-C6-HSL incubated at pH 13 for 1 h; lane 6, 3-oxo-C6-HSL incubated at pH 12 for 1 h; lane 7, 3-oxo-C6-HSL incubated at pH 7.
The concentrations of 3-oxo-C8-HSL and 3-oxo-C6-HSL were estimated by serially diluting each concentrated extract and identifying the greatest dilution that contained detectable amounts of each compound. These spots were compared to spots made by serially diluted synthetic autoinducers (Fig. 5B). From these data, we estimated that the culture contained 16 μM 3-oxo-C8-HSL and 0.6 μM 3-oxo-C6-HSL.
We also tested the production of autoinducers by strain R10(pCF372)(pCF218), which overexpresses TraR and therefore constitutively overexpresses TraI (17), as well as strain KYC6, which contains a null mutation in traM and therefore expresses the tra regulon at elevated levels (15). The culture supernatants of these strains contained all of the bioactive compounds detected above (data not shown). Furthermore, these compounds were synthesized in ratios similar to those in R10(pCF372). The culture supernatant of strain R10(pCF372)(pCF218) contained approximately 1,100 μM 3-oxo-C8-HSL and 25 μM 3-oxo-C6-HSL, while that of KYC6 contained 800 μM 3-oxo-C8-HSL and 50 μM 3-oxo-C6-HSL. Therefore, artificial overexpression of the tra regulon increased the production of all detectable autoinducers but did not greatly alter the ratios of their concentrations. Since 3-oxo-C6-HSL and C6-HSL inhibit tra gene expression only when they are present at concentrations higher than that of 3-oxo-C8-HSL (Table 2), the concentrations found in A. tumefaciens supernatants are unlikely to interfere with expression of the tra regulon.
As described above, two very polar bioactive compounds present in culture supernatants were detected in these TLC assays. Neither of these compounds is likely to be 3-oxo-C4-HSL, because synthetic 3-oxo-C4-HSL was poorly detected in this assay and formed an extremely diffuse spot (data not shown). We hypothesized that the two polar compounds could be a consequence of spontaneous hydrolysis of the HSL ring of two of the autoinducers described above. To test this, we treated synthetic 3-oxo-C8-HSL and 3-oxo-C6-HSL with alkali at pH 12 or 13 for 1 h at room temperature and chromatographed and bioassayed the resulting compounds. Incubation at pH 13 destroyed both compounds (Fig. 5C, lane 3 and 5). However, hydrolysis of 3-oxo-C8-HSL caused the appearance of a bioactive compound having an Rf identical to the that of most polar compound found in A. tumefaciens culture supernatants (Fig. 5C, lanes 2, 3, and 4). Hydrolysis of 3-oxo-C6-HSL caused the appearance of an even more polar compound (Fig. 5C, lanes 5 and 6) that did not comigrate with any bioactive compound in lane 4. Since alkali opens the HSL ring, these polar compounds are probably 3-oxo-C8-homoserine and 3-oxo-C6-homoserine. These compounds are either detectably active in this bioassay or else undergo lactonization during the drying of the TLC plates, forming bioactive acyl-HSLs. These data are reminiscent of the data of Zhang and colleagues, who reported that an acyclic methyl ester of 3-oxo-C8-HSL is bioactive (46).
DISCUSSION
We have tested the ability of two strains of A. tumefaciens to discriminate between the autoinducer 3-oxo-C8-HSL and a variety of related compounds. The strain expressing wild-type levels of TraR was far more strongly induced by its cognate autoinducer than by any other tested compound and was completely nonresponsive to all but four analogs. In contrast, an isogenic strain that constitutively overexpressed TraR was far more sensitive to these compounds and seemed to be less discriminatory, since 29 of the 33 compounds tested were stimulatory and several of these showed a half-maximal responses at only 3 to 30-fold-higher concentrations than that of the native autoinducer. Furthermore, in the strain expressing wild-type levels of TraR, many analogs were potent inhibitors of induction, while in a strain that overexpressed TraR, inhibition was not detected.
The narrow substrate specificities of wild-type strains might be interpreted to mean that only 3-oxo-C8-HSL and a few closely related compounds can bind TraR at the concentrations used. However, this possibility is inconsistent with our finding that many of the same compounds are potent antagonists, since this antagonism is presumably a consequence of competition for autoinducer binding sites. We therefore prefer a model in which TraR can bind a wide variety of autoinducer analogs but in which only a small subset can cause a conformational change in TraR necessary to convert it to an active form.
As described above, the strain expressing wild-type levels of TraR was stimulated only by compounds that closely resemble the cognate autoinducer (compounds D, F, G, and AB). Furthermore, the most effective antagonists (compounds C, L, M, N, P, Q, U, and V) also closely resemble the cognate autoinducer. However, there was little if any overlap in performance between effective agonists and the most effective antagonists. All agonists had acyl chains of seven carbon residues or more, and all had 3-oxo substituents. Therefore, TraR tolerated acyl groups one carbon shorter or up to four carbons longer than the cognate autoinducer and tolerated a triple bond at the 7-8 position but did not tolerate other alterations. In contrast, with one exception, the strongest antagonists lacked the 3-oxo substituent and had methylene residues, hydroxyl residues, or 2-3 unsaturated bonds at this position. The single best antagonist (compound M) is identical to the best autoinducer (compound E) except for the fact that compound M lacks the 3-oxo group. The exception to this pattern is compound C, which has a 3-oxo group but has a six-carbon fatty acyl group. Since antagonism is probably a consequence of competitive binding, we conclude that the 3-oxo group is completely dispensable for TraR binding but that it plays an important role in converting TraR into an active conformation.
Overproduction of TraR did not lead to constitutive expression of a target promoter. Rather, promoter activity still required an autoinducer, but the number of active compounds was drastically increased. Overexpression of TraR therefore potentiated its ability to activate transcription. Perhaps these data can best be explained by postulating that autoinducers increase the affinity of TraR either for other TraR monomers or for tra box DNA (or conceivably for some other macromolecule such as RNA polymerase). If so, autoinducer analogs may cause similar but smaller increases in affinity. Small increases in affinity would be insufficient to drive activation of wild-type TraR pools but would be sufficient to cause activation when TraR is overproduced. To help illustrate this point, we propose that 3-oxo-C8-HSL may act by decreasing the Kd for TraR dimerization from, perhaps, 10−4 to 10−7 M, while 3-oxo-C6-HSL may decrease the Kd to 10−6 M. According to this hypothetical example, a low concentration of TraR (for example, 10−7 M TraR monomers) would permit significant dimerization by 3-oxo-C8-HSL but not by 3-oxo-C6-HSL while a higher concentration of TraR (for example, 10−5 M TraR monomers) would permit significant dimerization by both autoinducers. If autoinducers decrease the Kd for TraR-DNA or TraR-RNA polymerase interactions, very similar models can be proposed.
There are at least two indications that active TraR is di- or oligomeric, as was previously suggested for LuxR (13). First, a truncated TraR-like protein lacking a DNA binding domain exerts dominant negative effects on tra gene expression, suggesting that it forms inactive heteromultimers (31, 47). Similar data have been reported for LuxR (7). Second, several putative TraR-binding sites show a strong dyad symmetry (16), suggesting that one monomer of a dimer contacts one arm of the dyad and that a second monomer makes identical contacts on the opposite arm.
Our observations are reminiscent of the data of Sitnikov and coworkers (41), who found that 3-hydroxy-C4-HSL failed to activate lux genes in V. fischeri but did so when the lux operon was cloned in E. coli, where LuxR may have been overexpressed. In the same study, C10-HSL was an antagonist of lux genes in V. fischeri but was an agonist in E. coli. Similarly, Schaefer and colleagues reported that 15 of 17 autoinducer analogs inhibited bioluminescence in V. fischeri (in the weakly bioluminescent strain B-61; see reference 13), while in a separate study, many of the same compounds did not inhibit bioluminescence in a recombinant strain of E. coli carrying a reconstituted lux regulon (37). Kuo and colleagues also demonstrated that C8-HSL is an antagonist in V. fischeri (28). LuxR was overexpressed in these E. coli strains, and this may have increased autoinducer sensitivity in a fashion similar to that observed for TraR in our study.
Similar studies have been carried out with an E. coli strain expressing LasR (32). In this study, LasR showed a broad substrate specificity, comparable to the response we observed using the TraR-overexpressing strain. Many of these compounds competed against radiolabeled 3-oxo-C12-HSL in a binding assay, but none interfered with induction of a target promoter. It is possible that LasR would have shown a narrower autoinducer specificity and would have been more effectively antagonized had it been expressed at wild-type levels. This may have important clinical implications, where autoinducer antagonists may be useful in treating Pseudomonas infections.
The ability of wild-type A. tumefaciens to discriminate between 3-oxo-C8-HSL and all other tested compounds should prevent induction by noncognate autoinducers released by other bacterial species. In fact, the only noncognate autoinducer that efficiently induced the tra regulon at low concentrations (3-oxo-C7-HSL) has an odd number of carbon atoms and is therefore unlikely to be found in nature. On the other hand, the finding that many compounds actively inhibited induction suggests that other bacterial species in the rhizosphere may interfere with autoinduction and thereby inhibit Ti plasmid conjugation. Whether this has important ecological consequences is not known. By analogy, one can imagine that 3-oxo-C6-HSL synthesized by A. tumefaciens may well perturb autoinduction by other bacterial species.
TraI can synthesize detectable amounts of several autoinducers in addition to 3-C8-HSL (Fig. 5). It is noteworthy that at least two of these compounds can inhibit induction of wild-type strains (Table 1). However, these compounds are synthesized at levels lower than that of 3-oxo-C8-HSL and are therefore unlikely to interfere with induction.
ACKNOWLEDGMENTS
We thank the following Ithaca College chemistry students: L. David Finger for the synthesis of compounds H, AD, AE, and AG; Michael Kiefer for the synthesis of compounds Q, X, Z, AA, and AB; A. Damon for the synthesis of compounds S and W; and Anthony Atti, Adam Brownstein, Shomesh Doddi, J. P. Kirby, George Lemieux, Robert Lewis, Patrick Sarmiere, Jonathan Sparks, and Robert Tarsa for synthesis of compounds D, F, O, P, R, U, V, AC, and AF, respectively.
This study was supported by NIH grant GM42893.
REFERENCES
- 1.Adar Y Y, Ulitzur S. GroESL proteins facilitate binding of externally added inducer by LuxR protein-containing Escherichia coli cells. J Biolumin Chemilumin. 1993;8:261–266. doi: 10.1002/bio.1170080506. [DOI] [PubMed] [Google Scholar]
- 2.Bassler B L, Silverman M R. Intercellular communication in marine Vibrio species: density-dependent regulation of the expression of bioluminescence. In: Hoch J A, Silhavy T J, editors. Two-component signal transduction. Washington, D.C: ASM Press; 1995. pp. 431–445. [Google Scholar]
- 3.Beck von Bodman S B, Farrand S K. Capsular polysaccharide biosynthesis and pathogenicity in Erwinia stewartii require induction by an N-acylhomoserine lactone autoinducer. J Bacteriol. 1995;177:5000–5008. doi: 10.1128/jb.177.17.5000-5008.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beck-von Bodman S, Hayman G T, Farrand S K. Opine catabolism and conjugal transfer of the nopaline Ti plasmid pTiC58 are coordinately regulated by a single repressor. Proc Natl Acad Sci USA. 1992;89:643–647. doi: 10.1073/pnas.89.2.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cao J G, Meighen E A. Biosynthesis and stereochemistry of the autoinducer controlling luminescence in Vibrio Harveyi. J Bacteriol. 1993;175:3856–3862. doi: 10.1128/jb.175.12.3856-3862.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chhabra S R, Stead P, Bainton N J, Salmond G P, Stewart G S, Williams P, Bycroft B W. Autoregulation of carbapenem biosynthesis in Erwinia carotovora by analogues of N-(3-oxohexanoyl)-L-homoserine lactone. J Antibiot. 1993;46:441–454. doi: 10.7164/antibiotics.46.441. [DOI] [PubMed] [Google Scholar]
- 7.Choi S H, Greenberg E P. Genetic evidence for multimerization of LuxR, the transcriptional activator of Vibrio fischeri luminescence. Mol Mar Biol Biotechnol. 1992;1:408–413. [Google Scholar]
- 8.Cronan J E, Jr, Rock C O. Biosynthesis of membrane lipids, P. 612–636. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Washington, D.C: ASM Press; 1996. [Google Scholar]
- 9.Dessaux Y, Petit A, Tempé J. Opines in Agrobacterium biology. In: Verma D P S, editor. Molecular signals in plant-microbe interactions. Boca Raton, Fla: CRC Press; 1992. pp. 109–136. [Google Scholar]
- 10.Dessaux Y, Petit A, Ellis J G, Legrain C, Demarez M, Wiame J M, Popoff M, Tempé J. Ti plasmid-controlled chromosome transfer in Agrobacterium tumefaciens. J Bacteriol. 1989;171:6363–6366. doi: 10.1128/jb.171.11.6363-6366.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eberhard A, Burlingame A, Eberhard C, Kenyon G, Nealson K H, Oppenheimer N J. Structural identification of autoinducer of Photobacterium fischeri luciferase. Biochemistry. 1981;20:2444–2449. doi: 10.1021/bi00512a013. [DOI] [PubMed] [Google Scholar]
- 12.Eberhard A, Longin T, Widrig C A, Stranick S J. Synthesis of the lux gene autoinducer in Vibrio fischeri is positively autoregulated. Arch Microbiol. 1991;155:294–297. [Google Scholar]
- 13.Eberhard A, Widrig C A, McBath P, Schineller J. Analogs of the autoinducer of bioluminescence in Vibrio fischeri. Arch Microbiol. 1986;146:35–40. doi: 10.1007/BF00690155. [DOI] [PubMed] [Google Scholar]
- 14.Eberl L, Winson M K, Sternberg C, Stewart G S, Christiansen G, Chhabra S R, Bycroft B, Williams P, Molin S, Givskov M. Involvement of N-acyl-L-homoserine lactone autoinducers in controlling the multicellular behaviour of Serratia liquefaciens. Mol Microbiol. 1996;20:127–136. doi: 10.1111/j.1365-2958.1996.tb02495.x. [DOI] [PubMed] [Google Scholar]
- 15.Fuqua C, Burbea M, Winans S C. Activity of the Agrobacterium Ti plasmid conjugal transfer regulator TraR is inhibited by the product of the traM gene. J Bacteriol. 1995;177:1367–1373. doi: 10.1128/jb.177.5.1367-1373.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fuqua C, Winans S C. Conserved cis-acting promoter elements are required for density-dependent transcription of Agrobacterium tumefaciens conjugal transfer genes. J Bacteriol. 1996;178:435–440. doi: 10.1128/jb.178.2.435-440.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fuqua W C, Winans S C. A LuxR-LuxI type regulatory system activates Agrobacterium Ti plasmid conjugal transfer in the presence of a plant tumor metabolite. J Bacteriol. 1994;176:2796–2806. doi: 10.1128/jb.176.10.2796-2806.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fuqua W C, Winans S C, Greenberg E P. Census and consensus in bacterial ecosystems: the LuxR-LuxI family of quorum-sensing transcriptional regulators. Annu Rev Microbiol. 1996;50:727–751. doi: 10.1146/annurev.micro.50.1.727. [DOI] [PubMed] [Google Scholar]
- 19.Givskov M, de Nys R, Manefield M, Gram L, Maximilien R, Eberl L, Molin S, Steinberg P, Kjelleberg S. Eucaryotic interference with homoserine lactone-mediated prokaryotic signalling. J Bacteriol. 1996;178:6618–6622. doi: 10.1128/jb.178.22.6618-6622.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gray K M, Pearson J P, Downie J A, Boboye B E A, Greenberg E P. Quorum sensing in the symbiotic nitrogen-fixing bacterium Rhizobilim leguminosarum: autoinduction of stationary phase and rhizosphere-expressed genes. J Bacteriol. 1996;178:372–376. doi: 10.1128/jb.178.2.372-376.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hanzelka B L, Greenberg E P. Evidence that the N-terminal region of the Vibrio fischeri LuxR protein constitutes an autoinducer-binding domain. J Bacteriol. 1995;177:815–817. doi: 10.1128/jb.177.3.815-817.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hanzelka B L, Greenberg E P. Quorum sensing in Vibrio fischeri: evidence that S-adenosylmethionine is the amino acid substrate for autoinducer synthesis. J Bacteriol. 1996;178:5291–5294. doi: 10.1128/jb.178.17.5291-5294.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hwang I, Li P-L, Zhang L, Piper K R, Cook D M, Tate M E, Farrand S K. TraI, a LuxI homologue, causes production of conjugation factor, the Ti plasmid N-acyl-homoserine lactone autoinducer. Proc Natl Acad Sci USA. 1994;91:4639–4643. doi: 10.1073/pnas.91.11.4639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaniga K, Delor I, Cornellis G R. A wide-host-range suicide vector for improving reverse genetics in gram-negative bacteria: inactivation of the blaA gene of Yersinia enterocolitica. Gene. 1991;109:137–141. doi: 10.1016/0378-1119(91)90599-7. [DOI] [PubMed] [Google Scholar]
- 25.Kaplan H B, Greenberg E P. Diffusion of autoinducer is involved in regulation of the Vibrio fischeri luminescence system. J Bacteriol. 1985;163:1210–1214. doi: 10.1128/jb.163.3.1210-1214.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kell D B, Kaprelyants A S, Grafen A. Pheromones, social behaviour and functions of secondary metabolism in bacteria. Trends Ecol Evol. 1995;10:126–129. doi: 10.1016/s0169-5347(00)89013-8. [DOI] [PubMed] [Google Scholar]
- 27.Kuo A, Blough N V, Dunlap P V. Multiple N-acyl-l-homoserine lactone autoinducers of luminescence in the marine symbiotic bacterium Vibrio fischeri. J Bacteriol. 1994;176:7558–7565. doi: 10.1128/jb.176.24.7558-7565.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuo A, Callahan S M, Dunlap P V. Modulation of luminescence operon expression by N-octanoyl-l-homoserine lactone in ainS mutants of Vibrio fischeri. J Bacteriol. 1996;178:971–976. doi: 10.1128/jb.178.4.971-976.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miller J. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1972. [Google Scholar]
- 30.Moré M I, Finger L D, Stryker J L, Fuqua C, Eberhard A, Winans S C. Enzymatic synthesis of a quorum-sensing autoinducer through the use of defined substrates. Science. 1996;272:1655–1658. doi: 10.1126/science.272.5268.1655. [DOI] [PubMed] [Google Scholar]
- 31.Oger P, Kim K S, Sackett R L, Piper K R, Farrand S K. Octopine-type Ti plasmids code for a mannopine-inducible dominant-negative allele of traR, the quorum-sensing activator that regulates Ti plasmid conjugal transfer. Mol Microbiol. 1998;27:277–288. doi: 10.1046/j.1365-2958.1998.00671.x. [DOI] [PubMed] [Google Scholar]
- 32.Passador L, Tucker K D, Guertin K R, Journet M P, Kende A S, Iglewski B H. Functional analysis of the Pseudomonas aeruginosa autoinducer PAI. J Bacteriol. 1996;178:5995–6000. doi: 10.1128/jb.178.20.5995-6000.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pearson J P, Gray K M, Passador L, Tucker K D, Eberhard A, Iglewski B H, Greenberg E P. Structure of the autoinducer required for expression of Pseudomonas aeruginosa virulence genes. Proc Natl Acad Sci USA. 1994;91:197–201. doi: 10.1073/pnas.91.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Piper K R, Beck von Bodman S, Farrand S K. Conjugation factor of Agrobacterium tumefaciens regulates Ti plasmid transfer by autoinduction. Nature. 1993;362:448–450. doi: 10.1038/362448a0. [DOI] [PubMed] [Google Scholar]
- 35.Puskas A, Greenberg E P, Kaplan S, Schaefer A L. A quorum-sensing system in the free-living photosynthetic bacterium Rhodobacter sphaeroides. J Bacteriol. 1997;179:7530–7537. doi: 10.1128/jb.179.23.7530-7537.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Salmond G P C, Bycroft B W, Stewart G S A B, Williams P. The bacterial ‘enigma’: cracking the code of cell-cell communication. Mol Microbiol. 1995;16:615–624. doi: 10.1111/j.1365-2958.1995.tb02424.x. [DOI] [PubMed] [Google Scholar]
- 37.Schaefer A L, Hanzelka B L, Eberhard A, Greenberg E P. Quorum sensing in Vibrio fischeri: probing autoinducer-LuxR interactions with autoinducer analogs. J Bacteriol. 1996;178:2897–2901. doi: 10.1128/jb.178.10.2897-2901.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schaefer A L, Val D L, Hanzelka B L, Cronan J E, Jr, Greenberg E P. Generation of cell-to-cell signals in quorum sensing: acyl homoserine lactone synthase activity of a purified Vibrio fischeri LuxI protein. Proc Natl Acad Sci USA. 1996;93:9505–9509. doi: 10.1073/pnas.93.18.9505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schripsema J, de Rudder K E E, van Vliet T B, Lankhorst P P, de Vroom E, Kijne J W, van Brussel A A N. Bacteriocin small of Rhizobium leguminosarum belongs to the class of N-acyl-l-homoserine lactone molecules, known as autoinducers and as quorum sensing co-transcription factors. J Bacteriol. 1996;178:366–371. doi: 10.1128/jb.178.2.366-371.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shaw P D, Ping G, Daly S L, Cha C, Cronan J E, Jr, Rinehart K L, Farrand S K. Detecting and characterizing N-acyl-homoserine lactone signal molecules by thin-layer chromatography. Proc Natl Acad Sci USA. 1997;94:6036–6041. doi: 10.1073/pnas.94.12.6036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sitnikov D M, Schineller J B, Baldwin T O. Transcriptional regulation of bioluminescence genes from Vibrio fischeri. Mol Microbiol. 1995;17:801–812. doi: 10.1111/j.1365-2958.1995.mmi_17050801.x. [DOI] [PubMed] [Google Scholar]
- 42.Stevens A M, Dolan K M, Greenberg E P. Synergistic binding of the Vibrio fischeri LuxR transcriptional activator domain and RNA polymerase to the lux promoter region. Proc Natl Acad Sci USA. 1994;91:12619–12623. doi: 10.1073/pnas.91.26.12619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tempé J, Petit A, Holsters M, Van Montagu M, Schell J. Thermosensitive step associated with transfer of Ti plasmid during conjugation: possible relation to transformation in crown gall. Proc Natl Acad Sci USA. 1977;74:2848–2849. doi: 10.1073/pnas.74.7.2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Winson M K, Camara M, Latifi A, Foglino M, Chhabra S R, Daykin M, Bally M, Chapon V, Salmond G P C, Bycroft B W, Lazdunski A, Stewart G S A B, Williams P. Multiple N-acyl-L-homoserine lactone signal molecules regulate production of virulence determinants and secondary metabolites in Pseudomonas aeruginosa. Proc Natl Acad Sci USA. 1995;92:9427–9431. doi: 10.1073/pnas.92.20.9427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wirth R, Muscholl A, Wanner G. The role of pheromones in bacterial infections. Trends Microbiol. 1996;4:96–103. doi: 10.1016/0966-842X(96)81525-3. [DOI] [PubMed] [Google Scholar]
- 46.Zhang L, Murphy P J, Kerr A, Tate M E. Agrobacterium conjugation and gene regulation by N-acyl-L-homoserine lactones. Nature. 1993;362:446–448. doi: 10.1038/362446a0. [DOI] [PubMed] [Google Scholar]
- 47.Zhu J, Winans S C. Activity of the quorum-sensing regulator TraR of Agrobacterium tumefaciens is inhibited by a truncated, dominant defective TraR-like protein. Mol Microbiol. 1998;27:289–297. doi: 10.1046/j.1365-2958.1998.00672.x. [DOI] [PubMed] [Google Scholar]