Abstract
Objectives
While several indicators have been studied, the association of body roundness index (BRI) with non-alcoholic fatty liver disease (NAFLD) remains unclear. We aimed to explore the association between BRI and ultrasound-defined NAFLD.
Methods
The sample dataset was extracted from the National Health and Nutrition Examination Survey (NHANES) during the period of 2017–2018. The diagnosis of NAFLD was determined based on the controlled attenuated parameter (CAP≥248 dB/m) score of liver ultrasound transient elastography (LUTE). Participants with excessive alcohol use and viral hepatitis were excluded. To delve deeper into the relationship, Multivariable logistic regression with adjustment for confounding variables and smoothing curve analysis was used to investigate the association and nonlinear relationships between BRI and NAFLD.
Results
Among 4210 individuals aged 20 years or older included in the study, 28.2 % had NAFLD. Compared to the first tertile, BRI notably increased the risk of NAFLD 3.53-fold [95 % confidence interval (CI) = 2.73–4.57] in the second tertile and 7.00-fold (95%CI = 5.29–9.27) in the third tertile after adjusting for multiple covariates (P for trend <0.001). Furthermore, when BRI was treated as a continuous variable, one unit of increment in BRI was associated with 41 % higher odds of NAFLD [adjusted odds ratio (aOR) = 1.41; 95%CI = 1.34–1.48; P < 0.001]. The associations of BRI with NAFLD persisted in all subgroup analyses. A smoothing curve fitting demonstrated that the relationship between BRI and NAFLD was a nonlinear connection. The risk of NAFLD increased significantly when BRI was lower than 4.82, after which the curve showed a modest ascent.
Conclusion
Higher BRI was consistently associated with an increased risk of NAFLD in US adults. BRI is a risk factor for NAFLD, and there is an imperative to give more attention to lowering the BRI.
Keywords: Body roundness index, Non-alcoholic fatty liver disease, Ultrasonography, NHANES
1. Introduction
It is estimated that 25%–30 % of the world's population suffers from nonalcoholic fatty liver disease (NAFLD), which has recently surpassed viral hepatitis as the leading cause of chronic liver disease [1,2]. NAFLD is characterized by excessive fat infiltration into the liver in the absence of alcohol abuse or other secondary causes. NAFLD encompasses a spectrum of histopathological features that ranges from simple steatosis, non-alcoholic steatohepatitis, progressive cirrhosis, to end-stage liver disease or hepatocellular carcinoma [[3], [4], [5]]. Associations between NAFLD and conditions like obesity, visceral obesity, hypertension, type 2 diabetes mellitus (T2DM), chronic kidney disease, metabolic syndrome, and cardiovascular disease have been well-documented [6,7]. Adiposity and obesity are undoubtedly associated with chronic diseases such as liver cirrhosis and NAFLD [8]. The gold standard for the diagnosis of NAFLD is liver biopsy, but it is invasive, and expensive and has significant variability in the samples taken. Clinically, in order to evaluate fatty liver, ultrasonography is recommended as the first-line modality [9]. In transient elastography with FibroScan®, the controlled attenuation parameter (CAP) can be used to estimate hepatic steatosis with high sensitivity [10,11]. Vibration-controlled transient elastography (VCTE) coupled with controlled attenuation parameter (CAP) is a noninvasive method for evaluating hepatic steatosis [12].
Body mass index (BMI) has been the most widely adopted weight-related anthropometric measure in the past decade. Obesity is known to be a major modifiable risk factor for NAFLD. There has been a strong correlation between obesity and NAFLD, and visceral obesity is arguably the most important predictor of the disease [13]. However, BMI is not an accurate predictor of body fat distribution, since it does not distinguish between lean and fat mass [14]. A body roundness index (BRI) was developed by Thomas et al., in 2013 to predict body fat and visceral adipose tissue volume [15]. In comparison with traditional body composition indices, BRI is a better indicator of body fat percentage and visceral fat than waist circumference (WC), BMI, and hip circumference [15].
BRI has previously been evaluated in terms of its ability to predict NAFLD, however, the relationship between the BRI and NAFLD is still not clear in US adults.
While the BRI has been evaluated for its potential to predict NAFLD, the relationship between BRI and NAFLD in US adults remains ambiguous. Especially against the backdrop of extensive research into the relationship between weight and health, it's unclear if the BRI, as a relatively new metric, offers unique insights into the risk assessment for NAFLD. Considering the strong correlation between obesity and NAFLD, and the more accurate representation of body fat distribution offered by BRI in comparison to BMI, we hypothesize that BRI may be a significant predictor of NAFLD in US adults. This study intends to shed light on the potential association between BRI and the prevalence of NAFLD in the population. The primary objective of this study is to bridge this knowledge gap by utilizing the publicly accessible NHANES database to examine the association between BRI and NAFLD. We anticipate that this research will not only elucidate the role of BRI in predicting NAFLD but also provide fresh perspectives for public health intervention strategies targeting NAFLD. Therefore, the major goal of this study was to assess this relationship using data from the publicly accessible National Health and Nutrition Examination Survey (NHANES) database, which could bring new insights on NAFLD health intervention.
2. Methods
2.1. Study design and population
NHANES is a continually updated, cross-sectional, continuing national representative survey that collects information on nutrition and health conditions in the US, including demographic, dietary, and examination measures, laboratory, and questionnaire data. For access to NHANES data, please go to www.cdc.gov/nchs/nhanes/. Given its comprehensive nature, the NHANES database is frequently employed in liver disease research. The survey was approved by the Centers for Disease Control and Prevention (CDC) ethics review board, and all participants signed informed consent. The NHANES data for 2017 and 2018 were used for this study since liver ultrasound transient elastography (LUTE) was available during this cycle.
In our study, all participants undergoing LUTE CAP measurement between 2017 and 2018 were included. FibroScan was used to determine the objective value of hepatic steatosis in the current study. The median CAP score was expressed in dB/m values. Hepatic steatosis (HS) was defined as CAP value greater than 248 dB/m in this study. Diagnoses of NAFLD are made based on the presence of hepatic steatosis [16]. Thus, NAFLD was diagnosed based on the presence of HS and in the absence of excessive alcohol use (>20 g/day for males and >10 g/day for females) and viral hepatitis (hepatitis B virus/hepatitis C virus) [11]. Participants' height and weight were measured in light clothes and without shoes. A standing position was used to measure participants' height. Calibrated platform scales were used to measure body weight. The WC was measured at the umbilicus level after normal expiration with an un-stretchable tape measure. BRI was calculated based on previous algorithm as follows [15]:
Initially, we assessed a cohort of 5717 individuals with data on both BRI and their NAFLD status. From this group, we omitted participants under the age of 20 (N = 1056) and those with a documented history of cancer or malignancy (N = 451). Consequently, our final analysis encompassed 4210 participants: 1187 with NAFLD and 3023 without NAFLD (Fig. 1).
Fig. 1.
A flow chart diagram of the study participants and exclusions.
2.2. Definition of other variables and outcome measure
The BRI was treated as an exposure variable in this study. The covariates were chosen based on previous research and their biological plausibility. For the continuous variables, these were age, BMI, WC, family poverty income ratio, eGFR, albumin, total cholesterol, HbA1c (%), HDL-C, creatinine, uric acid, aspartate aminotransferase (AST), and alanine transaminase (ALT). As for the categorical variables, these comprised of sex, race, educational attainment, marital status, smoking habits, presence of asthma, cardiovascular disease (CVD), diabetes, hypertension, hyperlipidemia, and metabolic syndrome (MetS). BMI was calculated as weight (kg) divided by the square of height (m). Based on their BMI, participants were divided into three groups: normal (BMI<25 kg/m2), overweight (25≤BMI≤30 kg/m2), and obese (BMI>30 kg/m2). In the NHANES database, WC is measured using a non-stretchable tape horizontally around the abdomen just above the highest point of the right iliac crest, taken at the end of a normal exhalation and recorded to the nearest 0.1 cm. The family poverty income ratio (PIR) represents a family's income relative to the U.S. Census Bureau's poverty threshold. A PIR less than 1 indicates income below the poverty line, while a PIR greater than 1 signifies income above it. Serum albumin levels are measured using a bromocresol green method or a similar colorimetric method. HbA1c is assessed using High-Performance Liquid Chromatography (HPLC) or other standardized methods to capture the percentage of hemoglobin that is glycated. Uric acid in serum is often assessed using a colorimetric method, typically based on the reaction with uricase. Both of AST and ALT are typically measured enzymatically in serum. The level of enzymatic activity indicates the amount of each enzyme in the sample. The race was grouped into 4 categories: Mexican American, non-Hispanic Black, non-Hispanic White, and others. The eGFR was calculated based on the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation [17], and was grouped into <60 ml/min/1.73 m2, 60–90 ml/min/1.73 m2, and≥90 ml/min/1.73 m2. A participant's level of education was classified as below high school, above high school, or high school. Marital status was categorized as married or unmarried. Smoking status was recorded as now (smoked more than 100 cigarettes in life and smoke some days or every day), former (smoked more than 100 cigarettes in life and smoked not at all now), or never smoking (smoked less than 100 cigarettes in life). The asthmatic individuals were defined as those who answered 'yes' to the following question: ‘‘has a doctor or other health professional ever told you that you have asthma?’’ or use antiasthmatic drug. A history of CVD was defined as self-reported ever receiving a diagnosis of angina, coronary heart disease, myocardial infarction/heart attack, congestive heart failure, or stroke. The definition of diabetes is self-reported doctor-diagnosed diabetes, medications to lower blood glucose, or HbA1c of less than 6.5 %. History of hypertension was defined as self-reported hypertension diagnosis, a diastolic blood pressure ≥90 mmHg, a systolic blood pressure ≥140 mmHg or use of anti-hypertensive medications. The definition of MetS was based the joint statement of the International Diabetes Federation (IDF) in 2009 [18]. The study's endpoint was the presence of NAFLD, determined via the CAP score—a significant metric in LUTE captured by the FibroScan® 502 V2 Touch.
2.3. Statistical analysis
Based on the NHANES recommendation and guidelines, an appropriate sampling weight was calculated and accounted for complex multistage survey design strategies in the analysis. Continuous variables were expressed as survey-weighted mean (95 % CI), and categorical variables were presented as a survey-weighted percentage (95 % CI). Participants were classified into three tertiles according to the BRI, with the first tertile used as the reference group. The variance inflation factor (VIF) was used to check for multi-collinearity, and variables with a VIF greater than 10 were excluded from the model. Covariates were selected as potential confounders in the final models if they changed the estimates of BRI on risk of NAFLD by more than 10 % or were notably associated with NAFLD [19]. The multivariate logistic regression models included the unadjusted crude model, minimally adjusted Model 1 (only sex, age, and family poverty income ratio were adjusted), and fully adjusted Model 2 (sex, age, family poverty income ratio, diabetes, hypertension, hyperlipidemia, eGFR, ALT, AST, HDL cholesterol, and MetS were adjusted) [20]. We further performed additional analyses. BRI as a continuous variable was subjected to logistic regression using these models. Analysis of non-linear relationships was conducted using smooth curve fittings and threshold effect analysis. A two-piecewise linear regression model using a smoothing function was further used to evaluate whether BRI had any threshold effect on risk of NAFLD. It was determined that a threshold level (or turning point) could be achieved using trial and error; turning points were selected along a predetermined interval, and the turning point that gave the greatest likelihood of the model was identified. We also compared a one-line linear regression model with a two-piecewise linear model using the log likelihood ratio. Subgroup analyses stratified by multiple factors were used to explore the stability of the main results. Additionally, the study calculated E-values as part of a sensitivity analysis to test the robustness of the presented results. E-values measure the minimal strength of an association between BRI (the exposure) and the risk of NAFLD (the outcome). Since a series of variables in the NHANES database have different degrees of missing, there is potential for bias. To create these imputed datasets, we used a mice software package to generate chained equations for ten datasets. We also performed sensitivity analyses to determine whether the created complete data differed significantly from the pre-imputation data. Package R version 3.4.3 (http://www.Rproject.org) and EmpowerStats software (http://www.empowerstats.net/en/) were used to analyze the data. A two-sided P value of less than 0.05 was considered statistically significant.
3. Results
3.1. Characteristics of participants
A total of 4210 participants were included in the study. Among these, 1187 (28.2 %) were diagnosed with NAFLD. The weighted demographic data of the participants are shown in Table 1. With increasing tertiles of BRI, participants were significantly older, had higher BMI, WC, ALT, total cholesterol, HbA1c, uric acid, were more likely to be female, Mexican American, high school, former smoking, asthma, CVD, diabetes, hypertension, hyperlipidemia, and MetS, but trend to have lower eGFR, albumin, and HDL cholesterol (all P for trend <0.05).
Table 1.
Baseline characteristics of included individuals according to body roundness index (BRI) tertiles, weighted.
| T1 | T2 | T3 | P for trend | |
|---|---|---|---|---|
| Age (years) | 40.70 (39.21,42.20) | 48.67 (47.14,50.20) | 50.09 (48.49,51.69) | <0.0001 |
| BMI (kg/m2) | 23.44 (23.22,23.66) | 29.07 (28.88,29.27) | 37.40 (36.82,37.97) | <0.0001 |
| Waist circumference (cm) | 83.95 (83.31,84.59) | 100.07 (99.27,100.86) | 119.58 (118.58,120.58) | <0.0001 |
| Family poverty income ratio | 3.12 (2.96,3.29) | 3.11 (2.93,3.29) | 2.91 (2.76,3.05) | 0.1163 |
| eGFR (ml/min/1.73 m2) | 100.19 (98.02,102.36) | 93.45 (91.09,95.80) | 93.62 (90.91,96.33) | 0.0012 |
| Albumin (g/l) | 41.93 (41.53,42.32) | 41.30 (40.97,41.63) | 39.71 (39.27,40.15) | <0.0001 |
| ALT (IU/L) | 20.19 (18.72,21.66) | 24.48 (23.22,25.73) | 26.66 (24.86,28.46) | 0.0001 |
| AST (IU/L) | 22.53 (21.29,23.77) | 22.34 (21.51,23.17) | 22.39 (21.11,23.66) | 0.9493 |
| Total cholesterol (mg/dl) | 182.07 (178.35,185.79) | 195.94 (193.34,198.54) | 189.70 (184.12,195.29) | 0.0001 |
| HbA1c (%) | 5.36 (5.32,5.40) | 5.67 (5.61,5.73) | 5.97 (5.91,6.04) | <0.0001 |
| HDL cholesterol (mg/dl) | 58.74 (57.28,60.21) | 51.75 (50.63,52.88) | 48.87 (47.63,50.10) | <0.0001 |
| Creatinine (mg/dl) | 76.53 (75.06,78.01) | 78.47 (76.58,80.36) | 76.60 (74.27,78.94) | 0.3297 |
| Uric acid (mg/dl) | 4.95 (4.83,5.07) | 5.45 (5.33,5.56) | 5.80 (5.72,5.88) | <0.0001 |
| Sex | 0.0007 | |||
| Female | 47.53 (43.00,52.11) | 45.47 (40.13,50.91) | 57.80 (53.96,61.55) | |
| Male | 52.47 (47.89,57.00) | 54.53 (49.09,59.87) | 42.20 (38.45,46.04) | |
| Race | <0.0001 | |||
| Mexican American | 5.98 (3.61,9.75) | 11.07 (7.09,16.88) | 11.12 (7.91,15.42) | |
| Non-Hispanic Black | 12.78 (9.90,16.36) | 9.38 (6.31,13.74) | 13.11 (9.32,18.15) | |
| Non-Hispanic White | 61.40 (55.05,67.38) | 57.47 (50.31,64.34) | 63.19 (56.65,69.27) | |
| Other | 19.84 (15.25,25.38) | 22.08 (17.98,26.80) | 12.57 (9.81,15.98) | |
| Education level | 0.0005 | |||
| Less Than High School | 9.54 (7.72,11.74) | 11.85 (9.57,14.58) | 11.46 (9.14,14.28) | |
| High school or GED | 24.20 (18.78,30.59) | 27.15 (23.67,30.93) | 32.57 (30.00,35.24) | |
| Above high school | 66.26 (59.51,72.40) | 61.01 (56.25,65.56) | 55.97 (52.22,59.66) | |
| Marital status | 0.2243 | |||
| Unmarried | 40.03 (36.56,43.60) | 35.56 (30.96,40.45) | 37.61 (32.84,42.63) | |
| Married | 59.97 (56.40,63.44) | 64.44 (59.55,69.04) | 62.39 (57.37,67.16) | |
| Smoke | <0.0001 | |||
| Never | 61.00 (56.14,65.66) | 59.88 (55.45,64.15) | 53.33 (48.80,57.81) | |
| Former | 19.39 (16.48,22.67) | 23.07 (20.53,25.83) | 30.42 (26.17,35.02) | |
| Now | 19.61 (15.34,24.71) | 17.04 (14.38,20.08) | 16.25 (14.06,18.71) | |
| Asthma | 0.0168 | |||
| No | 85.70 (82.46,88.43) | 88.34 (84.47,91.35) | 82.21 (78.60,85.32) | |
| Yes | 14.30 (11.57,17.54) | 11.66 (8.65,15.53) | 17.79 (14.68,21.40) | |
| CVD | <0.0001 | |||
| No | 95.93 (93.65,97.41) | 93.73 (91.44,95.44) | 88.52 (85.13,91.21) | |
| Yes | 4.07 (2.59,6.35) | 6.27 (4.56,8.56) | 11.48 (8.79,14.87) | |
| Diabetes | <0.0001 | |||
| No | 96.74 (95.45,97.66) | 88.16 (85.73,90.23) | 74.79 (71.69,77.66) | |
| Yes | 3.26 (2.34,4.55) | 11.84 (9.77,14.27) | 25.21 (22.34,28.31) | |
| Hypertension | <0.0001 | |||
| No | 81.41 (78.43,84.07) | 58.80 (54.39,63.07) | 45.49 (40.39,50.70) | |
| Yes | 18.59 (15.93,21.57) | 41.20 (36.93,45.61) | 54.51 (49.30,59.61) | |
| Hyperlipidemia | <0.0001 | |||
| No | 55.39 (49.35,61.28) | 26.45 (22.97,30.24) | 23.87 (20.16,28.02) | |
| Yes | 44.61 (38.72,50.65) | 73.55 (69.76,77.03) | 76.13 (71.98,79.84) | |
| MetS | <0.0001 | |||
| No | 97.37 (95.67,98.42) | 71.42 (66.47,75.91) | 52.90 (47.91,57.83) | |
| Yes | 2.63 (1.58,4.33) | 28.58 (24.09,33.53) | 47.10 (42.17,52.09) |
3.2. BRI and the risk of NAFLD
Table 2 displays the relationship between BRI and NAFLD. In the crude model, after we grouped the BRI into tertiles, BRI were demonstrated to positively associate with NAFLD. After fully adjustment, compared with individuals in T1, BRI was still positively associated with NAFLD in T2 (OR = 3.53, 95 % CI = 2.73–4.57, P < 0.001) and T3 (OR = 7.00, 95 % CI = 5.29–9.27, P < 0.001), respectively. Moreover, after full adjusting for potential confounders, every one-unit increase in BRI was associated with 41 % increase in the OR of NAFLD.
Table 2.
Associations of the body roundness index (BRI) with the risk of non-alcoholic fatty liver disease (NAFLD).
| Exposure | Non-adjusted | Adjust I | Adjust II |
|---|---|---|---|
| BRI | 1.26 (1.22, 1.29) <0.0001 | 1.23 (1.19, 1.27) <0.0001 | 1.41 (1.34, 1.48) <0.0001 |
| BRI tertiles | |||
| T1 | 1.0 (Reference) | 1.0 (Reference) | 1.0 (Reference) |
| T2 | 2.50 (2.07, 3.02) <0.0001 | 2.21 (1.80, 2.72) <0.0001 | 3.53 (2.73, 4.57) <0.0001 |
| T3 | 4.00 (3.33, 4.81) <0.0001 | 3.27 (2.67, 4.01) <0.0001 | 7.00 (5.29, 9.27) <0.0001 |
| P for trend | <0.001 | <0.001 | <0.001 |
Non-adjusted model adjust for: None.
Adjust I model adjust for: sex, age, and family poverty income ratio.
Adjust II model adjust for: sex, age, family poverty income ratio, diabetes, hypertension, Hyperlipidemia, eGFR, ALT, AST, HDL cholesterol, and MetS.
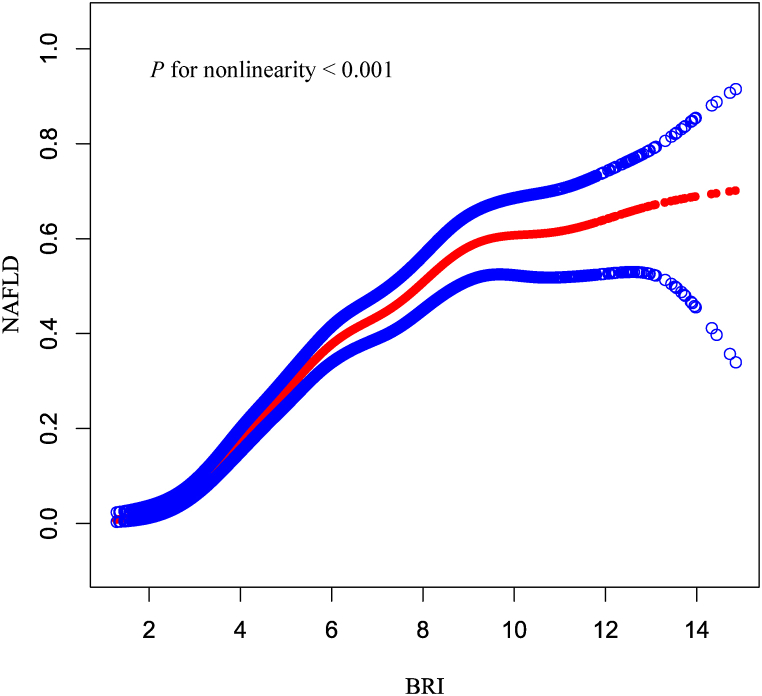
A smooth curve fitting approach and threshold effect analysis were used to detect the potentially non-linear relationship between BRI and NAFLD risk. There was a significant nonlinear correlation between BRI and NAFLD (P for nonlinearity <0.001) (Fig. 2). Based on the threshold effect analysis, BRI had an inflection point of 4.82. Two-piecewise linear regression model demonstrated that when BRI ≤4.82, for every one-unit increase in BRI, the risk of NAFLD increased by 1.63-fold, while when BRI >4.82, the risk of NAFLD increased by 26 % (aOR = 2.63, 95 % CI = 2.21–3.13, P < 0.001; aOR = 1.26, 95 % CI = 1.18–1.33, P < 0.001; respectively) (Table 3). For fitting the association between BRI and NAFLD, the segmented logistic regression model was better than the linear logistic regression model (P < 0.001).
Fig. 2.
Relationship between BRI and NAFLD risk by smooth curve fitting. Adjustment for: sex, age, family poverty income ratio, diabetes, hypertension, hyperlipidemia, eGFR, ALT, AST, HDL cholesterol, and MetS. The red line demonstrates the risk of NAFLD, and the blue ribbons illustrate its 95 % confidence interval.
Table 3.
Threshold analysis for the relationship between BRI and NAFLD.
| Models | Adjusted OR (95%CI) |
|---|---|
| Model I | |
| One line slope | 1.44 (1.37, 1.51) <0.001 |
| Model II | |
| Turning point (K) | 4.82 |
| < 4.8 | 2.63 (2.21, 3.13) <0.001 |
| > 4.8 | 1.26 (1.18, 1.33) <0.001 |
| OR between < 4.82 and > 4.82 | 0.48 (0.39, 0.58) <0.001 |
| Logarithmic likelihood ratio test | <0.001 |
Adjust for: sex, age, family poverty income ratio, diabetes, hypertension, hyperlipidemia, eGFR, ALT, AST, HDL cholesterol, and MetS.
3.3. Subgroup analyses and sensitivity analyses
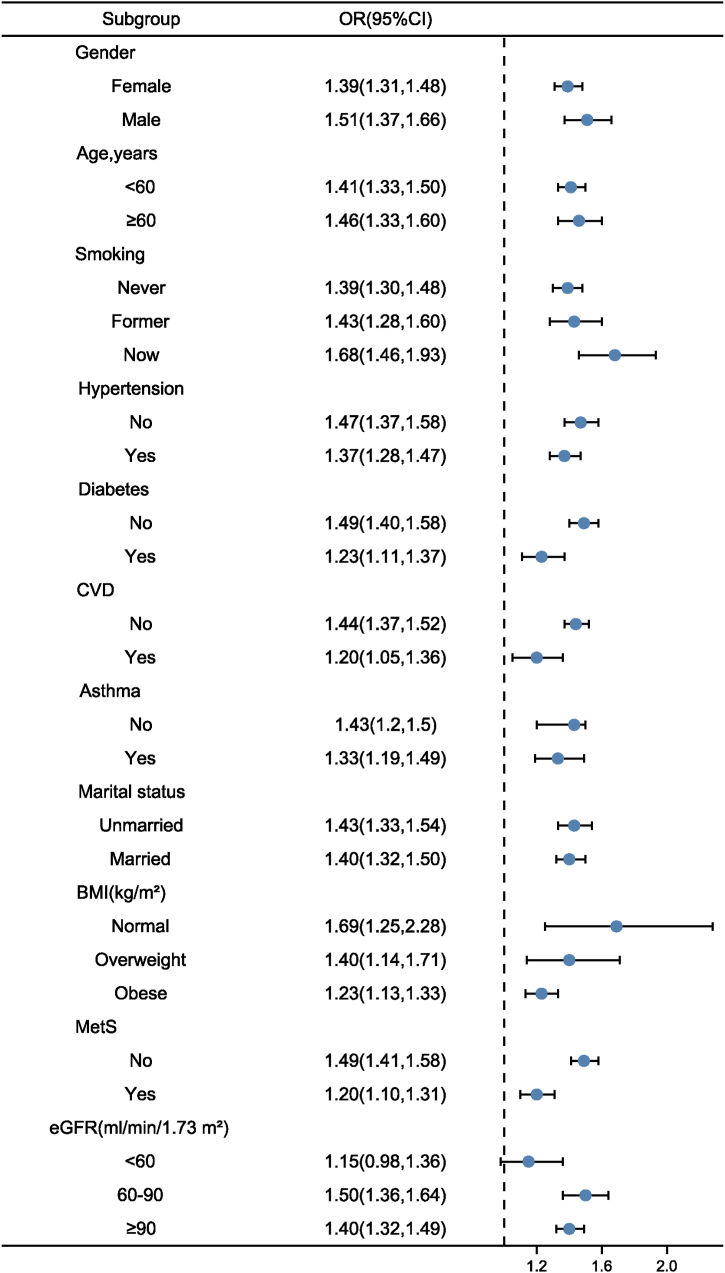
Subgroup analysis was performed to investigate the robustness of relationship between BRI and all-cause and NAFLD risk. The main results were particularly robust among all subgroups except for individuals with eGFR <60 ml/min/1.73 m2 (Fig. 3).
Fig. 3.
Subgroup analysis using potential confounders as the stratification factors.
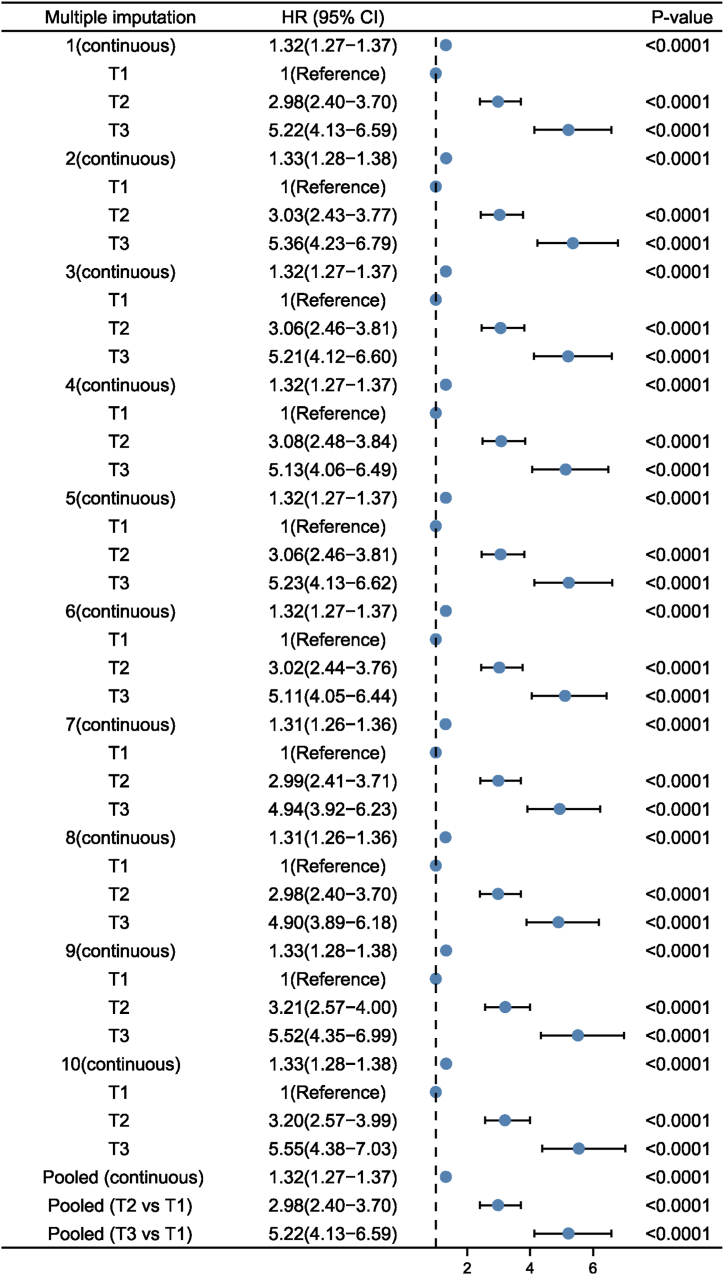
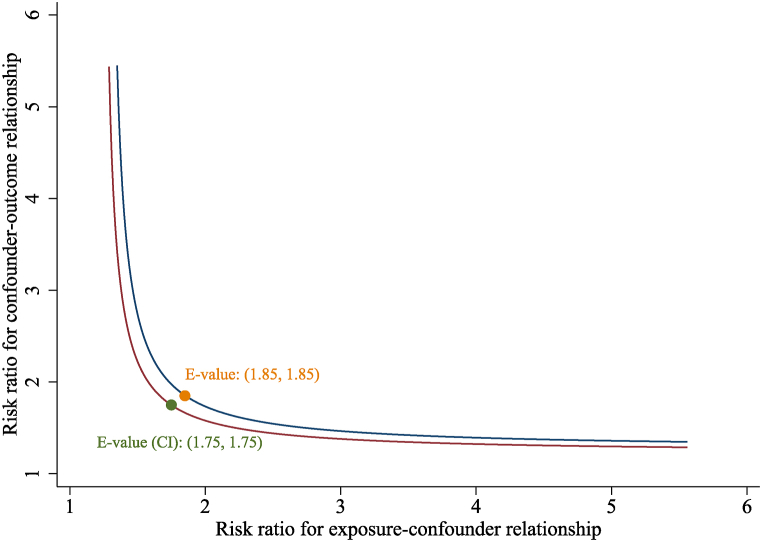
Sensitivity analyses using the ten created complete data did not greatly change the main findings (Fig. 4). The results remained robust after adjusting for multiple variables. The association of BRI with NAFLD remained significantly positive when BRI was treated as continuous variable (aOR = 1.32; 95%CI = 1.27–1.37). After full adjustment, compared with individuals in T1, BRI was still positively associated with NAFLD in T2 (aOR = 2.98, 95 % CI = 2.40–3.70, P < 0.001) and T3 (aOR = 5.22, 95 % CI = 4.13–6.59, P < 0.001), respectively. Moreover, the E-value (and its lower limit of 95%CI) for the relationship between BRI and NAFLD risk was 1.85 (1.75) (Fig. 5). Given that the E-value of 1.85 is much higher than any observed risk factors examined in the present study, it is implausible that an unmeasured confounder exists that can overcome the effect of BRI observed in the current analysis study. Therefore, the E-value and the above sensitivity analyses confirmed the robustness of the main results.
Fig. 4.
Pooled results of created complete data using multiple imputations with ten imputed datasets.
Fig. 5.
Sensitivity analyses via E-value for the lower 95 % CI and point estimate for NAFLD risk.
4. Discussion
In this study, we investigated the relationship between BRI and the risk of NAFLD in a large representative sample of US adults. Upon employing a stricter definition of NAFLD, our results highlighted that a higher BRI was notably associated with the risk of ultrasound-defined NAFLD. This main effect persisted even after adjustment for potential confounding variables. Interestingly, the associations of BRI with NAFLD were persisted in all subgroup analyses. The smoothing curve fitting demonstrated that the relationship between BRI and NAFLD was a nonlinear connection. The risk of NAFLD increased significantly when BRI was lower than 4.82, and then the curve slightly increased.
In spite of the fact that liver biopsy is regarded the gold standard in NAFLD evaluation, performing liver biopsies on the general population for steatosis and fibrosis prevalence estimation is neither practical nor feasible. LUTE (FibroScan®) measures steatosis and fibrosis in patients suffering from NAFLD in a noninvasive manner [21]. FibroScan transient elastography with CAP has yielded favorable results in liver steatosis, fibrosis, and NAFLD [[22], [23], [24]]. The prevalence of NAFLD is increasing worldwide. Fatty liver is strongly linked with obesity and is considered the hepatic manifestation of the metabolic syndrome. The main cause of obesity is the disproportionate growth of adipose tissue and lean body mass. In general, the BMI is considered a diagnostic indicator of obesity and may show the distribution of fat throughout the body [25]. The traditional anthropometric indices, however, failed to distinguish between fat and muscle [26]. A new anthropometric index BRI that determines body fat percentages by measuring waist circumference and height was developed. The BRI is effective in predicting fat distribution for percent body fat [15]. BRI has been shown to improve body fat prediction and has been identified as an alternative indicator to evaluate diabetes, insulin resistance, MetS, and hyperuricemia [[27], [28], [29]]. Supporting this, recent study showed that BRI provides a relative accurate prediction for the percentage of body fat as well as total percentage of visceral adipose tissue. In our study, we found that BRI was positively associated with NAFLD. Therefore, our findings could provide details for future randomized controlled trials to explore whether lowering BRI would result in decreased risk of NAFLD. Consequently, early identification and appropriate intervention treatment could potentially reduce the excess risk of NAFLD. To date, only one other cross-sectional study has explored the BRI-NAFLD connection. In previous study, the authors found BRI (OR = 5.484 for male and OR = 3.482 for female) was associated with a higher risk of NAFLD. However, they failed to reveal a nonlinear association between them. In contrast, our study spotlighted this nonlinear relationship, with NAFLD risk surging when BRI is below 4.82 and plateauing thereafter. Nonetheless, further research is warranted to unravel this nonlinear relationship more comprehensively. Furthermore, the results were robust in multiple subgroup analysis and sensitivity analyses. The main results kept unchanged among nearly all subgroups. Sensitivity analyses results showed that created complete data did not differ significantly from raw data. Besides, E-value 1.85 indicates that an unmeasured confounder associated with both exposure and outcome by an OR of at least 1.85 times would eliminate the observed associations. Therefore, all the results confirmed the robustness of our main findings.
The present study had several strengths. First, this was a cross-sectional study with large sample size based on a nationally representative survey among US adults. Secondly, multiple subgroup analyses and multiple imputation results did not diminish the power of the relationship between BRI and NAFLD. Thirdly, multiple possible confounders were available, and we adjusted for them in different models. Furthermore, the results of the subgroup analyses, sensitivity analysis via E-value demonstrated that the association between BRI and NAFLD risk was robust to unmeasured confounders.
However, it is important to acknowledge several inherent limitations in this cross-sectional study. The measurement of BRI only taken at the beginning of the study, and this may not adequately capture potential fluctuations or long-term changes in BRI over time, which could provide a more comprehensive understanding about its association with NAFLD. Additionally, the diagnosis of NAFLD primarily relied on transient elastography findings. While this method is non-invasive and convenient, it lacks the definitive diagnostic accuracy of a liver biopsy, which is often considered the gold standard for diagnosing liver diseases and provides more detailed histological data. However, obtaining liver biopsies is impractical in epidemiological studies, particularly when involving asymptomatic individuals, due to its invasive nature and potential complications. Despite our efforts to adjust for multiple confounders, there is always a risk of unmeasured or residual confounding in observational studies. This means that there may be variables not considered that could influence both the BRI and NAFLD, potentially biasing our findings. Although our conclusions were consistent across different subgroups and were supported by various sensitivity analyses, and the E value adds confidence to the robustness of our findings, it is important to approach these conclusions with caution. The cross-sectional nature of our study inherently limits our ability to establish causation, and these limitations provide important context and suggest areas for future research.
5. Conclusion
In a large, nationally representative survey of US adults, our observations revealed that higher BRI was associated with an increased risk of NAFLD. The risk of NAFLD increased significantly when BRI was lower than 4.82, and then the curve slightly increased. Further studies will be needed to confirm whether lowering BRI will reduce the risk of NAFLD.
Declaration of funding
None.
Data availability statement
A publicly available dataset was analyzed in this study. The National Health and Nutrition Examination Survey dataset are publicly available at https://www.cdc.gov/nchs/nhanes/index.htm.
CRediT authorship contribution statement
Enfa Zhao: Writing – original draft, Validation, Software, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Xiaolin Wen: Writing – original draft, Visualization, Validation, Supervision, Methodology, Investigation, Formal analysis, Conceptualization. Wenqian Qiu: Writing – original draft, Visualization, Validation, Supervision, Resources, Project administration, Investigation, Funding acquisition, Conceptualization. Chaoxue Zhang: Writing – review & editing, Validation, Supervision, Resources, Project administration, Investigation, Funding acquisition, Data curation, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors thank the National Center for Health Statistics of the Centers for Disease Control and Prevention for sharing the National Health and Nutrition Examination Survey (NHANES) data.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e23429.
Appendix A. Supplementary data
The following is/are the supplementary data to this article.
References
- 1.Younossi Z.M., Koenig A.B., Abdelatif D., Fazel Y., Henry L., Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64(1):73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 2.Younossi Z.M. Non-alcoholic fatty liver disease - a global public health perspective. J. Hepatol. 2019;70(3):531–544. doi: 10.1016/j.jhep.2018.10.033. [DOI] [PubMed] [Google Scholar]
- 3.McPherson S., Hardy T., Henderson E., Burt A.D., Day C.P., Anstee Q.M. Evidence of NAFLD progression from steatosis to fibrosing-steatohepatitis using paired biopsies: implications for prognosis and clinical management. J. Hepatol. 2015;62(5):1148–1155. doi: 10.1016/j.jhep.2014.11.034. [DOI] [PubMed] [Google Scholar]
- 4.Ekstedt M., Franzén L.E., Mathiesen U.L., Thorelius L., Holmqvist M., Bodemar G., Kechagias S. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology. 2006;44(4):865–873. doi: 10.1002/hep.21327. [DOI] [PubMed] [Google Scholar]
- 5.Nasr P., Ignatova S., Kechagias S., Ekstedt M. Natural history of nonalcoholic fatty liver disease: a prospective follow-up study with serial biopsies. Hepatol Commun. 2018;2(2):199–210. doi: 10.1002/hep4.1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sheka A.C., Adeyi O., Thompson J., Hameed B., Crawford P.A., Ikramuddin S. Nonalcoholic steatohepatitis: a review. JAMA. 2020;323(12):1175–1183. doi: 10.1001/jama.2020.2298. [DOI] [PubMed] [Google Scholar]
- 7.Ciardullo S., Perseghin G. Statin use is associated with lower prevalence of advanced liver fibrosis in patients with type 2 diabetes. Metabolism. 2021;121 doi: 10.1016/j.metabol.2021.154752. [DOI] [PubMed] [Google Scholar]
- 8.Yin Y., Li Y., Shao L., Yuan S., Liu B., Lin S., Yang Y., Tang S., Meng F., Wu Y., Chen Y., Li B., Zhu Q., Qi X. Effect of body mass index on the prognosis of liver cirrhosis. Front. Nutr. 2021;8 doi: 10.3389/fnut.2021.700132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hernaez R., Lazo M., Bonekamp S., Kamel I., Brancati F.L., Guallar E., Clark J.M. Diagnostic accuracy and reliability of ultrasonography for the detection of fatty liver: a meta-analysis. Hepatology. 2011;54(3):1082–1090. doi: 10.1002/hep.24452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kwak M.S., Chung G.E., Yang J.I., Yim J.Y., Chung S.J., Jung S.Y., Kim J.S. Clinical implications of controlled attenuation parameter in a health check-up cohort. Liver Int. 2018;38(5):915–923. doi: 10.1111/liv.13558. [DOI] [PubMed] [Google Scholar]
- 11.Karlas T., Petroff D., Sasso M., Fan J.G., Mi Y.Q., de Lédinghen V., Kumar M., Lupsor-Platon M., Han K.H., Cardoso A.C., Ferraioli G., Chan W.K., Wong V.W., Myers R.P., Chayama K., Friedrich-Rust M., Beaugrand M., Shen F., Hiriart J.B., Sarin S.K., Badea R., Jung K.S., Marcellin P., Filice C., Mahadeva S., Wong G.L., Crotty P., Masaki K., Bojunga J., Bedossa P., Keim V., Wiegand J. Individual patient data meta-analysis of controlled attenuation parameter (CAP) technology for assessing steatosis. J. Hepatol. 2017;66(5):1022–1030. doi: 10.1016/j.jhep.2016.12.022. [DOI] [PubMed] [Google Scholar]
- 12.Sasso M., Beaugrand M., de Ledinghen V., Douvin C., Marcellin P., Poupon R., Sandrin L., Miette V. Controlled attenuation parameter (CAP): a novel VCTE™ guided ultrasonic attenuation measurement for the evaluation of hepatic steatosis: preliminary study and validation in a cohort of patients with chronic liver disease from various causes. Ultrasound Med. Biol. 2010;36(11):1825–1835. doi: 10.1016/j.ultrasmedbio.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 13.Kim D., Chung G.E., Kwak M.S., Seo H.B., Kang J.H., Kim W., Kim Y.J., Yoon J.H., Lee H.S., Kim C.Y. Body fat distribution and risk of incident and regressed nonalcoholic fatty liver disease. Clin. Gastroenterol. Hepatol. 2016;14(1) doi: 10.1016/j.cgh.2015.07.024. 132-8.e4. [DOI] [PubMed] [Google Scholar]
- 14.Kang S.M., Yoon J.W., Ahn H.Y., Kim S.Y., Lee K.H., Shin H., Choi S.H., Park K.S., Jang H.C., Lim S. Android fat depot is more closely associated with metabolic syndrome than abdominal visceral fat in elderly people. PLoS One. 2011;6(11) doi: 10.1371/journal.pone.0027694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thomas D.M., Bredlau C., Bosy-Westphal A., Mueller M., Shen W., Gallagher D., Maeda Y., McDougall A., Peterson C.M., Ravussin E., Heymsfield S.B. Relationships between body roundness with body fat and visceral adipose tissue emerging from a new geometrical model. Obesity. 2013;21(11):2264–2271. doi: 10.1002/oby.20408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adams L.A., Angulo P., Lindor K.D. Nonalcoholic fatty liver disease. CMAJ (Can. Med. Assoc. J.) 2005;172(7):899–905. doi: 10.1503/cmaj.045232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levey A.S., Stevens L.A., Schmid C.H., Zhang Y.L., Castro A.F., 3rd, Feldman H.I., Kusek J.W., Eggers P., Van Lente F., Greene T., Coresh J. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alberti K.G., Eckel R.H., Grundy S.M., Zimmet P.Z., Cleeman J.I., Donato K.A., Fruchart J.C., James W.P., Loria C.M., Smith S.C., Jr. Harmonizing the metabolic syndrome: a joint interim statement of the international diabetes federation task force on epidemiology and prevention; national heart, lung, and blood institute; American heart association; world heart federation; international atherosclerosis society; and international association for the study of obesity. Circulation. 2009;120(16):1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 19.Jaddoe V.W., de Jonge L.L., Hofman A., Franco O.H., Steegers E.A., Gaillard R. First trimester fetal growth restriction and cardiovascular risk factors in school age children: population based cohort study. Bmj. 2014;348:g14. doi: 10.1136/bmj.g14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee S.W. Regression analysis for continuous independent variables in medical research: statistical standard and guideline of Life Cycle Committee. Life Cycle. 2022;2:e3. [Google Scholar]
- 21.Chalasani N., Younossi Z., Lavine J.E., Charlton M., Cusi K., Rinella M., Harrison S.A., Brunt E.M., Sanyal A.J. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67(1):328–357. doi: 10.1002/hep.29367. [DOI] [PubMed] [Google Scholar]
- 22.Liu X., Shen H., Chen M., Shao J. Clinical relevance of vitamins and carotenoids with liver steatosis and fibrosis detected by transient elastography in adults. Front. Nutr. 2021;8 doi: 10.3389/fnut.2021.760985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou J., Long Y., Ding N., Su Y. Association between bedtime at night and nonalcoholic fatty liver disease diagnosed by liver ultrasound transient elastography. Diabetes Res. Clin. Pract. 2022;184 doi: 10.1016/j.diabres.2022.109195. [DOI] [PubMed] [Google Scholar]
- 24.Kim H.M., Kim B.S., Cho Y.K., Kim B.I., Sohn C.I., Jeon W.K., Kim H.J., Park D.I., Park J.H., Joo K.J., Kim C.J., Kim Y.S., Heo W.J., Choi W.S. Elevated red cell distribution width is associated with advanced fibrosis in NAFLD. Clin. Mol. Hepatol. 2013;19(3):258–265. doi: 10.3350/cmh.2013.19.3.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Elagizi A., Kachur S., Lavie C.J., Carbone S., Pandey A., Ortega F.B., Milani R.V. An overview and update on obesity and the obesity paradox in cardiovascular diseases. Prog. Cardiovasc. Dis. 2018;61(2):142–150. doi: 10.1016/j.pcad.2018.07.003. [DOI] [PubMed] [Google Scholar]
- 26.Myint P.K., Kwok C.S., Luben R.N., Wareham N.J., Khaw K.T. Body fat percentage, body mass index and waist-to-hip ratio as predictors of mortality and cardiovascular disease. Heart. 2014;100(20):1613–1619. doi: 10.1136/heartjnl-2014-305816. [DOI] [PubMed] [Google Scholar]
- 27.Zhao Q., Zhang K., Li Y., Zhen Q., Shi J., Yu Y., Tao Y., Cheng Y., Liu Y. Capacity of a body shape index and body roundness index to identify diabetes mellitus in Han Chinese people in Northeast China: a cross-sectional study. Diabet. Med. 2018;35(11):1580–1587. doi: 10.1111/dme.13787. [DOI] [PubMed] [Google Scholar]
- 28.Feng J., He S., Chen X. Body adiposity index and body roundness index in identifying insulin resistance among adults without diabetes. Am. J. Med. Sci. 2019;357(2):116–123. doi: 10.1016/j.amjms.2018.11.006. [DOI] [PubMed] [Google Scholar]
- 29.Zhang N., Chang Y., Guo X., Chen Y., Ye N., Sun Y. A Body Shape Index and Body Roundness Index: two new body indices for detecting association between obesity and hyperuricemia in rural area of China. Eur. J. Intern. Med. 2016;29:32–36. doi: 10.1016/j.ejim.2016.01.019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
A publicly available dataset was analyzed in this study. The National Health and Nutrition Examination Survey dataset are publicly available at https://www.cdc.gov/nchs/nhanes/index.htm.