Abstract
Introduction
Aspergillus endocarditis is a rare fungal infection associated with a poor prognosis. Most cases of Aspergillus endocarditis involve prosthetic valves, with native valve involvement being rarely reported.
Case presentation
A 53-year-old asian female patient presented with fever, chills, dyspnea, generalized fatigue, and significant weight loss one month after undergoing left lower lobectomy for a pulmonary abscess. Echocardiogram showed a large mobile vegetation with a broad base on the anterior leaflet of the mitral valve, resembling atrial myxoma. Despite negative blood cultures, circulating DNA of Aspergillus fumigatus was detected by metagenome Next Generation Sequencing, prompting the initiation of empiric antifungal therapy with voriconazole. Emergency surgery, involving thorough debridement and mitral valve replacement, was successfully performed. Indefinite fungal suppression therapy with oral voriconazole is continued to mitigate the risk of recurrence. The patient survived with no signs of Aspergillus disease recurrence for four years.
Clinical discussion
Diagnosis of Aspergillus endocarditis requires a high index of suspicion and is often delayed due to consistently negative results from blood cultures. Non-culture-based methods, particularly metagenome Next-Generation Sequencing, play a crucial role in early diagnosis and therapeutic decision-making. Surgical debridement and valve replacement are imperative for survival in cases of Aspergillus endocarditis. Voriconazole should be considered the primary fungicidal agent for its treatment. Moreover, lifelong fungal suppression therapy is strongly recommended for all survivors to ensure long-term survival and minimize the risk of recurrence.
Conclusion
Despite grim prognosis associated with Aspergillus endocarditis, patients can attain long-term survival through meticulous surgical debridement and lifelong antifungal therapy.
Keywords: Aspergillus endocarditis, Next-generation sequencing, Antifungal therapy, Case report
Highlights
-
•
Aspergillus endocarditis with native valve involvement is seldom reported in the literature.
-
•
The vegetation exhibits a large mobile mass with a broad base and a smooth pseudocapsule, resembling atrial myxoma.
-
•
Metagenome Next-Generation Sequencing may play a pivotal role in early diagnosis and therapeutic decision-making.
-
•
Lifelong fungal suppression with Voriconazole is recommended for all survivors to mitigate the risk of recurrence.
1. Introduction
Fungal microorganisms are uncommon culprits, responsible for 2–4 % of all cases of infective endocarditis [1]. Among these, Aspergillus spp. accounts for 24 % of all fungal endocarditis cases, with Aspergillus fumigatus being the most prevalent species [2]. While most cases of Aspergillus endocarditis involve prosthetic valves, reports of native valve involvement are exceedingly rare [1]. Herein, we presented a case of native valve fungal endocarditis caused by Aspergillus fumigatus, the patient was successfully treated through a combination of surgical debridement and antifungal therapy. This case report has been presented in accordance with the SCARE Criteria [3].
2. Case report
A 53-year-old asian female patient was admitted to a district hospital due to a chronic cough with purulent sputum persisting for two months. Chest computed tomography (CT) scan showed a giant pulmonary abscess in the left lower lobe. The patient was HIV negative, other potential risk factors including intravenous drug use, immunosuppression, and history of hematopoietic stem cell or solid organ transplantation were also absent. The patient underwent thoracoscopic lobectomy, and tissue culture of the resected lung yielded negative results. Her recovery was uneventful, and she was discharged on the tenth Postoperative Day (POD). However, one month after hospital discharge, the patient became febrile with shortness of breath and palpitation. She was promptly transferred to a local hospital, where initial empiric antibiotics including cefuroxime, vancomycin, and imipenem were administered, but the fevers persisted. Multiple sets of blood cultures were obtained, but all remained negative. An echocardiogram revealed the presence of “a large vegetation with severe mitral regurgitation”. Consequently, the patient was transferred to our hospital for further evaluation and treatment.
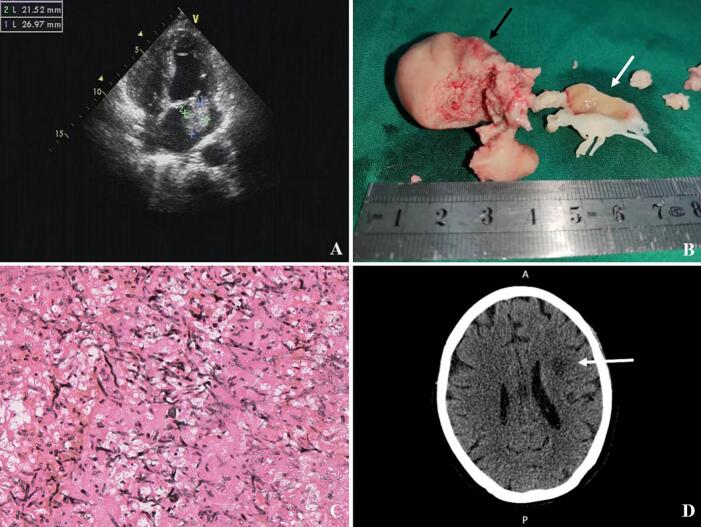
On admission, the patient complaint of fever, chills, dyspnea, generalized fatigue and a 5 kg weight loss over a 4-week period. Her body temperature was 39.2 °C, with pulse rate of 110 beats per minute, blood pressure of 95/65 mmHg, and respiratory rate of 20 breaths per minute. Physical examination revealed a systolic murmur loudest at the apex and radiating to the left axilla. There were no peripheral signs of endocarditis. Laboratory evaluation showed a high white blood cell count of 18 × 109/L with 90 % neutrophils and a low hemoglobin level of 7.8 g/dl. Liver enzymes, serum creatinine, and electrolytes were within normal ranges. Serum β (1,3)-D-glucan was significantly elevated at 1248.4 pg/ml, while serum procalcitonin remained normal at 0.04 ng/ml. Due to the negative results of previous blood cultures, metagenome Next Generation Sequencing (NGS) of peripheral blood was performed, which revealed that 176 out of 2,284,907 reads from the specimen mapped to the genome of Aspergillus fumigatus. Additionally, 4 and 3 reads corresponded to Arcanobacterium haemolyticum and Serratia marcescens, respectively. Transthoracic echocardiogram indicated the presence of a large broad-based vegetation (2.2 × 2.7 cm in diameter) on the anterior leaflet of the mitral valve, accompanied by severe eccentric mitral regurgitation (see Fig. 1A). Transesophageal echocardiogram captured a clear view of the reciprocating movement of the mass through the mitral valve orifice, resembling atrial myxoma (Video 1). The patient was promptly initiated on empiric antifungal therapy with intravenous voriconazole at a dose of 200 mg every 12 h.
Fig. 1.
A:Preoperative transthoracic echocardiogram showing a large vegetation in the left atrium. B: Surgical extracted specimens: Huge fragile vegetation with smooth psuedocapsule (black arrow), Infected anterior leaflet of mitral valve (white arrow). C: Histopathology of mitral vegetation showing tissue invasion by massive branching fungal hyphae (GMS staining, ×40). D:Brain CT showing ill-defined hypodensities suspicious for acute infarction of the corona radiata adjacent to the left lateral ventricle (white arrow).
Emergent surgical removal of the vegetation and mitral valve replacement was performed for this patient. Intraoperative exploration demonstrated large fragile vegetation with smooth pseudocapsule located on the anterolateral commissure and A1 area of the mitral valve. Obvious swelling and purulent effusions were detected on the entire anterior leaflet, chordae tendineae and both papillary muscles (see Fig. 1B). Extensive resection of the infected valve and thorough debridement of the vegetations were successfully carried out, preserving the posterior mitral leaflet and subvalvular structures. A 27-mm Carbomedics standard mechanical valve was implanted. Postoperatively, antifungal therapy was continued with intravenous voriconazole and Caspofungin (50 mg/day, following a loading dose of 70 mg). Operative histopathology revealed tissue invasion by branching fungal hyphae (see Fig. 1C), and the surgical specimen culture confirmed the presence of Aspergillus fumigatus. The patient was extubated on POD 2. On POD 4, she developed moderate right-sided hemiparesis. A brain CT scan revealed an acute ischemic infarction of the corona radiata adjacent to the left lateral ventricle, most likely caused by septic emboli (see Fig. 1D). As the ischemic lesion was primarily focal, additional invasive assessments such as cerebral angiography or thrombus aspiration were not conducted. Standard medical treatment, including warfarin and antifungal agents, was administered. The patient's fever rapidly subsided, and she was discharged on POD 17, with continued indefinite suppression of Aspergillus using oral voriconazole at a dose of 200 mg twice a day.
The patient consistently adhered to regular annual follow-ups for 4 years, during which time she continued to show significant improvement in her right-sided weakness and a noticeable increase in body weight. There were no indications of Aspergillus disease recurrence. However, due to economic constraints, the oral voriconazole dosage was reduced to 200 mg per day starting from the second year post-surgery, and it has been maintained indefinitely to ensure lifelong fungal suppression.
3. Discussion
Diagnosis of Aspergillus endocarditis requires high index of suspicion and is frequently delayed, with the diagnosis being confirmed postmortem in up to one-third of cases [4]. Typical clinical indicators encompass the presence of a large valve vegetation, early onset of systemic embolic events, and resistance to empiric broad-spectrum antibiotic therapy. The identification of the pathogen typically relies on tissue cultures obtained from cardiac specimens, as blood cultures almost invariably yield negative results [5]. Non-culture-based methods have been developed to facilitate early detection of the fungus in the bloodstream. These methods include the β-D-glucan test, the galactomannan (GM) test, real-time polymerase chain reaction (PCR) targets, and Next-Generation Sequencing (NGS) [6]. β-D-glucan is a common component of the outer cell walls of many fungal species, thus not specific to Aspergillus. In contrast, galactomannan is a major constituent of the Aspergillus cell wall. It is released into the host's serum during hyphal growth and serves as a highly sensitive biomarker for invasive aspergillosis. However, it is noteworthy that false-positive serum GM results can occur in non-Aspergillus fungal diseases [[7], [8], [9]] and in cases where certain antibiotics are concurrently administered [10,11]. Compared to the GM test, serum PCR targeting Aspergillus DNA demonstrates greater specificity and a higher positive predictive value (79.6 % vs 27 %) [12]. The accurate species information provided by metagenome NGS is of considerable value for guiding the selection of appropriate antifungal agents and facilitating early decisions pertaining to surgical intervention.
Optimal management of Aspergillus endocarditis requires combination of both surgical debridement and antifungal therapy [13]. Surgical intervention plays a pivotal role in eradication of bulky vegetations and mitigating the risk of relapse and embolic events [14]. Notably, the mortality rate can reach up to as high as 96 % among patients treated solely with medical therapy [5]. With the landmark trial published in 2002, voriconazole was established as the primary fungicidal agent for invasive aspergillosis. Voriconazole demonstrated superior responses, improved survival rates, and fewer severe side effects compared to the standard therapy involving amphotericin B [15]. Alongside voriconazole, lipid formulation of amphotericin B is also recommended for initial antifungal therapy according to current guidelines delineated by the Infectious Diseases Society of America (IDSA) [16].
Endocarditis caused by Aspergillus species presents the most unfavorable prognosis among all fungal organisms. Ellis et al. reported a mortality rate of 63 % (41 out of 65 cases) for Aspergillus endocarditis cases during 1965–1995 [17]. Shockingly, the mortality rate soared to 68 % (24 out of 35 cases) for patients diagnosed between 2003 and 2009, despite remarkable advancements in surgical techniques and the advent of new antifungal medications [4]. One of the primary concerns for survivors of fungal endocarditis is the considerable risk of relapse, with an overall recurrence rate of 30 % [17]. Notably, the incubation period for relapse of Aspergillus fumigatus endocarditis can extend up to 20 years [18]. It is hypothesized that the residual Aspergillus may persist in a quiescent state, forming protective biofilms to evade both humoral and cellular immune responses while developing resistance to antifungal agents. Hence, it is strongly recommended that survivors of Aspergillus endocarditis undergo lifelong fungal suppression therapy to mitigate the risk of recurrence.
4. Conclusion
Despite the grim prognosis associated with Aspergillus endocarditis, patients can attain long-term survival through meticulous surgical debridement and lifelong antifungal therapy.
The following is the supplementary data related to this article.
Intraoperative transoesophageal echocardiogram showing the mobile nature of the giant vegetation, resembling atrial myxoma.
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Ethical approval
Ethical approval for this study was provided by the Ethical Committee of Guangdong Provincial People's Hospital. Reference number: KY2020-610-01.
Funding
This work was supported by the National Natural Science Foundation of China (82200518), Outstanding Young Talent Trainee Program of Guangdong Provincial People's Hospital (KY012023331), Science and Technology Projects in Guangzhou (2023A04J0490), and Guangdong Provincial Medical Science and Technology Research Fund Project (A2022140). The funders had no role in study design, data collection, data analysis, data interpretation, and the writing of the report.
Author contribution
Xin Zang: surgeon, conceptualization, data collection and analysis, writing the paper.
Jin-Lin Wu: conceptualization, data collection and analysis.
Xiao-Dong Zeng: conceptualization, data collection and analysis.
Jian Liu: conceptualization, data collection and analysis.
Hui-Ming Guo: surgeon, conceptualization and supervision.
Ji-Mei Chen: conceptualization and supervision.
Guarantor
Xin Zang accepts full responsibility for the work, had access to the data, and controlled the decision to publish.
Research registration number
Not applicable.
Conflict of interest statement
All authors declare no conflicts of interest regarding the publication of this paper.
References
- 1.Gould F.K., Denning D.W., Elliott T.S., et al. Guidelines for the diagnosis and antibiotic treatment of endocarditis in adults: a report of the working party of the British Society for antimicrobial chemotherapy. J. Antimicrob. Chemother. 2012;67(2):269–289. doi: 10.1093/jac/dkr450. [DOI] [PubMed] [Google Scholar]
- 2.Baddour L.M., Wilson W.R., Bayer A.S., et al. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation. 2015;132(15):1435–1486. doi: 10.1161/CIR.0000000000000296. [DOI] [PubMed] [Google Scholar]
- 3.Agha R.A., Franchi T., Sohrab C., Mathew G., Kirwan A., Thomas A., et al. The SCARE 2020 guideline: updating consensus surgical case report (SCARE) guidelines. Int. J. Surg. 2020;84:226–230. doi: 10.1016/j.ijsu.2020.10.034. [DOI] [PubMed] [Google Scholar]
- 4.McCormack J., Pollard J. Aspergillus endocarditis 2003-2009. Med. Mycol. 2011;49(Suppl. 1):S30–S34. doi: 10.3109/13693786.2010.498449. [DOI] [PubMed] [Google Scholar]
- 5.Kalokhe A.S., Rouphael N., El C.M., et al. Aspergillus endocarditis: a review of the literature. Int. J. Infect. Dis. 2010;14(12):e1040–e1047. doi: 10.1016/j.ijid.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 6.Ammannaya G., Sripad N. Fungal endocarditis: what do we know in 2019? Kardiol. Pol. 2019;77(7–8):670–673. doi: 10.33963/KP.14869. [DOI] [PubMed] [Google Scholar]
- 7.Wheat L.J., Hackett E., Durkin M., et al. Histoplasmosis-associated cross-reactivity in the BioRad Platelia aspergillus enzyme immunoassay. Clin. Vaccine Immunol. 2007;14(5):638–640. doi: 10.1128/CVI.00479-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dalle F., Charles P.E., Blanc K., et al. Cryptococcus neoformans Galactoxylomannan contains an epitope(s) that is cross-reactive with aspergillus galactomannan. J. Clin. Microbiol. 2005;43(6):2929–2931. doi: 10.1128/JCM.43.6.2929-2931.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fekkar A., Brun S., D’Ussel M., et al. Serum cross-reactivity with aspergillus galactomannan and cryptococcal antigen during fatal disseminated Trichosporon dermatis infection. Clin. Infect. Dis. 2009;49(9):1457–1458. doi: 10.1086/644499. [DOI] [PubMed] [Google Scholar]
- 10.Zandijk E., Mewis A., Magerman K., et al. False-positive results by the platelia aspergillus galactomannan antigen test for patients treated with amoxicillin-clavulanate. Clin. Vaccine Immunol. 2008;15(7):1132–1133. doi: 10.1128/CVI.00022-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Viscoli C., Machetti M., Cappellano P., et al. False-positive galactomannan platelia aspergillus test results for patients receiving piperacillin-tazobactam. Clin. Infect. Dis. 2004;38(6):913–916. doi: 10.1086/382224. [DOI] [PubMed] [Google Scholar]
- 12.Imbert S., Gauthier L., Joly I., et al. Aspergillus PCR in serum for the diagnosis, follow-up and prognosis of invasive aspergillosis in neutropenic and nonneutropenic patients. Clin. Microbiol. Infect. 2016;22(6):561–562. doi: 10.1016/j.cmi.2016.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walsh T.J., Anaissie E.J., Denning D.W., et al. Treatment of aspergillosis: clinical practice guidelines of the Infectious Diseases Society of America. Clin. Infect. Dis. 2008;46(3):327–360. doi: 10.1086/525258. [DOI] [PubMed] [Google Scholar]
- 14.Tattevin P., Revest M., Lefort A., et al. Fungal endocarditis: current challenges. Int. J. Antimicrob. Agents. 2014;44(4):290–294. doi: 10.1016/j.ijantimicag.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 15.Herbrecht R., Denning D.W., Patterson T.F., et al. Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N. Engl. J. Med. 2002;347(6):408–415. doi: 10.1056/NEJMoa020191. [DOI] [PubMed] [Google Scholar]
- 16.Patterson T.F., Thompson G.R., Denning D.W., et al. Practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2016;63(4):e1–e60. doi: 10.1093/cid/ciw326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ellis M.E., Al-Abdely H., Sandridge A., et al. Fungal endocarditis: evidence in the world literature, 1965-1995. Clin. Infect. Dis. 2001;32(1):50–62. doi: 10.1086/317550. [DOI] [PubMed] [Google Scholar]
- 18.Escande W., Fayad G., Modine T., et al. Culture of a prosthetic valve excised for streptococcal endocarditis positive for aspergillus fumigatus 20 years after previous A fumigatus endocarditis. Ann. Thorac. Surg. 2011;91(6):e92–e93. doi: 10.1016/j.athoracsur.2011.01.102. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Intraoperative transoesophageal echocardiogram showing the mobile nature of the giant vegetation, resembling atrial myxoma.