Summary
Carbon capture, utilization, and storage (CCUS) technology is widely recognized as a key solution for mitigating global climate change. Consequently, it has received significant attention from countries worldwide. However, carbon dioxide corrosion poses a significant challenge to CCUS and represents a bottleneck to the large-scale development and application of this technology. To mitigate this issue, this review starts with a discussion of corrosion problems in CCUS. Later, the fundamentals of the carbon dioxide corrosion mechanism are introduced. Then, the influences of various factors that affect the corrosion are highlighted, such as water content, pH, flow rate, etc. Afterward, we summarize the commonly used methods for corrosion protection, with a particular focus on inhibitor, given their eco-friendly and effective nature. Lastly, challenges and prospects are discussed to motivate future studies on developing novel, high-performance green inhibitor and studying the corresponding protection mechanisms, hoping to make some contributions to carbon emission reduction.
Subject areas: Chemistry, chemical engineering, corrosion
Graphical abstract

Chemistry; Chemical engineering; Corrosion
Introduction
As the use of fossil fuels continues, CO2 emissions are increasing significantly. Moreover, fossil fuels remain the predominance of energy source consumption for at least in the next 30 years, although renewable energy technologies such as solar and wind energy have obtained the remarkable growth.1 As a result, the energy-related CO2 emissions will continue to increase. This trend will lead to more severe greenhouse effects, resulting in a rise in global average temperature, sea level, and rapid climate changes,2 which are major contributors to global warming, posing a significant threat to our planet’s health. Reducing CO2 emissions is crucial in mitigating the effects of climate change and protecting our planet’s fragile ecosystem. To combat this issue, the countries all over the world are vigorously advocating the implementation of various measures for energy conservation and emission reduction, with a particular focus on carbon capture, utilization, and storage (CCUS).3 CCUS technology is highly anticipated as a key solution in the quest for carbon neutrality.4 It is recognized as an effective approach to reduce CO2 emissions and holds the potential to significantly mitigate global warming.5 In fact, the related technology of CCUS has already been used for enhanced oil recovery in the oil fields for many years. The combination of CCUS and the fossil fuel power plant can achieve a clean, reliable, and sustainable energy supply. Furthermore, CCUS has brought the additional advantage of potential energy and resource recovery by employing CO2 as the raw material. Hence, CCUS technology is promising to serve as the foundation for constructing a zero or negative carbon emission system, presenting a prospective pathway toward mitigating carbon emissions and realizing a sustainable future.6,7,8 A large number of anthropogenic CO2 emissions mainly arise from carbon-intensive sources including fossil fuel power plants, oil refineries, cement industry, iron & steel industry, non-ferrous metals manufacturing, and building materials production, as well as the petrochemical and chemical industries.9 Therefore, the large-scale application of CCUS in these industries is very imperative. In the whole CCUS chain (Figure 1), CO2 is captured from carbon-intensive sources, compressed and purified, and then transported (usually in supercritical state) to an appropriate site for sequestration and permanent storage or to specific places for utilization in the enhanced oil recovery and converting the captured CO2 into valuable products.10 Corrosion issues tend to occur in every aspect of CCUS. Hence, the corrosion is a serious challenge in safety aspect for the whole CCUS.
Figure 1.
Main constituent parts of CCUS.
CO2 capture technology is the key part in CCUS and the most expensive and energy-intensive technical part. Currently, there are various CO2 capture technologies available, including physical absorption, chemical absorption, pressure swing adsorption, temperature swing adsorption, membrane separation, cryogenic separation, and others. Pre-combustion capture converts captured gas components into H2 and CO2 through chemical reaction. Post-combustion capture is to purify the flue gas first and then install a CO2 separation and capture device in the flue gas channel (Figure 2).11,12,13,14 Among various methods, the alcohol amine method is susceptible to corrode carbon steel. , H3O+, and protonated amine are the root causes of the corrosivity of the ethanolamine solution.15 When CO2 is absorbed, the precipitated dissociates throughout the absorption system along with the absorption liquid, leading to overall and localized corrosion of equipment. During the capture process, any contamination or impurities can lead to corrosion.
Figure 2.
Carbon capture technology roadmap.
It is worth noting that it is only a wasted resource if the collected CO2 cannot be efficiently and safely transported to the suitable storage locations or end use for producing the valuable chemicals. The transportation of CO2 can be implemented by rail, trucks, and ships (liquid phase) or via the pipeline (dense phase) in terms of the CO2 phase. Compared to CO2 capture process, the issue of CO2 corrosion during transportation should be even more not allowed to overlook.
Corrosion not only depletes resources but also leads to substantial economic losses. According to the reports of corrosion cost and preventive strategies in the United States, the annual direct cost incurred by corrosion is a staggering $276 billion, accounting for approximately 3.1 percent of the United States' gross domestic product in 1998.16 Additionally, the China Corrosion Survey Report also highlights that corrosion results in annual economic losses equivalent to approximately 5% of China’s gross domestic product. Hence, CO2 corrosion poses a significant challenge for the large-scale application of CCUS. However, by employing suitable material selection, corrosion monitoring, and preventive measures, and through continuous research and innovation, this issue can be effectively mitigated, ensuring the reliability and continuous operation of CCUS systems. Hence, it is crucial to investigate and analyze the formation mechanism and influencing factors.
Previous research primarily focuses on corrosion mechanism, the selection of corrosion-resistant materials, and the impact of impurities and corrosion behavior in supercritical CO2.17,18,19,20,21,22,23,24,25,26 Mubarak et al.27 concerned about the corrosion of oil and gas well casing and tubing and corrosion mitigation techniques. Obot et al.28 studied the challenges and progress of corrosion inhibitor testing under extreme conditions in the oil and gas industries. Zhang et al.29 discussed the superhydrophobic anti-corrosion coatings for metallic materials. Li et al.30 comprehensively elucidated corrosion-related behavior of alloys in supercritical CO2 environments. Kairy et al.31 reviewed corrosion of pipeline steel in dense-phase CO2 containing impurities. Sheetal et al.32 concluded corrosion inhibitor system through N-heterocyclic compounds.
The research and reviews of corrosion have largely focused on oil and gas industries, prevention measures, and corrosion-related behavior and mechanism independently, while few summarize these aspects together. In practice, understanding the mechanism can help better study how to inhibit corrosion. This work systematically reviews the recent research on corrosion in CO2 systems and its influencing factors, as well as anti-corrosion methods, with a particular focus on the application of corrosion inhibitor. Firstly, we give an overview of CO2 corrosion in CCUS. After that, corrosion mechanisms that have been widely accepted and new mechanism proposed in recent years will be provided. Plus, the research on influencing factors of CO2 corrosion is summarized based on recent studies. Then, the research of anti-corrosion technology in CCUS is discussed and demonstrated. Finally, the future research tendency and direction will be proposed. This study provides a thorough review of the literature to make some contribution to the action of carbon reduction. The anti-corrosion technology has a far-reaching influence and holds great potential to make breakthrough in CO2 reduction. The purpose of this paper is to arouse people’s attention to CO2 corrosion; otherwise, corrosion will become an obstacle to the large-scale application of CCUS. Looking forward to this paper can provide some salutary views for future investigations of CO2 corrosion mechanism and anti-corrosion measures and as well promote related scholars to achieve better improvements and extensive industrial application of CCUS.
Carbon dioxide corrosion mechanism
To better mitigate CO2 corrosion, it is essential to comprehend the corrosion mechanisms associated with CO2. This section focuses on the corrosion mechanism of CO2. Here, we laid out some possible reaction mechanisms that have been put forward.
It is well known that CO2 gas is non-corrosive. However, once CO2 combines with water to form carbonic acid, it can corrode metals. CO2 increases the corrosion rate of metals owing to the occurrence of cathodic reaction—hydrogen evolution. The carbonic acid solution undergoes an electrochemical reaction with the metal, and the reaction process is given in the following.33
The overall reaction is
| (Equation 1) |
Anodic reaction is
| (Equation 2) |
Cathodic reaction is
| (Equation 3) |
| (Equation 4) |
| (Equation 5) |
| (Equation 6) |
The corrosion process of CO2 on the metal surface in the water-rich environment involves the formation of a Fe3C framework within the steel substrate during the initial dissolution stage (Figure 3B), and the FeCO3 grains deposited on the surface (Figure 3C); then and diffused inwards and reacted with the steel matrix to form the intermediate layer and the inner layer (Figure 3D).
Figure 3.
Schematic diagram of CO2 corrosion process on carbon steel surface.9
The layer of iron carbonate corrosion products affects most of the anodic reactions, but the cathodic reaction is not affected too much. Due to the presence of minor phases, making the corrosion product layer becomes an electronic conductor.34
As mentioned earlier, CO2 is non-corrosive, stable, and inert, and therefore CO2 itself does not pose a threat to metal part of CCUS. However, because of the fuels and capture technologies used for different industrial CO2 emitters, the collected CO2 including CO2 streams at any process of the CCUS chain always more or less contain certain number of impurities, such as H2O, O2, H2S, SO2, SO3, NO2, and organics. The existence of these impurities will induce serious corrosion problem to metal part of CCUS chain. Hence, it is very necessary to ascertain the influences of impurities on the corrosion. Moreover, the in-depth understanding of CO2 corrosion mechanism is a requisite for further seeking the effective protection measures of CO2 corrosion.
Direct reduction of carbonic acid
Bockris et al.35 suggested that, under CO2 conditions, the intermediate medium causing Fe → Fe2+ (FeCO3) is OH−. Dewaard and Milliams36 investigated the mechanism of CO2 corrosion very early on. They measured the relationship between the corrosion rate of steel in carbonic acid and the partial pressure of CO2 and concluded that the corrosion rate may be related to the concentration of undissolved carbonic acid rather than the concentration of dissociated acid (pH). In this regard, they proposed a mechanism of cathodic reaction in line with this proposal: after initial adsorption on the metal surface, the non-dissociated carbonic acid molecules are directly reduced. The research findings of Ogundele et al.37 and Linter et al.38 closely align with the mechanism. Wiȩckowski et al.39 further confirmed this mechanism through the research results of cyclic voltage of static. In addition, they also observed the direct (activated) reduction of both H2CO3 molecules and ions.
For example, in environments containing CO2/O2 solution, CO2 combines with water to form H2CO3, which then ionizes to produce , H+, and . This leads to H+ reaction with the matrix Fe to generate Fe2+. Subsequently, Fe2+ and combine on the metal surface, resulting in the formation of a dense FeCO3 layer (Figure 4A). In the presence of both O2 and CO2, the metal surface becomes coated with a dense and reducible FeCO3 layer. Over time, FeCO3 gradually converts to Fe3+ in the presence of O2, thus forming porous Fe2O3 (Figure 4B). Thereafter O2 infiltrates into the product film, resulting in the formation of Fe2O3, which disrupts the originally dense FeCO3 layer on the surface (Figure 4C). During the conversion of FeCO3 crystal to Fe2O3, micro-pores are formed (Figure 4D). Because of the lower pH relative to the surrounding solution, metal ions undergo hydrolysis and split into H+ within these micro-pores. The acidified environment in the micro-pores intensifies the dissolution of FeCO3 and leads to the generation of CO2.
Figure 4.
Damage mechanism of CO2 product film by O240
(A) Formation of dense FeCO3.
(B) Formation of porous Fe2O3: 4FeCO3+O2→2Fe2O3+4CO2.
(C) O2 permeates and destroys FeCO3 layer.
(D) Formation of dome-like structure.
(E) Formation of oxygen concentration cell.
(F) Break of thin part of dome-like film top.
However, the H2 generated from the cathodic polarization reaction of H+ remains trapped in the gaps. The presence of the corrosion product film above the gaps hinders the escape of these gases, resulting in an elevated pressure within the gaps and the formation of a dome-like structure in the product film (Figure 4D). Within the micro-pore, the cathode reaction continues to consume oxygen, leading to a decrease in oxygen concentration and the establishment of an oxygen concentration cell (Figure 4E). The region with lower oxygen concentration beneath the film serves as the anode, while the area with higher oxygen concentration on the substrate surface functions as the cathode. The anode reaction leads to an increase in Fe2+ concentration, and the hydrolysis of Fe2+ further amplifies the acidity within the micro-pore. The reduced pH accelerates metal dissolution, resulting in crevice or local corrosion. The thin portion of the dome-like film on top ruptures, releasing gas and Fe2+ into the solution due to the continued pressure buildup within the micro-pore (Figure 4F). At this stage, the damaged film gap, along with other substrate surfaces, exhibits a galvanic effect, causing FeCO3 to infiltrate the active corrosion area and deposit in the micro-pore region beneath the film.
But Nesic et al.41 pointed out that some of the basic assumptions of the direct reduction mechanism in the previous article35 were problematic; because the first-order reaction of OH− was reported only in the context of pH < 4, so it is not reliable for Dewaard and Milliams to directly infer higher pH. They observed different anodic mechanisms for pH < 4 and for pH > 5. In the intermediate area there seems to be a transition from one mechanism to another. Furthermore, Nesic et al.42 confirmed that at pH 5 the reduction of H2CO3 dominated as the cathodic reaction and summarized a reaction. This reaction is characterized by a limiting current, which is governed by a slow chemical step, namely, the hydration of CO2. When the current exceeds the limiting current for H+ or H2CO3 reduction, the primary cathodic reaction shifts to the direct reduction of water:
| (Equation 7) |
Buffering effect
The above cathodic reaction has been widely accepted. Nevertheless, numerous scholars have put forward a new viewpoint: buffering effect.43,44,45,46,47 In those studies, it was quantitatively demonstrated that the limiting currents could be adequately explained even without considering H2CO3 as an electroactive species. This was elucidated by the homogeneous dissociation of H2CO3 inside the diffusion boundary layer, subsequently followed by the reduction of H+ that provides a parallel reaction pathway to the direct reduction of H2CO3. It becomes more comprehensible when considering the local concentration of chemical species near the metal surface. In this context, the homogeneous dissociation of H2CO3, followed by electrochemical reduction of the produced H+ ions, provides a parallel reaction pathway to the direct H2CO3 reduction reaction. This mechanism is not unique to CO2 corrosion but also exists in acetic acid corrosion of low-carbon steel.48 The aforementioned formula is as follows:
| (Equation 8) |
| (Equation 9) |
| (Equation 10) |
That suggests that carbonic acid is merely a “reservoir” of hydrogen ions and its presence only increases the observed limiting current densities by buffering the H+ concentration at the metal surface.43 Carbonic acid only affects the limiting cathodic current but has no effect on the charge transfer current, and the charge transfer current only responds to changes in pH, indicating that the reduction of hydrogen ions is the primary cathodic reaction.49 Direct reduction mechanism is based on the assumption that H2CO3 is an electrochemical reaction material and is reduced during corrosion, while buffering mechanism emphasizes that H2CO3 is uniformly decomposed. It should be stressed that the two roles of H2CO3 (as a buffer and as an electroactive categories) are not mutually exclusive; they are two entirely different and completely independent processes.46 The buffering effect plays a crucial role in the CO2 corrosion. However, these mechanisms are not mutually exclusive, and the corrosion process of CO2 may encompass all of these scenarios.
Influencing factors on carbon dioxide corrosion
CO2 corrosion is a complex electrochemical process influenced by a range of factors, including water content, pH, flow rate, temperature, pressure, Cl− concentration, oil phase environment, and so on. Table 1 shows the influencing factors and their effects.
Table 1.
Influencing factors and effects
| Influencing factors | Effect |
|---|---|
| Water content | The corrosion rate of carbon steel materials tends to accelerate when the water content in CO2 increases. |
| pH | The higher the pH is, the lower the hydrogen ion content is, and the corrosion rate of carbon steel will also be reduced. |
| Flow rate | High flow rate can accelerate the rate at which the corrosion medium reaches the surface of metal pipes, potentially generating pressure that can disrupt the initially stable and compact corrosion product film, thereby increasing the corrosion rate. |
| Temperature | High-temperature environment can promote electrochemical reaction rates and accelerate CO2 corrosion. |
| Pressure | Within a certain pressure range, the corrosive ability of carbonated water formed by CO2 and formation of water gradually increases with increasing pressure. |
| Cl− | Cl− has minimal impact on uniform corrosion but primarily affects localized corrosion, such as pitting corrosion. |
| Oil phase environment | Oil can alter the structure and chemical composition of the corrosion product film, thereby playing a role in inhibiting corrosion. |
For instance, due to the high cost of complete purification, the captured CO2 fluid usually contains corrosive impurities, which can interact with CO2, forming more corrosive compounds and exacerbating corrosion.50 Pipeline corrosion can be attributed to a variety of physical and chemical factors, including environmental conditions, materials used, and more (Figure 5). Corrosion of pipeline steel often results in decreased strength and ductility, ultimately leading to pipeline ruptures or leaks. This can bring about environmental contamination and pose safety threats to personnel and equipment.52
Figure 5.
Causes of pipeline corrosion
Figures reproduced from Ossai et al.51: Copyright 2015, Elsevier.
Another scenario occurs during ballast voyages in cargo tanks, where the entire tank is filled with inert gas, which can often become saturated with water vapor. Throughout the voyage, this water vapor can condense and absorb any remaining sulfur-containing compounds, along with CO2 and nitrous oxides, giving rise to the formation of diverse acids that aggressively attack the steel, posing a threat to both the environment and the safety of the crew.16
Water content
Water content is the key factor in metal corrosion. As widely recognized, carbon steel does not corrode in the presence of dry CO2. The variation of the corrosion rates increases with increasing relative humidity.53 Excessive water content will lead to electrochemical corrosion, which will seriously corrode the pipeline, shorten the service life of the pipeline, and cause economic losses. In s-CO2 containing with SO2 environment, reducing the water content is a more effective solution than reducing the sulfur dioxide content to mitigate corrosion.54
In s-CO2 transportation pipeline, the corrosion rate increases with the increase of water content, and exist the critical water content. In oil pipeline, when the water content is higher than 50%, the corrosion rate is significantly enhanced, and the corrosion form changes from uniform corrosion to local corrosion due to the uneven wetting of crude oil and water.55 Water solubility increases with higher temperatures and pressures within the studied range.56 In high water conditions with increased temperature, pressure, CO2 partial pressure, Ca2+, Cl−, and Mg2+ content, the corrosion products of 20# steel gradually increase. Consequently, the resulting corrosion product film becomes less compact, rendering it less effectively prevent corrosion.50 It should be noticed that the water content should be rigorously controlled when transporting CO2 at low temperatures and relatively low pressures.57 Therefore, meticulous monitoring and control of moisture content, coupled with the implementation of appropriate corrosion control measures, are of paramount importance in ensuring the long-term reliability of equipment and pipelines.
pH
It is well known that pH will not only affect the composition of corrosion products but also affect the structure of corrosion product film, thus affecting the corrosion rate. As the pH gradually increases, it reduces the concentration of H+, inhibits the cathodic reaction of H+ depolarization during the metal corrosion process, promotes the formation of an oxidation protective film on the surface of carbon steel, and consequently reduces the corrosion rate of the metal.58 Based on Figures 6A and 6B, the corrosion rate can be reduced by adding methyl diethanolamine (MDEA) as it increases the pH to reduce the solubility of FeCO3.59 Additionally, lower pH will accelerate the corrosion of Fe thin films at the presence of CO2.62
Figure 6.
Effect of pH on corrosion
(A) Potentiodynamic polarization.
(B) Variation of 1/Rp with time.
(C) Bare steel potentiodynamic polarization curves.
(D) Sulfur-coated steel potentiodynamic polarization curves.
(E) Order of reactions.
(F) Dominant cathodic reactions.
Figures reproduced from: (A and B) (Ajayi et al.59) Copyright 2021, John Wiley and Sons; (C and D) (Wang et al.60) Copyright 2021, John Wiley and Sons; (E and F) (Zhu et al.61) Copyright 2019, Springer Nature.
In CO2-Cl− medium with the presence of elemental sulfur, the deposition of elemental sulfur on the electrode surface can cause severe local corrosion, particularly under lower pH because the presence of sulfur and CO2 accelerates corrosion through direct reactions with the metal substrate or through hydrolysis to form acids.60 Figures 6C and 6D show the polarization curves of the bare electrode and sulfur-coated electrode at different pH levels. The presence of sulfides is more likely to induce surface cracking and unevenness.63 Hence, it is also essential to take into account the impact of sulfides on the corrosion process. Qin et al.64 and Fatah et al.65 also point out that sulfide will induce the generation of corrosion leading to cracking.
For low-Cr steels, the cathodic reaction is dominated by the reduction of H2CO3 and is controlled by activation when pH is greater than 5. However, at pH levels below approximately 3.5, H+ reduction becomes dominant and the reaction is controlled by diffusion.61 Figure 6E illustrates the order of reactions with respect to pH in CO2 corrosion of four low-Cr steels, representing the degree of influence of pH on the cathodic corrosion current. In this regard, it is suggested to ensure the stability of pH as much as possible, as fluctuations may lead to unexpected results. The dominant cathodic reactions at different pH levels are described in Figure 6F. Therefore, comprehending and controlling the pH are crucial factors in ensuring the long-term reliability and safety of equipment and pipelines.
Flow rate
Flow rate is also an important factor affecting CO2 corrosion rate, for it changes the dominant corrosion type from general corrosion to local corrosion.66 Generally, the corrosion rate will increase with the increase of the flow rate.67 This is mainly because the high-speed flow accelerates the transfer of ions, which hinders the formation of corrosion product film and damages the corrosion product film already formed. The increase of flow rate increased the constant phase element of the electric double layer capacitance, reduced the charge transfer resistance, and finally accelerated the corrosion rate of steel.68 However, some studies showed that the corrosion rate was reduced with the increase of flow rate.69,70
With the increase of flow rate, the corrosion current density increases and the impedance value decreases, and the migration rate of corrosive medium on the metal surface was accelerated; thus, the formation of FeCO3 on the metal surface was inhibited.58 And the concentration of Fe2+ in the solution near the steel surface is reduced, the deposition of FeCO3 is inhibited, and then the concentration of Cr(OH)3 in the corrosion film is increased, thus reducing the corrosion rate.69
Compared with static corrosion, flowing corrosion can reduce the formation of polycrystalline layer.71 Nevertheless, increasing the velocity of s-CO2 has no significant effect on the overall/local corrosion rate under various dynamic conditions.70 And increasing the CO2 gas flow rate caused a clear reduction in the CO2 corrosion; since the CO2 gas is not as corrosive as the liquid, the corrosion rate decreases by the increased gas hold up.72 Simultaneously, consideration must be given to the variation of flow rate under high-temperature and high-pressure conditions. Also, it is important to care about the phenomenon of increased gas-liquid mass transfer at high flow rates. Choosing appropriate protective measures becomes crucial in mitigating the adverse effects of high flow rates on corrosion.
Temperature
Temperature affects the morphology, grain size, and density of the corrosion product film, resulting in corrosion of the substrate to varying degrees. One of the reasons elevated temperatures can exacerbate corrosion is that they lead to higher cathodic reaction kinetics.73 However, the corrosion rate does not necessarily increase uniformly with rising temperature. As shown in Figure 7A, the corrosion rates of S135 and G105 increase at first and then decrease with the increase of temperature. In CO2/H2S coexistence system, high temperature makes the corrosion of carbon steel worse and produces high concentration of CO2 and loose corrosion product film. However, with the increase of temperature, dense and uniform FeS products are formed on the surface of steel, which can inhibit corrosion.74
Figure 7.
Effect of temperature on corrosion
(A) Corrosion rates of S135 and G105.
(B) Localized corrosion rate of 20 G steel.
(C) Evolution of corrosion rates with time.
(D1-D3) SEM cross-section micrographs of corrosion products.
Figures reproduced from: (A) (Gao et al.74) Copyright 2022, MDPI; (B) (He et al.75) Copyright 2022, Elsevier; (C and D) (Eškinja et al.76) Copyright 2022, Elsevier.
Indeed, increasing the temperature will facilitate the formation of the corrosion product film. Furthermore, in the context of a steam injection pipeline environment, the temperature’s impact on the kinetics of corrosion product film formation outweighs its influence on the thermodynamics of catalytic reactions.75 Due to the corrosion product film’s excellent anti-corrosive properties, this leads to a decrease in the corrosion rate with rising temperatures, as demonstrated in Figure 7B. Magdalena et al. also confirmed that higher temperatures (90°C) result in the formation of a thicker corrosion product film (FeCO3), which effectively reduces the corrosion rate, as depicted in Figure 7C.76 In addition, it is evident that increasing the temperature results in higher oxygen content and lower carbon content in the scale, which can account for the composition of the protective layer as shown in Figure 7D1-D3. The similar conclusion is also drawn by some scholars.77,78
Furthermore, temperature variations can also influence corrosion mechanisms. For instance, under high-temperature conditions, it may induce other corrosion mechanisms such as thermal corrosion or high-temperature hydrogen embrittlement. In summary, temperature significantly impacts the corrosion rate of CO2. In general, increasing temperature accelerates corrosion, but it also affects the formation of corrosion products. Its specific effects vary depending on environmental conditions and materials. Understanding the influence of temperature on corrosion aids in selecting appropriate materials and corrosion prevention measures, thereby enhancing engineering solutions.
Pressure
Pressure is one of the significant factors influencing CO2 corrosion, and it interacts with other factors such as temperature, CO2 concentration, and the type of metal. Generally speaking, under high-pressure conditions, CO2 is more prone to dissolve into the liquid phase, thereby increasing the concentration of CO2 in the corrosion medium and accelerating the corrosion reaction. As shown in Figures 8A–8D, a number of studies have reached similar conclusions.79,80,81 Pressure has a greater influence at a constant temperature.83 Additionally, localized pressure differentials can lead to the movement of gas-liquid interfaces, resulting in localized corrosion or pitting.
Figure 8.
Effect of pressure on corrosion
(A) Comparison of corrosion rates.
(B and C) Corrosion rates of J55 and L80 steel tubes.
(D) General and localized corrosion rates of sulfur-resistant steel.
(E) Solubility (molar ppm) of H2O in CO2.
Figures reproduced from: (A) (Tian et al.79) Copyright 2022, Elsevier; (B and C) (Ma80) Copyright 2022, Springer Nature; (D) (Wang et al.81) Copyright 2022, Elsevier; (E) (Li et al.82) Copyright 2023, Elsevier.
In s-CO2 phase, the corrosion was more serious with the increase of PH2S, while the corrosion rate of water phase decreased.84 There was a threshold pressure (about 10 MPa) in the transported s-CO2 pipeline, beyond which the corrosion rate increased significantly with the pressure.82 In Figure 8E, it can be observed that the solubility of H2O in s-CO2 exhibits two distinct trends, which are divided by the phase transition point. When in gaseous CO2, the solubility of H2O decreases logarithmically with increasing pressure. However, once the pressure surpasses a certain value where CO2 enters the liquid/supercritical state, the solubility of H2O increases rapidly with pressure and stabilizes at a relatively high value. Under the condition that H2S/CO2 coexist, the corrosion rate increases with the increase of H2S partial pressure.84
In the high water content period of oil-water mixed transportation pipeline, with the increase of CO2 partial pressure, the corrosion rate of 20# steel in the mixed pipeline gradually increases.50 In magnetite (Fe3O4) high-density cement-casing steel system, CO2 and corrosive substances tend to migrate to the cement-casing interface under high pressure, which leads to an increase in corrosion rate compared with that under normal pressure.85 Sun et al.86 also found that the corrosion potential of L80 steel shifted positively with the increase of PCO2. The influence of PCO2 on corrosion potential and current is partly attributed to the pH change of CO2 solution in corrosive medium. From this perspective, it becomes evident that the influence of pressure is intricately interlinked with other factors. These complex relationships need to be elucidated to gain a better understanding of the impact of pressure. This facilitates the adoption of corresponding protective measures to ensure the long-term reliability and safety of equipment and pipelines under different pressure conditions.
Cl−
As one of the main anions, Cl− has an aggressive effect on carbon steel. It is generally believed that Cl− has little effect on uniform corrosion, while Cl− mainly affects local corrosion such as pitting corrosion.87 The enrichment of Cl− accelerates local corrosion, and the protective capacity of the passive film decreases with the increase of Cl− concentration.88 However, Cl− is not the main factor contributing to the increased corrosivity of the solution.89 As shown in Figures 9A–9D, the synergistic effect between adequate Cl− concentration and fluid flow can promote initiation and propagation of pitting corrosion of carbon steel. In comparison, pitting behavior has been obviously limited at an extremely low surface coverage ratio induced by a combined action of an excessive Cl− concentration and fluid flow.
Figure 9.
Effect of Cl− on corrosion
(A–D) Reverse action mechanism.
(E) Localized corrosion rate and average pit depths.
(F) Average pitting depth and localized corrosion rate.
Figures reproduced from: (A–D) (Mou et al.89) Copyright 2022, Elsevier; (E) (Yue et al.90) Copyright 2022, Elsevier; (F) (Sun et al.91) Copyright 2019, Elsevier.
The presence of Cl− can alter the corrosion mechanism, leading to more intricate reaction pathways. For example, the increase of Cl− (at low concentration) enhances the synergistic effect with O2, while the high content of Cl− (3,000–5,000 mg/L) can reduce the solubility of CO2/O2 and replace the adsorption of corrosive media and ions on the substrate surface, thus inhibiting the cathodic process of corrosion reaction.92 As shown in Figure 9E, high Cl− content (81,000 ppm) will lead to the precipitation of chloride-rich products, thus accelerating the general/local corrosion, inhibiting the formation of nanocrystalline NiFe2O4, and reducing the corrosion resistance.90
In addition, because the radius of Cl is very small, it can penetrate into the metal surface through the defects in the corrosion layer and accumulate in the pits of the iron oxide layer.93 The chemical reaction formula of Cl− and Fe ions are as follows:
| (Equation 13) |
| (Equation 14) |
| (Equation 15) |
Although Cl− does not participate in the electrode reaction, acidification promotes the local dissolution of Fe metal, thus intensifying the corrosion. Moreover, Cl− would affect the stress corrosion cracking (SCC) of 13Cr stainless steel and increase the risk of material failure, and SCC sensitivity decreased first and then increased with the increase of Cl− concentration.94
The general corrosion rate of carbon steel is influenced by the Cl− content and exposure time. The corrosion rate tends to decrease with longer soaking time, while Cl− content shows an increase in the total penetration rate of carbon steel.18,95,96 While in the process of coating protection, the presence of enriched chloride ions at the coating/substrate interface can compromise the effectiveness of the coating, as it can catalyze the corrosion process and promote the dissolution of the carbon steel substrate through a catalytic mechanism.91 In Figure 9F, the pitting depth and localized corrosion rate of the substrate in H2S-containing environments are quantified.
In summary, the role of chloride ions in CO2 corrosion is complex and often elevates the risk of corrosion. Therefore, in practical applications, it is advisable to choose alloy materials with higher resistance to chloride ion corrosion, utilize chloride ion inhibitors, or optimize coatings to mitigate the impact of chloride ions on corrosion and thereby protect equipment and pipelines.
Other factors
Apart from the aforementioned factors, there are additional factors that can also influence CO2 corrosion. For example, the existence of oil can change the structure and chemical composition of the corrosion product film, thus playing a certain role in corrosion inhibition. In addition, the addition of Ca2+ promoted the dissolution of scale and reduced the corrosion resistance of steel to CO2, the mechanical properties, and wear resistance.97 Moreover, Kahyarian et al.98 found that CO2 and/or its related carbonate species are directly involved in the metal dissolution reaction. The presence of CO2 significantly influences the kinetics and the mechanism of the iron dissolution reaction. Besides, Paolinelli et al.99 revealed that pre-corrosion decreased the inhibitor efficiency, but its impact depended on the microstructure.
In summary, there are numerous factors that influence CO2 corrosion. In practice, it is crucial to comprehensively consider these factors and select appropriate corrosion control methods based on the specific application environment and metal materials. This ensures the reliability and safety of equipment and structures, reduces risks, and enhances overall reliability.
Prevention and control approaches to carbon dioxide corrosion
Currently, the commonly used methods for corrosion protection include the use of corrosion inhibitors, coatings, and alloys. It is worth noting that inhibitors, owing to their remarkable effectiveness and cost-effectiveness, are currently the primary focus of research in this field.
Corrosion inhibitor
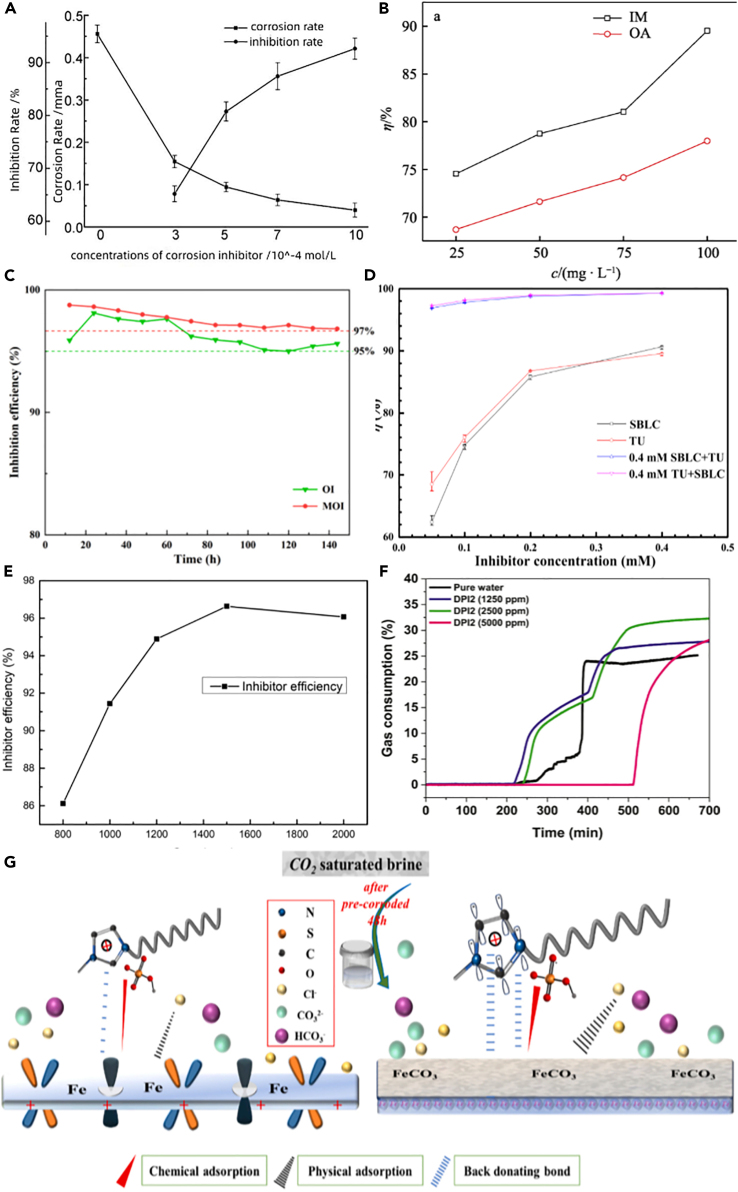
Corrosion inhibitor is the most commonly used method to inhibit corrosion. The mechanisms can be summarized as electrochemical mechanism and physical-chemical mechanism. The former is based on the corrosion electrochemical process of a certain step that is blocked and slows down the corrosion rate. The latter is based on the metal surface to produce adsorption or into the film and slows down the corrosion rate, which is widely employed in the protection of metallic materials. For example, Zheng et al.100 synthesized two imidazoline (IM) derivatives oleic imidazoline (OI) and Mercapto-oleic imidazoline (MOI) with high efficiency and confirmed that the inhibition efficiency of MOI was as high as 95.58% at 20 ppm as shown in Figure 10C. Both inhibitors are attached to the metal surface through chemisorption, while the inhibition efficiency of MOI is better than that of OI because sulfhydryl groups in MOI can be used as powerful adsorption sites, which effectively improves the adsorption capacity of MOI. Coincidentally, Li et al.104 obtained a bimannich-based TZBM containing a thiazole ring by synthesizing Mannich base, which effectively inhibits the corrosion of containing Cl−+H2S + CO2 environment as illustrated in Figure 10E. Figure 11A represent the synthetic of TZBM. The inhibitor molecules adsorbed on the surface block the corrosive medium. The corrosion inhibitor polyethylene glycol-2 oleamide functions on the similar principle.107
Figure 10.
Effect diagram of corrosion inhibitor
(A) Corrosion rate and inhibition rate.101
(B) Corrosion inhibition efficiency.102
(C) Inhibition efficiency.
(D) Comparison of inhibition efficiency.
(E) Concentration with inhibitor efficiency.
(F) Gas consumption ratio curves.
(G) Corrosion inhibition mechanism.
Figures reproduced from: (C) (Zheng et al.100) Copyright 2022, Elsevier; (D) (Zhang et al.103) Copyright 2021, Elsevier; (E) (Zhuoke et al.104) Copyright 2021, BMC; (F) (Farhadian et al.105) Copyright 2023, Elsevier; (G) (Guo et al.106) Copyright 2022, Elsevier.
Figure 11.
Molecular structure diagram
(A) Synthetic of TZBM.
(B) Chemical structure of DPls.
(C) Molecular structure of DMIMHS.
(D) Molecular structures of SBLC and TU.
Figures reproduced from: (A) (Zhuoke et al.104) Copyright 2021, BMC; (B) (Farhadian et al.105) Copyright 2023, Elsevier; (C) (Guo et al.106) Copyright 2022, Elsevier; (D) (Zhang et al.103) Copyright 2021, Elsevier.
Abdolreza et al.105 developed the newly synthesized dual-purpose inhibitors (DPIs). Figure 11B display the chemical structure of DPIs. As exhibited in Figure 10F, DPI2 exhibited the highest inhibition activity on the nucleation step of hydrate crystals by delaying their formation. The strong adsorption of DPI2 on the metal surface makes the protective layer formed to protect the metal from corrosion. Furthermore, DPI2 exhibits a high level of adsorption on the metal surface, which allows for the formation of a protective layer on the mild steel.
Guo et al.106 used an imidazolium-based ionic liquid, 1-dodecyl-3-methyl imidazolium hydrogen sulfate (DMIMHS), as corrosion inhibitor and tested its performance. Figure 11C shows the molecular structure of DMIMHS. The addition of DMIMHS significantly improved the inhibitive properties for pre-corroded carbon steel. The proposed adsorption mechanism of DMIMHS on Fe and FeCO3 surfaces is illustrated in Figure 10G.
Combining different types of inhibitors, namely hybrid inhibitors, has been found to enhance their inhibitory effectiveness compared to using a single inhibitor. Furthermore, employing electrochemical methods provides a deeper understanding of the mechanisms involved in CO2 corrosion. This, in turn, facilitates the development of more precise and effective inhibitors. This method is instrumental for researchers in gaining insights into the corrosion processes and improving the performance of inhibitors. For instance, Wei et al.101 used linoleic acid and tetraethylenepentamine (TEP) as reactive monomers to synthesize a highly effective IM inhibitor (GIM). The results in Figure 10A show that the addition of GIM corrosion inhibitor reduces the corrosion rate of J55 steel sheet by increasing the charge transfer resistance on the metal substrate surface, thus reducing the corrosion current density, effectively slowing down the electrochemical reaction on the metal surface. The IM inhibitor was completely protonated under the condition of CO2 saturation of 3 wt %NaCl (pH∼4.1), which increased the solubility/dispersibility of the inhibitor in brine and enhanced the molecular adsorption ability of positively charged IM on metal surface.108
The combination of different types of inhibitors, known as hybrid inhibitors, has garnered attention. This approach enhances the effectiveness of inhibitors and makes them adaptable to various environmental conditions. Liu et al.102 studied the synergistic corrosion inhibition effect of IM and oleic acid (OA) on N80 steel in formation of water containing CO2-saturated oil field. As shown in Figure 10B, when IM and OA were added separately, the corrosion inhibition efficiency was 82.89% and 78.51%, respectively, at the mass concentration of 100 mg/L. The corrosion inhibition efficiency was increased to 98.07% after the mixture ratio of IM-OA was 25:75. The structure of the mixed film is changed after the two compounds are mixed, which makes the inhibitor film more compact, so as to achieve better corrosion inhibition effect.
Zhang et al.103 studied the synergistic inhibition of S-benzyl-L-cysteine (SBLC) and thiourea (TU). What can be seen in Figure 10D is that the inhibitory effect of the mix inhibitor is significantly higher than that of individual SBLC or TU at the same concentration, owing to the intermolecular interaction between SBLC and TU resulting in the formation of a denser adsorption film on the surface of carbon steel, which showed significant synergistic inhibition. Figure 11D shows the molecular structures of SBLC and TU.
In recent years, out of consideration for the environment, there has been a significant focus on environmentally friendly and green CO2 corrosion inhibitors. For example, Berdimurodov et al.109 put forward a point that using carbon dots (CDs) in corrosion protection is an ecological and environmentally efficient method because CDs have good water solubility, biocompatibility, low toxicity, excellent antibacterial properties, chemical stability, high thermal activity, and nonflammability. The lone electron pairs promote CDs to become efficient corrosion inhibitors. It is hoped that, in the near future, CDs can play an important role in corrosion inhibitors, for it is more effective at low concentrations and easy to synthesize.
Indeed, CO2 corrosion inhibitors offer several advantages, including environment friendly, cost-effectiveness, and compatibility. By comparison, CO2 corrosion inhibitors do have certain disadvantages, including limited effectiveness, application challenges, and dependency on concentration. When selecting and implementing CO2 corrosion inhibitors for corrosion protection in specific applications, it is important to consider various factors, such as metal type, performance requirements, cost considerations, and environmental impact.
Above all, the forefront of corrosion inhibitor research is centered on the exploration of novel and environmentally friendly inhibitors, the utilization of hybrid inhibitors, and delving deeper into electrochemical investigations. These advancements hold the promise of enhancing control over CO2 corrosion, reducing equipment degradation, and mitigating adverse environmental impacts.
Coating protection
Coatings offer several advantages, including excellent corrosion resistance, long-term protection, and adaptability. Considering these advantages, coatings play a crucial role in safeguarding structures and equipment against corrosion. The underlying mechanisms primarily involve isolation and shielding principles. The former is achieved by forming an isolating layer that separates the metal surface from CO2 gas, thus reducing the reactivity between the corrosion medium and the metal. For example, Peng et al.110 used the water-based modified epoxy resin as the anti-corrosion material to improve the anti-corrosion performance of oil well cement. Compared with cement stone without modified epoxy resin, the modified epoxy resin mainly improved the corrosion resistance of cement paste by filling pores to improve the compactness of cement paste structure and forming a three-dimensional network polymer film to isolate acidic media.
The latter provides a sacrificial shielding layer that is more susceptible to damage than the metal itself, confining corrosion damage to the coating rather than the metal surface, similar to the principle of sacrificial anodes. Commonly used coatings include nickel-phosphorus (Ni-P) coatings, zinc coatings, polymer coatings, and organic coatings. Achieving optimal protective performance often depends on the selection of coating materials, which is influenced by factors such as the application’s environmental conditions and the type of metal.
For Ni-P coating, it showed good corrosion resistance in CO2 environment, especially in the presence of high chloride ions. But in H2S and CO2-H2S environment as shown in Figure 12A, the coexistence of them led to CO2 enhancing H2S corrosion effect, and the synergistic effect accelerated the degradation of the coating.91 The addition of H2S accelerates the diffusion process of electrolyte/coating interface and promotes the penetration of electrolyte through the coating, resulting in serious local corrosion and coating peeling.119 Nevertheless, as displayed in Figure 12B, the morphology of Ni-Cr-Mo coating has no change after corrosion in the simulated solution environment of CO2, H2S, and their mixture, with higher corrosion resistance.111 The Ni-Cr-Mo coating has low cost and excellent performance. It is feasible to apply it to the corrosion protection of petroleum industry.
Figure 12.
Coating protection effect
(A) Weight loss and average corrosion rate.
(B) Corrosion current density.
(C) Potentiodynamic polarization curves.
(D) Corrosion rates and corrosion inhibition efficiencies.
(E) Tafel curves.
(F) Corrosion rate and time.
(G) Nyquist plots.
(H1-H2) Bode diagram.
(I) Potentiodynamic polarization curves.
Figures reproduced from: (A) (Sun et al.91) Copyright 2019, Elsevier; (B) (Wang et al.111) Copyright 2016, Elsevier; (C) (Li et al.112) Copyright 2020, Elsevier; (D) (Li et al.113) Copyright 2021, Elsevier; (E) (Zhang et al.114) Copyright 2022, Elsevier; (F) (Zhu et al.115) Copyright 2023, Elsevier; (G) (Luo et al.116) Copyright 2022, Elsevier; (H) (Wang et al.117) Copyright 2021, John Wiley and Sons; (I) (Al Shenawa et al.118) Copyright 2021, Taylor & Francis.
To enhance the corrosion resistance and improve the passivation properties of the coating, Li et al.112 applied an electroless Ni-Mo-P/Ni-P composite coating on N80 carbon steel, which involved adding molybdenum and undergoing heat treatment. As depicted in Figure 12C, heat-treated coatings exhibit lower corrosion activity, as evidenced by the leftward shift of the curves (indicating smaller current densities) compared to as-deposited coatings. In addition, the concentration of Ca2+ has a significant impact on the corrosion of N80 steel, whereas the Ni-P coating shows excellent corrosion resistance and is hardly affected by Ca2+, as demonstrated in Figure 12D.113 Furthermore, the addition of iron to the Ni-P coating can improve its flatness, increase grain size, induce crystallization, and facilitate the transformation of Ni(OH)2 to NiO in the passivation film, as exhibited in Figure 12E.114 However, it is not recommended for use in highly acidic environments. This is because the iron within Ni-Fe-P coatings can undergo dissolution due to the presence of H+ ions, resulting in the deterioration of the coating’s structure and eventual detachment from the substrate surface.
The addition of other elements or compounds to Ni-P coatings is also a promising research direction. This approach has the potential to enhance their performance, reduce adverse environmental effects, and broaden their applicability through alloying. For example, Zhu et al.115 electroplated Ni coating on pipeline steel surface and incorporated CuO nanoparticles to impart antibacterial functionality. As shown in Figure 12F, the corrosion rate was decreased due to the reduced probability of corrosion reaction by the presence of nano-CuO and its antibacterial effect against sulfate-reducing bacteria. This research provides new insights for addressing microbial corrosion issues in oil and gas pipelines.
Graphene oxide (GO) holds immense potential as an efficient coating, which is typically combined with other materials, such as polymers or nanoparticles, to create composite inhibitors. This further enhances the performance in mitigating CO2 corrosion and provides long-lasting protection. For instance, Luo et al.116 modified GO nanosheets with the photocatalytic heterostructure TiO2-ZnO and fabricated TiO2-ZnO-GO (T-Z-G) ternary nanofillers to block the intrusion of corrosive media through the layer structure of GO. As illustrated in Figure 12G, the coating has excellent anti-fouling, anti-corrosion, anti-corrosion, and barrier properties and can be applied to CO2 storage and transportation protection in CCUS process.
Oppong Boakye et al.120 investigated polymer coating modified with GO and duplex electroless Ni-P with polytetrafluroethylene (PTFE) coating for the infrared corrosion behavior. At 120°C, the polymer coating with added GO nanosheets displayed reduced wetting ability and effectively suppressed the corrosion effects of the substrate. However, none of the coatings provided protection at 250°C in the H2S/CO2 environment. Therefore, when applying this coating, it is important to consider practical environmental temperature, in order to prevent coating failure. But Wang et al.117 fabricated a novel superhydrophobic nickel-reduced graphene oxide (sNi-rGO) coating on N80 steel substrate. As shown in Figure 12H, the sNi-rGO coating has excellent corrosion resistance in simulated well fluid. The coating still maintained superhydrophobicity and corrosion resistance under the condition of high-temperature and high-pressure CO2.
Research into the incorporation of ceramic materials within coatings remains an active and dynamic field. The fundamental concept involves the amalgamation of ceramic particles or ceramic materials within a coating to enhance its resistance. For example, Al Shenawa et al.118 analyzed the effect of salt water corrosion in filled and unfilled ceramic filled polymer coatings before and after supercritical CO2 exposure. As revealed in Figure 12I, after exposure to CO2, the filled and unfilled systems had similar corrosion properties, while the filled coating had better wear resistance.
Coating and cathodic protection are effective measures to prevent pipeline corrosion. Although coating can provide excellent corrosion protection, it can degrade over time or become ineffective due to factors such as water absorption, blistering, and damage. Thus, it is crucial to carefully select appropriate coating materials, surface treatment processes, optimal working temperatures, suitable coating thicknesses, and other relevant factors based on the specific situation to achieve the best possible protection. Furthermore, regular inspection and maintenance of coatings, corrosion rate monitoring, and training for operational and maintenance personnel are also indispensable. A comprehensive consideration of these points will aid in reducing the risks associated with corrosion, ultimately extending the lifespan of pipelines.
Alloy protection
Alloy protection refers to a kind of protection means through element control, lattice control, and matching with metal production process. Selecting appropriate corrosion-resistant materials can also effectively reduce the corrosion risk of CO2 and improve the stability of equipment. For example, the addition of aluminum in low-Cr steel helped to form a dense corrosion product layer, greatly reducing the local corrosion sensitivity (Figure 13D).123 Figure 13C indicates that the corrosion resistance of carbon steel such as N80 can be improved by adding small amounts of alloy elements.122
Figure 13.
Alloy protection efficiency
(A) Corrosion rates.
(B) Average density.
(C) Corrosion rates comparison.
(D) Corrosion rate.
(E) Schematic formation mechanism.
Figures reproduced from (A, B, and E) (Wei et al.121) Copyright 2019, Elsevier; (C) (Wu et al.122) Copyright 2013, Elsevier; (D) (Gao et al.123) Copyright 2022, Emerald Publishing Limited.
The enhancement of corrosion resistance in Cr (chromium) alloys through alloy design and optimization constitutes a pivotal component of alloy protection strategies. However, under static conditions, the incorporation of a small amount of Cr cannot facilitate the formation of a robust Cr(OH)3 protective layer.121 As shown in Figures 13A and 13B, 6.5Cr steel has good corrosion resistance because the corrosion products of 6.5Cr under dynamic and static conditions are single-layer chromium-rich layer composed of amorphous FeCO3 and Cr(OH)3. The good local corrosion resistance of Cr-containing steel is attributed to the in situ formation of the initial amorphous Cr-rich layer. Figure 13E displayed schematic diagram of the formation mechanism of corrosion product scale on 3Cr steel in dynamic s-CO2-saturated aqueous phase.
Kai et al.124 studied the effect of surface finishing on the oxidation behavior of a Fe-21Cr-32Ni alloy in s-CO2. The high Cr diffusion led to the transition from internal to external oxidation by favoring the formation of a thin/protective Cr-rich oxide layer. Furthermore, it reduced the permeability of carbon into the oxides, thus improving the oxidation and carburization resistance of the ground specimen. In the context of high-purity research-grade CO2, the corrosion resistance of the Ni-based alloy Haynes 230 proved to be excellent.125 This superiority can be attributed to the formation of a remarkably thin, consistently uniform, and protective layer enriched with chromium oxide on its surface.
The effectiveness of alloy protection against CO2 corrosion is significant; however, its high cost and complex processing are important factors that limit its development. Additionally, the maintenance and repair of the alloy are typically more intricate compared to conventional materials, and one must also consider compatibility when it comes into contact with other metals. Therefore, the research focus in the future should be on the development of low-cost and easy-to-process alloys to enable large-scale application. Simultaneously, there is a need for a focus on material modeling and simulation calculations to predict the corrosion behavior of the alloy under various conditions, thereby expediting the development of high-performance environmentally friendly alloy materials.
Conclusion and outlook
CCUS is an effective approach to reducing carbon emissions, but the issue of CO2 corrosion cannot be overlooked. This review elucidates CO2 corrosion problems, including its mechanism and influencing factors, and summarizes anti-corrosion methods. It particularly underscores the distinctive advantages of inhibitors.
Unfortunately, the field of corrosion-resistant materials still faces challenges, such as high cost and difficulty in processing. Although it can efficiently mitigate corrosion, further research and improvement are required to ensure its economic feasibility. While the cost of coating protection is relatively low, there are still some areas that require improvement, such as long-term durability, applicability, and sustainability.
Another promising direction is developing novel green inhibitors. Inhibitor is currently one of the most commonly applied methods, offering good corrosion resistance and superior economy. However, improper discharge may result in detrimental environmental consequences due to its corrosive nature. Currently, nitrogen-based compounds like IMs serve as effective inhibitors with good anti-corrosion performance, and they represent an essential research direction for the future. Moreover, the high adaptability and effectiveness demonstrated by mixed inhibitors have sparked considerable research interest and represent one of the key areas for future investigations. Developing high-performance and environmentally friendly corrosion inhibitors to enhance stability and optimize usage methods is a top priority.
In the future, the authors recommend CO2 corrosion protection research could focus on the following areas.
Although significant progress has been made in corrosion inhibitor research worldwide, the overall progress is still relatively limited. The development of green corrosion inhibitors is crucial for future research. The focus should be on preparing environmentally friendly and efficient corrosion inhibitors while explaining their compliance with green standards and corrosion inhibition mechanisms. Computer-based molecular simulation methods have been applied in corrosion inhibitor research, providing a useful tool for future molecular design. As oil field production technology and the environment continue to evolve, the stability and durability of corrosion inhibitor performance are challenged under harsh conditions; specific research on the application of these coatings in oil field production technology and actual environments should be carried out to determine their effectiveness in practical situations. Coatings gradually soften with increasing temperatures, so future research can focus on developing coatings with high temperature and pressure resistance, acid resistance, and compactness to improve permeability resistance.
Acknowledgments
The authors would like to thank the support from Capacity Building Projects in Local Universities of Science and Technology Commission of Shanghai Municipality (NO. 22010501500).
Declaration of interests
The authors declare no competing interests.
Contributor Information
Ting Yan, Email: yt81725@126.com.
Wei-Guo Pan, Email: pweiguo@163.com.
References
- 1.Godin J., Liu W., Ren S., Xu C.C. Advances in recovery and utilization of carbon dioxide: A brief review. J. Environ. Chem. Eng. 2021;9 [Google Scholar]
- 2.Fleming R.J. An updated review about carbon dioxide and climate change. Environ. Earth Sci. 2018;77:262. [Google Scholar]
- 3.Kang J.-N., Wei Y.-M., Liu L.-c., Wang J.-W. Observing technology reserves of carbon capture and storage via patent data: Paving the way for carbon neutral. Technol. Forecast. Soc. Change. 2021;171 [Google Scholar]
- 4.Zhang Q., Wang Y., Liu L. Carbon Tax or Low-Carbon Subsidy? Carbon Reduction Policy Options under CCUS Investment. Sustainability. 2023;15:5301. [Google Scholar]
- 5.Huang L., Hou Z., Fang Y., Liu J., Shi T. Evolution of CCUS Technologies Using LDA Topic Model and Derwent Patent Data. Energies. 2023;16:2556. [Google Scholar]
- 6.Yang Y., Xu W., Wang Y., Shen J., Wang Y., Geng Z., Wang Q., Zhu T. Progress of CCUS technology in the iron and steel industry and the suggestion of the integrated application schemes for China. Chem. Eng. J. 2022;450 [Google Scholar]
- 7.Deng Q., Ling X., Zhang K., Tan L., Qi G., Zhang J. CCS and CCUS Technologies: Giving the Oil and Gas Industry a Green Future. Front. Energy Res. 2022;10:72–75. [Google Scholar]
- 8.Pal M., Karaliūtė V., Malik S. Exploring the Potential of Carbon Capture, Utilization, and Storage in Baltic Sea Region Countries: A Review of CCUS Patents from 2000 to 2022. Processes. 2023;11:605. [Google Scholar]
- 9.Zhang K., Sun Y., Wang C.-J., Ge H.-J., Zhu Y.-J., Wang H.-Y. Research on CO2 Corrosion and Protection in Carbon Capture, Utilization and Storage. Surf. Technol. 2022;51:43–52. [Google Scholar]
- 10.Che X., Yi X., Dai Z., Zhang Z., Zhang Y. Application and Development Countermeasures of CCUS Technology in China’s Petroleum Industry. Atmosphere. 2022;13:1757. [Google Scholar]
- 11.Horn J., Zbacnik R. Post-Combustion Carbon Capture Technologies. Chem. Eng. 2015;122 [Google Scholar]
- 12.Olabi A.G., Wilberforce T., Sayed E.T., Shehata N., Alami A.H., Maghrabie H.M., Abdelkareem M.A. Prospect of Post-Combustion Carbon Capture Technology and Its Impact on the Circular Economy. Energies. 2022;15:8639. [Google Scholar]
- 13.Jiang L., Liu W., Wang R., Gonzalez-Diaz A., Rojas-Michaga M.F., Michailos S., Pourkashanian M., Zhang X., Font-Palma C. Sorption direct air capture with CO2 utilization. Prog. Energy Combust. Sci. 2023;95 [Google Scholar]
- 14.Jiang L., Gonzalez-Diaz A., Ling-Chin J., Roskilly A.P., Smallbone A.J. Post-combustion CO2 capture from a natural gas combined cycle power plant using activated carbon adsorption. Appl. Energy. 2019;245:1–15. [Google Scholar]
- 15.Lu S., Gong Y., Liu L., Kang G., Chen X., Liu M., Zhang J., Wang F. Advances in corrosion happened in CO2 absorption by alkanolamine (In Chinese) Mod. Chem. Ind. 2022;42:76–80. [Google Scholar]
- 16.Koch G., Brongers M., Thompson N., Virmani Y., Payer J. 2001. Corrosion Cost and Preventive Strategies in the United States. [Google Scholar]
- 17.Liao K., Leng J., Cheng Y.F., He T., He G., Zhao S., Liu X., Huang Q. Effect of H2S concentrations on corrosion failure of L245NS steel in CO2-O2-H2S system. Process Saf. Environ. Protect. 2022;168:224–238. [Google Scholar]
- 18.Zhang Y., Pang X., Qu S., Li X., Gao K. Discussion of the CO2 corrosion mechanism between low partial pressure and supercritical condition. Corrosion Sci. 2012;59:186–197. [Google Scholar]
- 19.Li Y., Liu X., Wang C., Hu Q., Wang J., Ma H., Zhang N. Research progress on the corrosion behavior of gaseous CO2 transportation pipelines containing impurities. Acta Metall. Sin. 2021;57:283–294. [Google Scholar]
- 20.Li K., Zeng Y. Long-term corrosion and stress corrosion cracking of X65 steel in H2O-saturated supercritical CO2 with SO2 and O2 impurities. Construct. Build. Mater. 2023;362 [Google Scholar]
- 21.Yang Y., Mao S., Yang Q., Xu Z., Ren Y., Wu W. Effect of stress on corrosion behavior of martensitic and austenitic steels in supercritical carbon dioxide at 550°C and 20 MPa. J. Supercrit. Fluids. 2023;192 [Google Scholar]
- 22.Sun C., Yan X., Sun J., Pang J., Zhao W., Lin X. Unraveling the effect of O2, NO2 and SO2 impurities on the stress corrosion behavior of X65 steel in water-saturated supercritical CO2 streams. Corrosion Sci. 2022;209 [Google Scholar]
- 23.Gui Y., Liang Z., Wang S., Zhao Q. Corrosion behavior of T91 tubing in high temperature supercritical carbon dioxide environment. Corrosion Sci. 2023;211 [Google Scholar]
- 24.Sun C., Wang Y., Sun J., Lin X., Li X., Liu H., Cheng X. Effect of impurity on the corrosion behavior of X65 steel in water-saturated supercritical CO2 system. J. Supercrit. Fluids. 2016;116:70–82. [Google Scholar]
- 25.Sun C., Sun J., Wang Y., Sui P., Lin X., Liu H., Cheng X., Zhou M. Effect of impurity interaction on the corrosion film characteristics and corrosion morphology evolution of X65 steel in water-saturated supercritical CO2 system. Int. J. Greenh. Gas Control. 2017;65:117–127. [Google Scholar]
- 26.Kang Y., Leng X., Zhao L., Bai B., Wang X., Chen H. Review on the Corrosion Behaviour of Nickel-Based Alloys in Supercritical Carbon Dioxide under High Temperature and Pressure. Crystals. 2023;13:725. [Google Scholar]
- 27.Mubarak G., Verma C., Barsoum I., Alfantazi A., Rhee K.Y. Internal corrosion in oil and gas wells during casings and tubing: Challenges and opportunities of corrosion inhibitors. J. Taiwan Inst. Chem. Eng. 2023;150 [Google Scholar]
- 28.Obot I.B., Sorour A.A., Malede Y.C., Chen T., Wang Q., Aljeaban N. A review study on the challenges and progress of corrosion inhibitor testing under extreme conditions in the oil and gas industries. Geoenergy Sci. Eng. 2023;226 [Google Scholar]
- 29.Zhang D., Xiao L., Xiong G., He Q., Pan Z., Ma G. Recent progress of zeolitic imidazolate frameworks (ZIFs) in superhydrophobic and anticorrosive coatings for metals and their alloys. J. Coat. Technol. Res. 2023;20:1157–1177. [Google Scholar]
- 30.Li K., Zhu Z., Xiao B., Luo J.L., Zhang N. State of the art overview material degradation in high-temperature supercritical CO2 environments. Prog. Mater. Sci. 2023;136 [Google Scholar]
- 31.Kairy S.K., Zhou S., Turnbull A., Hinds G. Corrosion of pipeline steel in dense phase CO2 containing impurities: A critical review of test methodologies. Corrosion Sci. 2023;214 [Google Scholar]
- 32.Sheetal, Batra R., Singh A.K., Singh M., Thakur S., Pani B., Kaya S. Advancement of corrosion inhibitor system through N-heterocyclic compounds: a review. Corrosion Eng. Sci. Technol. 2023;58:73–101. [Google Scholar]
- 33.Mansoori H., Young D., Brown B., Singer M. Influence of calcium and magnesium ions on CO2 corrosion of carbon steel in oil and gas production systems - A review. J. Nat. Gas Sci. Eng. 2018;59:287–296. [Google Scholar]
- 34.Li W., Brown B., Young D., Nešić S. Investigation of Pseudo-Passivation of Mild Steel in CO2 Corrosion. Corrosion. 2014;70:294–302. [Google Scholar]
- 35.Bockris J.O.M., Drazic D., Despic A.R. The electrode kinetics of the deposition and dissolution of iron. Electrochim. Acta. 1961;4:325–361. [Google Scholar]
- 36.Dewaard C., Milliams D.E. Carbonic Acid Corrosion of Steel. Corrosion. 1975;31:177–181. [Google Scholar]
- 37.Ogundele G.I., White W.E. Some Observations on Corrosion of Carbon Steel in Aqueous Environments Containing Carbon Dioxide. Corrosion. 1986;42:71–78. [Google Scholar]
- 38.Linter B.R., Burstein G.T. Reactions of pipeline steels in carbon dioxide solutions. Corrosion Sci. 1999;41:117–139. [Google Scholar]
- 39.Wiȩckowski A., Ghali E., Szklarczyk M., Sobkowski J. The behaviour of iron electrode in CO2− saturated neutral electrolyte—I. Electrochemical study. Electrochim. Acta. 1983;28:1619–1626. [Google Scholar]
- 40.Lin H. Research progress of pipeline corrosion in CO2/CO2-O2 environment (In Chinese) Energy Chem. Indust. 2019;40:11–17. [Google Scholar]
- 41.Nesic S., Drazic D., Thevenot N., Crolet J. NACE International; 1996. Electrochemical Properties of Iron Dissolution in the Presence of CO2 - Basics Revisited. [Google Scholar]
- 42.Nesic S., Postlethwaite J., Olsen S. An Electrochemical Model for Prediction of Corrosion of Mild Steel in Aqueous Carbon Dioxide Solutions. Corrosion. 1996;52:280–294. [Google Scholar]
- 43.Kahyarian A., Brown B., Nesic S. NACE - International Corrosion Conference Series; 2018. Mechanism of CO2 Corrosion of Mild Steel: A New Narrative. [Google Scholar]
- 44.Remita E., Tribollet B., Sutter E., Vivier V., Ropital F., Kittel J. Hydrogen evolution in aqueous solutions containing dissolved CO2: Quantitative contribution of the buffering effect. Corrosion Sci. 2008;50:1433–1440. [Google Scholar]
- 45.Kahyarian A., Nesic S. On the mechanism of carbon dioxide corrosion of mild steel: Experimental investigation and mathematical modeling at elevated pressures and non-ideal solutions. Corrosion Sci. 2020;173 [Google Scholar]
- 46.Kahyarian A., Nesic S. A New Narrative for CO2 Corrosion of Mild Steel. J. Electrochem. Soc. 2019;166:C3048–C3063. [Google Scholar]
- 47.Kittel J., Ropital F., Grosjean F., Sutter E.M.M., Tribollet B. Corrosion mechanisms in aqueous solutions containing dissolved H2S. Part 1: Characterisation of H2S reduction on a 316L rotating disc electrode. Corrosion Sci. 2013;66:324–329. [Google Scholar]
- 48.Tran T., Brown B., Nešić S., Tribollet B. Investigation of the Electrochemical Mechanisms for Acetic Acid Corrosion of Mild Steel. Corrosion. 2014;70:223–229. [Google Scholar]
- 49.Tran T., Brown B., Nesic S. NACE - International Corrosion Conference Series; 2015. Corrosion of Mild Steel in an Aqueous CO2 Environment - Basic Electrochemical Mechanisms Revisited. [Google Scholar]
- 50.Yang, Z., Shi, L., Zou, M., Wang, C. Factors Influencing the CO2 Corrosion Pattern of Oil–Water Mixed Transmission Pipeline during High Water Content Period. Atmosphere. 2022;13:1687. [Google Scholar]
- 51.Ossai C.I., Boswell B., Davies I.J. Pipeline failures in corrosive environments – A conceptual analysis of trends and effects. Eng. Fail. Anal. 2015;53:36–58. [Google Scholar]
- 52.Li K., Cao G., Shan G., Zhang N., Liu X., Zhai S., Bai Y. Optimization of Anti-Plugging Working Parameters for Alternating Injection Wells of Carbon Dioxide and Water. Processes. 2022;10:2447. [Google Scholar]
- 53.Xiang Y., Wang Z., Yang X., Li Z., Ni W. The upper limit of moisture content for supercritical CO2 pipeline transport. J. Supercrit. Fluids. 2012;67:14–21. [Google Scholar]
- 54.Hua Y., Barker R., Neville A. The influence of SO2 on the tolerable water content to avoid pipeline corrosion during the transportation of supercritical CO2. Int. J. Greenh. Gas Control. 2015;37:412–423. [Google Scholar]
- 55.Ye Z., Ding T., Zhou X., Ju M., Yi R., Jiang W., Cui X., Lin X., Sun C., Sun J. Corrosion Behavior of Carbon Steel in Crude Oil-Water-Gas Multiphase Environments with CO2 and H2S. J. Mater. Eng. Perform. 2022;31:7673–7685. [Google Scholar]
- 56.Vitali M., Corvaro F., Marchetti B., Terenzi A. Thermodynamic challenges for CO2 pipelines design: A critical review on the effects of impurities, water content, and low temperature. Int. J. Greenh. Gas Control. 2022;114 [Google Scholar]
- 57.de Visser E., Hendriks C., Barrio M., Mølnvik M.J., de Koeijer G., Liljemark S., Le Gallo Y. Dynamis CO2 quality recommendations. Int. J. Greenh. Gas Control. 2008;2:478–484. [Google Scholar]
- 58.Fang X.J., Liu L., Yang Z.G., Zhang Y.Q. Corrosion Behavior and Mechanism of Oil Casing Steel in CO2 Salt Solution. Mater. Sci. Forum. 2021;6114:534–538. [Google Scholar]
- 59.Ajayi F.O., Lyon S. Mitigation of top- and bottom-of-the-line CO2 corrosion in the presence of acetic acid (I): pH control using methyl diethanolamine. Mater. Corros. 2021;72:1177–1188. [Google Scholar]
- 60.Wang F., Li J., Qu C., Yu T., Li Y., Zhu S., Yang B., Cheng F. Corrosion Mechanism of L360 Pipeline Steel Coated with S8 in CO2-Cl− System at Different pH Values. Metals. 2021;11:1975. [Google Scholar]
- 61.Zhu J.-y., Xu L.-n., Lu M.-x., Chang W. Cathodic reaction mechanisms in CO2 corrosion of low-Cr steels. Int. J. Miner. Metall. Mater. 2019;26:1405–1414. [Google Scholar]
- 62.Wright R.F., English R., Egbu J.C., Baltrus J., Ziomek-Moroz M., Ohodnicki P.R. Fe Thin Film-Coated Optics for Corrosion Monitoring: Optical and Electrochemical Studies. JOM. 2021;73:655–664. [Google Scholar]
- 63.Santos B.A.F., Serenario M.E.D., Souza R.C., Oliveira J.R., Vaz G.L., Gomes J., Bueno A.H.S. The electrolyte renewal effect on the corrosion mechanisms of API X65 carbon steel under sweet and sour environments. J. Petrol. Sci. Eng. 2021;199 [Google Scholar]
- 64.Qin M., Li J., Chen S., Qu Y. Experimental study on stress corrosion crack propagation rate of FV520B in carbon dioxide and hydrogen sulfide solution. Results Phys. 2016;6:365–372. [Google Scholar]
- 65.Fatah M.C., Ismail M.C., Ari-Wahjoedi B., Kurnia K.A. Effects of sulphide ion on the corrosion behaviour of X52 steel in a carbon dioxide environment at temperature 40°C. Mater. Chem. Phys. 2011;127:347–352. [Google Scholar]
- 66.Wei L., Pang X., Gao K. Effect of flow rate on localized corrosion of X70 steel in supercritical CO2 environments. Corrosion Sci. 2018;136:339–351. [Google Scholar]
- 67.Liu A.Q., Bian C., Wang Z.M., Han X., Zhang J. Flow dependence of steel corrosion in supercritical CO2 environments with different water concentrations. Corrosion Sci. 2018;134:149–161. [Google Scholar]
- 68.Niu Q., Li Z., Cui G., Wang B. Effect of Flow Rate on the Corrosion Behavior of N80 Steel in Simulated Oil Field Environment Containing CO2 and HAc. Int. J. Electrochem. Sci. 2017;12:10279–10290. [Google Scholar]
- 69.Wang B., Wang Y., Li Q., Hu L., Chang W., Li H., Lu M. Effect of Turbulent Flow on Corrosion Behavior of 6.5Cr Steel in CO2-Containing Environment. Int. J. Electrochem. Sci. 2021;16 [Google Scholar]
- 70.da Silva de Sá J., Ma W., Owen J., Hua Y., Neville A., Ponciano Gomes J.A., Barker R. Effect of Flow Rate on the Corrosion Behavior of API 5L X80 Steel in Water-Saturated Supercritical CO2Environments. Corrosion. 2022;78:58–67. [Google Scholar]
- 71.Wang Z., Zhao Y., Liu M., Shen H., Fang Q., Yao J. Investigation of the effects of small flow rate and particle impact on high temperature CO2 corrosion of N80 steel. Corrosion Sci. 2022;209 [Google Scholar]
- 72.Hasan B.O., Aziz S.M. Corrosion of carbon steel in two phase flow (CO2 gas-CaCO3 solution) controlled by sacrificial anode. J. Nat. Gas Sci. Eng. 2017;46:71–79. [Google Scholar]
- 73.Stergioudi F., Baxevani A., Florou C., Michailidis N., Nessi E., Papadopoulos A.I., Seferlis P. Corrosion Behavior of Stainless Steels in CO2 Absorption Process Using Aqueous Solution of Monoethanolamine (MEA) Corrosion Mater. Degrad. 2022;3:422–438. [Google Scholar]
- 74.Gao K., Shang S., Zhang Z., Gao Q., Ma J., Liu W. Effect of Temperature on Corrosion Behavior and Mechanism of S135 and G105 Steels in CO2/H2S Coexisting System. Metals. 2022;12:1848. [Google Scholar]
- 75.He G., Zou Q., Liao K., Leng J., Zhao S. Corrosion mechanism of high temperature and O2 content in steamed CO2/O2/SO2 system and failure behavior of 20G steel on steam-injection pipelines. Process Saf. Environ. Protect. 2022;163:528–542. [Google Scholar]
- 76.Eškinja M., Moshtaghi M., Hönig S., Zehethofer G., Mori G. Investigation of the effects of temperature and exposure time on the corrosion behavior of a ferritic steel in CO2 environment using the optimized linear polarization resistance method. Results Materials. 2022;14 [Google Scholar]
- 77.Zhang Y., Xu M., Song J., Wang C., Wang X., Hamad B.A. Study on the corrosion change law and prediction model of cement stone in oil wells with CO2 corrosion in ultra-high-temperature acid gas wells. Construct. Build. Mater. 2022;323 [Google Scholar]
- 78.Ren X., Wang H., Wei Q., Lu Y., Xiao B., Xie J. Electrochemical behaviour of N80 steel in CO2 environment at high temperature and pressure conditions. Corrosion Sci. 2021;189 [Google Scholar]
- 79.Tian C., Pang Z., Liu D., Wang X., Hong Q., Chen J., Zhang Y., Wang H. Micro-action mechanism and macro-prediction analysis in the process of CO2 huff-n-puff in ultra-heavy oil reservoirs. J. Petrol. Sci. Eng. 2022;211 [Google Scholar]
- 80.Ma W. Corrosion Behavior of Gas Storage Well Pipe Strings in Corrosive H2S–CO2 Environment. J. Fail. Anal. Preven. 2022;22:368–376. [Google Scholar]
- 81.Wang Y., Wang B., He S., Zhang L., Xing X., Li H., Lu M. Unraveling the effect of H2S on the corrosion behavior of high strength sulfur-resistant steel in CO2/H2S/Cl− environments at ultra high temperature and high pressure. J. Nat. Gas Sci. Eng. 2022;100 [Google Scholar]
- 82.Li K., Zeng Y. Advancing the mechanistic understanding of corrosion in supercritical CO2 with H2O and O2 impurities. Corrosion Sci. 2023;213 [Google Scholar]
- 83.Orozco-Agamez J., Santos L.F., Moreno J.A., Alviz-Meza A., Leon A.Y., Pena-Ballesteros D. Influence of the Oxygen Content, Pressure and Temperature in the Api N-80 Corrosion for Applications of Ccs-eor Processes. Chem. Eng. Trans. 2022;96 [Google Scholar]
- 84.Huang X., Zhou L., Li Y., Du Z., Zhu Q., Han Z. The synergistic effect of temperature, H2S/CO2 partial pressure and stress toward corrosion of X80 pipeline steel. Eng. Fail. Anal. 2023;146:107079. [Google Scholar]
- 85.Wang S., He X., Wang S., Yao M., Zhang J., Wu M., Liu L., Zhang X., Xiang D. Experimental Investigation of CO2- and Fe3O4-Assisted Corrosion at the Cement–Casing Interface. Steel Res. Int. 2022;93:2200209. [Google Scholar]
- 86.Sun X., Cui H., Li Z., He R., Liu Z., Lu L. Effect of Service Environmental Parameters on Electrochemical Corrosion Behavior of L80 Casing Steel. Materials. 2021;14 doi: 10.3390/ma14195575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Videm K. NACE - International Corrosion Conference Series; 2000. The Anodic Behaviour of Iron and Steel in Aqueous Solutions with CO2, HCO3-, CO32- and Cl- [Google Scholar]
- 88.Zhu M., Zhang Q., Yuan Y.F., Guo S.Y., Pan J. Effects of Cl− and AC on the Corrosion Behavior of 2507 Super Duplex Stainless Steel in a Simulated Concrete Pore Solution. J. Mater. Eng. Perform. 2020;29:8431–8440. [Google Scholar]
- 89.Mou L., Zhang S., Li J., Liu B., Yan X., Zhao J., Zhang J., Zhang Y. Comprehending of the reverse effect of Cl− concentration and fluid flow on pitting corrosion of carbon steel. Vacuum. 2022;206 [Google Scholar]
- 90.Yue X., Ren Y., Huang L., Zou S., Zhang L., Hua Y. The role of Cl- in the formation of the corrosion products and localised corrosion of 15Cr martensite stainless steel under an CO2-containing extreme oilfield condition. Corrosion Sci. 2022;194 [Google Scholar]
- 91.Sun C., Zeng H., Luo J.-L. Unraveling the effects of CO2 and H2S on the corrosion behavior of electroless Ni-P coating in CO2/H2S/Cl– environments at high temperature and high pressure. Corrosion Sci. 2019;148:317–330. [Google Scholar]
- 92.Leng J., Cheng Y.F., Liao K., Huang Y., Zhou F., Zhao S., Liu X., Zou Q. Synergistic effect of O2-Cl− on localized corrosion failure of L245N pipeline in CO2-O2-Cl− environment. Eng. Fail. Anal. 2022;138:106332. [Google Scholar]
- 93.Wang Q., Wu W., Li Q., Zhang D., Yu Y., Cao B., Liu Z. Under-deposit corrosion of tubing served for injection and production wells of CO2 flooding. Eng. Fail. Anal. 2021;127 [Google Scholar]
- 94.Zhao J.-J., Liu X.-B., Hu S., Han E.-H. Effect of Cl− Concentration on the SCC Behavior of 13Cr Stainless Steel in High-Pressure CO2 Environment. Acta Metall. Sin. 2019;32:1459–1469. [Google Scholar]
- 95.Hua Y., Shamsa A., Barker R., Neville A. Protectiveness, morphology and composition of corrosion products formed on carbon steel in the presence of Cl− , Ca2+ and Mg2+ in high pressure CO2 environments. Appl. Surf. Sci. 2018;455:667–682. [Google Scholar]
- 96.Song W., Yang G., Liao B., Li J., Ma Y., Hao Y. Effect of Cl− Concentration on the Corrosion Behavior of 16Mn Steel in Saturated H2S/CO2 Solution. J. Wuhan Univ. Technol. Mat. Sci. Edit. 2018;33:1205–1215. [Google Scholar]
- 97.Bacca K.R.G., Lopes N.F., Batista G.d.S., dos Santos C.A., Costa E.M.d. Electrochemical, mechanical, and tribological properties of corrosion product scales formed on X65 steel under CO2 supercritical pressure environments. Surf. Coat. Technol. 2022;446 [Google Scholar]
- 98.Kahyarian A., Brown B., Nesic S. Electrochemistry of CO2 corrosion of mild steel: Effect of CO2 on iron dissolution reaction. Corrosion Sci. 2017;129:146–151. [Google Scholar]
- 99.Paolinelli L.D., Pérez T., Simison S.N. The effect of pre-corrosion and steel microstructure on inhibitor performance in CO2 corrosion. Corrosion Sci. 2008;50:2456–2464. [Google Scholar]
- 100.Zheng Z., Hu J., Eliaz N., Zhou L., Yuan X., Zhong X. Mercaptopropionic acid-modified oleic imidazoline as a highly efficient corrosion inhibitor for carbon steel in CO2-saturated formation water. Corrosion Sci. 2022;194 [Google Scholar]
- 101.Wei X., Shi X., Zeng W., Wen X., Liu D., Li Q., Yu X. Synthesis of High Efficient lmidazoline Derivatives and Their Corrosion lnhibition on J55 Steel in Saturated CO2 Solution (In Chinese) Chin. J. Synth. Chem. 2021;29:132–136. [Google Scholar]
- 102.Liu C., Chen X., Yang J., Wang X. Synergistic Corrosion lnhibition Effect of Oleic Acid lmidazoline and Oleic Acid in Saturated CO2 Salt Solution (In Chinese) Surf. Technol. 2022;51:291–299. [Google Scholar]
- 103.Zhang Q.H., Li Y.Y., Zhu G.Y., Lei Y., Wang X., Liu H.F., Zhang G.A. In-depth insight into the synergistic inhibition mechanism of S-benzyl-L-cysteine and thiourea on the corrosion of carbon steel in the CO2-saturated oilfield produced water. Corrosion Sci. 2021;192 [Google Scholar]
- 104.Zhuoke L., Jun C., Ting M., Dan N. Synthesis of bimannich base with thiazole and its corrosion inhibition effect on H2S and CO2 at high temperature. BMC Chem. 2021;15:59. doi: 10.1186/s13065-021-00784-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Farhadian A., Zhao Y., Naeiji P., Rahimi A., Berisha A., Zhang L., Rizi Z.T., Iravani D., Zhao J. Simultaneous inhibition of natural gas hydrate formation and CO2/H2S corrosion for flow assurance inside the oil and gas pipelines. Energy. 2023;269 [Google Scholar]
- 106.Guo Y., Xue J., Zhang J., Chen Q., Fan L., Tang C., Ren K., Fu A., Bi Q. Effect of corrosion products on the inhibitory performance of imidazolium ionic liquid toward carbon steel in CO2-saturated NaCl brine. Colloids Surf. A Physicochem. Eng. Asp. 2022;651 [Google Scholar]
- 107.Palimi M.J., Tang Y., Alvarez V., Kuru E., Li D.Y. Green corrosion inhibitors for drilling operation: New derivatives of fatty acid-based inhibitors in drilling fluids for 1018 carbon steel in CO2-saturated KCl environments. Mater. Chem. Phys. 2022;288 [Google Scholar]
- 108.Shamsa A., Barmatov E., Hughes T.L., Hua Y., Neville A., Barker R. Hydrolysis of imidazoline based corrosion inhibitor and effects on inhibition performance of X65 steel in CO2 saturated brine. J. Petrol. Sci. Eng. 2022;208 [Google Scholar]
- 109.Berdimurodov E., Verma D.K., Kholikov A., Akbarov K., Guo L. The recent development of carbon dots as powerful green corrosion inhibitors: A prospective review. J. Mol. Liq. 2022;349 [Google Scholar]
- 110.Peng Z., Lv F., Feng Q., Zheng Y. Enhancing the CO2-H2S corrosion resistance of oil well cement with a modified epoxy resin. Construct. Build. Mater. 2022;326 [Google Scholar]
- 111.Wang Q.-Y., Wang X.-Z., Luo H., Luo J.-L. A study on corrosion behaviors of Ni–Cr–Mo laser coating, 316 stainless steel and X70 steel in simulated solutions with H2S and CO2. Surf. Coat. Technol. 2016;291:250–257. [Google Scholar]
- 112.Li J., Sun C., Roostaei M., Mahmoudi M., Fattahpour V., Zeng H., Luo J.-L. Characterization and corrosion behavior of electroless Ni-Mo-P/Ni-P composite coating in CO2/H2S/Cl− brine: Effects of Mo addition and heat treatment. Surf. Coating. Technol. 2020;403 [Google Scholar]
- 113.Li J., Sun C., Roostaei M., Mahmoudi M., Fattahpour V., Zeng H., Luo J.-L. Role of Ca2+ in the CO2 corrosion behavior and film characteristics of N80 steel and electroless Ni–P coating at high temperature and high pressure. Mater. Chem. Phys. 2021;267 [Google Scholar]
- 114.Zhang Z., He Y., Bai Y., Song R., He Y., Liu B., Li H., Shangguan J. Influence of iron element on the structure and corrosion resistance of Ni-P coatings in different corrosive environments. Colloids Surf. A Physicochem. Eng. Asp. 2022;655 [Google Scholar]
- 115.Zhu L., Tang Y., Cao S., Jiang J., Wu C., Zhao K. Enhanced anti-microbial corrosion of nano-CuO-loaded Ni coatings on pipeline steels in simulation environment of natural gas transportation pipeline. Ceram. Int. 2023;49:5543–5549. doi: 10.1039/d3ra06940k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Luo H., Wang C., Liu S., Liu S., Fan W., Wang Z., Xie D., Wang H. A novel self-cleaning functional composite coating with extraordinary anti-corrosion performance in high pressure CO2 conditions. Compos. Sci. Technol. 2022;228 [Google Scholar]
- 117.Wang B., Zhao Y., Ran C., Qin Z., Chi Y., Liu E. Fabrication of Superhydrophobic Nickel-Reduced Graphene Oxide Coating with Corrosion Resistance in High-Temperature and High-Pressure CO2 Environment. Adv. Eng. Mater. 2021;24:2101417. [Google Scholar]
- 118.Al Shenawa A., Argade G., Chilukuri A., D’Souza N.A., Nasrazadani S., Scharf T., Banerjee R. Effect of supercritical CO2 on salt water corrosion and wear resistance of bismaleimide coating filled with organophilic montmorillonite clay. J. Adhes. Sci. Technol. 2021;35:2301–2318. [Google Scholar]
- 119.Li J., Sun C., Zeng H., Luo J.L. Insights into the Electrochemical Corrosion Behavior and Mechanism of Electroless Ni-P Coating in the CO2/H2S/Cl- Environment. Corrosion. 2020;403:126416. [Google Scholar]
- 120.Oppong Boakye G., Straume E.O., Kovalov D., Karlsdottir S.N. Wear-reducing nickel-phosphorus and graphene oxide-based composite coatings: Microstructure and corrosion behavior in high temperature geothermal environment. Corrosion Sci. 2022;209 [Google Scholar]
- 121.Wei L., Gao K. Understanding the general and localized corrosion mechanisms of Cr-containing steels in supercritical CO2-saturated aqueous environments. J. Alloys Compd. 2019;792:328–340. [Google Scholar]
- 122.Wu Q., Zhang Z., Dong X., Yang J. Corrosion behavior of low-alloy steel containing 1% chromium in CO2 environments. Corrosion Sci. 2013;75:400–408. [Google Scholar]
- 123.Gao Z., Liu Y., Wang C., Yang H., Xu L., Qiao L. The study on the influence of aluminum on the CO2 corrosion resistance of 3%Cr steel. Anti-corrosion Methods & Mater. 2022;69:177–182. [Google Scholar]
- 124.Chen K., Liu Z., Guo X., Wang H., Shen Z., Zeng X. Effect of surface finishing on the oxidation characteristics of a Fe-21Cr-32Ni alloy in supercritical carbon dioxide. Corrosion Sci. 2022;195 [Google Scholar]
- 125.Mahaffey J., Adam D., Brittan A., Anderson M., Sridharan K. Corrosion of Alloy Haynes 230 in High Temperature Supercritical Carbon Dioxide with Oxygen Impurity Additions. Oxid. Met. 2016;86:567–580. [Google Scholar]