Abstract
The exploitation of natural products and their analogues in the field of pharmacology has been regarded as of great importance. It can be attributed to the fact that these scaffolds exhibit diverse chemical properties, distinct biological activities and zenith specificity in their biochemical processes, enabling them to act as favorable structures for lead compounds. The synthesis of natural products has been a crafty and hard-to-achieve task. Steglich esterification reaction has played a significant role in that area. It is a mild and efficient technique for constructing ester linkages. This technique involves the establishment of ester moiety via a carbodiimide-based condensation of a carboxylic acid with an alcohol, thiol or an amine catalyzed by dimethyl aminopyridine (DMAP). Specifically, labile reagents with multiple reactive sites are esterified efficiently with the classical and modified Steglich esterification conditions, which accounts for their synthetic utility. This review encloses the performance of the Steglich esterification reaction in forging the ester linkage for executing the total synthesis of natural products and their derivatives since 2018.
1. Introduction
Ester linkages are omnipresent in nature, constructed by the condensation of a carboxylic acid and an alcohol in the presence of acid catalysts or coupling agents. It falls under the category of oleochemicals, which is defined as nature-acquired chemicals. The ester moiety can be detected in numerous biological compounds except in proteins (functional biopolymers) and DNA/RNA (information storing) because of their lability towards hydrolysis. The esters have been employed for a number of synthetic applications as well. To mention a few esterification products having vast applications in modern life, including biofuels (biodiesel), varnishes, paints, solvents (ethyl acetate/methyl acetate), coatings, plastics, pesticides, herbicides, fungicides and in pharmaceutical industries. The most frequent utilization of esters is found in food preservatives, flavorings, perfume additives and fragrances in cosmetics and soaps [[1], [2], [3], [4]].
Esterification reaction is generally performed in three ways i.e., either by reaction of acid anhydride and alcohol, alcohol and acid chloride or alcohol and carboxylic acid. The ester linkage can be introduced via a number of methodologies including; Yamaguchi esterification based on alcoholysis of heterogeneous anhydride; Mitsunobu coupling assisted by triphenylphosphines; Baeyer–Villiger oxidation comprising of NADPH-mediated carbonyl conversion to lactones; Corey– Nicolaou reaction to convert hydroxy acids into lactones; Trost, based on Ru chemistry; Fischer esterification involving acid catalysts and Steglich esterification comprising of coupling agents [[5], [6], [7], [8], [9], [10]]. All these esterification methodologies employ different activating reagents and reaction conditions. The appropriate selection of the esterification technique requires understanding the transformation sustainability of starting materials towards required linkage under provided conditions.
Among these above-mentioned techniques, the Steglich esterification reaction is an efficient method to convert acid and alcohol into esters under neutral and mild conditions. It was developed by Steglich and Neises in 1978 [11]. The reaction conditions employed for this reaction include coupling agents i.e., N, N′-dicyclohexylcarbodiimide (DCC), N, N′-diisopropylcarbodimide (DIC) and N-ethyl-N’- (3-dimethylaminopropyl) carbodiimide (EDC) with or without the catalytic amount of dimethyl aminopyridine (DMAP) in a suitable solvent (Scheme 1) [11]. The general Steglich esterification reaction is presented as follows.
Scheme 1.
General Steglich esterification reaction.
The general mechanism of Steglich esterification is represented as.
The first step involves the reaction between carbodiimide and carboxylic acid mediated by ion pair interaction giving off O-acyl urea as an intermediate. This intermediate then transfers the acyl group to dimethyl aminopyridine (DMAP) which reacts with an alcohol to give corresponding esters. DMAP acts as an active reagent to transfer the acyl group. Without DMAP or active reagents for acyl transfer, irreversible reactions occur generating side products i.e., N-acyl urea or possible anhydrides leading towards poor or diminished yields of desired products (Scheme 2) [12].
Scheme 2.
Reaction mechanism of Steglich esterification.
Due to their biological significance, the Steglich esterification reaction has been extremely advantageous in synthesizing natural products [[13], [14], [15]]. The derivatives and analogues of natural products have also been synthesized via Steglich esterification, possessing striking medicinal properties i.e., anticancerous, antimicrobial, antiviral and antiinflammatory etc. [[16], [17], [18]]. Fig. 1 contains the structures and biological significance of some important natural compounds and derivatives of natural compounds ((−)-Herdmanine D 1, Gallinamide A 2, Betulin derivative 3, Leoligin 4) [[19], [20], [21], [22]].
Fig. 1.
Structures of some biologically active compounds ((−)-Herdmanine D 1, Gallinamide A 2, Betulin derivative 3, Leoligin 4).
This review aims to provide the significance of Steglich esterification in the synthesis of natural products and their derivatives reported since 2018.
2. Literature review
2.1. Peptide-based natural product synthesis
Natural products containing peptides and cyclotides are important because of their medicinal and pharmacological significance [23,24]. The increased resistance of bacterial strains against antibiotics has prompted researchers towards a number of trials to get their hands on novel biologically active antibacterial compounds. Ilamycin and its derivatives were obtained from Streptomyces insulates (A-165-Z1) and were found active against Mycobacterium phlei and Mycobacterium 607 [25,26]. Greve et al., in 2022, proposed the total synthesis of Ilamycin and its derivatives by employing asymmetric hydrogenation, Claisen rearrangement and Steglich esterification as key steps [27]. The synthesis commenced from commercially available nitrotyrosine 5 which was converted to amino acid building block 6 over a few steps. Further, commercially available (R)-Roche ester 7 was employed to obtain required N-methylated-hydroxyleucine 8 over a few steps. Further, Alloc-protected glycine 9 underwent modified Steglich protocol to produce compound 11 in the presence of N, N′-dicyclohexylcarbodiimide (DCC), dimethyl aminopyridine (DMAP), compound 10 and a solvent ratio of DCM/DMF. In the next step, Lindlar hydrogenation was employed mediated by Lindlar catalyst followed by Claisen rearrangement in the presence of zinc chloride and lithium diisopropylamide (LDA) to obtain amino acid 12 in 87 % yield. The synthesis of linear peptide started with the coupling of synthesized hydroxyleucine derivative 8 with Leu-OMe followed by deprotection of Cbz-protecting group along with Alloc-Ala-OH coupling to furnish 78 % of tripeptide 13. The next step involved Pd-catalyzed Alloc removal with TPPTS ligand along with nitrotyrosine followed by TBTU/DIPEA-assisted activation of 6 and coupling with N-MeLeu to obtain 77 % of compound 14. Further, 87 % of linear heptapeptide 15 was obtained by ring-closing reaction along with Alloc-protecting group cleavage. The heptapeptide 15 was converted to aldehyde 16 over a few steps. The aldehyde 16 was transformed to hemiaminal 17 via oxidation in 40 % yield and carboxylic acid 18 in 39 % yield via Pinnick conditions of sodium chlorate, sodium hydrogen phosphate and 2-methyl-2-butene as presented in Scheme 4. The synthesized derivatives were evaluated against bacterial strains, among which the derivative with hemiaminal structure was found to exhibit an excellent MIC activity of 50 nM against M. tuberculosis and 0.26 nM against M. marinum and M. smegmatis (Scheme 3, Scheme 4).
Scheme 4.
Synthesis of compounds 17 and 18.
Scheme 3.
Synthesis of compound 15.
Marine organisms have been a great source of bioactive compounds including cyanobacteria as part of this family. Hormoscilla sp. is a green filamentous cyanobacteria which produces anaenamide natural products obtained from the reef system of Anae Island in Guam [28]. Brumley et al. in 2020, have proposed the total synthesis of anaenamides A, B and biosynthetic intermediate anaenoic acid [24]. The synthesis was initiated from compound 19 which produced compound 20 over a few steps. The next step involved the Steglich esterification between compound 20 and compound 21 in the presence of DCC, DMAP and DCM at 0 °C to rt followed by deprotection in the presence of Pd/C in ethanol to forge anaenoic acid 22 in 48 % yield over 2 steps. Further, the anaenoic acid was reacted with compounds 23 and 25 to construct anaenamide A 24 and B 26 respectively, each in 53 % yield. The synthesized compounds were evaluated against HCT116 cancer cells and exhibited moderate cytotoxicity (Scheme 5).
Scheme 5.
Total synthesis of anaenamides A 24 and B 26.
2.2. Lactam-based natural product synthesis
Dysoxylum species has a folklore background due to its usage in malarial treatment in old Chinese times and production of anticancerous agents, Hangkonoides [29]. One of the members of dysoxylum, Dysoxylum hongkongense produces biologically active dysoxylactam A [30]. In 2020, Reddy et al. provided the total synthesis of dysoxylactam A in 17 linear steps with Steglich easterification, ring-closing metathesis and iterative aldol reactions as key steps to give a 9.5 % overall yield of the desired product [31]. The synthesis was actualized from dibutyl boron triflate-mediated aldol reaction of Evans propionate 27 and 4-pentene-1-al 28 to give 89 % aldol product 29. The aldol product 29 was transformed into polypropionate fragment 30 over a few steps. The EDCl-mediated Steglich esterification was employed as a next step in the presence of N-Boc-l-valine followed by treatment with 5-hexenoic acid and Hunig's base in the presence of HATU to give 79 % of bis-olefin amides 31. The macrolactam ring was completed with the final step of ring-closing metathesis in the presence of Grubbs II catalyst and subsequent hydrogenation to furnish dysoxylactam A 32 in 72 % yield over 2 steps (Scheme 6).
Scheme 6.
Total synthesis of dysoxylactam A 32.
2.3. Antibiotic-based natural product synthesis
Among antibiotics, β-lactam antibiotics are considered more significant. Cephalosporin is one of the β-lactam antibiotics which exhibit good potential as an antibacterial agent. Woodward et al. proposed a scheme for the total synthesis of cephalosporin C in 2021 [32]. The synthesis commenced from compound 34 which was converted to β-lactam 35 over a few steps. The next step involved coupling of compounds 35 with 36 followed by the functionalization of β-lactam ring by employing the Michael reaction to give compound 37. It was followed by Steglich esterification with 38 which took place in the presence of DCC and pyridine to furnish compound 39. The obtained compound 39 underwent a series of deprotection steps assisted with acetic acid to furnish cephalosporin C 40 (Scheme 7).
Scheme 7.
Total synthesis of cephalosporin C 40.
Laterocidines and brevicidine are obtained from Brevibacillus laterosporus strains [33]. These are found to be potent antibiotics against ESKAPE family of bacterial strains with considerable MIC values. Laboratory synthesis is preferred to avoid the laborious fermentation process for the acquisition of these products. Ayed et al. provided the total synthesis of laterocidines and brevicidine in 2021 [34]. For the total synthesis of laterocidine, Fmoc-Asp-OAll 41 was employed as a commencing reagent. The Fmoc-Asp-OAll was loaded with a rink amide resin in the presence of BOP/DIPEA to provide 42. In the next step, allyl ester cleavage was performed with palladium tetrakis triphenylphosphine and PhSiH3 followed by macrolactamization assisted with BOP/DIPEA to give macrocyclic product 43. The next step was Steglich esterification, for which DIC and DMAP were employed to obtain the desired esterified product 44 with Alloc-Gly-OH. The required laterocidine 45 was obtained from compound 44 over a few steps in 2 % overall yield (Scheme 8).
Scheme 8.
Synthesis of laterocidine 45.
In 2023, Ayed et al. employed a similar strategy to synthesize another member of laterocidine family, named as relacidine A. The structure of relacidine A 46 is presented in Fig. 2 [35].
Fig. 2.
Structure of relacidine A 46.
2.4. Terpenoid-based natural product synthesis
Terpenoids exist in the form of mono, di or triterpenoid, meroterpenoid, indole terpenoids and various others [36]. A meroterpenoid consists of phenolic and quinone moieties combined with sesquiterpene i.e., yahazunol. Yahazunol and natural products related to it consist of meroterpenoid skeleton. These are naturally occurring compounds extracted from Dictyopteris undulata and Dysidea genus, occurring as sponges [37,38]. (−)-Yahazunol demonstrated antimicrobial activity and (+)-yahazunol exhibited anticancerous activity against some human cancer cell lines i.e., A-549 and MDA-MB-231 [39]. Some of yahazunol related natural products i.e., isozonarol, zonarol, isozonarone and zonarone also showed significant biological activity such as antifungal towards Rhizoctonia solani, Sclerotinia sclerotiorum and Phytophthora cinnamomic [40]. In 2018, Zhang et al. provided the total synthesis of (+)-yahazunol and relevant natural products by employing (−)-sclareol 47 as an inexpensive and commercially available starting material [41]. The first step involved oxidative degradation of (−)-sclareol 47 into 8-O-acetylhomodrimanic acid 48 in 42 % yield. Next, the Steglich esterification was employed to obtain thiohydroxamic ester 50 from 8-O-acetylhomodrimanic acid 48 and 2-mercaptopyridine N-oxide 49 in the presence of EDC and DMAP. Over a few steps, (+)-8-O-acetylyahazunol 51 was obtained over a few steps which was subjected to LiAlH4-mediated hydrolysis to give (+)-yahazunol 52 in 93 % yield. Treatment of (+)-yahazunol 52 with MnO2 furnished 83 % of (+)-yahazunone 53. Exo- and endo-cyclic olefins 54a and 54b (86 %) were obtained by SOCl2/Et3N based oxidation. Further, (−)-zonarol (55a) and (−)-isozonarol (55b) were obtained in a combined yield of 50 % by reduction of (−)-zonarone 54a and (−)-isozonarone 54b in the presence of sodium dithionite and DCM. Lastly, (+)-yahazunol 52 was also converted to 94 % of (+)-chromazonarol 56 in the presence of trifluoro acetic acid and DCM. The synthesized compounds were subjected to antifungal screening which proved (+)-yahazunone 53 and (+)-chromazonarol 56 as potent antifungal agents with EC50 values of 28.7 and 24.1 μM respectively (Scheme 9).
Scheme 9.
Synthesis of (+)-yahazunol 52 and related analogues.
Cembranoid is a family of furanobutenolide-derived polycyclic diterpenoid. The total synthesis of four members of this family have been achieved. Havellockate is a member of this family obtained from Sinularia granosa in 1998 [42]. Hafemen et al., in 2022 aimed at total synthesis of havellockate by engaging Julia−Kocienski olefination, propiolic acid mediated esterification, Zn-mediated Barbier allylation, Cu-catalyzed aerobic oxidation and Steglich esterification as key steps [43]. The synthesis commenced with the acquisition of aldehyde 58 from enone 57 and sulfone 60 from acyl oxazolidinone 59 over a few steps. These two fragments were made to couple via Julia−Kocienski olefination in the presence of KHMDS in THF followed by desilylation to forge a diol 61 with >20:1 E:Z selectivity in 57 % yield over two steps. Steglich esterification has opted for the next step in the presence of propiolic acid, DIC and DMAP in DCM to furnish an ester 62. The ester 62 was subjected to heating in xylene to provide Diels-Alder product 63 by [4 + 2] cycloaddition in 51 % yield over two steps having dr > 20:1 which leads to the final product havellockate 64, over a couple of steps (Scheme 10).
Scheme 10.
Total synthesis of havellockate 64.
(−)-Scabrolide A is a furanobutenolide-derived diterpenoid obtained from soft corals of Sinularia [44]. In 2020, Hafeman et al. disclosed its first total synthesis by employing enone-olefin reactions, Diels-Alder reaction and Steglich esterification [45]. The synthesis was commenced from enone 65 which was converted to dihydroxy vinyl cyclopentene 66 and a dialdehyde 67 which was converted to ynoic acid 68. The next step involved Steglich esterification between dihydroxy vinyl cyclopentene 66 and ynoic acid 68 in the presence of DIC, DMAP and DCM to give ester 69 in 79 % yield. The Diels-Alder reaction was employed as a next step, followed by vanadium-mediated epoxidation to give epoxide 70 in 94 % yield. The epoxide 70 was transformed to (−)-scabrolide A 71 over a few steps. In 2023, Hafeman et al. provided an account of all the approaches and key advancements employed to accomplish the synthesis of scabrolide A and its related natural product, inferring this class to be a relevant and substantial research area (Scheme 11) [46].
Scheme 11.
Total synthesis of (−)-scabrolide A 71.
(+)-Darwinolide is a diterpenoid extracted from marine sources. This natural product express activity against biofilm phase of methicillin-resistant S. aureus. In 2019, Siemon and co-workers proposed the total synthesis of (+)-darwinolide by forging Steglich esterification, an aldol fragment coupling, Ireland-Claisen rearrangement, ring-closing metathesis and organocatalytic meso-desymmetrization as key steps [47]. The total synthesis was begotten with Steglich esterification between 72 and compound 73 in the presence of 10 mol% of DMAP, DCC and dichloromethane accompanied with Ireland-Claisen rearrangement in the presence of LiHMDS to give carboxylic acid 74 in 70 % yield with diastereomeric ratio of 14:1. The carboxylic acid functionality was converted to aldehyde 77 through a two-step sequence to give 70 % of product. Furthermore, the compound 78 was converted to compound 79 over a few steps. Aldol reaction of compound 79 with aldehyde 77 in the presence of NaHMDS accompanied by 5 steps gave compound 80 in 50 % yield. In the next step, ring-closing metathesis was performed to obtain 54 % of compound 82. Over a series of steps, the target compound (+)-darwinolide 84 was furnished in 18 % yield (Scheme 12).
Scheme 12.
Synthesis of (+)-darwinolide 84.
Kauranes are plant-based diterpenes possessing a number of pharmacological properties i.e., antibacterial, antiinflammatory and anticancerous. These are also considered active agents against leishmaniasis which is a group of zoonotic and anthroponotic diseases caused by 20 protozoan parasite species of the genus Leishmania. The database of around 360 kauranes was generated and studied by structure-based and ligand-based analysis to identify bioactive kauranes. Among these, only 7 kauranes were found potentially active. Out of these seven kauranes, two are ranked best and are obtained from Wedelia chinensis [48]. In 2021, Herrera-Acevedo et al. reported the synthesis of these biologically active kauranes [49]. For the synthesis of these kauranes, commercially available precursors were envisaged from Xylopia frutescens, which produced kaurane-type diterpenoids. These kaurane-type diterpenoids were utilized as synthetic precursors. The synthesis was materialized from (R)-(−)-carvone 85 which underwent chemoselective monohydroxylation in the presence of Cu–Al Ox and tert-butoxide followed by selective hydrogenation in the presence of Wilkinsin's catalyst to forge 2-oxo-menth-6-en-5β-ol 86 in 91 % yield. For the next step, Steglich esterification was actualized to obtain ester adduct 88 with coupling of compounds 86 and 87 in 79 % yield. A selective 1,2-reduction was employed to obtain 2β-hydroxy-menth-6-en-5β-yl ent-kaurenoate 89 in the presence of sodium borohydride and cerium chloride in 82 % yield (Scheme 13).
Scheme 13.
Synthesis of diterpenoid ester adduct 89.
Further, 2 kauranes (92, 93) and their derivatives (92a, 93a) were envisioned by esterification of cinnamic acid derivatives 90a and 90b with compound 91a and 92b in 78–79 % yield. The synthesized kauranes were evaluated for their potential against LmPTR1 enzyme inhibition which provided 89 and 93 most potent leishmaniasis inhibitor with IC50 value of 10 μM against L. major (Scheme 14). [49].
Scheme 14.
Synthesis of diterpenoid ester adducts.
2.5. Dihydropyrone-based natural product synthesis
Brevipolide M belongs to pyrone-based natural compounds which have been found to exhibit numerous biological activities. In 2022, Liu et al. aimed at total synthesis of Brevipolide M by employing ring-closing metathesis, Wittig olefination and Steglich esterification in 17.8 % overall yield in 13 linear steps [50]. The synthesis was actualized from d-mannofuranose diacetonide 94 which was transformed to compound 95 over a few steps. The compound 95 was esterified via Steglich conditions in the presence of (E)-4-methoxy cinnamic acid, DMAP, DIC and anhydrous DCM to give ester 96 in 81 % yield. The next step involved acetyl chloride-mediated isopropylidene removal to give 95 % of diol 97. The diol underwent another Steglich reaction with vinyl acetic acid, DIC, DMAP and DCM to give 61 % of ester 98. Finally, Brevipolide M 99 was obtained by ring-closing metathesis followed by DBU-assisted isomerization (Scheme 15).
Scheme 15.
Synthesis of brevipolide M 99.
Pyrone diterpenes are biologically active natural compounds obtained from fungal strains [51]. Merchant et al. utilized radical-based inter- and intramolecular chemistry to provide access to the pyrone diterpenes, a class of natural products, in 2018 [52]. By employing the electrochemically mediated oxidative radical polycyclization and radical decarboxylative cross couplings, four natural products i.e., higginsianin A, subglutinols A/B and sesquicillin A have been synthesized. The synthesis of higginsianin A commenced from polyene 100 which underwent Mn-assisted radical polycyclization in the presence of Cu(sal)2, potassium acetate, acetic acid and ethyl acetate followed by diastereoselective H-PHOX-mediated Tsuji-allylation to give compound 101. Next, compound 101 was treated with L-selectride along with V-directed Sharpless epoxidation to produce a diastereomeric mixture of 102a and 102b in 1.2:1 dr. The next step involved esterification by employing TPAP/NMO and DIC/MeOH-mediated Steglich esterification method followed by allylic oxidation and TESOTf-assisted quenching along with hydroboration in the presence of BH3 and hydrolysis to provide a mixture of epimers 103 in 1.3:1 diastereomeric ratio. The acid 103 was converted to higginsianin A 104 over a few steps (Scheme 16). The divergent synthesis protocol employed by Merchant et al. was also utilized for the synthesis of a few other pyrone diterpenes named as subglutinol B 105, subglutinol A 106 and sesquicillin A 107 as presented in Fig. 3.
Scheme 16.
Synthesis of higginasianin A 104.
Fig. 3.
Structures of pyrone diterpenes subglutinol B 105, subglutinol A 106 and sesquicillin A 107.
2.6. Lactone-based natural product synthesis
(+)-Longirabdiol, (−)-longirabdolactone, and (−)-effusin are ent-kaurane diterpenoids obtained from Isodon genus [53]. These are structurally diverse and biologically active natural products exhibiting potent potential as antiinflammatory, antibacterial and antineoplastic properties [53]. Zhang et al. have furnished the first total synthesis of these ent-kauranes by materializing tandem decarboxylative alkenylation, Giese reaction, Steglich reaction and vinyl radical cyclization as key steps in 2019 [54]. The synthesis was commenced from ethyl 2-cyclohexanonecarboxylate 108 which is commercially available as rac-20 and provided compound 109 over a few steps. The compound 109 underwent the Steglich esterification in the presence of DIC, DMAP and N-hydroxyphthalimide (NHPI) to provide ester 110 in 88 % yield over 2 steps. After optimization of radical alkenylation or cyclization step, the ester 110 was converted to γ-lactone 114 in the presence of 7 mol% CuCl2, 7 mol% of 111, 7 mol% of 112 and 3 equivalents of 113 in 60 % yield. The γ-lactone 114 was transformed to (+)-longirabdiol 115 over a few steps which underwent DMP-mediated oxidation to furnish (−)-longirabdolactone 116 in 76 % yield. Next, (+)-longirabdiol 115 was being proceeded through the Steglich esterification accompanying Dess-Martin oxidation to establish (−)- effusin 117 in 65 % yield (Scheme 17).
Scheme 17.
Synthesis of (+)-longirabdiol 115, (−)-longirabdolactone 116, and (−)- effusin 117.
Berkeleylactone A is a potent antibiotic, obtained from co-culturing of Penicillium camembertii and Penicillium fuscum [55]. In 2019, Ferko et al. provided the total synthesis of berkeleylactone A by employing ring closing metathesis, Steglich esterification, sulfa-Michael addition and chemoselective reduction to achieve target compound in 9.5 % overall yield consisting of 10 steps [56]. The synthesis was initiated by weinreb amide 118 which underwent addition with cyclohexenyl magnesium bromide 119 followed by reduction with borane dimethylsulfide in the presence of 10 mol% catalyst (R)-2-methyl-CBS-oxazaborolidine along with TBS-mediated hydroxyl group protection to give 93 % of compound 120. The next step involved N-bromosuccinimide-assisted Achmatowicz oxidation together with pyridine catalyzed isomerization of double bond to give 69 % of aldehyde 121. Further, the aldehyde 121 underwent oxidation with sodium chlorite and amylene followed by Steglich esterification with alcohol 122 in the presence of DCC and DMAP to provide compound 123 in 67 % yield. Next, compound 124 was obtained in 89 % yield by Grubbs–II–mediated ring closing metathesis followed by TBS deprotection with TFA. Further, Potassium (R)-oxirane-carboxylate 125 was employed to give acid 126 in 85 % yield by epoxide opening reaction in the presence of sodium hydride followed by triethyl silane. The last step involved two-step coupling of compounds 124 and acid 126 to furnish target compound berkeleylactone A 127 in 92 % yield with high diastereoselectivity of >20:1. The synthesized compound was screened for antibacterial activity and exhibited potency against S. aureus MB17 (Scheme 18).
Scheme 18.
Synthesis of berkeleylactone A 127.
The synthesis of some new berkeleylactones have also been provided by Schriefer et al. in 2023 [57]. The synthesis was initiated from halide 128 and epoxide 129 which were transformed into protected alcohol 130. The alcohol 130 underwent Steglich esterification in the presence of EDC.HCl, DMAP and dichloromethane to acquire quantitative yield of 132. Berkeleylactone E 133 and K 134 were obtained from ester 132 by TFA-mediated deprotection. Further, compound 130 underwent TBDPSP-assisted protection of OH group and deprotection of MOM group to get ether 134. Next, Steglich esterification was employed in the presence of carboxylic acid 131 catalyzed with EDC.HCl along with hydrogenation to obtain macrolide 135 in 98 % yield over 2 steps. Berkeleylactone M 136 was obtained from macrolide 135 by TBAF global deprotection in 66 % yield and Berkeley—lactone O 137 was obtained in 57 % yield. The article also covers the synthesis of some other berkeleylactones by employing a similar strategy (Scheme 19).
Scheme 19.
Synthesis of different berkeleylactones.
(₋)-Cleistenolide is a naturally occurring α,β-unsaturated δ-lactone derived from Cleistochlamys kirkii [58]. The (₋)-cleistenolide and its analogues possess antiproliferative potential against a number of human cancer cell lines [59]. The purpose of analogue synthesis of natural products is to enhance their potential as antiproliferative agents which is achieved by introducing a number of functionalities. The synthesis of (₋)-cleistenolide analogues was reported by Benedekovic et al. in 2021 which commenced from triol 138 [60]. The triol 138 was converted to secondary alcohol 139 over a few steps. The secondary alcohol 139 was made to couple with carboxylic acid 140 under Steglich conditions of DCC and DMAP to facilitate ester 141 in 88 % yield. The synthesized esters 141 were acylated in the presence of acetic anhydride and ferric chloride to afford mono-O-acetylated derivative 142 in 95 % yield. For the synthesis of di-O-acetylated derivatives, acetyl bromide with zinc triflate as a catalyst were employed along with cinnamic acid derivative, acetic anhydride and ferric chloride to furnish the target analogue 143 in 63 % yield. By employing different acid derivatives, a number of analogues can be synthesized. The synthesized analogues were studied for antiproliferative activities against seven human cancer cell lines in comparison with cleistenolide. The best activity was exhibited by flouro-cinnamic acid derivative against HeLa cell lines with IC50 value of 0.04 μM (Scheme 20).
Scheme 20.
Synthesis of (₋)-cleistenolide analogues.
2.7. Chromanone-based natural product synthesis
Violacin A and its analogues are naturally occurring chromanones obtained from fermentation broth of Equus burchelli [61]. These are effective antiinflammatory agents and have been analyzed by antiinflammatory mechanism. The inhibitory effects of these compounds have been estimated by using Raw 264.7 macrophage of lipopolysaccharide (LPS). The synthesis of violacin and its analogues have been provided by Liu et al. in 2019 [62]. The key steps for achieving the synthesis involve Baker-Venkatamaran rearrangement promoted by NaH, regioselective etherification and Friedel-Crafts acylation. The construction of violacin skeleton was actualized from commercially available orcinol 144 which provided 2,4-dihydroxy-6-methyl-acetophenone 145. Next, etherification of dihydric phenol 146 was achieved by treating it with acetic acid and BF3.OEt2 to achieve compound 147. Further, compound 147 underwent O-benzylation with potassium carbonate, benzyl chloride and sodium iodide to obtain compound 148. Compound 148 underwent the Steglich esterification with ketone 145 in the presence of DCC, DMAP and DCM to give compound 149. The ester underwent cyclization with sodium hydride along with deprotection by employing soft Lewis acid PdCl2(CH3CN)2 in acetone accompanying the hydrogenolysis in the presence of Pd–C and hydrogen gas to furnish the target compound violacin A 150. Furthermore, the compound 152 was synthesized from dihydric phenol 151 by treating it with an alkyl halide, sodium iodide and potassium carbonate accompanied by the Steglich esterification and NaH-mediated cyclization to get 152 in 92 % yield. Next, compound 152 was treated with K2CO3 in methanol to obtain 153 in 75 % yield. The compound 152 gave 82 % of analogue 154 by trimethylsilyl treatment and 92 % of analogue 155 was obtained by treatment with PdCl2(CH3CN)2 in acetone. The same strategy was employed to synthesize various other analogues as well. The synthesized analogues were subjected to analyze the production of NO in macrophages. Some of the synthesized compounds exhibited potent anti-inflammatory activity, even better than violacin A (Scheme 21).
Scheme 21.
Synthesis of violacin A 150 and its analogues.
2.8. Alkaloid-based natural product synthesis
Rauvolfia serpentina is a flower that belongs to Apocynaceae family. It is a potent source of a drug, named reserpine, which is used for hypertension treatment. Owing to its medicinal properties, many research groups have undertaken this structure to accomplish its total synthesis. Its first total synthesis was achieved by Woodward and co-workers, in 2022, by forging Meerwein– Ponndorf–Verley reduction, Jones oxidation, Bischler–Napieralski reaction and Steglich esterification as key steps [63]. The reaction of quinone 156 and diene 157 comprised the first step to provide compound 158 in 27 % yield. Over a few steps, the compound 158 was converted to (159) which underwent the Steglich esterification in the presence of DCC, PvOH and pyridine to provide esterified product 160 in 50 % yield. The next step involved coupling with 3,4,5-trimethoxybenzoyl chloride 161 in the presence of sodium methoxide in methanol to furnish target compound (±)-reserpine 162 in 45 % yield (Scheme 22).
Scheme 22.
Synthesis of (±)-reserpine 162.
Schwarzinicines A and B are isolated from Ficus schwarzii. These are 1,4-diarylbutanoid–phenethylamine alkaloids attributed with ethnopharmacological and vasorelaxant properties [64]. Lee et al. provided the total synthesis of schwarzinicines A and B by employing Claisen condensation and reductive amination to envisage the core skeleton of target compounds with overall yield of 9.1 % and 3.5 % respectively [65]. The synthesis commenced from commercially available (3,4-dimethoxyphenyl) propionic acid 163 which underwent Steglich esterification with 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC) in the presence of DMAP and DCM to yield 82 % of esterified product 164. Further, the Claisen condensation of 3,4-dimethoxyphenyl) acetonitrile 165 and ester 164 in the presence of organolithium reagent followed by nitrile hydrolysis in acetic acid with subsequent reductive amination in the presence of NaBH(OAc)3 with 3,4-dimethoxyphenethylamine 166 to forge schwarzinicines A 167 in 79 % yield. Schwarzinicines B 168 was obtained by N-methylation with MeI from 167 in 38 % yield. The synthesized alkaloids underwent for evaluation of vasorelaxant effects on phenylephrine-precontracted aortic rings of rat and exhibited EC50 value in the range of 1.19–2.98 μM (Scheme 23).
Scheme 23.
Synthesis of schwarzinicines A 167 and B 168.
A cis-fused decahydroquinoline (DHQ) containing marine alkaloids, known as lepadins, are obtained from flatworms and tunicates [66]. Lepadins exhibit a wide range of biological activities such as lepadins A and B are anticancerous, lepadin B is active against receptor blocking of neuronal nicotinic acetylcholine, lepadins D-F exhibit antimalarial activity and lepadin I inhibit the action of butyrylcholine esterase. The significance of lepadins has led towards strivings on achieving the total synthesis of DHQ core [67,68]. In 2021, Ma et al. provided the synthetic methodology comprised of five steps to achieve the DHQ core by intramolecular [3 + 2] cycloaddition and aza-Achmatowicz rearrangement [69]. The synthesis was initiated by furfuryl amide 169 which furnished DHQ core structure 170 over a few steps. The next step involved one carbon homologation by Wittig reaction in the presence of Ph3PCH2OCH3Cl and NaHMDS along with subsequent hydrolysis with trichloroacetic acid to produce a mixture of diastereomeric products (5S)-171a and (5R)-171b in 1:1.9 ratio with a combined yield of 72 %. The next step involved Julia− Kocienski olefination in the presence of NaHMDS and tetrazole sulfone 172 with subsequent hydrolysis to give alcohol 173. The ent-lepadin I 175 was obtained in 34 % yield by employing the Steglich esterification with acid 174 followed by deprotection in the presence of TFA. Next, phosphonate-mediated olefination was utilized to give 177a and 177b. The lepadin B 178 was obtained from 177a by TBAF-assisted deprotection in 96 % yield. The 177a was converted to lepadin A 180 in 70 % yield over two steps by deprotection and BF3.OEt2 treatment. Further, 177b underwent TBAF-mediated acylation with 179 to give 47 % of lepadin C 181 (Scheme 24).
Scheme 24.
Synthesis of lepadin A 180, B 178, and C 181.
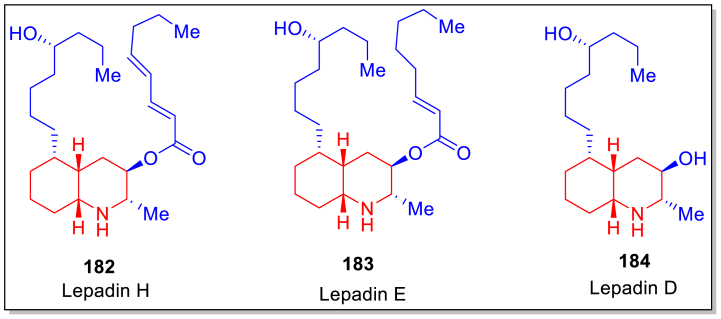
The structures of lepadin D 184, lepadin E 183 and lepadin H 182 are presented in Fig. 4, whose synthesis was also achieved from 139a by employing Julia−Kocienski olefination, Pd-catalyzed hydrogenation, Mitsunobu reaction and cesium acetate-mediated mesylate substitution (Fig. 4).
Fig. 4.
Structures of lepadin H 182, E 183 and D 184.
2.9. Macrolide-based natural product synthesis
Macrocyclic scaffolds are omnipresent in naturally occurring compounds [70] whose synthesis can be achieved by employing ring-closing metathesis and Steglich esterification [71,72]. Thiacladospolide is a sulfur containing natural product obtained from marine sources. These are biologically important as they act against plant pathogen named Colletotrichum glecosporioides with 1–2 μg/ml MIC value and gram-negative and gram-positive bacteria [73]. The total synthesis of thiocladospolide A and its C2-epimer was designed by Swami et al. in 2022 [74]. They employed sulfa-michael addition, ring-closing metathesis and Steglich esterification reaction as key steps to achieve 12 % overall yield in straightforward nine steps. The synthesis commenced from TBS protection of cis-but-2-ene-1,4-diol 185 followed by Swern oxidation in the presence of (COCl)2, DMSO and triethylamine to furnish secondary alcohol 187 in 83 % yield over 2 steps. TBS deprotection of alcohol 187 in the presence of TBAF followed by Jones oxidation in acetone provided 74 % of acid 188. The Steglich esterification of acid 188 with a known alcohol 187 in the presence of DCC, DMAP and dichloromethane furnished compound 190. Further, l-serine 191 was converted to alcohol 192 over a few steps. Finally, a Sulfa-Michael addition was proceeded between the compound 190 and alcohol 192 in the presence of triethylamine in DCM followed by Grubbs-II mediated ring-closing metathesis along with hydrogenation forging the final compounds thiocladospolide A 193 and its C2-epimer 193a in 76 % and 78 % respective yields (Scheme 25).
Scheme 25.
Synthesis of thiocladospolide A 193 and its C2-epimer 193a.
The total synthesis of a queen specific compound has been reported by Machara et al., in 2018 [75]. This compound is specifically present in the bodies of Silvestritermes minutus that regulate a number of female body functions i.e., fertility signals to nestmates and supporting the dominance of reproduction etc. [76]. The synthesis begets with the (S)-glycidyl tosylate 194, an optically pure substance, which underwent allyl magnesium bromide-mediated epoxide opening with Li2CuCl4 in a catalytic amount along with Grignard reaction in the presence of butyl magnesium bromide and copper catalyst to give 78 % of secondary alcohol 195. Next, the Steglich esterification reaction was utilized with alcohol 195 in the presence of DCC, DMAP and hex-5-enoic acid followed by synthesis of macrolide in the presence of HG-II catalyst to furnish mixture of 3-E 196 and 4-Z 197 macrolides in 41 % yield. Furthermore, the synthesis of 6-E and 7-Z macrolides also commenced from (S)-glycidyl tosylate 194 which underwent butyl magnesium bromide-mediated epoxide opening with Li2CuCl4 in a catalytic amount along with Grignard reaction in the presence of allyl magnesium bromide and copper catalyst to give 40 % of secondary alcohol 198. The Steglich protocol in the presence of DCC, DMAP and hex-5-enoic acid along with HG-II mediated metathesis and morpholinoethyl isocyanide-mediated quenching was employed with alcohol 166 to give target compounds 199 and 200. The synthesized compounds were characterized by CIMS fragmentation and FTIR spectra (Scheme 26).
Scheme 26.
Synthesis of macrolides 196, 197, 199 and 200.
Nonenolide is a ten-membered macrolide, also known as decanolide, obtained from Cordyceps militaries BCC2816, which is an entomopathogenic fungus [77]. Nonenolide has been proven to be a potent antimalarial agent against Plasmodium falciparum K1, a multidrug-resistant bacterium. Sudina et al. proposed the total synthesis of nonenolide in 10.4 % overall yield constituting 12 linear steps by employing MacMillan α-hydroxylation, the Steglich esterification and ring-closing metathesis in 2018 [78]. The synthesis commenced from the construction of fragment 202 and 204 from 201 and 203 over a few steps. Next, the fragment 202 and 204 were coupled via the Steglich esterification mediated by DCC and DMAP to obtain 84 % of ester 205. Further, lactone ring of nonenolide was constructed by TBAF-mediated silyl group deprotection along with ring-closing metathesis with Grubbs catalyst followed by DDQ-based PMB group removal to furnish 93 % of nonenolide 206 (Scheme 27).
Scheme 27.
Synthesis of nonenolide 206.
Oxacyclododecindione, 14-deoxyoxacyclododecindione and 13-hydroxy isomer are twelve-membered macrolactones. Exserohilum rostratum is a fungal source of these secondary metabolites [79]. The oxacyclododecindiones are exceptionally good antiinflammatory agents and can prove to be a scaffold in the field of pharmacology [80]. But, exhaustive procedure and low yield of fermentation make it less applicable for medicinal field. To provide a back-up for this problem, seven new analogues of oxacyclododecindione-type macrolactones have been synthesized and evaluated for their antiinflammatory activities. Weber et al. forged the synthesis of seven new analogues of oxacyclododecindione-type macrolactones in 2020 [81]. The synthesis was actualized from δ-valerolactone 207 which gave 94 % of lactol (±)-208 via double methylation in the presence of methyl iodide followed by subsequent reduction with DIBAL-H. Trityl-protected alcohol (±)-209 was obtained from lactol (±)-208 by ring-opening in the presence of a methyl lithium reagent. The next step involved selective Steglich esterification of secondary alcohol (±)-209 in the presence of DCC, DMAP, acid 210 and DCM to give ester (±)-211 in 83 % yield. The analogues (±)-212 and (±)-213 were obtained from ester (±)-211 over a few steps (Scheme 28).
Scheme 28.
Synthesis of oxacyclododecindione-type macrolactones (±)-212 and (±)-213.
Furthermore, the synthesis of analogue (±)-218 commenced from 2-bromobutyric acid (±)-214 which underwent the Steglich esterification in the presence of allyl bromide and DCC followed by treatment with triphenylphosphine to give salt (±)-215 in 94 % yield. The next step involved deprotonation of salt (±)-215 together with Wittig olefination in the presence of aldehyde (±)-216 to provide 67 % of unsaturated ester (±)-217. The analogue (±)-218 was obtained from unsaturated ester (±)-217 over a few steps (Scheme 29). In 2023, Seipp et al. disclosed a stereoselective synthesis of (13R,14S,15R)-13-hydroxy-14-deoxyoxacyclododecindione by employing Steglich esterification and confirmed its structure by analytical techniques [82].
Scheme 29.
Synthesis of oxacyclododecindione-type macrolactone (±)-218.
Isolated from a soil sample of a volcanic island, (+)-prunustatin is an anticancer agent produced by Streptomyces violaceoniger specie. It exhibits anticancerous activity by downregulation of molecular chaperone, GRP78, that helps tumors to survive against chemotherapy [83]. Chojnacka et al. (2018) provided the total synthesis of (+)-prunustatin by employing Steglich esterification, MNBA Shiina couplings, organoboron-mediated prenylation and carbonyldiimidazole-based coupling [84]. The synthesis commenced by l-lactic acid 219 which underwent acetylation in the presence of acyl halide and acetic acid to give compound 220 in 92 % yield. Next, modified Steglich esterification was employed to protect tertiary butyl group mediated by EDCl, DMAP and DCM to provide 84 % of ester 221. Further, the northern fragment 222 was synthesized from ester 221 over a few steps. The southern fragment 224 was prepared from d-phenylalanine 223 over a few steps. A MNBA-mediated Shiina coupling was employed for coupling the northern fragment 222 and southern fragment 224 followed by MOM and t-butyl group deprotection in the presence of TFA and Cbz removal to provide a quantitative yield of macrocycle 225. Next, 3-nitrosalicylic acid 226 in an unprotected form, activated by CDI followed by coupling with macrocycle 225 along with reduction of nitro group mediated by 30 wt% Pd/C and Cossy's protocol based on N-formyl saccharin to furnish (+)-prunustatin A (+)-227 in 70 % yield (Scheme 30).
Scheme 30.
Synthesis of (+)-prunustatin A 227.
2.10. Carbasugar-based natural product synthesis
Carbasugars are a subclass of carbohydrates having a minor difference of ring oxygen replaced with a methylene group and thus being more stable than carbohydrates. Pericosines (A-E) and Gabosines are carbasugars obtained from a fungus Perconia byssoides (OUPS–N133) and culture broth of Streptomyces filipensis respectively [85,86]. These compounds are considered significant in medicinal field owing to their applications. Bidus et al. provided the total synthesis of these carbasugars in 2020 [87]. The synthesis begun from d-ribose 228 which was converted to 229 over a few steps. Next, the anti-229 was consumed by employing mild conditions of MeI and Ag2O and recovered syn-229 was methylated followed by deprotection in the presence of BCl3 in DCM to achieve 85 % of (+)-Pericosine C 230 and 54 % of (+)-Pericosine B 231. Further, carbocyclic intermediate 229 was employed for the synthesis of COTC 234 and 7-chloro-analogue of (+)-Gabosine C 235. For that purpose, carbocyclic intermediate 229 was first reduced with DIBAL-H to obtain mixture of 232. In the next step, Steglich esterification was employed for esterification of 232 in the presence of crotonic acid, DCC and DMAP to obtain 52 % of ester 233 with 9 % of the unreacted substrate. Finally, 26 % of (+)-COTC 234 and 40 % of 7-chloro-7-deoxy-(+)-gabosine C 235 were obtained by Swern oxidation followed by deprotection with BCl3 in DCM (Scheme 31).
Scheme 31.
Synthesis of (+)-Pericosine C 230 and (+)-Pericosine B 231, (+)-COTC 234 and 7- chloro-7-deoxy-(+)-gabosine C 235.
2.11. Fatty acid-based natural product synthesis
Chatenaytrienin-2 is annonaceous acetogenins, obtained from Annonaceae family, which exhibits growth inhibitory and anticancerous effects [88]. In 2019, Kunkalkar et al. executed the total synthesis of chatenaytrienin-2247 by implementation of Sonogashira cross coupling reaction and ring closing metathesis to achieve 36.5 % overall yield of final product [89]. The total synthesis started off from Styryl methyl ketone 236 which underwent a few steps to give alcohol 237. The bromo decanol 238 was subjected to DMP-mediated oxidation along with Mannich reaction together with Pinnick oxidation to give 73 % of α-methelenic acid 239. Next, the Steglich esterification was performed with alcohol 237 and α-methelenic acid 239 in the presence of DCC, DMAP and DCM to give 91 % of ester 240. Further, Hoveyda-Grubbs-assisted ring closing metathesis was carried out to yield 93 % of the cyclic product 241. The next step involved the synthesis of ene-diyne fragment 245 in 76 % yield by alkylation of compound 242 with the help of 1-bromododecane 243 followed by DMP-interceded oxidation and treatment with Wittig salt 244. The final step involved coupling of compounds 241 and ene-diyne 245 in the presence of 5 mol% of ligand 246 and 2.5 mol% of [PdCl(allyl)]2 catalyst, CuI and cesium carbonate followed by Lindlar reduction to furnish target compound chatenaytrienin-2247 in 59 % yield over two steps (Scheme 32).
Scheme 32.
Synthesis of chatenaytrienin-2247.
2.12. Glycolipid-based natural product synthesis
An immunity booster glycolipid, diphosphatidyltrehalose (diPT), is obtained from a fever-causing Salmonella Typhi gram-negative bacterium [90]. Mishra et al. proposed total synthesis of diphosphatidyltrehalose (diPT) in 2019 [91]. The synthesis started from α, α-trehalose 248 which was converted to bisphosphoramidite 249 over a few steps in good yield. Next, acyl glycerol 251 was synthesized over a few steps from octyne 250. A Steglich protocol with (R, S)-252 was employed in the presence of DCC, DMAP and DCM to give diglyceride 253 in 97 % yield. The intermediate 254 was obtained in quantitative yield by BF3·CH3CN-mediated deprotection of diglyceride 253. Subsequently, coupling of intermediate 254 and bisphosphoramidite 249 was performed in the presence of DCI in DCM followed by clean deprotection with DBU along with hydrogenolysis in the presence of Pd/C and Pd(OH)2/C (2:3) in a CH2Cl2/MeOH/water mixture furnished target compound diPT 255 in 67 % yield (Scheme 33).
Scheme 33.
Synthesis of glycolipid 255.
2.13. Sterol-based natural product synthesis
Steroids are a peremptory class of naturally occurring organic compounds possessing a wide range of biological properties. These steroids are also known as mesogens because of their occurrence as liquid crystals. The liquid crystals exhibit a number of applications in different fields [92]. Bhat et al. proposed the synthesis of target compounds by employing Steglich esterification as a key step in 2020 [93]. The first step involved coupling of Stigmasterol 256 with 4-bromo benzoic acid 257 via Steglich esterification in the presence of DCC, DMAP and dichloromethane to obtain sterol 4-bromo benzoates 258. The mercapto alkanes were nucleophilically substituted with sterol 4-bromo benzoates 258 to realize 4-mercapto alkyl benzoate 259. Just like the synthesis of Stigmasterol mesogens, Cholesterol and Ergosterol mesogens were also synthesized by employing same strategy. The synthesized mesogens were subjected for mesomorphic studies which revealed that stigmasterol and ergosterol derivatives (259a-259f) were smectogenic. Further the cholesterol derivatives exhibited cholesteric, smectic and blue phase with brief temperature range. The studies further revealed that replacement of sulfur with oxygen in cholesterol derivatives provides a low clearing temperature (Scheme 34).
Scheme 34.
Synthesis of Stigmasterol mesogens.
2.13.1. Miscellanous
Nelliella nelliiformis is a Pacific bryozoan, known for the source of secondary metabolites nelliellosides A and B. These bioactive natural compounds belong to nucleoside family which have been recognized as potent antibacterial, antiviral and anticancerous drugs [94]. Bracegirdle et al. reported the synthesis of nelliellosides A and B by utilizing the Steglich esterification in 2019 [95]. The synthesis commenced from adenosine 260 which underwent OH group protection in the presence of 2,2-dimethoxypropane, p-TsOH and acetic acid to give compound 261. The next step involved the Steglich esterification with pyrrole-2-carboxylic acid 263 and pyrrole-3-carboxylic acid 262 in the presence of DCC, DMAP and DCM accompanied by deprotection to give nelliellosides A 265 and nelliellosides B 264. To synthesize the derivatives of hypoxanthine and guanine nucleoside, an exact similar strategy was employed (Scheme 35).
Scheme 35.
Synthesis of derivatives of nellielloside A 265 and B 264.
The (indole-N-isoprenyl)-tryptophan-valine diketopiperazine is a natural product possessing antifungal properties which was obtained from Ascomycota phylum. This natural product exhibits a variety of medicinal properties depending upon amino acid derivative attached to it i.e., tryptophan causes it to be herbicide and insecticide active in addition to antiviral, anticancerous and antioxidant [96]. In 2019, Bai et al. reported its first total synthesis along with the synthesis of stereoisomers [97]. The synthesis was initiated from tryptophan 266, which underwent NH protection in the presence of (Boc)2 to give intermediate 267. The next step involved condensation reaction with valine methyl ester via the Steglich protocol to provide compound 268. Next, Boc deprotection was being performed in the presence of TFA in dichloromethane followed by cyclization in the presence of ammonium hydroxide to furnish target compound 269. Depending upon the configuration of starting compound, configuration of final compound is obtained. On a similar note, four stereoisomers of target compound were also obtained by employing an analogous pathway as employed for its total synthesis. The synthesized compounds exhibited a broad spectrum of antifungal properties with a rate of 15 % at 50 mg/L (Scheme 36).
Scheme 36.
Synthesis of diketopiprazine 269.
In 2019, Zaghouani et al. provided a total synthesis scheme to obtain 11.6 % overall yield of (±)-fumimycin in concise seven steps [98]. The key steps of this synthesis were Steglich esterification, intramolecular aza Friedel–Crafts reaction, regioselective chlorination and Suzuki-Miyaura cross coupling reaction. The synthesis commenced from Steglich esterification of 3,4-dimethoxy phenyl 271 and pyruvic acid 270 in the presence of 1.5 equivalents of DCC, DMAP and dichloromethane to give 82 % of pyruvate 272. The next step involved intramolecular aza Friedel–Crafts reaction followed by regioselective chlorination in the presence of 5 mol% of Palau' Chlor catalyst to furnish 87 % of product 273. The Suzuki-Miyaura cross coupling reaction was employed for next step along with Cbz deprotection and fumaryl coupling to forge 274. The final step involved the hydrolysis to furnish target compound (±)-fumimycin 275 in 42 % yield. By altering the substitution of starting compounds, the analogues of (±)-fumimycin can also be obtained with the same scheme (Scheme 37).
Scheme 37.
Synthesis of (±)-fumimycin 275.
Bryostatin is a natural product obtained from a marine source named, Bugula Neritina. It is known for its pharmacological and medicinal properties and has undergone for trials for a number of diseases like ischemic stroke, Niemann-Pick, multiple sclerosis, fragile X disorder and Charcot-Marie-Tooth syndrome. Due to its indispensable potential in medicinal field, researchers have approached this compound to achieve its total synthesis. But they have achieved only the synthesis of bryostatin analogues so far which behave similar to bryostatin and are more active biologically. The synthesis of bryostatin analogue, reported by Wender et al. in 2020, commenced from neopentyl glycol 276 which was converted to compound 277 over a few steps [99]. Next, the compound 277 underwent deprotection followed by Steglich esterification with monobenzyl glutarate in the presence of DCC and DMAP to give ester 278. The next step included Sharpless asymmetric dihydroxylation to introduce a diastereoselective mixture of diols 279 with a 1.5:1 diastereomeric ratio. Further, mixtures of diastereomers were separated by treating the mixture with aq. HF in acetonitrile followed by hydroxyl group protection with TBSCl in the presence of DMAP and DCM to obtain 280 in 38 % yield over three steps. Further, the compound 280 underwent hydrogenolysis followed by Yamguchi lactonization with 2,4,6-trichlorobenzoyl chloride in the presence of triethylamine and DMAP along with deprotection of silyl group to furnish bryostatin analogue 281 in 75 % yield (Scheme 38).
Scheme 38.
Synthesis of bryostatin analogue 281.
In 2020, Wood et al. aimed at [2 + 2+2] cycloaddition of benzoquinones and diynes catalyzed by rhodium and exemplified it with a short total synthesis of justicidone [100]. The synthesis was initialized with a Sonogashira coupling of aryl iodide 282 and propargyl alcohol 283 to give 89 % of compound 284. Further, the Steglich esterification of compound 284 with propiolic acid in the presence of DCC, DMAP and dichloromethane furnished ester 285 in 99 % yield. The final step involved Rh-catalyzed optimized protocol to provide target compound 287 in 42 % yield (Scheme 39).
Scheme 39.
Synthesis of justicidone 287.
3. Conclusion
The synthesis of natural products which include alkaloids, peptides, lactams, terpenoids, chromanones, macrolides, glycolipids, pyrones, fatty acids and carbasugars has been a tedious task since the advancement of synthetic chemistry. A number of key steps and procedures play vital part in achieving this task. Steglich reaction is one of those procedures. To conclude, this review article has provided an efficacious role of the Steglich reaction towards the total synthesis of simple to complex natural products. The key points of this reaction are mild reaction conditions, best yields, facile procedure for esterification of complex compounds with difficult separation procedures, requiring further research to be done in that area. The synthesis of representative classes of natural products with necessary conditions has been mentioned in this article. This review will prove to be helpful for the selection of Steglich esterification conditions to achieve the total synthesis of natural products and synthetically important compounds.
Data availability statement
Data included in the article and referenced in article. No data associated with this study has been deposited into a publicly available repository.
CRediT authorship contribution statement
Saba Munawar: Writing - original draft, Investigation. Ameer Fawad Zahoor: Supervision, Project administration, Conceptualization. Syed Makhdoom Hussain: Methodology, Investigation. Sajjad Ahmad: Resources, Methodology, Investigation. Asim Mansha: Resources, Methodology, Investigation. Bushra Parveen: Resources, Methodology, Investigation. Kulsoom Ghulam Ali: Resources, Methodology, Investigation. Ahmad Irfan: Resources, Investigation.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests.
Acknowledgements
A. Irfan extends his appreciation to the Deanship of Scientific Research at King Khalid University for funding through large group Research Project under grant number RGP2/276/44.
List of Abbreviations used
- DMAP
Dimethyl aminopyridine
- DCC
N, N′-dicyclohexylcarbodiimide
- DIC
N, N′-diisopropylcarbodimide
- EDC
N-ethyl-N’- (3-dimethylaminopropyl) carbodiimide
- LDA
Lithium diisopropylamide
- MIC
Minimum inhibitory concentration
- HATU
Hexafluorophosphate Azabenzotriazole Tetramethyl Uronium
- DIPEA
N, N-Diisopropylethylamine
- TFA
Trifluoroacetic acid
- THF
Tetrahydrofuran
- DCM
Dichloromethane
- LiAlH4
Lithium Aluminium Hydride
- DCI
Dicyanoimidazole
- DBU
Diazabicyclo Undec Ene
- p-TsOH
p-Toluenesulfonic acid
- Boc
tert-butyloxycarbonyl
- NCS
N-Chlorosuccinimide
- TBSCl
tert-Butyldimethysilyl chloride
- DiPT
Diphosphatidyltrehalose
- MNBA
2-Methyl-6-nitrobenzoic anhydride
- CDI
1,1′-Carbonyldiimidazole
- TBAF
Tetra-n-butylammonium fluoride
- TPAP
Tetra-n-propyl Ammonium Perruthenate
- NMO
N-Methylmorpholine-N-Oxide
- TESOTf
Triethylsilyl trifluoromethanesulfonate
- KHMDS
Potassium bis(trimethylsilyl)amide
References
- 1.Zhou Y.J., Buijs N.A., Zhu Z., Qin J., Siewers V., Nielsen J. Production of fatty acid-derived oleochemicals and biofuels by synthetic yeast cell factories. Nat. Commun. 2016;7:1–9. doi: 10.1038/ncomms11709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Groeneveld G., Kuijer S., Puit M.D. Preparation of cyanoacrylate derivatives and comparison of dual action cyanoacrylate formulations. Sci. Justice. 2014;54:42–48. doi: 10.1016/j.scijus.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 3.Shin Y.J., Shin J., Jang H., Son H., Kwon Y. Decursinol chloroacrylates useful as fungicides. Appl. Bio. Chem. 2022;65:53–61. doi: 10.1186/s13765-022-00720-y. [DOI] [Google Scholar]
- 4.Sά A.G.A., Meneses A. C. de, Araújo P. H. H. de, Oliveira D. de. A review on enzymatic synthesis of aromatic esters used as flavor ingredients for food, cosmetics and pharmaceuticals industries. Trends Food Sci. Technol. 2017;69:95–105. doi: 10.1016/j.tifs.2017.09.004. [DOI] [Google Scholar]
- 5.Dekić M.S., Selimović E.S. New natural products from Asphodelus albus MILL. Essential Oil. Chem. Biodiversity. 2021;18:1–8. doi: 10.1002/cbdv.202100103. [DOI] [PubMed] [Google Scholar]
- 6.Majhi S. Applications of Yamaguchi method to esterification and macrolactonization in total synthesis of bioactive natural products. ChemistrySelect. 2021;6:4178–4206. doi: 10.1002/slct.202100206. [DOI] [Google Scholar]
- 7.Munawar S., Zahoor A.F., Ali S., Javed S., Irfan M., Irfan A., Kotwica-Mojzych K., Mojzych M. Mitsunobu reaction: a powerful tool for the synthesis of natural products: a review. Molecules. 2022;27:1–67. doi: 10.3390/molecules27206953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fürst M.J.L.J., Gran-Scheuch A., Aalbers F.S., Fraaije M.W. Baeyer−Villiger monooxygenases: tunable oxidative biocatalysts. ACS Catal. 2019;9:11207–11241. doi: 10.1021/acscatal.9b03396. [DOI] [Google Scholar]
- 9.Li J.J. Name Reactions. Springer; Cham: 2014. Corey–nicolaou macrolactonization; pp. 178–179. [DOI] [Google Scholar]
- 10.Khan Z., Javed F., Shamair Z., Hafeez A., Fazal T., Aslam A., Zimmerman W.B., Rehman F. Current developments in esterification reaction: a review on process and parameters. J. Ind. Eng. Chem. 2021;103:80–101. doi: 10.1016/j.jiec.2021.07.018. [DOI] [Google Scholar]
- 11.Neises B., Steglich W. Simple method for the esterification of carboxylic acids. Angew. Chem., Int. Ed. Engl. 1978;17:522–524. doi: 10.1002/anie.197805221. [DOI] [Google Scholar]
- 12.Tsakos M., Schaffert E.S., Clement L.L., Villadsen N.L., Poulsen T.B. Ester coupling reactions – an enduring challenge in the chemical synthesis of bioactive natural products. Nat. Prod. Rep. 2015;32:605–632. doi: 10.1039/c4np00106k. [DOI] [PubMed] [Google Scholar]
- 13.Saha S., Auddy S.S., Chatterjee S., Sen P., Goswami R.K. Late-stage functionalization: total synthesis of beauveamide A and its congeners and their anticancer activities. Org. Lett. 2022;24:7113–7117. doi: 10.1021/acs.orglett.2c02699. [DOI] [PubMed] [Google Scholar]
- 14.Scheeff S., Rivière S., Ruiz J., Dedenbach S., Menche D. Modular total synthesis of iso-archazolids and archazologs. J. Org. Chem. 2021;86:10190–10223. doi: 10.1021/acs.joc.1c00946. [DOI] [PubMed] [Google Scholar]
- 15.Liniger M., Neuhaus C.M., Altmann K.H. Ring-closing metathesis approaches towards the total synthesis of rhizoxins. Molecules. 2020;25:4527. doi: 10.3390/molecules25194527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nurieva E.V., Zefirov N.A., Temnyakova N.S., Kuznetsov S.A., Zefirova O.N. C(7)-Derivatives of colchicine with guanosine and biphenyl moieties: molecular modeling, synthesis, and tubulin clustering effect in cancer cells. Russ. Chem. Bull. 2020;69:2222—2227. doi: 10.1007/s11172-020-3025-y. [DOI] [Google Scholar]
- 17.Kiefer A., Bader C.D., Held J., Esser A., Rybniker J., Empting M., Müller R., Kazmier U. Synthesis of new cyclomarin derivatives and their biological evaluation towards Mycobacterium tuberculosis and Plasmodium falciparum. Chem. Eur J. 2019;25:8894–8902. doi: 10.1002/chem.201901640. [DOI] [PubMed] [Google Scholar]
- 18.Karagöz A.C., Reiter C., Seo E.J., Gruber L., Hahn F., Leidenberger M., Klein V., Hampel F., Friedrich O., Marschall M., Kappes B., Efferth T., Tsogoeva S.B. Access to new highly potent antileukemia, antiviral and antimalarial agents via hybridization of natural products (homo)egonol, thymoquinone and artemisinin. Bioorg. Med. Chem. 2018;26:3610–3618. doi: 10.1016/j.bmc.2018.05.041. [DOI] [PubMed] [Google Scholar]
- 19.Sharma P., Sharma N., Kashyap G., Bhagat S. First total synthesis of the marine-derived antiinflammatory natural product (–)-Herdmanine D through a Steglich esterification. Synlett. 2022;33:62–65. doi: 10.1055/a-1672-3000. [DOI] [Google Scholar]
- 20.Linder T., Papaplioura E., Ogurlu D., Geyrhofer S., Hummelbrunner S., Schachner D., Atanasov A.G., Mihovilovic M.D., Dirsch V.M., Schnürch M. Investigation of Leoligin derivatives as NF-κB inhibitory agents. Biomedicines. 2022;10:62–76. doi: 10.3390/biomedicines10010062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rzepka Z., Bębenek E., Chrobak E., Wrześniok D. Synthesis and anticancer activity of indole-functionalized derivatives of Betulin. Pharmaceutics. 2022;14:2372–2388. doi: 10.3390/pharmaceutics14112372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tang A.H., Payne R.J. Second generation synthesis of the antiinfective natural product gallinamide A. Tetrahedron. 2023 doi: 10.1016/j.tet.2023.133445. [DOI] [Google Scholar]
- 23.Rahmadani A., Masruhim M.A., Rijai L., Hidayat A.T., Supratman U., Maharani R. Total synthesis of cyclohexadepsipeptides exumolides A and B. Tetrahedron. 2021;83:1–5. doi: 10.1016/j.tet.2021.131987. [DOI] [Google Scholar]
- 24.Brumley D.A., Gunasekera S.P., Chen Q.Y., Paul V.J., Luesch H. Discovery, total synthesis, and sar of anaenamides A and B: anticancer cyanobacterial depsipeptides with a chlorinated pharmacophore. Org. Lett. 2020;22:4235–4239. doi: 10.1021/acs.orglett.0c01281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakayama Y., Takita T., Ozawa H., Umezawa H., Tahara K. Studies on ilamycin. J. Antibiot. 1962;15:49–50. doi: 10.11554/antibioticsa.15.1_49. [DOI] [Google Scholar]
- 26.Takita T., Ohi K., Maeda K., Okami Y., Umezawa H. New antibiotics, ilamycins. J. Antibiot. 1962;15:46–48. doi: 10.11554/antibioticsa.15.1_46. [DOI] [PubMed] [Google Scholar]
- 27.Greve J., Mogk A., Kazmaier U. Total synthesis and biological evaluation of modified illamycin derivatives. Mar. Drugs. 2022;20:632. doi: 10.3390/md20100632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brumley D., Spencer K.A., Gunasekera S.P., Sauvage T., Biggs J., Paul V.J., Luesch H. Isolation and characterization of anaephenes A−C, alkylphenols from a filamentous cyanobacterium (hormoscilla sp., oscillatoriales) J. Nat. Prod. 2018;81(12):2716−–2721. doi: 10.1021/acs.jnatprod.8b00650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Han M.L., Zhao J.X., Liu H.C., Ni G., Ding J., Yang S.P., Yue J.M. Limonoids and Triterpenoids from Dysoxylum mollissimum var. glaberrimum. J. Nat. Prod. 2015;78:754–761. doi: 10.1021/np500967k. [DOI] [PubMed] [Google Scholar]
- 30.Liu C.P., Xie C.Y., Zhao J.X., Ji K.L., Lei X.X., Sun H., Lou L.G., Yue J.M., Dysoxylactam A. A macrocyclolipopeptide reverses P-Glycoprotein-Mediated multidrug resistance in cancer cells. J. Am. Chem. Soc. 2019;141:6812–6816. doi: 10.1021/jacs.9b02259. [DOI] [PubMed] [Google Scholar]
- 31.Reddy R.B., Yu B. Total synthesis of macrocyclic dysoxylactam. A. Chem. Asian J. 2020;15:2467–2469. doi: 10.1002/asia.202000482. [DOI] [PubMed] [Google Scholar]
- 32.Woodward R.B., Heusler K., Gosteli J., Naegeli P., Oppolzer W., Ramage R., Ranganathan S., Vorbruggen H. Total synthesis of cephalosporin C. J. Am. Chem. Soc. 1996;88:852–853. doi: 10.1021/ja00956a051. [DOI] [Google Scholar]
- 33.Li Y.X., Zhong Z., Zhang W.P., Qian P.Y. Discovery of cationic nonribosomal peptides as Gram-negative antibiotics through global genome mining. Nat. Commun. 2018;9:3273. doi: 10.1038/s41467-018-05781-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ayed K.A., Ballantine R., Zhong Z., Li Y.X., Cochraneb S.A., Martina N.I. Total Synthesis of the brevicidine and laterocidine family of lipopeptide antibiotics. Chem. Sci. 2021;13:3563–3570. doi: 10.26434/chemrxiv.13660949.v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ayed K.A., Losada D.Z., Machushynets N.V., Terlouw B., Elsayed S.S., Schill J., Trebosc V., Pieren M., Medema M.H., Wezel G.P.v., Martin N.I. Total synthesis and structure assignment of the relacidine lipopeptide antibiotics and preparation of analogues with enhanced stability. ACS Infect. Dis. 2023;9(4):739–748. doi: 10.1021/acsinfecdis.3c00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hauser N., Imhof M.A., Eichenberger S.S., Kündig T., Carreira E.M. Total synthesis of shearinines D and G: a convergent approach to indole diterpenoids. Angew. Chem., Int. Ed. 2022;134 doi: 10.1002/anie.202112838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ochi M., Kotsuki H., Muraoka K., Tokoroyama T. The structure of yahazunol, a new sesquiterpene-substituted hydroquinone from the Brown seaweed Dictyopteris undulata okamura. Bull. Chem. Soc. Jpn. 1979;52:629–630. doi: 10.1246/bcsj.52.629. [DOI] [Google Scholar]
- 38.Perez-Garcia E., Zubıa E., Ortega M.J., Carballo J.L. Merosesquiterpenes from two sponges of the genus Dysidea. J. Nat. Prod. 2005;68:653–658. doi: 10.1021/np040237z. [DOI] [PubMed] [Google Scholar]
- 39.Djura P., Stierle D.B., Sullivan B., Faulkner D.J. Some metabolites of the marine sponges Smenospongia aurea and Smenospongia (.ident.Polyfibrospongia) echina. J. Org. Chem. 1980;45:1435–1441. doi: 10.1021/jo01296a019. [DOI] [Google Scholar]
- 40.Kurata K., Taniguchi K., Suzuki M. Cyclozonarone, a sesquiterpene-substituted benzoquinone derivative from the brown alga Dictyopteris undulata. Phytochemistry (Elsevier) 1996;41:749–752. doi: 10.1016/0031-9422(95)00651-6. [DOI] [Google Scholar]
- 41.Zhang S., Wang X., Hao J., Li D., Csuk R., Li S. Expediently scalable synthesis and antifungal exploration of (+)-Yahazunol and related meroterpenoids. J. Nat. Prod. 2018;81:2010–2017. doi: 10.1021/acs.jnatprod.8b00310. [DOI] [PubMed] [Google Scholar]
- 42.Anjaneyulu A.S.R., Venugopal M.J.R.V., Sarada P., Clardy J., Lobkovsky E. Havellockate. A novel seco and spiro lactone diterpenoid from the Indian ocean soft coral Sinularia granosa. Tetrahedron Lett. 1998;39:139–142. doi: 10.1016/S0040-4039(97)10470-1. [DOI] [Google Scholar]
- 43.Hafeman N.J., Chan M., Fulton T.J., Alexy E.J., Loskot S.A., Virgil S.C., Stoltz B.M. Asymmetric total synthesis of havellockate. J. Am. Chem. Soc. 2022;144:20232–20236. doi: 10.1021/jacs.2c09583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li Y., Pattenden G. Novel macrocyclic and polycyclic norcembranoid diterpenes from Sinularia species of soft coral: structural relationships and biosynthetic speculations. Nat. Prod. Rep. 2011;28:429–440. doi: 10.1039/C0NP00029A. [DOI] [PubMed] [Google Scholar]
- 45.Hafeman N.J., Loskot S.A., Reimann C.E., Pritchett B.P., Virgil S.C., Stoltz B.M. The total synthesis of (–)-Scabrolide A. J. Am. Chem. Soc. 2020;142(19):8585–8590. doi: 10.1021/jacs.0c02513. [DOI] [PubMed] [Google Scholar]
- 46.Hafeman N.J., Loskot S.A., Reimann C.E., Pritchett B.P., Virgil S.C., Stoltz B.M. Total synthesis of (−)-scabrolide A and (−)-yonarolide. Chem. Sci. 2023;14:4745–4758. doi: 10.1039/d3sc00651d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Siemon T., Steinhauer S. Synthesis of (+)-Darwinolide. Angew. Chem. Int. Ed. 2019;114 doi: 10.1002/anie.201813142. [DOI] [PubMed] [Google Scholar]
- 48.Sarwar M., Xia Y.-X., Liang Z.-M., Tsang S.W., Zhang H.-J. Mechanistic pathways and molecular targets of plant-derived anticancer ent-kaurane diterpenes. Biomolecules. 2020;10:144. doi: 10.3390/biom10010144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Herrera-Acevedo C., Flores-Gaspar A., Scotti L., Mendonça-Junior F.J.B., Scotti M.T., Coy-Barrera E. Identification of kaurane-type diterpenes as inhibitors of leishmania pteridine reductase I. Molecules. 2021;26:3076–3099. doi: 10.3390/molecules26113076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu Y., Zhao Z., Hu C., Zhao C., Liu J., Du Y. Chiron approach for the total synthesis of brevipolide M. Synlett. 2022;33:478–482. doi: 10.1055/a-1730-9857. [DOI] [Google Scholar]
- 51.Uchida R., Imasato R., Yamaguchi Y., Masuma R., Shiomi K., Tomoda H., Omura S. New insecticidal antibiotics, hydroxyfungerins A and B, produced by metarhizium sp. FKI-1079. J. Antibiot. 2005;58:804. doi: 10.1038/ja.2005.107. [DOI] [PubMed] [Google Scholar]
- 52.Merchant R.R., Oberg K.M., Lin Y., Novak A.J.E., Felding J., Baran P.S. Divergent synthesis of pyrone diterpenes via radical cross coupling. J. Am. Chem. Soc. 2018;140:7462–7465. doi: 10.1021/jacs.8b04891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sun H.D., Huang S.X., Han Q.B. Diterpenoids from Isodon species and their biological activities. Nat. Prod. Rep. 2006;23:673–698. doi: 10.1039/B604174D. [DOI] [PubMed] [Google Scholar]
- 54.Zhang J., Li Z., Zhuo J., Cui Y., Han T., Li C. A tandem decarboxylative cyclization/alkenylation strategy for the total syntheses of (+)-Longirabdiol, (−)-Longirabdolactone, and (−)- effusin. J. Am. Chem. Soc. 2019;141:8372–8380. doi: 10.1021/jacs.9b03978. [DOI] [PubMed] [Google Scholar]
- 55.Stierle A.A., Stierle D.B., Decato D., Priestley N.D., Alverson J.B., Hoody J., McGrath K., Klepacki D. The berkeleylactones, antibiotic macrolides from fungal coculture. J. Nat. Prod. 2017;80:1150–1160. doi: 10.1021/acs.jnatprod.7b00133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ferko B., Zeman M., Formica M., Veselý S., Doháošová J., Moncol J., Olejníková P., Berkes D., Jakubec P., Dixon D.J., Caletkova O. Total synthesis of berkeleylactone A. J. Org. Chem. 2019:1–9. doi: 10.1021/acs.joc.9b00850. [DOI] [PubMed] [Google Scholar]
- 57.Schriefer M.G., Schobert R. Divergent synthesis of six recent berkeleylactones. J. Nat. Prod. 2023;86:423–428. doi: 10.1021/acs.jnatprod.3c00053. [DOI] [PubMed] [Google Scholar]
- 58.Samwel S., Mdachi S.J.M., Nkunya M.H.H., Irungu B.N., Moshi M.J., Moulton B., Luisi B.S. Nat. Prod. Commun. A novel antiplasmodial 3′,5′-diformylchalcone and other constituents of Friesodielsia obovata. 2007;2:737–741. doi: 10.1177/1934578X0700200706. [DOI] [PubMed] [Google Scholar]
- 59.Benedeković G., Popsavin M., Kovaĉević I., Kojić V., Rodić M., Popsavin V. Synthesis, antiproliferative activity and SAR analysis of (−)-cleistenolide and analogues. Eur. J. Med. Chem. 2020;202 doi: 10.1016/j.ejmech.2020.112597. [DOI] [PubMed] [Google Scholar]
- 60.Benedeković G., Popsavin M., Kovaĉević I., Kojić V., Kesić J., Farkas S., Popsavin V. Design, synthesis and cytotoxic activity of new 6-O-aroyl (₋)-cleistenolide derivatives. Tetrahedron. 2021;96 doi: 10.1016/j.tet.2021.132385. [DOI] [Google Scholar]
- 61.Ma J., Cao B., Chen X., Xu M., Bi X., Guan P., Jiang Y., Xu J., Han L., Huang X., Violacin A. A new chromanone produced by Streptomyces violaceoruber and its antiinflammatory activity. Bioorg. Med. Chem. Lett. 2018;5:947–951. doi: 10.1016/j.bmcl.2018.01.051. [DOI] [PubMed] [Google Scholar]
- 62.Liu Q., Mu Y., An Q., Xun J., Ma J., Wu W., Xu M., Xu J., Han L., Huang X. Total synthesis and antiinflammatory evaluation of violacin A and its analogues. Bioorg. Chem. 2019 doi: 10.1016/j.bioorg.2019.103420. [DOI] [PubMed] [Google Scholar]
- 63.Woodward R.B., Bader F.E., Bickel H., Frey A.J., Kierstead R.W. The total synthesis of (±)-Reserpine. J. Am. Chem. Soc. 1956;78:2023–2025. doi: 10.1016/0040-4020(58)88022-9. [DOI] [Google Scholar]
- 64.Krishnan P., Lee F.K., Yap V.A., Low Y.Y., Kam T.S., Yong K.T., Ting K.N., Lim K.H., Schwarzinicines A.-G. 1,4-diarylbutanoid-phenethylamine conjugates from the leaves of Ficus schwarzii. J. Nat. Prod. 2020;83(1):152–158. doi: 10.1021/acs.jnatprod.9b01160. [DOI] [PubMed] [Google Scholar]
- 65.Lee F.K., Krishnan P., Muhamad A., Low Y.Y., Kam T.S., Ting K.N., Lim K.H. Concise synthesis of the vasorelaxant alkaloids schwarzinicines A and B. Nat. Prod. Res. 2021 doi: 10.1080/14786419.2021.1903005. [DOI] [PubMed] [Google Scholar]
- 66.Steffan B. Lepadin A, a decahydroquinoline alkaloid from the tunicate clavelina-lepadiformis. Tetrahedron. 1991;47:8729–8732. doi: 10.1016/S0040-4020(01)96194-0. [DOI] [Google Scholar]
- 67.Kubanek J., Williams D.E., Desilva E.D., Allen T., Andersen R.J. Cytotoxic alkaloids from the flatworm prostheceraeus-villatus and its tunicate prey clavelina-lepadiformis. Tetrahedron Lett. 1995;36:6189–6192. doi: 10.1016/0040-4039(95)01246-E. [DOI] [Google Scholar]
- 68.Tsuneki H., You Y.R., Toyooka N., Sasaoka T., Nemoto H., Dani J.A., Kimura I. Marine alkaloids (−)-Pictamine and (−)-Lepadin B block neuronal nicotinic acetylcholine receptors. Biol. Pharm. Bull. 2005;28:611–614. doi: 10.1248/bpb.28.611. [DOI] [PubMed] [Google Scholar]
- 69.Ma F., He C., Wang E., Tong R. Collective asymmetric total syntheses of marine decahydroquinoline alkaloid lepadins A−E, H, and ent-I. Org. Lett. 2021;23:6583–6588. doi: 10.1021/acs.orglett.1c02435. [DOI] [PubMed] [Google Scholar]
- 70.Gade N.R., Iqbal J. Natural product inspired topology directed synthesis of hybrid macrocyclic compounds: a simple approach to natural product analogues. ChemistrySelect. 2018;3:6262–6266. doi: 10.1002/slct.201801117. [DOI] [Google Scholar]
- 71.Brütsch T.M., Cotter E., Agell D.L., Horcajo M.R., Davies C., Pfeiffer B., Pagani S., Berardozzi S., Díaz J.F., Miller J.H., Altmann K.H. Synthesis and structure-activity relationship studies of C(13)-desmethylene-(−)-zampanolide analogs. Chem. 2023:1–24. doi: 10.26434/chemrxiv-2023-mfxxz-v2. [DOI] [PubMed] [Google Scholar]
- 72..
- 73.Zhang F.Z., Li X.M., Yang S.Q., Meng L.H., Wang B.G., Thiocladospolides A.-D. 12-Membered macrolides from the mangrove-derived endophytic fungus cladosporium cladosporioides MA-299 and structure revision of pandangolide 3. J. Nat. Prod. 2019;82:1535. doi: 10.1021/acs.jnatprod.8b01091. [DOI] [PubMed] [Google Scholar]
- 74.Swami P., Mali M., Dhulshette B., Ghosh S. Total synthesis of thiocladospolide A and its C2-epimer. Synth. Met. 2022;54:683–688. doi: 10.1055/a-1652-3714. [DOI] [Google Scholar]
- 75.Machara A., Křivánek J., Dolejŝová K., Havlíĉková J., Bednárová L., Hanus R., Majer P., Kyjaková P. Identification and enantiodivergent synthesis of (5Z,9S)-Tetradec-5- en-9-olide, a queen-specific volatile of the termite Silvestritermes minutus. J. Nat. Prod. 2018;81:2266–2274. doi: 10.1021/acs.jnatprod.8b00632. [DOI] [PubMed] [Google Scholar]
- 76.Fougeyrollas R., Křivánek J., Roy V., Dolejŝová K., Frechault S., Roisin Y., Hanus R., Sillam-Dussès D. Asexual queen succession in termites. Mol. Ecol. 2017;26:3295–3308. doi: 10.1111/mec.14095. [DOI] [PubMed] [Google Scholar]
- 77.Rukachaisirikul V., Pramjit S., Pakawatchai C., Isaka M., Supothina S. 10-Membered macrolides from the insect pathogenic fungus Cordyceps militaris BCC 2816. J. Nat. Prod. 2004;67:1953–1955. doi: 10.1021/np0401415. [DOI] [PubMed] [Google Scholar]
- 78.Sudina P.R., Motati D.R., Seema A. Stereocontrolled total synthesis of nonenolide. J. Nat. Prod. 2018;81:1399–1404. doi: 10.1021/acs.jnatprod.8b00001. [DOI] [PubMed] [Google Scholar]
- 79.Lin P.-C., Wu Y.-Z., Bao T.-W., Wang Y.-N., Shang X.-Y., Lin S. A new cytotoxic 12-membered macrolactone from the endophytic fungus Exserohilum rostratum LPC-001. J. Asian Nat. Prod. Res. 2018;20:1093–1100. doi: 10.1080/10286020.2018.1518322. [DOI] [PubMed] [Google Scholar]
- 80.Richter J., Sandjo L.P., Liermann J.C., Opatz T., Erkel G. 4-Dechloro-14-Deoxy-Oxacyclododecindione and 14-deoxy-oxacylododecindione, two inhibitors of inducible connective tissue growth factor expression from the imperfect fungus Exserohilum rostratum. Bioorg. Med. Chem. 2015;23:556–563. doi: 10.1016/j.bmc.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 81.Weber C., Vierengel N., Walter T., Behrendt T., Lucas T., Erkel G., Opatz T. Total synthesis and biological evaluation of seven new AntiInflammatory oxacyclododecindione-type macrolactones. Org. Biomol. Chem. 2020;18:5906–5917. doi: 10.1039/D0OB00958J. [DOI] [PubMed] [Google Scholar]
- 82.Seipp K., Groß J., Kiefer A.M., Erkel G., Opatz T. Total synthesis and biological evaluation of the AntiInflammatory (13R,14S,15R)-13-Hydroxy-14-deoxyoxacyclododecindione. J. Nat. Prod. 2023;86:924–938. doi: 10.1021/acs.jnatprod.2c01145. [DOI] [PubMed] [Google Scholar]
- 83.Thomas S., Sharma N., Gonzalez R., Pao P.-W., Hofman F.M., Chen T.C., Louie S.G., Pirrung M.C., Schönthal A.H. Repositioning of verrucosidin, a purported inhibitor of chaperone protein GRP78, as an inhibitor of mitochondrial electron transport chain complex I. PLoS One. 2013;8:1–14. doi: 10.1371/journal.pone.0065695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chojnacka M.W., Batey R.A. Total synthesis of (+)-Prunustatin A: utility of organotrifluoroborate-mediated prenylation and Shiina MNBA esterification and macrolactonization to avoid a competing Thorpe−Ingold effect accelerated transesterification. Org. Lett. 2018;20:5671–5675. doi: 10.1021/acs.orglett.8b02396. [DOI] [PubMed] [Google Scholar]
- 85.Numata A., Iritani M., Yamada T., Minoura K., Matsumura E., Yamori T., Tsuruo T. Novel antitumor metabolites produced by a fungal strain from a sea hare. Tetrahedron Lett. 1997;38:8215–8218. doi: 10.1016/S0040-4039(97)10198-8. [DOI] [Google Scholar]
- 86.Tatsuta K., Tsuchiya T., Mikami N., Umezawa S., Umezawa H., Naganawa H. KD16-U1, A new metabolite of Streptomyces: isolation and structural studies. J. Antibiot. 1974;27:579–586. doi: 10.7164/antibiotics.27.579. [DOI] [PubMed] [Google Scholar]
- 87.Biduś N., Banachowicz P., Buda S. Application of a tandem seleno-michael/aldol reaction in the total syntheses of (þ)-Pericosine B, (þ)-Pericosine C, (þ)-COTC and 7- chloro-analogue of (þ)-Gabosine C. Tetrahedron. 2020;76:1–8. doi: 10.1016/j.tet.2020.131397. [DOI] [Google Scholar]
- 88.Oberlies N.H., Croy V.L., Harrison M.L., McLaughlin J.L. The annonaceous acetogenin bullatacin is cytotoxic against multidrug-resistant human mammary adenocarcinoma cells. Cancer Lett. 1997;115:73. doi: 10.1016/S0304-3835(97)04716-2. [DOI] [PubMed] [Google Scholar]
- 89.Kunkalkar R.A., Fernandes R.A. A protecting-group-free total synthesis of chatenaytrienin-2. J. Org. Chem. 2019:1–7. doi: 10.1021/acs.joc.9b01952. [DOI] [PubMed] [Google Scholar]
- 90.Reinink P., Buter J., Mishra V.K., Ishikawa E., Cheng T.-Y., Willemsen P.T.J., Porwollik S., Brennan P.J., Heinz E., Mayfield J.A., Dougan G., van Els C.A., Cerundolo V., Napolitani G., Yamasaki S., Minnaard A.J., McClelland M., Moody D.B., Van Rhijn I. Discovery of trehalose phospholipids reveals functional convergence with mycobacteria. J. Exp. Med. 2019;216:757–771. doi: 10.1084/jem.20181812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mishra V.K., Buter J., Blevins M.S., Witte M.D., Rhijn I.V., Moody D.B., Brodbelt J.S., Minnaard A.J. Total synthesis of an immunogenic trehalose phospholipid from Salmonella Typhi and elucidation of its sn-regiochemistry by mass spectrometry. Org. Lett. 2019;21:5126–5131. doi: 10.1021/acs.orglett.9b01725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bahadur B., Tilton M. Liquid crystals – applications and uses. J. Soc. Inf. Disp. 1994;63:1–2. doi: 10.1889/1.1984911. [DOI] [Google Scholar]
- 93.Bhat S.V., Swamynathan K., Kumar S. Synthesis and mesomorphic characterization of novel liquid crystals derived from bioactive natural sterols. J. Mole. Liquids. 2021;326 doi: 10.1016/j.molliq.2020.115216. [DOI] [Google Scholar]
- 94.Shelton J., Lu X., Hollenbaugh J.A., Cho J.H., Amblard F., Schinazi R.F. Metabolism, biochemical actions, and chemical synthesis of anticancer nucleosides, nucleotides, and base analogs. Chem. Rev. 2016;116 doi: 10.1021/acs.chemrev.6b00209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bracegirdle J., Gordon D.P., Harvey J.E., Keyzers R.A. Kinase-inhibitory nucleoside derivatives from the pacific bryozoan Nelliella nelliiformis. J. Nat. Prod. 2020;83:547–551. doi: 10.1021/acs.jnatprod.9b01231. [DOI] [PubMed] [Google Scholar]
- 96.Ma Y.-M., Liang X.-A., Kong Y., Jia B. Structural diversity and biological activities of indole diketopiperazine alkaloids from fungi. J. Agric. Food Chem. 2016;64:6659–6671. doi: 10.1021/acs.jafc.6b01772. [DOI] [PubMed] [Google Scholar]
- 97.Bai H., Cui P., Zang C., Li S. Enantioselective total synthesis, divergent optimization and preliminary biological evaluation of (indole-N-alkyl)-Diketopiperazines. Bioorg. Med. Chem. Lett. 2019;29 doi: 10.1016/j.bmcl.2019.126718. [DOI] [PubMed] [Google Scholar]
- 98.Zaghouani M., Bögeholz L.A.K., Mercier E., Wintermeyer W., Roche S.P. Total synthesis of (±)-Fumimycin and analogues for biological evaluation as peptide deformylase inhibitors. Tetrahedron. 2019;75:3216–3230. doi: 10.1016/j.tet.2019.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wender P.A., Sloane J.L., Luu-Nguyen Q.H., Ogawa Y., Shimizu A.J., Ryckbosch S.M., Tyler J.H., Hardman C. Unlocking the drug potential of the bryostatin family: recent advances in product synthesis and biomedical applications. J. Org. Chem. 2020;85:15116–15128. doi: 10.1021/acs.joc.0c01988. [DOI] [PubMed] [Google Scholar]
- 100.Wood J.M., Juńior E.N., da S., Bower J.F. Rh-catalyzed [2 + 2 + 2] cycloadditions with benzoquinones: de novo access to naphthoquinones for lignan and type II polyketide synthesis. Org. Lett. 2019;22:265–269. doi: 10.1021/acs.orglett.9b04266. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in the article and referenced in article. No data associated with this study has been deposited into a publicly available repository.