Abstract
Background
Topically‐applied fluoride varnishes have been used extensively as an operator‐applied caries‐preventive intervention for over three decades. This review updates the first Cochrane review of fluoride varnishes for preventing dental caries in children and adolescents, which was first published in 2002.
Objectives
To determine the effectiveness and safety of fluoride varnishes in preventing dental caries in children and adolescents, and to examine factors potentially modifying their effect.
Search methods
We searched the Cochrane Oral Health Group's Trials Register (to 13 May 2013), the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2013, Issue 4), MEDLINE via OVID (1946 to 13 May 2013), EMBASE via OVID (1980 to 13 May 2013), CINAHL via EBSCO (1980 to 13 May 2013), LILACS and BBO via the BIREME Virtual Health Library (1980 to 13 May 2013), ProQuest Dissertations and Theses (1861 to 13 May 2013), and Web of Science Conference Proceedings (1945 to 13 May 2013). A search for ongoing trials was undertaken on ClinicalTrials.gov on 13 May 2013. There were no restrictions on language or date of publication in the search of the electronic databases.
Selection criteria
Randomised or quasi‐randomised controlled trials with blind outcome assessment used or indicated, comparing topically‐applied fluoride varnish with placebo or no treatment in children up to 16 years during at least one year. The main outcome was caries increment measured by the change in decayed, missing and filled tooth surfaces in both permanent (D(M)FS) and primary (d(e/m)fs) teeth.
Data collection and analysis
At least two review authors assessed all search results, extracted data and undertook risk of bias independently. Study authors were contacted for additional information. The primary measure of effect was the prevented fraction, that is the difference in mean caries increments between the treatment and control groups expressed as a percentage of the mean increment in the control group. The caries increments nearest to three years were used from each included study. Random‐effects meta‐analyses were performed where data could be pooled. Potential sources of heterogeneity were examined in random‐effects meta‐regression analyses. Adverse effects information was collected from the included trials.
Main results
Twenty‐two trials with 12,455 participants randomised (9595 used in analyses) were included. For the 13 that contributed data for the permanent tooth surfaces meta‐analysis, the pooled D(M)FS prevented fraction estimate comparing fluoride varnish with placebo or no treatment was 43% (95% confidence interval (CI) 30% to 57%; P < 0.0001). There was substantial heterogeneity, confirmed statistically (P < 0.0001; I2 = 75%), however this body of evidence was assessed as of moderate quality. The pooled d(e/m)fs prevented fraction estimate was 37% (95% CI 24% to 51%; P < 0.0001) for the 10 trials that contributed data for the primary tooth surfaces meta‐analysis, also with some heterogeneity (P = 0.009; I2 = 59%). Once again this body of evidence was assessed as of moderate quality. No significant association between estimates of D(M)FS or d(e/m)fs prevented fractions and the pre‐specified factors of baseline caries severity, background exposure to fluorides, application features such as prior prophylaxis, concentration of fluoride, frequency of application were found. There was also no significant association between estimates of D(M)FS or d(e/m)fs prevented fractions and the post hoc factors: whether a placebo or no treatment control was used, length of follow‐up, or whether individual or cluster randomisation was used, in the meta‐regression models. A funnel plot of the trials in the main meta‐analyses indicated no clear relationship between prevented fraction and study precision. In both methods, power is limited when few trials are included. There was little information concerning possible adverse effects or acceptability of treatment.
Authors' conclusions
The conclusions of this updated review remain the same as those when it was first published. The review suggests a substantial caries‐inhibiting effect of fluoride varnish in both permanent and primary teeth, however the quality of the evidence was assessed as moderate, as it included mainly high risk of bias studies, with considerable heterogeneity.
Plain language summary
Fluoride varnishes for preventing dental caries in children and adolescents
Review question
The main question addressed by this review is how effective the use of fluoride varnish for the prevention of caries in children and adolescents is compared to placebo (a treatment without the active ingredient i.e. fluoride) or no treatment.
Background
Tooth decay (dental caries) is a significant health problem worldwide. It affects not only the vast majority of adults but also children, from 60% to 90% of them. In other words, six to nine children in every 10 are affected by tooth decay. Levels of tooth decay vary both between and within different countries, but it is generally true that children in lower socio‐economic groups (measured by income, education and employment) have greater levels of tooth decay. Untreated tooth decay causes progressive destruction of the tops of teeth (crowns) and this is often accompanied by severe pain and suffering. Repairing and replacing decayed teeth is extremely costly in terms of time and money and is a major drain on the resources of healthcare systems.
The prevention of dental caries in children and adolescents is regarded as a priority for dental services and considered more cost‐effective than its treatment. Fluoride is a mineral that prevents tooth decay. Fluoride is added to the water supply in many areas. It can also be applied directly to teeth in the form of fluoride varnish. This is applied to first (baby) and permanent teeth (depending on the age of the child) usually by a dental professional from two to four times a year. Because it stays on the surface of the tooth for relatively long periods of time it releases fluoride in an efficient and effective way.
Study characteristics
This review of existing studies was carried out by the Cochrane Oral Health Group and the evidence is current up to 13 May 2013. In this updated review there are now 22 trials published between 1975 and 2012 in which a total of 12,455 children were randomised to treatment with either fluoride varnish or placebo/no treatment. Study duration ranged from one to five years among included trials (12 of these lasted two years).
Key results
The evidence produced has been found to be of moderate quality due to issues with trial designs. However in the 13 trials that looked at children and adolescents with permanent teeth the review found that the young people treated with fluoride varnish experienced on average a 43% reduction in decayed, missing and filled tooth surfaces. In the 10 trials looking at the effect of fluoride varnish on first or baby teeth the evidence suggests a 37% reduction in decayed, missing and filled tooth surfaces. There was little information concerning possible adverse effects or acceptability of treatment.
Quality of the evidence
The evidence presented is of moderate quality due to issues with trial designs.
Summary of findings
for the main comparison.
| Fluoride varnish compared with placebo/no treatment for preventing caries in children and adolescents | ||||||
|
Patient or population: Children and adolescents Settings: School/clinic Intervention: Fluoride varnish Comparison: No treatment/placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (trials) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| No treatment/placebo | Fluoride varnish | |||||
|
Permanent tooth surfaces D(M)FS increment PF ‐ nearest to 3 years (14 trials) The duration of the trials ranged from 1 to 5 years with most trials (10) being of 2 to 3 years duration |
Mean increment in control group 0.171 | The corresponding mean increments in the intervention group is 0.10 (95% CI 0.07 to 0.12) | PF = 0.43 (95% CI 0.30 to 0.57) | 6478 (13) | ⊕⊕⊕⊝ moderate2 | |
| Mean increment in control group 2.37 | The corresponding mean increments in the intervention group is 1.35 (95% CI 1.02 to 1.70) | PF = 0.43 (95% CI 0.30 to 0.57) | 6478 (13) | ⊕⊕⊕⊝ moderate2 | ||
| Mean increment in control group 7.72 | The corresponding mean increments in the intervention group is 4.40 (95% CI 3.32 to 5.40) | PF = 0.43 (95% CI 0.30 to 0.57) | 6478 (13) | ⊕⊕⊕⊝ moderate2 | ||
|
Deciduous tooth surfaces d((e)/m)fs increment PF ‐ nearest to 3 years (10 trials) The duration of the trials ranged from 1 to 2.5 years with most trials (7) being of 2 years duration |
Mean increment in control group 0.893 | The corresponding mean increments in the intervention group is 0.56 (95% CI 0.44 to 0.68) | PF = 0.37 (95% CI 0.24 to 0.51) | 3804 (10 ) |
⊕⊕⊕⊝ moderate4 | |
| Mean increment in control group 1.65 | The corresponding mean increments in the intervention group is 1.04 (95% CI 0.81 to 1.25) | PF = 0.37 (95% CI 0.24 to 0.51) | 3804 (10 ) |
⊕⊕⊕⊝ moderate4 | ||
| Mean increment in control group 13.8 | The corresponding mean increments in the intervention group is 8.69 (95% CI 6.76 to 10.49) | PF = 0.37 (95% CI 0.24 to 0.51) | 3804 (10 ) |
⊕⊕⊕⊝ moderate4 | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) CI: confidence interval; PF: prevented fraction | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate Very low quality: We are very uncertain about the estimate | ||||||
1 The mean increments in the control group ranged from 0.17 to 7.72, median 2.37
2 The quality of the evidence was downgraded due to considerable heterogeneity; 9 trials were at high and 4 trials at unclear risk of bias. However this body of evidence showed a consistent, large clinically important effect and we have upgraded the quality of evidence to moderate
3 The mean increments in the control group ranged from 0.89 to 13.8, median 1.65
4 The quality of the evidence was downgraded due to considerable heterogeneity; 5 trials were at high and 5 trials at unclear risk of bias. However this body of evidence showed a consistent, large clinically important effect and we have upgraded the quality of evidence to moderate
Background
Description of the condition
Dental caries is a highly prevalent chronic disease afflicting a significant proportion of the world population, including around 60% to 90% of school‐aged children and the vast majority of adults (Petersen 2004). In general, dental caries levels vary considerably between and within different countries, but children in the lower socio‐economic status (SES) groups have higher caries levels than those in the upper SES groups (Chen 1995; Reisine 2001). Untreated caries causes progressive destruction of the crowns of the teeth, often accompanied by severe pain and suffering. The repair and replacement of carious teeth is excessively time consuming and costly, representing a major drain of resources for healthcare systems.
Description of the intervention
Professionally‐applied fluoride varnishes, developed in the 1960s as a preventive intervention for dental caries, have been extensively used in Europe, Scandinavia and Canada and their use in other countries seems to be increasing, including the United States, where they can be used off‐label as caries preventive agents (Bawden 1998; Beltrán‐Aguilar 2000; Kallestal 1999; WHO 1994). The use of fluoride varnishes is considered appropriate for at risk tooth surfaces in caries susceptible individuals and for moderate and high caries prevalence child populations in community‐based preventive programmes (Petersson 1997). Varnishes were originally developed to prolong the contact time between fluoride and dental enamel, as they adhere to the tooth surface for longer periods (12 hours or more) in a thin layer, and prevent the immediate loss of fluoride after application, thus acting as slow‐releasing reservoirs of fluoride making acute toxicity unlikely (Ogaard 1994). Although various different formulations of fluoride varnishes are available, there are two main preparations commercially known as Duraphat and Fluor Protector. Duraphat contains 5% sodium fluoride (NaF), or 22,600 parts per million fluoride ions (ppm F), in a natural resin carrier with some alcohol included as a solvent. Fluor Protector contains 0.9% difluorosilane by weight (1000 ppm F) in polyurethane‐based varnish and sets to a thin transparent film (originally developed in 1975 by Arends and Schuthof with a fluoride concentration of 0.7%, the fluoride concentration was changed to 0.1% in 1987). Varnishes are usually applied with small brushes, syringes, or cotton pellets, with or without prior dental prophylaxis, at the frequency of two to four times a year. They are considered safe, despite the high fluoride concentration (in Duraphat for example), because the amount of varnish usually applied to treat one child is only 0.5 ml on average (Petersson 1993; Ripa 1990), which delivers 3 to 11 mg of fluoride ion per dose. This is far below the probable toxic dose of 5 mg/kg body weight (Whitford 1992), even with the potential exposure (ingestion) varying from 3.5 to 11.3 mg of fluoride ion (Johnston 1994).
Numerous clinical trials evaluating the caries preventive effect of fluoride varnishes in children in both permanent and primary teeth have been reported, and these have been the subject of several narrative reviews (Beltrán‐Aguilar 2000; Clark 1982; De Bruyn 1987; Petersson 1993; Petersson 1997; Primosch 1985; Seppa 1991; Yanover 1982) and of systematic reviews and meta‐analyses (Bader 2004; Carvalho 2010; Clark 1985; Helfenstein 1994; Petersson 2004; Rozier 2001; Strohmenger 2001). It is evident from these reviews and meta‐analyses that fluoride varnishes are caries‐inhibitory agents. However, they either failed to fully report the quantitative approaches used for data synthesis, or did not include a comprehensive search for individual trials or a formal evaluation of the risk of bias in included trials, despite obvious drawbacks in study design and methods in the trials. Some reviews included trials, mainly carried out in the 1970s, that had adopted the 'split‐mouth' design for example (i.e. used half‐mouth controls). There is a general agreement against the use of the within‐subject paired design for fluoride varnish trials in the literature; a major drawback is that the possibility of significant contamination of control sites cannot be excluded, regardless of the adhesiveness of the material to the tooth surface in the first hours after application (Clark 1982; De Bruyn 1987; Petersson 1993).
How the intervention might work
The most important anti‐caries effect of fluoride is considered to result from its local action on the tooth/plaque interface, through promotion of remineralisation of early caries lesions and by reducing tooth enamel solubility (Featherstone 1988). Enamel demineralisation is markedly inhibited if fluoride is present at the time of the acid challenge because fluoride diffuses with the acid from plaque into the enamel and acts at the crystal surface to reduce mineral loss. When the pH rises following demineralisation, fluoride can combine with dissolved calcium and phosphate ions to precipitate or grow fluorapatite‐like crystalline material within the tooth. Fluoride enhances this mineral gain and provides a material which is more resistant to subsequent acid attack (ten Cate 1999). This occurs with all forms and concentrations of topical fluoride although to a variable extent. Regular use of fluoride toothpaste or mouthrinse results in sustained elevated fluoride concentrations in the oral fluids during the demineralisation/remineralisation cycle, but with higher concentration topical fluoride vehicles (such as varnishes and gels), calcium fluoride is precipitated on the enamel surface and in the plaque. This calcium fluoride acts as a fluoride reservoir which is released when the oral pH falls (Horowitz 1996; Ogaard 1994).
Thus, varnishes deliver fluoride to the surface of enamel and to subsurface carious lesions, where it forms deposits of calcium fluoride and provides a reservoir of fluoride ions (Ogaard 1994). The greatest release occurs during the first three weeks after application, with more gradual release thereafter (Shen 2002).
Why it is important to do this review
The prevention of dental caries in children and adolescents is generally regarded as a priority for dental services and considered more cost‐effective than its treatment (Burt 1998). Fluoride therapy has been the centrepiece of caries‐preventive strategies since the introduction of water fluoridation schemes over five decades ago (Murray 1991). These were introduced when caries was highly prevalent and severe, and when even modest prevention activities led to considerable reductions in disease levels. In the last 30 years, with the substantial decline in dental caries rates in many western countries, an increase in dental fluorosis levels in some countries, and intensive research on the mechanism of action of fluoride highlighting the primary importance of its topical effect, greater attention has been paid to the appropriate use of other fluoride‐based interventions (Featherstone 1988; Featherstone 1999; Glass 1982; Marthaler 1996; O'Mullane 1994; Ripa 1991).
The use of topically‐applied fluoride products in particular, which are much more concentrated than the fluoride in drinking water, has increased over recent decades. By definition, the term 'topically‐applied fluoride' is used to describe those delivery systems which provide fluoride to exposed surfaces of the dentition, at elevated concentrations, for a local protective effect, and are therefore not intended for ingestion. Fluoride‐containing toothpastes (dentifrices), mouthrinses, gels and varnishes are the modalities most commonly used at present, either alone or in combination. Various products are marketed in different countries and a variety of caries preventive programmes based on these have been implemented. Toothpastes are by far the most widespread form of fluoride usage (Murray 1991a; Ripa 1991) and although the reasons for the decline in the prevalence of dental caries in children from different countries has been the subject of much debate (de Liefde 1998; Krasse 1996; Marthaler 1996; Marthaler 2004; Nadanovsky 1995), it has been mainly attributed to the gradual increase in, and regular home use of fluoride in toothpaste (Bratthall 1996; Glass 1982; Marthaler 1994; O'Mullane 1994; Ripa 1991; Rolla 1991).
At the same time, the lower caries prevalence now prevailing in many countries and the widespread availability of fluoride from multiple sources have raised the question of whether topically‐applied fluorides are still effective in reducing caries, and safe, mainly in terms of the potential risk of fluorosis (mottled enamel). This is particularly important as nearly all child populations in high‐income countries are exposed to some source of fluoride, notably in toothpaste, and adverse effects may be rare (such as acute fluoride toxicity) or more subtle (such as mild dental fluorosis) (Marthaler 2004; Murray 1991a).
The evidence on the effect of topically‐applied fluoride products on the prevention of dental caries in children has been extensively reviewed in traditional narrative reviews. A number of reviews focusing on the evaluation of specific fluoride active agents within specific delivery systems have used a quantitative meta‐analytical approach to synthesise trials results (Ammari 2003; Bartizek 2001; Chaves 2002; Clark 1985; Helfenstein 1994; Johnson 1993; Petersson 2004; Stamm 1984; Stamm 1995; Steiner 2004; Strohmenger 2001; Twetman 2004; van Rijkom 1998). However, there has been no systematic investigation evaluating and comparing the effects of the main modalities of topically‐applied fluoride and examining formally the main factors that may influence their effectiveness.
This review is one in a series of systematic reviews of topical fluoride interventions and assesses the effectiveness of fluoride varnishes for the prevention of dental caries in children. It is an update of the review first published in 2002, which suggested a substantial caries‐inhibiting effect of fluoride varnish in both the permanent and primary teeth of children, but based largely on a small number of relatively old trials of variable methodological quality (Marinho 2002).
Objectives
(1) To determine the effectiveness and safety of fluoride varnishes in preventing dental caries in the child/adolescent population. (2) To examine whether the effect of fluoride varnishes is influenced by the initial level of caries severity. (3) To examine whether the effect of fluoride varnishes is influenced by the background exposure to fluoride in water (or salt), toothpastes, or reported fluoride sources other than the study option(s). (4) To examine whether the effect of fluoride varnishes is influenced by fluoride concentration or application features, such as frequency of use and prophylaxis.
Methods
Criteria for considering studies for this review
Types of studies
Randomised or quasi‐randomised controlled trials using or indicating blind outcome assessment, in which fluoride varnish is compared concurrently to a placebo or no treatment group during at least one year. We excluded randomised or quasi‐randomised controlled trials using within‐group paired comparison designs (e.g. split‐mouth trials), or with open outcome assessment or no indication of blind outcome assessment, or lasting less than one year, or controlled trials where random or quasi‐random allocation was not used or indicated.
Types of participants
Children or adolescents aged 16 or less at the start of the study (irrespective of initial level of dental caries, background exposure to fluorides, dental treatment level, nationality, setting where intervention is received or time when it started). Studies where participants were selected on the basis of special (general or oral) health conditions were excluded.
Types of interventions
Topical fluoride in the form of varnishes only, using any fluoride agent, at any concentration (ppm F), amount or duration of application, and with any technique of application, prior or post‐application. However, frequency of application should have been at least once a year. The control group is placebo or no treatment resulting in the following comparison: Fluoride varnish compared with a placebo or no treatment.
Studies where the intervention consisted of any other caries preventive agent or procedure (e.g. other fluoride‐based measures, chlorhexidine, sealants, oral hygiene interventions, xylitol chewing gums) used in addition to fluoride varnish were excluded.
Types of outcome measures
The primary outcome measure in this review was caries increment, as measured by change from baseline in the number of decayed, (missing) and filled permanent surfaces / number of decayed, (extracted/missing) and filled primary surfaces (D(M)FS / d(e/m)fs). Caries is defined here as being recorded at the dentine level of diagnosis. If caries data only reported caries at both dentine and enamel lesions combined then this was used in the analysis (seeData collection and analysis for the different ways of recording caries and reporting the D(M)FT/S / d(m)ft/s scores in permanent and primary teeth in clinical trials of caries preventive interventions).
The following outcomes were considered relevant: coronal dental caries and dental fillings, in both the permanent and the primary dentitions, tooth loss, dental pain, specific adverse effects (oral allergic reactions, mucosal irritation, adverse symptoms such as nausea, gagging, vomiting), use of health service resources (such as visits to dental care units, length of dental treatment time). Studies reporting no dental caries data, reporting only on plaque/gingivitis, calculus, dentine hypersensitivity or fluoride physiological outcome measures (fluoride uptake by enamel or dentine, salivary secretion levels, etc.) were excluded.
Search methods for identification of studies
For the identification of trials included or considered for this review, we developed detailed search strategies for each database searched. These were based on the search strategy developed for MEDLINE (OVID) but revised appropriately for each database. The search strategy used a combination of controlled vocabulary and free text terms and was linked with the Cochrane Highly Sensitive Search Strategy (CHSSS) for identifying randomised trials (RCTs) in MEDLINE: sensitivity maximising version (2008 revision) as referenced in chapter 6.4.11.1 and detailed in box 6.4.c of the Cochrane Handbook for Systematic Reviews of Interventions version 5.1.0 (updated March 2011) (Higgins 2011). Details of the current MEDLINE search strategy are provided in Appendix 3. The search of EMBASE was linked to the Cochrane Oral Health Group filter for identifying RCTs, and the searches of LILACS and BBO were linked to the Brazilian Cochrane Center filter.
Electronic searching (databases and registers)
We searched the following electronic databases:
The Cochrane Oral Health Group's Trials Register (to 13 May 2013) (Appendix 1)
The Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2013, Issue 4) (Appendix 2)
MEDLINE via OVID (1946 to 13 May 2013) (Appendix 3)
EMBASE via OVID (1980 to 13 May 2013) (Appendix 4)
CINAHL via EBSCO (1980 to 13 May 2013) (Appendix 5)
LILACs via BIREME Virtual Health Library (1980 to 13 May 2013) (Appendix 6)
BBO via BIREME Virtual Health Library (1980 to 13 May 2013) (Appendix 6)
ProQuest Dissertations and Theses (1861 to 13 May 2013) (Appendix 7)
Web of Science Conference Proceedings (1945 to 13 May 2013) (Appendix 8).
No restrictions were placed on language or date of publication in the search of the electronic databases.
Ongoing trials
A search of the National Institutes of Health registry and results service (ClinicalTrials.gov) was undertaken on 13 May 2013 (Appendix 9).
Reference searching
All eligible trial reports, previous meta‐analyses and review articles were scanned for relevant references. For the original version of this review reference lists of relevant chapters from preventive dentistry text books on topically‐applied fluoride interventions had also been consulted.
Handsearching
Some handsearching was carried out for the original version of this review, on journals identified as having the highest yield of eligible RCTs / controlled clinical trials (CCTs):
Community Dentistry and Oral Epidemiology (1990 to 1999)
British Dental Journal (1999 to 2000)
Caries Research (1999 to 2000)
Community Dentistry and Oral Epidemiology (1999 to 2000)
Journal of the American Dental Association (1999 to 2000)
Journal of Dental Research (1999 to 2000)
Journal of Public Health Dentistry (1999 to 2000)
European Journal of Oral Sciences (1999 to 2000).
For the update of this review, only handsearching done as part of the Cochrane Worldwide Handsearching Programme was carried out. See the Cochrane Masterlist of journals and issues searched to date for more information.
Personal contact/correspondence
For the original review, we contacted experts in the field of preventive dentistry to identify any unpublished trials or trials which may not be indexed by the major databases. A letter was sent to the author(s) of each included study published during the 1980s and 1990s in order to obtain information on possible unpublished trials eligible for inclusion. All the authors of trials who had been contacted in order to clarify reported information to enable assessment of eligibility or obtain missing data were also asked for unpublished trials.
Based on information extracted mainly from included trials, a list of manufacturers of fluoride varnishes was created for locating unpublished trials, and three fluoride varnish manufacturers were contacted in October 2000 and in December 2012. Information on any unpublished trials was requested from Colgate Oral Pharmaceuticals, Ivoclar North America and Pharmascience.
Data collection and analysis
Selection of studies
The screening for eligibility was done in duplicate by at least two review authors for all potential studies identified from all searches performed.
Trial reports thought to be potentially relevant in languages not known by the review authors were translated and the initial form completed by an author with reference to the translator. Attempts were made to contact authors of trials that could not be classified in order to ascertain whether inclusion criteria were met.
Data extraction and management
At least two review authors extracted data from all included studies in duplicate. Numerical data presented only in graphs and figures were extracted whenever possible. Attempts were made to contact authors through an open‐ended request in order to obtain missing information or for clarification whenever necessary.
Information related to study methodology that was extracted included: study duration (years of follow‐up); comparability of baseline characteristics ‐ methods used pre‐randomisation in sizing/balancing (stratification based on relevant variables) or used post‐randomisation in analysing/adjusting for possible differences in prognostic factors between groups; objectivity/reliability of primary outcome measurement (diagnostic methods and thresholds/definitions used and included, and monitoring of diagnostic errors); and any co‐intervention or contamination or both. Information on sponsoring institutions and manufacturers involved was also recorded.
Characteristics related to participants that were extracted included: age (mean and range) at start; caries severity at start (average DMFS/dmfs, DFS/dfs, or other measure); background exposure to other fluoride sources (toothpaste, water, etc.); year study began; location where study was conducted (country); setting where participants were recruited; and dental treatment level (F/DMF).
Characteristics of the intervention that were extracted included: methods (technique/device) of application, prior and post‐application; fluoride active agents and concentrations used; frequency and duration of application; and amount applied. Information on what the fluoride varnish was compared to (no treatment or placebo) was also recorded. These data are described in the Characteristics of included studies table.
Different ways of assessing/reporting caries increment (change from baseline as measured by the decayed‐missing‐filled (DMF) index) in the trials were recorded separately and/or combined according to the components of the index chosen and units measured (DMFT/S, or DFT/S, or DT/S, or FT/S); types of tooth/surface considered (primary/permanent teeth/surfaces, first molar teeth, approximal surfaces, etc.); state of tooth eruption considered (erupted and/or erupting teeth or surface); diagnostic thresholds used (cavitated/dentine lesions, non‐cavitated/incipient lesions); methods of examination adopted (clinical or radiographical or both, other); and approaches to account or not for reversals in caries increment adopted (in a net or observed caries increment respectively). In addition, caries increment data have been recorded at all reported time periods (at various follow‐ups).
As we were aware that caries increment could be reported differently in different trials, we developed a set of a priori rules to choose the primary outcome data (D(M)FS) for analysis from each study: DFS data would be chosen over DMFS data, and these would be chosen over DS or FS; data for 'all surface types combined' would be chosen over data for 'specific types' only; data for 'all erupted and erupting teeth combined' would be chosen over data for 'erupted' only, and these over data for 'erupting' only; data from 'clinical and radiological examinations combined' would be chosen over data from 'clinical' only, and these over 'radiological' only; data for dentinal/cavitated caries lesions would be chosen over data for enamel/non‐cavitated lesions; net caries increment data would be chosen over crude (observed) increment data; and follow‐up nearest to three years (often the one at the end of the treatment period) would be chosen over all other lengths of follow‐up, unless otherwise stated. When no specification was provided with regard to the methods of examination adopted, diagnostic thresholds used, groups of teeth and types of tooth eruption recorded, and approaches for reversals adopted, the primary choices described above were assumed.
The Characteristics of included studies table provides a description of all the main outcome data reported from each study with the chosen primary outcome measure featuring at the top. All other relevant outcomes identified as being assessed in the trials are also listed in this table.
Assessment of risk of bias in included studies
At least two review authors undertook the assessment of the risk of bias in all of the included trials independently. Disagreements were resolved by discussion or the involvement of another review author. This was carried out using The Cochrane Collaboration's tool for assessing risk of bias as outlined in the Cochrane Handbook for Systematic Reviews of Interventions version 5.1 (Higgins 2011), but according to pre‐defined criteria which were adapted and refined for the Cochrane topical fluoride reviews updates. Eight domains, namely sequence generation, allocation concealment, blinding of participants, blinding of outcome assessment, incomplete outcome data, selective outcome reporting, baseline balance, and free from contamination or co‐intervention, were assessed according to the tool. Each domain included one or more specific entries in a 'Risk of bias' table. Within each entry, information reported in the study was described and a judgement relating to the risk of bias for that entry was assigned. Where the study clearly reported the methodology, a judgement of 'low risk of bias' or ' high risk of bias' was made. Where trial methodology was unclear, a domain was judged at 'unclear risk of bias' unless and until further information became available. After taking into account the additional information provided by the authors of the trials, the overall risk of bias in included trials was assessed over all eight domains. Studies were graded into the following categories.
Low risk of bias (plausible bias unlikely to seriously alter the results: all eight domains assessed as at low risk of bias).
Moderate risk of bias (plausible bias that raises some doubt about the results: at least one domain assessed as at unclear risk of bias, but none at high risk of bias).
High risk of bias (plausible bias that seriously weakens confidence in the results: at least one domain assessed as at high risk of bias).
Measures of treatment effect
Prevented fraction (PF) was the measure of treatment effect presented for caries increment. The prevented fraction is calculated as the mean increment in the control group minus the mean increment in the intervention group divided by the mean increment in the control group. For an outcome such as caries increment (where discrete counts are considered to approximate to a continuous scale and are treated as continuous outcome), this measure was considered more appropriate than the mean difference or standardised mean difference since it allowed combination of different ways of measuring caries increment and a meaningful investigation of heterogeneity between trials. It is also simple to interpret.
For outcomes other than caries increment, continuous data were to be analysed according to differences in mean treatment effects and their standard deviations. Dichotomous outcome data were analysed by calculating risk ratios (RRs).
Unit of analysis issues
Not all the cluster randomised trials reported results adjusted for the clustering present in the data. In such cases, we estimated the design effect with the intra‐class correlation coefficient (ICC) if reported or a value of 0.05 (Lawrence 2008; ICC = 0.045). This was then used to modify the numbers in the intervention and control groups by calculating the effective sample size.
Dealing with missing data
We decided that missing standard deviations for caries increments that were not revealed by contacting the original researchers would be imputed through linear regression of log standard deviations on log mean caries increments. This is a suitable approach for caries prevention trials since, as they follow an approximate Poisson distribution, caries increments are closely related (similar) to their standard deviations (van Rijkom 1998). This approach was undertaken wherever possible. Where caries increment data were not reported but baseline and final mean caries scores were reported instead, mean caries increments were calculated and standard deviation of the increments estimated using a correlation coefficient between the baseline and final values of 0.5.
Assessment of heterogeneity
Heterogeneity was assessed by inspection of a graphical display of the estimated treatment effects from the trials along with their 95% confidence intervals and by formal tests of homogeneity undertaken prior to each meta‐analysis (Thompson 1999). This was also quantified by the I2 statistic and classified according to the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). A rough guide to interpretation: 0% to 40% might not be important, 30% to 60% may represent moderate heterogeneity, 50% to 90% may represent substantial heterogeneity and 75% to 100% considerable heterogeneity.
Assessment of reporting biases
Funnel plots (plots of effect estimates versus the inverse of their standard errors (SE)) were drawn where there were sufficient trials (> 10). Asymmetry of the funnel plot may indicate publication bias and other biases related to sample size, though may also represent a true relationship between trial size and effect size. A formal investigation of the degree of asymmetry was planned using the method proposed by Egger 1997 and Harbord 2005.
Data synthesis
The meta‐analyses were conducted as inverse variance weighted averages. PF variances were estimated using the formula presented in Dubey 1965. Random‐effects meta‐analyses were performed throughout. The prevented fraction data PF (SE) were entered using the GIV option. Primary and permanent teeth were analysed separately throughout.
Dichotomous outcome data were analysed by calculating RRs. Again random‐effects models were used to calculate a pooled estimate of effect.
Dealing with studies with more than one intervention arm
In the trials with more than one relevant intervention group and a common control group, such as those comparing different active fluoride agents or concentrations of fluoride ions to a placebo group, summary statistics from the trials (number of children analysed, mean caries increments and standard deviations) from all relevant intervention groups were combined in order to obtain a measure of treatment effect. This enables the inclusion of all relevant data in the primary meta‐analysis, although may slightly compromise any secondary investigations of dose response.
Subgroup analysis and investigation of heterogeneity
Three potential sources of heterogeneity were specified a priori, and these formed part of the primary objectives of the review. We hypothesised that: (1) the effect of fluoride varnishes differs according to the baseline levels of caries severity, (2) the effect of fluoride varnishes differs according to exposure to other fluoride sources (in water, in toothpastes, etc.) and (3) the effect of fluoride varnishes differs according to characteristics of use (fluoride concentration or application features, such as frequency of use and prophylaxis).
For this update it was also hypothesised that trials could be categorised according to whether the teeth which the intervention had been applied to were within two years of eruption. This is important as newly erupted teeth are thought to be at higher risk of caries. If sufficient number of trials were included, the association of these factors with estimated effects (PF) would be examined by performing random‐effects meta‐regression analyses in Stata version 12.0 (Stata Corporation, USA) using the program 'Metareg'.
To allow such investigation, relevant data were dealt with as follows: data on 'baseline levels of caries' were calculated from the study sample analysed (final sample) unless otherwise stated, and were averaged among all relevant study groups. Data on 'background exposure to other fluoride sources' combined data on the use of fluoride toothpaste and the consumption of fluoridated water (or salt) and were grouped into two categories: one for trials which were based on samples provided with non‐fluoride toothpaste and which were from non‐fluoridated areas (non‐exposed), and another for trials based on samples using fluoride toothpaste or trials in fluoridated communities or both. When use or non‐use of fluoride toothpaste was not clearly indicated in trials carried out in high‐income countries, it was assumed that fluoride toothpaste was widely used from the middle of the 1970s (Ripa 1989); this information was sought from authors (or obtained from other sources) when missing from trials carried out in other locations. When data on the year a study had begun were not provided, these were calculated as a 'probable date' by subtracting the duration of the study (in years) plus one extra year, from the publication date of the study.
Further potential sources of heterogeneity were investigated by meta‐regression ‐ for different types of control groups (placebo (PL) or no treatment (NT)), different types of randomisation (individual child or cluster) and time since eruption (permanent teeth only), but these post hoc analyses were reported as such, and findings should be treated with caution. It should be remembered that all the meta‐regressions have low power and the findings should not be interpreted as no effect.
Sensitivity analysis
We intended to undertake a sensitivity analysis including the trials with an overall assessment of low risk of bias, however there were no trials satisfying this criteria. We undertook a sensitivity analysis excluding trials where we imputed missing data such as standard deviations and the design effect in cluster randomised trials.
Results
Description of studies
Results of the search
Identification of reports/trials
The full search conducted as described in Search methods for identification of studies on 13 May 2013 has been used to construct the PRISMA flow chart shown in Figure 1.
1.
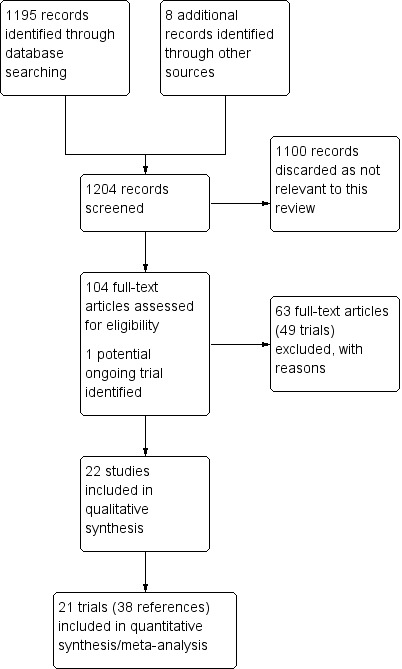
Study flow diagram from 2013 search
Selection of trials
For this update, 1204 reports were identified by the searches (from databases and other sources) and 104 full‐text articles were assessed as potentially eligible. These comprised 40 reports relating to 22 included trials (including the nine trials already identified as included in the initial review), 63 reports relating to 49 excluded trials (including the 33 trials already identified as excluded in the initial review) and one ongoing study which may be eligible (Figure 1).
Ongoing trials
We identified one ongoing trial which may be eligible (Macpherson 2012).
Included studies
SeeCharacteristics of included studies table for details of each study, a summary of some of the data is given in Additional Table 2.
1. Study details.
| Study | NT or placebo | Study duration (years) | Number randomised | Number analysed | Cluster RCT | Setting | Age (years) | Varnish manufacturer | F conc (ppmF) | Frequency per year |
| Arruda 2012 | NT | 1 | 379 | 210 | No | School | 7 to 14 | Cavity Shield | 22,600 | 2 |
| Borutta 1991 | Placebo | 2 | 400 | 360 | No | Unclear | 12 to 14 | Lawefluorid Bifluord | 22,600 56,300 | 2 & 4 |
| Borutta 2006 | NT | 2 | 288 | 200 | Yes | Nursery | 2 to 4 | Duraphat Fluoridin | 22,600 22,600 | 2 |
| Bravo 1997 | NT | 2 | 265* | 214 | Yes | School | 6 to 8 | Duraphat | 22,600 | 2 |
| Chu 2002 | Placebo | 2.5 | 146 | 123 | No | School | 3 to 5 | Duraphat | 22,600 | 4 |
| Clark 1985 | Placebo | 5 | 787 | 676 | No | School | 6 to 7 | Duraphat Fluor Protector | 22,600 7000 | 2 |
| Frostell 1991 | NT | 2 | 206 | 206 | No | Unclear | 4 | Duraphat | 22,600 | 2 |
| Glugwad 2011 | NT | 1 | 250 | 211 | No | Unclear | 6 to 7 | Cavity Shield | 22,600 | 3 times in 1 week |
| Hardman 2007 | NT | 2 | 2091 | 664 | Yes | School | 6 to 8 | Duraphat | 22,600 | 2 |
| Holm 1979 | NT | 2 | 250 | 225 | No | Clinic | Mean 3 | Duraphat | 22,600 | 2 |
| Holm 1984 | NT | 2 | 113 | 95 | No | Clinic | 6 | Duraphat | 22,600 | 2 |
| Koch 1975 | NT | 1 | 135 | 121 | No | Clinic | 15 | Duraphat | 22,600 | 2 |
| Lawrence 2008 | NT | 2 | 1275 | 1160 | yes | Clinic | 1 to 5 | Duroflor | 22,600 | 2 to 3 |
| Liu 2012 | Placebo | 2 | 252 | 240 | no | School | Mean 9.1 | Duraphat | 22,600 | 2 |
| Milsom 2011 | NT | 3 | 2967 | 2604 | Yes | School | 7 to 8 | Duraphat | 22,600 | 3 |
| Modeer 1984 | NT | 3 | 236 | 194 | No | Clinic | 14 | Duraphat | 22,600 | 4 |
| Salazar 2008 | Placebo | 1 | 200 | 148 | No | Clinic | 1 to 4 | Duraphat | 22,600 | 2 |
| Sköld 2005 | NT | 1 | 854 | 758 | No | School | 13 | Duraphat | 22,600 | 2, 3 & 8 |
| Tagliaferro 2011 | NT | 2 | 219 | 177 | No | School | 6 to 8 | Duraphat | 22,600 | 2 |
| Tewari 1990 | Placebo | 2.5 | 766* | 618 | No | Clinic | 6 to 12 | Duraphat | 22,600 | 2 |
| Weintraub 2006 | Placebo | 2 | 376 | 280 | No | Clinic | 1 to 4 | Duraphat | 22,600 | 1.5 |
| Yang 2008 | Placebo | 2 | 150 | 111 | No | Nursery | 3 | Fluor Protector | 5000 1000 | 2 |
* the number randomised was unclear so estimate from other studies of 19% used F = fluoride; NT = no treatment; RCT = randomised controlled trial
There are 22 trials included, published between 1975 and 2012. Six trials were conducted in Sweden (Frostell 1991; Holm 1979; Holm 1984; Koch 1975; Modeer 1984; Sköld 2005), three in Brazil (Arruda 2012; Salazar 2008; Tagliaferro 2011) and China (Chu 2002; Liu 2012; Yang 2008), two in Germany (Borutta 1991; Borutta 2006), Canada (Clark 1985; Lawrence 2008), India (Gugwad 2011; Tewari 1990), UK (Hardman 2007; Milsom 2011), and one in each of the following countries: Spain (Bravo 1997) and USA (Weintraub 2006). Ten trials had multiple publications. Eighteen of the included trials did not mention involvement with a fluoride varnish manufacturer, two acknowledged the supply of varnish (Arruda 2012; Weintraub 2006) and one acknowledged supply of equipment to apply the varnish (Borutta 2006). The only one which acknowledged partial financial support from a fluoride varnish manufacturer (Frostell 1991) also acknowledge support from a sugar company.
Two of the published trials (Hardman 2007; Weintraub 2006) included in this update were listed as ongoing trials in the last published version of this review.
Design and methods
All the included trials used parallel group designs (the split‐mouth trials were excluded), five being cluster randomised trials (Borutta 2006; Bravo 1997; Hardman 2007; Lawrence 2008; Milsom 2011). Six trials had more than one fluoride varnish treatment group compared to a placebo or no treatment (Borutta 1991; Borutta 2006; Clark 1985; Sköld 2005; Weintraub 2006; Yang 2008). With regard to type of control group used, 14 trials used a no treatment control group, and the remaining eight used a placebo control group, however five of these used an inactive treatment other than varnish ('placebo' solution/distilled water). The study duration (indicated by the total length of follow‐up as well as the treatment duration) ranged from one to five years among the included trials (12 of these lasted two years). Studies were of moderate size with seven trials allocating less than 100 children to relevant study groups. The total number of children participating in the 22 included trials (given by the sample analysed at the end of the trial period) was 9595, and ranged from 95 in the smallest trial to 2604 in the largest trial (although this was a cluster trial). Eleven trials conducted the trials in schools or nurseries, eight in clinics and the setting was unclear in the remaining three trials.
Participants
The ages of the children at the start of the trials ranged from 1 to 15 years, with similar numbers from both sexes (where these data were reported); 14 trials included participants who were over six years of age at the start, and eight trials included children from one to five (in which primary teeth have been assessed for caries development). Decayed, (missing) and filled permanent surfaces (D(M)FS) at baseline, reported in 11 of the trials, ranged from 0 to 29.2, and from 0 (ds) to 12.4 (dmfs) in the eight trials that reported data for primary dentition. With regard to 'background exposure to other fluoride sources', only three trials were conducted in water fluoridated communities (Holm 1984; Sköld 2005; Weintraub 2006) and only one (Borutta 1991) clearly reported no exposure to fluoride toothpastes; 13 trials reported some other exposure to fluoride (rinses, tablets), with one study mentioning fluoridated milk (Hardman 2007). Seven studies reported that both groups received oral hygiene advice or instruction (Arruda 2012; Chu 2002; Gugwad 2011; Lawrence 2008; Liu 2012; Tagliaferro 2011; Weintraub 2006).
Interventions
Teeth were usually painted with a fluoride varnish using a small brush (10 trials), in other trials the use of a probe or cotton swab was reported. The use of NaF‐based varnishes (Duraphat, Lawefluor, Bifluorid 12, 3M™ CavityShield™, Fluoridin, Difluorsilane (Fluor Protector) was reported in all trials. The fluoride concentration in 18 trials was 22,600 ppm F; the other trials ranged from 7000 ppm F (Difluorsilane) to 56,300 ppm F (6% NaF + 6% calcium fluoride (CaF) (Borutta 1991)). Two trials had arms with fluoride varnish applied with less than 5% fluoride (Clark 1985; Yang 2008). The application frequency of twice a year was tested in 17 trials and that of four times a year in only three trials (Borutta 1991; Chu 2002; Modeer 1984). One study applied the varnish three times in one week with no other applications (Gugwad 2011). The amount of varnish applied was usually of around 0.5 ml per child (reported in five trials). Where the actual application time was reported it ranged from 1 to 4 minutes. The performance of some form of tooth prophylaxis prior to administering the varnish was reported in seven trials (Clark 1985; Frostell 1991; Gugwad 2011; Holm 1984; Koch 1975; Modeer 1984; Sköld 2005), with four trials with no paste and three with a non‐fluoride paste (if with a fluoride paste the trial would have been excluded). The prior tooth cleaning was considered by the review authors as a possible part of the technique of varnish application and not as a separate intervention on its own.
Outcome measures
All 22 included trials reported caries increment data at the tooth surface level with D(M)FS reported in 13 trials, 11 trials reporting d(e/m)fs, two trials reporting both D(M)FS and d(e/m)fs (Gugwad 2011; Hardman 2007). Five of the 11 trials reported caries increment data at the tooth level (D(M)FT) and only three trials reported caries increment data for primary teeth at the tooth level (dmft). With regard to the components of the DMFS index used (and types of teeth/surface assessed), 13 trials reported DMFS data (five trials for first molars only and six trials for all tooth surface types) and the other two reported DFS data (one trial for posterior approximal surfaces only and another for first molar fissures only), one also reported DS and FS data separately. (No choice had to be made between DMFS or DFS data in any one trial, but DFS data were chosen over DS/FS data in one of the trials.) All trials reported D(M)FS data on specific teeth or tooth surfaces ‐ first molars, occlusal, mesio‐distal (approximal) and/or buco‐lingual ‐ but three of these did not report data on all tooth surfaces (whole mouth). D(M)FS data were reported at more than one follow‐up time in two trials only; follow‐up of two years was the most common among all trials.
Details of all the caries outcomes reported for each trial are given in the Characteristics of included studies table. The caries outcomes used in the meta‐analyses are described. Twenty studies included a visual examination, three with the International Caries Detection and Assessment System (ICDAS) (Arruda 2012; Liu 2012; Salazar 2008), three with fibre‐optic transillumination (FOTI) (Borutta 2006; Chu 2002; Hardman 2007), and variable use of a probe was reported including tactile criteria. X‐rays were used in addition to visual examination in three trials (Frostell 1991; Gugwad 2011; Koch 1975). Two trials diagnosed approximal caries in permanent molars only from X‐rays (Modeer 1984; Sköld 2005). Data at the dentine cavitation level of diagnosis were used in the analysis for 16 trials and that for non‐cavitated plus cavitated in six trials (Arruda 2012; Gugwad 2011; Lawrence 2008; Sköld 2005; Tewari 1990; Yang 2008;). In seven of the 16 trials with dentine level data, the increment of non‐cavitated lesions were also reported (Frostell 1991; Hardman 2007; Holm 1979; Koch 1975; Modeer 1984; Salazar 2008; Tagliaferro 2011). Caries increments on only selected teeth were reported in seven trials: primary anterior teeth (Chu 2002), and permanent molars (Clark 1985; Milsom 2011; Modeer 1984; Sköld 2005; Tagliaferro 2011).
Other dental caries data reported were: caries progression rate (Modeer 1984; Sköld 2005), proportion of children developing new caries (five trials in the permanent dentition, five trials in the primary dentition), proportion of teeth developing new caries and failures (carious teeth) over time (Holm 1984), and 'net' increment data taking account of reversals (Lawrence 2008).
Three studies provided data reporting no adverse effects (Salazar 2008; Sköld 2005; Weintraub 2006). One study reported oral health habits and diet (Arruda 2012) and costs (Bravo 1997).
Excluded studies
SeeCharacteristics of excluded studies table for the description of reasons for rejecting each study.
The 49 trials in this section were excluded for a variety of reasons and these have been categorised as related to the study design, intervention/comparison, participant or outcome as given below (some trials appear in more than one category).
Study design related
Study design inappropriate for review (split‐mouth trials): 12 trials (Billy‐Pryga 1983; Bodnar 1984; Kolehmainen 1979; Kolehmainen 1981; Murray 1977; Pashaev 1977; Riethe 1977; Ruszynska 1978; Salem 1979; Schmidt 1970; Seppä 1982; Suwansingha 2011).
Not RCT or quasi‐RCT or unlikely to be so: 23 trials (Grodzka 1982; Heuser 1968; Ivanova 1990; Ji 2007; Kunin 1991; Lagutina 1978; Lieser 1978; Maiwald 1974; Maiwald 1978; Mari 1988; Mari 1988a; Petersson 1998; Shobha 1987; Splieth 2000; Suntsov 1991; Suwansingha 2011; Todorashko 1983; Treide 1980; van Eck 1984; Wacińska‐Drabińska 1987; Wegner 1976; Winter 1975; Zimmer 1999).
No blind outcome assessment used/indicated: two trials (Ramos 1995; Wojtowicz 1986).
Intervention/comparison related
Other intervention with fluoride varnish: five trials (Dülgergil 2005; Hetzer 1973; Rodríguez Miró 1988; Schioth 1981; Slade 2011).
Other intervention with control group: two trials (Lindquist 1989; Ramos‐Gomez 2012).
Participants related
Medically/dentally compromised participants: two trials (Demito 2011; Hochstein 1975).
Outcomes related
Follow‐up < one year or one school year: 5 trials (Alves 1997; Autio‐Gold 2001; Suwansingha 2011; Tranaeus 2001; Xhemnica 2008).
Risk of bias in included studies
Allocation
Sequence generation
Eight of the included trials (Gugwad 2011; Lawrence 2008; Liu 2012; Milsom 2011; Modeer 1984; Salazar 2008; Tewari 1990; Weintraub 2006) were assessed at low risk of bias for this domain. Six of these used computer generated randomisation sequences, one used the lottery method (Gugwad 2011), and one used random number tables (Modeer 1984). The study by Hardman 2007 was cluster randomised using a computer generated sequence but recruitment into the study was done after randomisation of the clusters and the high rate of pre‐recruitment drop‐outs (56%) may have led to selection bias so this study was assessed as at unclear risk of bias for this domain. Six trials (Arruda 2012; Bravo 1997; Chu 2002; Frostell 1991; Holm 1979; Tagliaferro 2011) used quasi‐random allocation and were assessed at high risk of selection bias. The remaining seven trials provided insufficient information in the study report to enable a judgement to be made and so these were assessed at unclear risk of bias for this domain.
Allocation concealment
Allocation was concealed from the investigators in six trials which were assessed at low risk of bias (Gugwad 2011; Hardman 2007; Lawrence 2008; Milsom 2011; Salazar 2008; Weintraub 2006). Six trials reported insufficient information about allocation concealment but the poor randomisation methods used would have made adequate allocation concealment impossible in these trials (Arruda 2012; Bravo 1997; Chu 2002; Frostell 1991; Holm 1979; Tagliaferro 2011) and they were assessed at high risk of bias. In 10 of the included trials there was insufficient information, either in the study report or in response to our emails to study authors, to make a judgement about whether allocation concealment took place so these trials were assessed at unclear risk of bias.
In summary five trials were at low risk of selection bias (Gugwad 2011; Lawrence 2008; Milsom 2011; Salazar 2008; Weintraub 2006), six trials were at high risk of selection bias (Arruda 2012; Bravo 1997; Chu 2002; Frostell 1991; Holm 1979; Tagliaferro 2011) and the remaining 11 trials were at unclear risk of selection bias.
Blinding
Five trials were described as double blind and reported the use of a placebo (Arruda 2012; Borutta 1991; Clark 1985; Salazar 2008; Yang 2008), and we assumed that participants were blinded to allocated intervention and assessed these trials at low risk of performance bias. In a further three trials (Chu 2002; Tewari 1990; Weintraub 2006) the use of a placebo was reported and we assessed that participants were likely to be unaware of allocated treatment. These eight trials were assessed at low risk of performance bias. In the study by Gugwad 2011 participant blinding was unclear and this study was assessed at unclear risk of performance bias. In the remaining 13 trials, there was no placebo used and no participant blinding so we assessed these trials at high risk of performance bias.
Blinding of outcome assessors to allocated treatment group was clearly reported in 20 of the 22 included trials (91%) and these were assessed at low risk of detection bias. In two trials (Holm 1984; Lawrence 2008) blind outcome assessment was not reported, but deemed likely, and these were assessed at unclear risk of detection bias.
Incomplete outcome data
Seven of the included trials (Holm 1979; Lawrence 2008; Liu 2012; Milsom 2011; Tagliaferro 2011; Tewari 1990; Yang 2008) reported low overall rates of attrition, with numbers lost and reasons similar in each group, so these were assessed at low risk of attrition bias. Two trials (Hardman 2007; Modeer 1984) were assessed at high risk of attrition bias. In Hardman 2007 the overall rate of post‐randomisation attrition was high (664 out of 2091) and in Modeer 1984 there was a big difference in percentage of participants lost in each group and the main reason given, poor co‐operation, was unbalanced between the groups. The remaining 13 trials were assessed at unclear risk of attrition bias because either the attrition rate was high (Arruda 2012; Salazar 2008), but similar in both groups, or the reasons for attrition were not described (Borutta 2006; Chu 2002; Clark 1985; Gugwad 2011; Holm 1984; Koch 1975; Sköld 2005; Weintraub 2006), the reasons were not balanced between groups (Borutta 1991; Bravo 1997) or the numbers include in the outcome evaluation were not reported (Frostell 1991).
Selective reporting
Ideally we would like to compare the outcomes listed in each study protocol with the outcomes reported in the papers but this was seldom possible. Nineteen included trials were assessed at low risk of reporting bias (Borutta 1991; Bravo 1997; Chu 2002; Clark 1985; Frostell 1991; Hardman 2007; Holm 1979; Holm 1984; Koch 1975; Lawrence 2008; Liu 2012; Milsom 2011; Modeer 1984; Salazar 2008; Sköld 2005; Tagliaferro 2011; Tewari 1990; Weintraub 2006; Yang 2008) because the outcomes reported in the results section were all those listed in the methods of the paper. One trial was assessed at high risk of reporting bias (Borutta 2006) and outcomes were reported without estimates of variance. In the remaining two trials (Arruda 2012; Gugwad 2011) the risk of reporting bias was assessed as unclear because one or more measured outcomes were not reported.
Other potential sources of bias
Baseline imbalance
We also assessed whether there was a balance of important prognostic factors between the arms of the included trials. Eighteen trials (82%) were assessed at low risk of bias for this domain as the differences between groups in prognostic factors such as caries prevalence at baseline and toothbrushing habits or diet or both during the study were not clinically important. However, four trials were assessed at high risk of bias due to baseline imbalance (Arruda 2012; Borutta 2006; Holm 1979; Modeer 1984) for at least one important prognostic factor.
Contamination/co‐intervention
Thirteen trials (59%) were assessed at low risk of bias due to co‐intervention (Arruda 2012; Chu 2002; Gugwad 2011; Hardman 2007; Holm 1984; Koch 1975; Lawrence 2008; Liu 2012; Milsom 2011; Modeer 1984; Tagliaferro 2011; Tewari 1990; Weintraub 2006). In Sköld 2005, 95% of the study participants, including those in the no treatment control group, had at least one fluoride varnish treatment, so this trial was assessed at high risk of bias due to co‐intervention. In the remaining seven included trials there were some differences between the groups with regard to co‐interventions or contamination but the risk of bias from these was assessed as unclear.
Overall risk of bias
A summary of the risk of bias assessments for each domain across studies is shown in Figure 2 and for each study is shown in Figure 3 . None of the trials included in this review are assessed at low risk of bias for all domains. Most (15 trials, 68%) are at high risk of bias in at least one domain (Arruda 2012; Borutta 2006; Bravo 1997; Chu 2002; Frostell 1991; Hardman 2007; Holm 1979; Holm 1984; Koch 1975; Lawrence 2008; Liu 2012; Milsom 2011; Modeer 1984; Sköld 2005; Tagliaferro 2011) and the remaining seven trials are at unclear risk of bias due to the lack of clear information for at least one domain.
2.
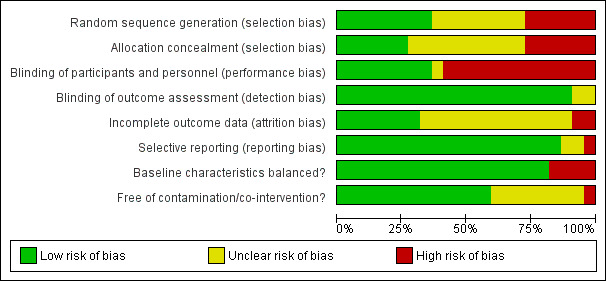
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included trials
3.
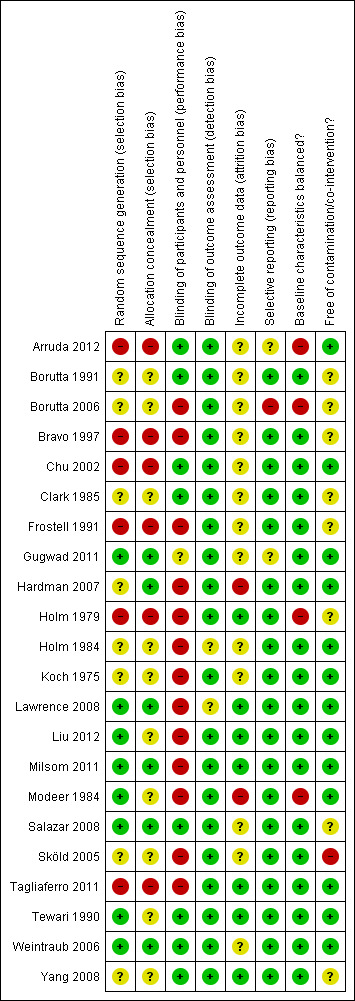
Risk of bias summary: review authors' judgements about each risk of bias item for each included study
Effects of interventions
See: Table 1
The data from 9595 children were included in the pooled meta‐analyses, 6478 in the meta‐analysis for permanent teeth and 3804 in the meta‐analysis for primary teeth (children from two trials were included in both).
Effect of fluoride varnish on caries increment
The effects of fluoride varnishes on caries increment were reported in a variety of different ways in the included trials. One study was not able to be included in the meta‐analysis because it was a cluster randomised trial with no standard deviations presented (Borutta 2006) and was judged as part of the qualitative data synthesis only (Figure 1). Data from the other trials have been extracted as appropriate to produce pooled estimates as described in the methods section. The results are reported separately for:
(1) Prevented fraction (PF)
decayed, (missing) and filled permanent surfaces prevented fraction (D(M)FS PF) (Analysis 1.1; 13 trials)
decayed, (missing) and filled permanent teeth prevented fraction (D(M)FT PF) (Analysis 1.2; five trials)
decayed, (extraction indicated/missing), and filled primary surfaces prevented fraction (d(e/m)fs PF) (Analysis 1.3; 10 trials) (Analysis 1.4; one trial)
decayed, (extraction indicated/missing), and filled primary teeth prevented fraction (d(e/m)ft PF) (Analysis 1.5; two trials) (Analysis 1.6; one trial).
1.1. Analysis.
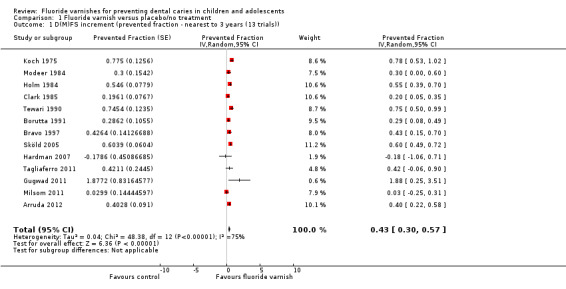
Comparison 1 Fluoride varnish versus placebo/no treatment, Outcome 1 D(M)FS increment (prevented fraction ‐ nearest to 3 years (13 trials)).
1.2. Analysis.
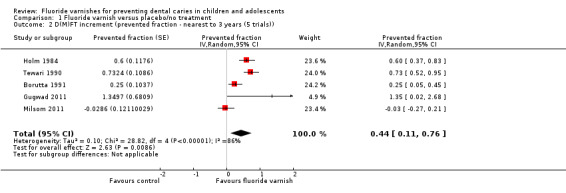
Comparison 1 Fluoride varnish versus placebo/no treatment, Outcome 2 D(M)FT increment (prevented fraction ‐ nearest to 3 years (5 trials)).
1.3. Analysis.
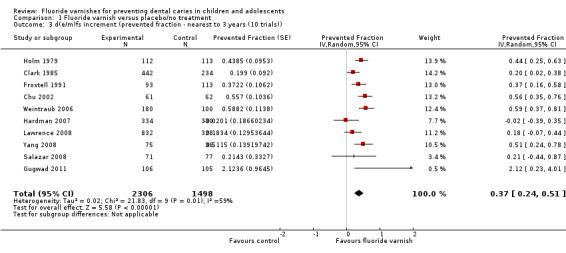
Comparison 1 Fluoride varnish versus placebo/no treatment, Outcome 3 d(e/m)fs increment (prevented fraction ‐ nearest to 3 years (10 trials)).
1.4. Analysis.
Comparison 1 Fluoride varnish versus placebo/no treatment, Outcome 4 d(e/m)fs increment (prevented fraction ‐ 2 years (incomplete data)).
| d(e/m)fs increment (prevented fraction ‐ 2 years (incomplete data)) | |||||
|---|---|---|---|---|---|
| Study | FV n | FV mean | NT n | NT mean | PF |
| Borutta 2006 | 136 | 2.01 | 64 | 4.87 | 58.7 |
1.5. Analysis.
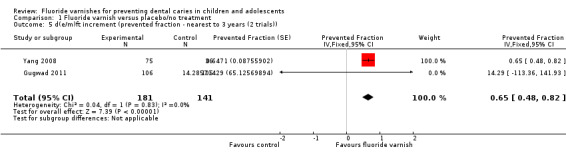
Comparison 1 Fluoride varnish versus placebo/no treatment, Outcome 5 d(e/m)ft increment (prevented fraction ‐ nearest to 3 years (2 trials)).
1.6. Analysis.
Comparison 1 Fluoride varnish versus placebo/no treatment, Outcome 6 d(e/m)ft increment (prevented fraction ‐ 2 years (incomplete data)).
| d(e/m)ft increment (prevented fraction ‐ 2 years (incomplete data)) | |||||
|---|---|---|---|---|---|
| Study | FV n | FV mean | NT n | NT mean | PF |
| Borutta 2006 | 136 | 0.97 | 64 | 2.24 | 56.7 |
(2) Developing one or more new caries lesions
DMFT (Analysis 1.7; six trials)
d(e/m)ft (Analysis 1.8; five trials).
1.7. Analysis.
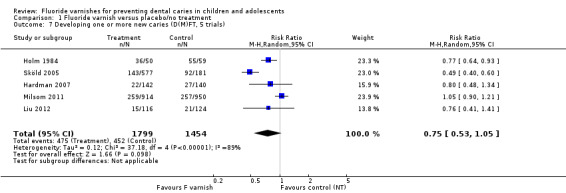
Comparison 1 Fluoride varnish versus placebo/no treatment, Outcome 7 Developing one or more new caries (D(M)FT, 5 trials).
1.8. Analysis.
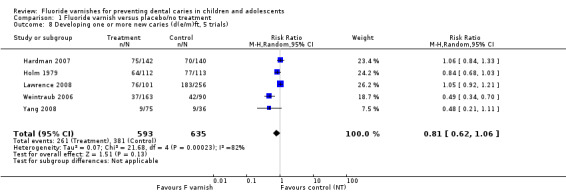
Comparison 1 Fluoride varnish versus placebo/no treatment, Outcome 8 Developing one or more new caries (d(e/m)ft, 5 trials).
Imputation of unreported results
In the original version of this review, unreported standard deviations (SD) were estimated from an analysis of the 179 available treatment arms for the series of topical fluoride reviews with complete information (as of October 1999). This resulted in a regression equation of: log (SD caries increment) = 0.64 + 0.55* log (mean caries increment), (R2 = 77%). This equation was applied to results of four trials (Clark 1985; Frostell 1991; Holm 1984; Modeer 1984) where the standard deviations were unreported.
Two trials (Gugwad 2011; Yang 2008) did not report caries increment data, reporting instead baseline and final mean caries score. Mean caries increments were calculated and standard deviations of the increments estimated using a correlation coefficient between the baseline and final values of 0.5.
Pooling of cluster randomised trials
In order to estimate the PF for the cluster randomised trials we calculated the effective sample size. One cluster randomised trial reported the results not accounting for clustering of the data (Bravo 1997). An intra‐class correlation coefficient of 0.05 (using the value reported in a similar trial (Lawrence 2008)) was used to estimate the design effect. This was then used to adjust the sample size of the control and intervention groups. One trial was not able to be included in the meta‐analysis because it was a cluster randomised trial with no standard deviations presented (Borutta 2006).
Effect on tooth surfaces permanent dentition: D(M)FS prevented fraction
For all 13 trials combined, the D(M)FS prevented fraction pooled estimate was 0.43 (95% confidence interval (CI) 0.30 to 0.57; P < 0.0001), suggesting a substantial benefit from the use of fluoride varnish. The confidence intervals are relatively wide and substantial heterogeneity in the results could be observed graphically (Chi2 = 48.38 on 12 degrees of freedom, P < 0.0001, I2 = 75%) (Analysis 1.1). The average treatment effect and its confidence interval do not directly provide information on the potential effectiveness of treatment when applied within an individual study setting. A 95% prediction interval was therefore calculated (Riley 2011). This ranged from ‐0.02 to 0.89, indicative of a benefit of fluoride varnish.
Meta‐regression and sensitivity analyses: D(M)FS prevented fraction
Meta‐regression results for potential effect modifiers specified a priori are given in Additional Table 3: Random‐effects meta‐regression analyses of prevented fractions: D(M)FS.
2. Random‐effects meta‐regression analyses of prevented fractions: D(M)FS.
| Objective | Characteristic | Number of trials | Slope estimate | 95% CI | Slope interpretation | P value |
| (2) | Mean baseline caries | 11 | 1.33% | (‐0.72% to 3.39%) | Increase per unit increase in mean baseline caries | 0.18 |
| (3) | Any fluorides | 10 | 11.47% | (‐46.42% to 69.35%) | Higher PF in presence of background fluorides | 0.66 |
| (3) | Dentifrice use | 8 | ‐19.88% | (‐74.50% to 34.74%) | Lower PF in presence of dentifrice use | 0.41 |
| (3) | Fluoridated water | 12 | 18.37% | (‐12.54% to 49.28%) | Higher PF in presence of water fluoridation | 0.22 |
| (4) | Concentration of fluoride > 5% | 13 | ‐26.61% | (‐78.30% to 25.08%) | Higher PF if concentration of fluoride is > 5% | 0.28 |
| (4) | Length of follow‐up | 13 | ‐12.22% | (‐35.71% to 11.27%) | Decrease per unit increase in length of follow‐up | 0.42 |
| (4) | Prior prophylaxis | 13 | 21.66% | (‐11.62% to 54.94%) | Higher PF in presence of prophylaxis | 0.18 |
| (4) | Frequency of application > twice per year | 13 | ‐4.85% | (‐24.27% to 14.57%) | Lower PF if application > twice per year | 0.59 |
| Time since eruption | 12 | ‐3.79% | (‐40.13% to 32.55%) | Lower PF if time since eruption < 2 years | 0.82 | |
| Placebo or no treatment control | 13 | 5.42% | (‐32.70% to 43.54%) | Increase in PF for no treatment control | 0.76 | |
| Design (individual versus cluster) | 13 | ‐29.85% | (‐69.49% to 9.78%) | Increase in PF for individual randomisation | 0.13 |
CI = confidence interval; D(M)FS = decayed, (missing) and filled permanent surfaces; PF = prevented fraction
Univariate meta‐regression suggested no significant association between estimates of D(M)FS prevented fractions and the pre‐specified factors: baseline caries severity, background exposure to fluoridated water, background exposure to fluoride toothpaste, or background exposure to any reported fluoride source, concentration of fluoride, length of follow‐up (duration of study), prior prophylaxis or frequency of application. Further univariate meta‐regression analyses showed no significant associations between estimates of D(M)FS prevented fractions and time since treated teeth had erupted (<= two years), whether a placebo or no treatment control was used, and whether individual randomisation or cluster randomised design was used.
In order to determine the influence of data imputation and approximation a sensitivity analysis was undertaken, restricting the pooling of trials to those that were fully reported and suitable for analysis (eight trials). The results of this gave rise to greater PF values than the results of the full meta‐analysis (PF = 0.55, 95% CI 0.42 to 0.68) and indicator of heterogeneity reduced from I2 = 75% to 62%.
Funnel plot: D(M)FS prevented fraction
A funnel plot of the 13 trials in the pooled analysis of D(M)FS prevented fractions indicated no clear asymmetry of prevented fraction and precision. The between‐study heterogeneity was large, and as such a formal bias detection tests was not undertaken.
Effect on whole teeth permanent dentition: D(M)FT prevented fraction
Five trials reported data which allowed the calculation of the D(M)FT prevented fraction. The pooled estimate of D(M)FT prevented fraction was 0.44 (95% CI 0.11 to 0.76; P = 0.009), suggesting a considerable benefit of fluoride varnish; the confidence intervals are wide, however (Analysis 1.2). There was, again, substantial heterogeneity between trials (Chi2 = 28.82 on 4 degrees of freedom, P < 0.0001, I2 = 86%).
Effect on tooth surfaces primary dentition: d(e/m)fs prevented fraction
Ten trials reported data which allowed the calculation of the d(e/m)fs prevented fraction. The pooled estimate of d(e/m)fs prevented fraction was 0.37 (95% CI 0.24 to 0.51; P < 0.0001), suggesting a substantial benefit of fluoride varnish in the primary dentition (Analysis 1.3). There was statistically significant heterogeneity between trials (Chi2 = 21.83 on 9 degrees of freedom, P = 0.009, I2 = 59%). A 95% prediction interval for the pooled trials was calculated and ranged from ‐0.01, 0.76, indicative of a benefit of fluoride varnish in the most part. One trial did not provide data in a format suitable for inclusion in the meta‐analysis (Borutta 2006) (Analysis 1.4).
Meta‐regression and sensitivity analyses: d(e/m)fs prevented fraction
Meta‐regression results for potential effect modifiers specified a priori are reported in Additional Table 4: Random‐effects meta‐regression analyses of prevented fractions: d(e/m)fs.
3. Random‐effects meta‐regression analyses of prevented fractions: d(e/m)fs.
| Characteristic | Number of trials | Slope estimate | 95% CI | Slope interpretation | P value | |
| (2) | Mean baseline caries | 8 | ‐1.00% | (‐4.81% to 2.80%) | Decrease per unit increase in mean baseline caries | 0.54 |
| (3) | Any fluorides | 7 | Not estimable (Collinearity) | |||
| (3) | Dentifrice use | 6 | Not estimable (Collinearity) | |||
| (3) | Fluoridated water | 8 | 20.64% | (‐36.33% to 77.61%) | Higher PF in presence of water fluoridation | 0.41 |
| (4) | Concentration of fluoride > 5% | 10 | ‐5.40% | (‐47.10% to 36.29%) | Higher PF if concentration of fluoride is > 5% | 0.77 |
| (4) | Length of follow‐up | 10 | ‐5.77% | (‐21.48% to 9.94%) | Decrease per unit increase in length of follow‐up | 0.28 |
| (4) | Prior prophylaxis | 10 | ‐8.81% | (‐47.72% to 30.11%) | Lower PF in presence of prophylaxis | 0.62 |
| (4) | Frequency of application > twice per year | 10 | 5.09% | (‐19.33% to 29.51%) | Lower PF if application > twice per year | 0.64 |
| Placebo or no treatment control | 10 | ‐13.99% | (‐47.60% to 19.62%) | Increase in PF for placebo | 0.37 | |
| Design (individual versus cluster) | 10 | ‐32.71% | (‐67.84% to 2.42%) | Increase in PF for individual randomisation | 0.064 |
CI = confidence interval; d(e/m)fs = decayed, (extracted/missing) and filled primary surfaces; PF = prevented fraction
Univariate meta‐regression suggested no significant association between estimates of d(e/m)fs prevented fractions and the pre‐specified factors: baseline caries severity and background exposure to fluoridated water. The effects of background exposure to fluoride toothpaste and background exposure to any reported fluoride source were inestimable due to collinearity in the data set. Further univariate meta‐regression analyses showed no significant association between estimates of d(e/m)fs prevented fractions and concentration of fluoride varnish, length of follow‐up (duration of study), frequency of application of varnish, whether a prophylaxis was undertaken prior to application of the varnish, use of a placebo rather than a no treatment control and which study design was used (individual randomisation or cluster randomisation).
In order to determine the influence of data imputation and approximation a sensitivity analysis was undertaken, restricting the pooling of trials to those that were fully reported and suitable for analysis (eight trials). The results of this differed only slightly from the results of the full meta‐analysis (PF = 0.45, 95% CI 0.29 to 0.62) and indicator of heterogeneity decreased to 52%.
Funnel plot: d(e/m)fs prevented fraction
A funnel plot of the pooled meta‐analysis of 10 trials reporting d(e/m)fs prevented fractions indicated no clear relationship between prevented fraction and precision (it appears symmetric). The between‐study heterogeneity was large, and as such a formal bias detection test was not undertaken.
Effect on whole teeth primary dentition: d(e/m)ft prevented fraction
Two trials reported data which allowed the calculation of the d(e/m)fs prevented fractions. The fixed‐effect pooled estimate was 0.65 (95% CI 0.48 to 0.82; P < 0.0001), suggesting a substantial benefit of fluoride varnish in the primary dentition (Analysis 1.5). There was no evidence of statistically significant heterogeneity between trials (Chi2 = 0.04 on 1 degree of freedom, P = 0.83, I2 = 0%). One study did not provide data in a format suitable for inclusion in the meta‐analysis (Borutta 2006) (Analysis 1.6).
Proportion developing new caries
Five trials reported results on the proportion of children developing one or more new caries (whole tooth) in the permanent dentition; five in the primary dentition. There was no evidence of effectiveness of fluoride varnish in the permanent dentition (RR = 0.75, 95% CI 0.53 to 1.05, P = 0.10) (Analysis 1.7), or the primary dentition (RR = 0.81, 95% CI 0.62 to 1.06, P = 0.13) (Analysis 1.8). There was substantial heterogeneity in both pooled analyses (Chi2 = 37.18 on 4 degrees of freedom, P < 0.0001, I2 = 89% and Chi2 = 21.68 on 4 degrees of freedom, P = 0.0002, I2 = 82%). However there was a statistically significant difference between the study design subgroups for both analyses, with the individual child randomisation subgroup showing a benefit.
Effect of fluoride varnish on other outcomes
Few trials reported data for other relevant outcomes.
Discussion
Summary of main results
The main question addressed by this review is how effective the use of fluoride varnish for the prevention of caries in children is compared to placebo or no treatment. In this updated review there are now 22 trials published between 1975 and 2012 in which a total of 12,455 children were randomised to treatment with either fluoride varnish or placebo/no treatment.
The evidence from meta‐analysis of the 13 trials assessing the effect of fluoride varnish on the permanent dentition is that the use of fluoride varnish is associated on average with a 43% (95% CI 30% to 57%) reduction in decayed, missing and filled tooth surfaces. The meta‐analysis of the 10 trials assessing the effect of fluoride varnish on the primary dentition suggests a 37% (95% CI 24% to 51%) reduction in decayed, missing and filled tooth surfaces. There was considerable statistical heterogeneity in both these estimates.
We explored this heterogeneity in addressing the second, third and fourth objectives of this review which were to examine whether there was any relationship between the caries‐preventive effectiveness of fluoride varnish and the initial level of caries severity, background exposure to fluoride (water supply, dentifrice, other fluoride sources), concentration of fluoride, frequency of application and whether prophylaxis was undertaken prior to the application of the varnish. The univariate meta‐regressions found no significant associations between any of these pre‐specified factors and the estimates of D(M)FS or d(m)fs prevented fractions, despite substantial variations between trials in these factors. As these meta‐regression analyses include only a few trials, they have limited power to detect such relationships. However, it is possible that these multiple variations between the included trials is a cause of the substantial heterogeneity associated with both estimates. We also found no significant associations for three factors posed post hoc: time since eruption (for permanent dentition), placebo or no treatment control and study design (individual randomisation versus cluster randomisation).
We performed a sensitivity analysis for the main meta‐analysis to take account of the uncertainty we have about the imputations for the missing standard deviations and to take the clustering into account where this had not been done in the cluster randomised trials. The sensitivity analysis showed results with larger effect estimate than the full meta‐analysis, with a similar level of heterogeneity.
Overall completeness and applicability of evidence
We found scarce information about the effects of fluoride varnishes on other outcomes such as the proportion of children developing caries or on acceptance of fluoride varnish treatment. Only three studies provided data, reporting no adverse effects. Even though fluoride varnishes are generally considered safe and well accepted, this lack of evidence makes it more difficult for clinicians and policy makers to weigh the benefits of fluoride varnishes in preventing caries against possible shortcomings of the procedure.
In the studies with more than one relevant intervention group and a common control group, such as those comparing different active fluoride agents or concentrations of fluoride ions to a placebo group, summary statistics from the studies (number of children analysed, mean caries increments and standard deviations) from all relevant intervention groups were combined in order to obtain a measure of treatment effect. This enabled the inclusion of all relevant data in the primary meta‐analyses, but has limited a secondary investigation of dose‐response.
Although we approached the manufacturers of fluoride varnishes requesting additional unpublished trial data, no such data were made available. However, we are aware that at least one additional study has been undertaken, and we have requested the data. If these data are made available to us it will be included in future updates of this review.
This review has evaluated the effects of fluoride varnish alone, versus either placebo or no treatment. We have excluded trials where fluoride varnish plus a complementary intervention, such as toothbrushing or provision of fluoride dentifrice are evaluated. Such trials would answer a different question which may be more relevant to current policy decisions.
The trials included in this review were conducted with participants at a range of caries risk as evidenced by the variability of the caries increments in the control groups. Trials were conducted in a variety of locations with variability in exposure to other sources of fluoride. The prevented fraction appears to be consistent across different populations, levels of caries risk and exposure to other factors. The absolute benefit from fluoride varnish will of course depend on the expected caries increment in the target population. Where expected caries increment is small the absolute benefit of fluoride varnish will be very small.
Quality of the evidence
None of the trials included in this review were assessed as at low risk of bias. In fact, 68% were assessed as at high risk of bias, with the remaining at unclear risk. In most of the trials allocation concealment was not reported. As with many long terms trials involving children, there was an average of 19% attrition in the included trials which was not clearly accounted for but is likely to be due to movement of families out of the study area. Overall the quality of the reporting of many of these trials was poor and we were unable to obtain further information from some trials because they were published many years ago.
There is substantial heterogeneity in the body of evidence which addresses the main question of this review. We were unable to find a conclusive explanation for this, but we note that there is substantial variability between the trials in this review with regard to factors which may influence the effect estimate in each study. While we have not been able to demonstrate a significant association between factors such as the initial level of caries severity, background exposure to fluoride (water supply, dentifrice, other fluoride sources), frequency of application, fluoride concentration and whether prophylaxis was undertaken prior to the application of the varnish, neither can we confidently exclude the possibility that one or more of these factors may account for the observed heterogeneity.
Potential biases in the review process
A sensitive search strategy was used to identify trials for inclusion in this review and there were no restrictions placed on publication status or language. Many references were translated in order to determine whether or not they reported trials eligible for inclusion in this review.
No clear relationship between prevented fraction and precision could be observed in the funnel plot of the 13 trials (it appeared asymmetric), but as for meta‐regression methods, power is limited when the number of trials is small. We cannot eliminate the possibility that bias may have influenced the results of this review.
Agreements and disagreements with other studies or reviews
The findings of this updated Cochrane review do not differ from those of the initial review, published first in 2002. The general direction of findings presented is in keeping with those of other reviews (Carvalho 2010; Petersson 2004) which also found evidence for the effectiveness of fluoride varnish. Carvalho 2010 evaluated the effectiveness of fluoride varnish in decreasing dental caries incidence in pre‐school children and added two RCTs to the body of evidence assessed in the 2002 version of this review. Azarpazhooh 2008 added four RCTs and three cohort trials to the body of evidence assessed in the previous version of this Cochrane review (Marinho 2002), to produce a body of evidence comprising 13 RCTs and three cohort trials. The review by Azarpazhooh 2008 concluded that there is "clear evidence of the efficacy of fluoride varnish in preventing dental caries in children and adolescents" but in the absence of a meta‐analysis, no estimate of the magnitude of the expected benefit was reported, and we are unhappy about the methodological quality of this review. The systematic review by Petersson 2004 based their conclusions on 15 included trials (both RCTs and CCTs) and reported a mean prevented fraction of 30% (0% to 69%) when fluoride varnishes were compared to placebo or no treatment.
This updated Cochrane review includes an additional 13 RCTs compared to the previous version (Marinho 2002). None of the additional included trials is included in the Petersson 2004 review, four are included in the Azarpazhooh 2008 review, and two are included in the Carvalho 2010 review. The large body of evidence contained in this updated Cochrane review provided the best available evidence of the effectiveness of fluoride varnish compared to either placebo or no treatment.
Authors' conclusions
Implications for practice.
This review has found that the application of fluoride varnishes two to four times a year, either in the permanent or primary dentition, is associated with a substantial reduction in caries increment. We found that this relative effect applies in populations with different levels of caries risk and exposure to other sources of fluoride. We also found no evidence that this relative effect was dependent on frequency of varnish application, length of follow‐up, whether a prophylaxis was undertaken prior to application of the varnish, concentration of fluoride in the varnish and use of a placebo rather than a no treatment control, although these results should be interpreted with caution. The review does not provide any information on the likelihood of side effects with this treatment and inconclusive information on acceptability.
Implications for research.
This review of 22 RCTs shows that fluoride varnish, compared to placebo or no treatment, is effective in the prevention of caries in children and adolescents. Despite the large number of trials identified, there is still a paucity of evidence from high quality randomised trials assessing the effectiveness of fluoride varnishes for the prevention of caries in children. It is also important that future trials should include the assessment of other relevant outcomes such as potential side effects (e.g. oral allergic reactions) and those related to acceptability of treatment. The reporting of caries at both the cavitated and non‐cavitated level would improve interpretation. Also future trials should consider evaluating the effects of complex interventions incorporating fluoride varnish with other caries preventive strategies, conducted in either the setting of a dental practice or a community site such as a school.
What's new
| Date | Event | Description |
|---|---|---|
| 18 February 2014 | Amended | Minor edit to additional reference. |
History
Protocol first published: Issue 3, 2000 Review first published: Issue 3, 2002
| Date | Event | Description |
|---|---|---|
| 9 July 2013 | New search has been performed | Searches updated May 2013. |
| 9 July 2013 | New citation required but conclusions have not changed | Review update completed with 22 included trials (9 in previously published version of the review). Changes in authorship. |
| 27 August 2008 | Amended | Converted to new review format. |
Acknowledgements
We would like to acknowledge the considerable amount of work undertaken by Julian Higgins, Stuart Logan and Aubrey Sheiham who were authors on the previous version of this review published in 2002. We would also like to thank the following investigators who provided additional information about their trials: M Bravo (University of Granada), A Borutta (Friedrich‐Schiller University), G Davies (Mancunian Community Health NHS Trust), G Frostell (Karolinska Institute), I Mejare (Sweden), A Treide (University of Leipzig), and J Weintraub (University of California). We would also like to thank the help and expertise of the following: R Wents and A Schreiber (German translations), H Pikhart (Russian translations), Chunjie Li (Chinese translations), L Fernandez‐Mauleffinch (Portuguese translation) and K Turai (Hungarian and Russian translations); B Anagnostelys, L Jones (Systematic Reviews Training Unit, London), A Littlewood, P Riley and L Fernandez‐Mauleffinch (Cochrane Oral Health Group), O Onwood and B Bhandari (QMUL, London). Finally, we would like to thank those who have provided comments and editorial input to this review: S Furness, J Leese and R Floate (Cochrane Oral Health Group), James Bader (University of North Carolina), Elizabeth Treasure (University of Cardiff), Chris Deery (Univeristy of Dundee), Derek Richards (NHS Forth Valley, Stirling), and Iain Pretty (The University of Manchester).
Appendices
Appendix 1. Cochrane Oral Health Group's Trials Register search strategy
((deminerali* or caries or carious or DMF* or fissure* or decay* or cavit* or "white spot*") AND (fluor* or "PPM F" or "PPMF" or "APF" or "NAF" or "sodium F" or "amine F" or "SNF2" or "stannous F" or acidulat* or "phosphat* fluorid*" or "fluorophosphat* sodium fluorid*" or "amine* fluorid*" or"stannous* fluorid*" or SMFP or "MFP" or monofluor*) AND (varnish* or paint* or laquer* or lacker* or lakk* or coating* or silane* or polyurethane* or duraphat* or "fluor protect*"))
Appendix 2. CENTRAL search strategy
#1 MeSH descriptor Tooth demineralization explode all trees #2 (carie in All Text or carious in All Text or caries in All Text or DMF* in All Text) #3 ( (dental in All Text or tooth in All Text or teeth in All Text or enamel in All Text or dentin* in All Text) and (decay* in All Text or cavit* in All Text or deminerali* in All Text or reminerali* in All Text or "white spot*" in All Text) ) #4 (#1 or #2 or #3) #5 MeSH descriptor Fluorides explode all trees #6 (fluoride* in All Text or fluor in All Text or "PPM F" in All Text or PPMF in All Text or APF in All Text or NAF in All Text or "sodium F" in All Text or "amine F" in All Text or SNF2 in All Text or "stannous F" in All Text or "phosphat* f" in All Text or "acidulat* F" in All Text or "acidulat* fluor*" in All Text or "phosphat* fluor*" in All Text or fluorophosphat* in All Text or "amin* fluor*" in All Text or "sodium* fluor*" in All Text or "stannous* fluor*" in All Text or SMFP in All Text or MFP in All Text or monofluor* in All Text) #7 (#5 or #6) #8 (varnish* in All Text or lacquer* in All Text or laquer* in All Text or lacker* in All Text or lakk* in All Text or polyurethane* in All Text) #9 (#7 and #8) #10 (duraphat in All Text or "fluor protector" in All Text or "bifluorid 12" in All Text or "cavity shield" in All Text or cavityshield in All Text or duraflor in All Text or Flulak in All Text or "omni varnish" in All Text or "prevident varnish" in All Text or clearshield in All Text or "clear shield" in All Text or allsolutions in All Text) #11 (#9 or #10) #12 (#4 and #11)
Appendix 3. MEDLINE (OVID) search strategy
1. exp Tooth demineralization/ 2. (carie or caries or carious or DMF$ or ((dental or tooth or teeth or enamel or dentin$) and (decay$ or cavit$ or deminerali$ or reminerali$ or white spot$))).mp. 3. 1 or 2 4. exp Fluorides/ 5. (fluoride$ or fluor or "PPM F" or PPMF or APF or NAF or "Sodium F" or "Amine F" or SNF2 or "Stannous F" or "phosphat$ F" or "acidulat$ F" or "acidulat$ fluor$" or "phosphat$ fluor$" or fluorphosphat$ or "amin$ fluor$" or "sodium$ fluor$" or "stannous$ fluor$" or SMFP or MFP or monofluor$).mp. 6. 4 or 5 7. (varnish$ or lacquer$ or laquer$ or lacker$ or lakk$ or polyurethane$).mp. 8. 6 and 7 9. (duraphat or "fluor protector" or "bifluorid 12" or "cavity shield" or cavityshield or duraflor or Flulak or "omni varnish" or "prevident varnish" or clearshield or "clear shield" or allsolutions).mp. 10. 8 or 9 11. 3 and 10 The above subject search was linked to the Cochrane Highly Sensitive Search Strategy (CHSSS) for identifying randomised trials in MEDLINE: sensitivity maximising version (2008 revision) as referenced in Chapter 6.4.11.1 and detailed in box 6.4.c of theCochrane Handbook for Systematic Reviews of Interventions version 5.1.0 [updated March 2011] (Higgins 2011).
1. randomized controlled trial.pt. 2. controlled clinical trial.pt. 3. randomized.ab. 4. placebo.ab. 5. drug therapy.fs. 6. randomly.ab. 7. trial.ab. 8. groups.ab. 9. or/1‐8 10. exp animals/ not humans.sh. 11. 9 not 10
The previous version of this review used the following search strategy for MEDLINE via SILVERPLATTER (search undertaken 2001):
[(CARIE* or (DENT* near CAVIT*) or TOOTH* DECAY* or DMF* or (explode "DENTAL‐CARIES"/ ALL SUBHEADINGS)) and ((FLUOR* or explode "FLUORIDES"/ ALL SUBHEADINGS) and ((VARNISH*) or (LACQUER* or LAQUER*) or (VERNIZ*) or (LACKER*) or (LAKK*) or (SILANE* or POLYURETHANE*)) or (DURAPHAT* or FLUOR PROTECTOR*)]
A search was undertaken for the fluoride series of reviews (considering varnish, gels, toothpastes, mouthrinses) in 1997, using the search strategy below for MEDLINE via SILVERPLATTER:
(a) [("DENTAL‐CARIES" explode all subheadings or "DENTAL‐CARIES‐ACTIVITY‐TESTS" all subheadings or "DENTAL‐CARIES‐SUSCEPTIBILITY" all subheadings or CARIE* or DMF*) and (("FLUORIDES" explode all subheadings or "FLUORIDES,‐TOPICAL" explode all subheadings or FLUOR* or AMF or AMINE F OR SNF2 OR STANNOUS F OR NAF OR SODIUM F OR APF OR SMFP OR MFP OR MONOFLUOR*) or ("CARIOSTATIC‐AGENTS" explode all subheadings or "DENTAL‐PROPHYLAXIS" explode all subheadings or "DENTIFRICES" explode all subheadings or "MOUTHWASHES" explode all subheadings or CARIOSTA* or PROPHYLA* or ANTICARI* or ANTI CARI* or VARNISH* or LACQUER* or DURAPHAT or GEL* or TOOTHPASTE* or TOOTH PASTE* or PASTE* or DENTIFRIC* or MOUTHRINS* or MOUTH RINS* or RINS* or MOUTHWASH* or MOUTH WASH*))]. (b) [((explode FLUORIDES/ all subheadings) or (explode FLUORIDES‐TOPICAL/ ALL SUBHEADINGS) or (FLUOR*) or (AMF or AMINE F OR SNF2 OR STANNOUS F OR NAF OR SODIUM F OR APF OR MFP OR SMFP OR MONOFLUOR* OR DURAPHAT)) and ((CARI*) or (DMF*) or (TOOTH*) or (TEETH*) or (DENT* in TI, in AB, in MESH)) or ((explode CARIOSTATIC‐AGENTS/ all subheadings) or (ANTICARI* or ANTI CARI*) or (explode MOUTHWASHES/ all subheadings) or (MOUTHWASH* or MOUTH WASH*) or (MOUTHRINS* or MOUTH RINS*) or (VARNISH* or LACQUER*))]
Appendix 4. EMBASE (OVID) search strategy
1. exp Tooth demineralization/ 2. (carie or caries or carious or DMF$ or ((dental or tooth or teeth or enamel or dentin$) and (decay$ or cavit$ or deminerali$ or reminerali$ or white spot$))).mp. 3. 1 or 2 4. exp Fluorides/ 5. (fluoride$ or fluor or "PPM F" or PPMF or APF or NAF or "Sodium F" or "Amine F" or SNF2 or "Stannous F" or "phosphat$ F" or "acidulat$ F" or "acidulat$ fluor$" or "phosphat$ fluor$" or fluorphosphat$ or "amin$ fluor$" or "sodium$ fluor$" or "stannous$ fluor$" or SMFP or MFP or monofluor$).mp. 6. 4 or 5 7. (varnish$ or lacquer$ or laquer$ or lacker$ or lakk$ or polyurethane$).mp. 8. 6 and 7 9. (duraphat or "fluor protector" or "bifluorid 12" or "cavity shield" or cavityshield or duraflor or Flulak or "omni varnish" or "prevident varnish" or clearshield or "clear shield" or allsolutions).mp. 10. 8 or 9 11. 3 and 10
The above subject search was linked to the Cochrane Oral Health Group filter for EMBASE via OVID:
1. random$.ti,ab. 2. factorial$.ti,ab. 3. (crossover$ or cross over$ or cross‐over$).ti,ab. 4. placebo$.ti,ab. 5. (doubl$ adj blind$).ti,ab. 6. (singl$ adj blind$).ti,ab. 7. assign$.ti,ab. 8. allocat$.ti,ab. 9. volunteer$.ti,ab. 10. CROSSOVER PROCEDURE.sh. 11. DOUBLE‐BLIND PROCEDURE.sh. 12. RANDOMIZED CONTROLLED TRIAL.sh. 13. SINGLE BLIND PROCEDURE.sh. 14. or/1‐13 15. ANIMAL/ or NONHUMAN/ or ANIMAL EXPERIMENT/ 16. HUMAN/ 17. 16 and 15 18. 15 not 17 19. 14 not 18
Appendix 5. CINAHL (EBSCO) search strategy
S1 (MH "Tooth demineralization+") S2 (carie or caries or carious or DMF* or cavit* or deminerali* or reminerali* or "white spot"*) S3 S1 or S2 S4 (MH "Fluorides+") S5 (fluoride* or fluor or "PPM F" or PPMF or APF or NAF or "Sodium F" or "Amine F" or SNF2 or "Stannous F" or "phosphat* F" or "acidulat* F" or "acidulat* fluor*" or "phosphat* fluor*" or fluorphosphat* or "amin* fluor*" or "sodium* fluor*" or "stannous* fluor*" or SMFP or MFP or monofluor*) S6 S4 or S5 S7 (varnish* or lacquer* or laquer* or lacker* or lakk* or polyurethane*) S8 S6 and S7 S9 (duraphat or "fluor protector" or "bifluorid 12" or "cavity shield" or cavityshield or duraflor or Flulak or "omni varnish" or "prevident varnish" or clearshield or "clear shield" or allsolutions) S10 S8 or S9 S11 S3 and S10
Appendix 6. LILACS/BBO (BIREME) search strategy
((Mh Fluorides or fluoride$ or fluoruro$ or fluoreto$) AND (varnish$ or barniz$ or verniz$ or laquer$ or lacquer or polyurethane)) [Words] and (Mh Dental caries or carie$ or carious) [Words]
The above subject search was linked to the Brazilian Cochrane Center filter for LILACs/BBO via BIREME:
Pt randomized controlled trial OR Pt controlled clinical trial OR Mh randomized controlled trials OR Mh random allocation OR Mh double‐blind method OR Mh single‐blind method) AND NOT (Ct animal AND NOT (Ct human and Ct animal)) OR (Pt clinical trial OR Ex E05.318.760.535$ OR (Tw clin$ AND (Tw trial$ OR Tw ensa$ OR Tw estud$ OR Tw experim$ OR Tw investiga$)) OR ((Tw singl$ OR Tw simple$ OR Tw doubl$ OR Tw doble$ OR Tw duplo$ OR Tw trebl$ OR Tw trip$) AND (Tw blind$ OR Tw cego$ OR Tw ciego$ OR Tw mask$ OR Tw mascar$)) OR Mh placebos OR Tw placebo$ OR (Tw random$ OR Tw randon$ OR Tw casual$ OR Tw acaso$ OR Tw azar OR Tw aleator$) OR Mh research design) AND NOT (Ct animal AND NOT (Ct human and Ct animal)) OR (Ct comparative study OR Ex E05.337$ OR Mh follow‐up trials OR Mh prospective trials OR Tw control$ OR Tw prospectiv$ OR Tw volunt$ OR Tw volunteer$) AND NOT (Ct animal AND NOT (Ct human and Ct animal)))and not (Ct ANIMAL AND NOT (Ct HUMAN and Ct ANIMAL)))
The previous version of this review used the following search strategy for LILACS via BIREME (search undertaken 1999):
[(fluor$ or ppmf or ppm f or amf or snf or naf or apf or mfp or smfp or monofluor$ or duraphat$) and (carie$ or dmf$ or cpo$ or tooth$ or teeth$ or dent$ or dient$ or anticarie$ or cario$ or mouthrins$ or mouth rins$ or rinse$ or bochech$ or enjuag$ or verniz$ or varnish$ or barniz$ or laca$ or gel or gels)] and [random$ or aleatori$ or acaso$ or azar$ or blind$ or mask$ or cego$ or cega$ or ciego$ or ciega$ or placebo$ or(clinic$ and (trial$ or ensaio$ or estud$)) or (control$ and (trial$ or ensaio$ or estud$))]
Appendix 7. Proquest Dissertations and Theses search strategy
all(fluoride*) AND all((varnish* OR laquer* or lacquer* or paint*))
Appendix 8. Web of Science Conference Proceedings search strategy
# 1 TS=(deminerali* or caries or carious or DMF* or fissure* or decay* or cavit* or "white spot*") # 2 TS=(varnish* or paint* or laquer* or lacker* or lakk* or coating* or silane* or polyurethane* or duraphat* or "fluor protect*") # 3 TS=(fluoride* or "PPM F" or "PPMF" or "APF" or "NAF" or "sodium F" or "amine F" or "SNF2" or "stannous F" or acidulat* or "phosphat* fluorid*" or "fluorophosphat* sodium fluorid*" or "amine* fluorid*" or"stannous* fluorid*" or SMFP or "MFP" or monofluor*) # 4 #1 and #2 and #3
Appendix 9. ClinicalTrials.gov search strategy
fluoride* and varnish*
Data and analyses
Comparison 1. Fluoride varnish versus placebo/no treatment.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Arruda 2012.
| Methods | Design: 2‐arm parallel group RCT Location: Tapiratiba, South Eastern Brazil Study started: January 2006 |
|
| Participants | Number randomised: 379 (198/181) Number analysed: 210 (113/97) Age range: At baseline 9.62 ± 1.36 and 9.63 ±1.36 years Background exposure to other fluoride: 58.4% and 59.7% of varnish and placebo groups attended a school with fluoridated water (0.7 ppm) |
|
| Interventions |
Comparison: FV versus PL
Group 1 (n = 113 ): 5% NaF varnish group (Cavity Shield ® = 22,600 ppm F from Omni oral pharmaceuticals), applied twice (baseline, 6 months), from single dose vials, applied by dentists in portable dental units under standard operating light illumination, with small brush, left to dry (duration NR) Group 2 (n = 97): Placebo provided by manufacturer in identical vials, pre‐numbered with the number assigned to each child Application method identical for all children (after toothbrushing teeth were dried and varnish/placebo applied using disposable brush, then varnish was air dried) Post‐op instructions: Nil solid foods for 4 hours, nil toothbrushing till next day Manufacturer provided both varnish and placebo |
|
| Outcomes | DFS CA + NCA (ICDAS code 1 (non‐cavitated lesions)) and preventive fraction assessed at baseline 6 and 12 months | |
| Notes | Sociodemographic and oral health questionnaire administered to all participants, with 7 day food frequency diary All children received oral health education, toothbrushing and caries examinations at baseline High sugar consumption in 53.1% and 71.1% of varnish and control groups respectively (P = 0.007) Toothbrushing at least once a day was reported by 104/113 (92%) and 80/97 (82%) of varnish and placebo groups (P = 0.04) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | "Children were identified by their school ID number. Randomisation was achieved on the basis of odd and even ID numbers by a coin toss.... children with odd ID numbers were assigned to one group and the IDs with even numbers were allocated to the other group" Comment: Quasi‐random. 2 cohorts enrolled, 1 in June 2006 and another in December 2006 |
| Allocation concealment (selection bias) | High risk | Unclear who performed the coin toss but allocation was determined once the first participant was enrolled |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "All parents, children and examiners were blinded to group allocation and intervention status (double blind design)" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "All parents, children and examiners were blinded to group allocation and intervention status (double blind design)" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 85/198 (43%) and 84/181 (46%) lost to follow‐up because children moved to other schools. Numbers high but numbers and reason similar in each group |
| Selective reporting (reporting bias) | Unclear risk | Caries assessed at 6 and 12 months but only reported at 12 months (increment and PF) |
| Baseline characteristics balanced? | High risk | Sugar consumption higher and toothbrushing less likely in placebo group |
| Free of contamination/co‐intervention? | Low risk | Exposure to fluoridated water similar in each group Compliance; only 57/198 (29%) and 43/181(24%) children in varnish and placebo groups received application twice at baseline and 6 months |
Borutta 1991.
| Methods | Design: 4‐arm RCT Location: Erfurt, Germany Study started: In/before 1988 |
|
| Participants | Number randomised: 400 Number analysed: 360 analysed at 2 years Age range: 12‐14 years Background exposure to other fluoride: No |
|
| Interventions | Comparison: FV (3 groups) + ptc versus PL + ptc Group 1 (n = 100): Bifluorid 12®: NaF + CaF (27,100 + 29,200 ppm F), applied twice a year Group 2 (n = 100): Bifluorid 12®: NaF + CaF (27,100 + 29,200 ppm F), applied 4 times a year Group 3 (n = 100): Lawefluor®: NaF (22,600 ppm F), applied 4 times a year Group 4 (n = 100): Placebo group, applied 4 times a year | |
| Outcomes | **2‐year DMFS increment ‐ (CA)cl + FOTI Reported at 2 years follow‐up: O‐DMFS; MD‐DMFS; BL‐DMFS; DMFT(CA) | |
| Notes | Baseline characteristics (DMFS, DMFT) 'balanced' Clinical caries assessment by 2 examiners; diagnostic threshold = CA; FOTI assessment (loss of translucency on transillumination) for approximal surfaces. State of tooth eruption included NR; inter‐examiner reproducibility checked for DMFS **Results presented separately by examiner (1 chosen by coin flip) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The aim of this randomised...." Comment: Not enough information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Double blind study" Comment: Use of placebo described |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Double blind study" Comment: Blind outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Overall drop‐out for length of follow‐up: 10% in 2 years. Drop‐outs by group: 10/100 FV1, 10/100 FV2, 10/100 FV3, 10/100 PL. Reasons for losses not reported, but "Groups kept at equal sizes for statistical reasons" Comment: Numbers lost were not unduly high for the length of follow‐up, and showed no differential loss between groups, but it is unclear why/how group sizes were kept equal at all times. It is also unclear if reasons for the missing data are acceptable and balanced. It is unclear to which sample caries data used in the analysis pertain to |
| Selective reporting (reporting bias) | Low risk | Outcomes reported: DMFS increment ‐ (CA) cl + FOTI, at 2 years follow‐up; O‐DMFS, MD‐DMFS, BL‐DMFS, DMFT (CA); drop‐outs Comment: Trial protocol not available. All pre‐specified outcomes (in Methods) were reported in the pre‐specified way |
| Baseline characteristics balanced? | Low risk | Prognostic factors reported: DMFT: 3.7(3.2) FV1, 3.7(2.8) FV2, 3.3(2.6) FV3, 3.5(2.7) PL; DMFS: 5.1(5.8) FV1, 5.1(4.5) FV2, 5.1(5.1) FV3, 5.4(5.5) PL Comment: Initial caries appears balanced between groups |
| Free of contamination/co‐intervention? | Unclear risk | Translation of report not detailed enough to make a categorical decision regarding contamination/co‐intervention |
Borutta 2006.
| Methods | Design: 3‐arm cluster RCT. 7 clusters were randomly allocated to study groups Location: Germany, 7 randomly selected day care centres (nurseries) in Erfurt/Thirugia where caries risk is high Study started: 2002/2003 |
|
| Participants | Number randomised: 288 (84, 113, 91) Number analysed: 200 (60, 76, 64) at 2 years Age range: 2‐4 years Exposure to other fluoride: All children used fluoride dentifrice daily (500 ppm F) under supervision of staff |
|
| Interventions |
Comparison: FV versus FV versus N/T*
Group 1: 5% Na varnish (Fluoridin N5 = 22,600 ppm F) applied twice a year, total of 4 applications
Group 2: 5% NaF varnish (Duraphat® = 22,600 ppm F) applied twice a year, total of 4 applications
Group 3: No treatment Both varnish groups combined Both varnishes applied by dental hygienist, after lunch and dental hygiene session. Cartridge and cannula (not product) supplied by manufacturer was used to apply varnishes All children received 6 monthly instruction including dietary advice, instruction and motivation for dental and oral hygiene. Children in varnish groups then had varnish applied |
|
| Outcomes | **2‐year dmfs increment ‐ (CA)cl + FOTI
Reported at 2 years follow‐up *2‐year dmft increment ‐ (CA)cl + FOTI Reported at 2 years follow‐up. No data on numbers with increment presented |
|
| Notes | Baseline characteristics (dmfs, dmft) described as balanced but large differences (baseline FV mean dmfs = 3.75, NT = 1.94). Unable to use data as no standard deviations and only 7 clusters. Caries reduction in both varnish groups was 56% to 57% | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "their allocation was random......" Comment: Not enough information but only 7 clusters over 3 groups |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No information on blinding of participants and personnel. No placebo used so risk of bias is high |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: " This was an examiner blind, clinically controlled 2 year study..." Comment: Blind outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Drop‐outs by group: 24/84 (29%) FV1, 76/113 (33%) FV2, 27/91 (30%) N/T. Reasons for losses not reported Comment: Numbers lost were high for the length of follow‐up (2 years), but showed no differential loss between groups. It is also unclear if reasons for the missing data are acceptable and balanced between groups |
| Selective reporting (reporting bias) | High risk | Outcomes reported: dmfs increment ‐ (CA)cl + FOTI, at 2 years follow‐up; dmft increment ‐ (CA)cl + FOTI, at 2 years follow‐up Comment: Trial protocol not available. All pre‐specified outcomes (in Methods) were reported in the pre‐specified way. No standard deviations presented |
| Baseline characteristics balanced? | High risk | Prognostic factors reported: dmft: (1.62) FV1, (1.53) FV2, (0.92) NT; dmfs: (4.22) FV1, (3.38) FV2, (1.94) N/T Comment: Initial caries does not appear balanced between groups |
| Free of contamination/co‐intervention? | Unclear risk | Translation of report not detailed enough to make a categorical decision regarding contamination/co‐intervention |
Bravo 1997.
| Methods | Design: 3‐arm cluster quasi‐RCT (1 arm is not eligible for inclusion in this review) Location: Granada, Spain Study started: 1990 |
|
| Participants | Number randomised: Not reported by group Number analysed: 214 analysed (in 15 schools) at 2* years (present for all examinations) Age range: 6‐8 years (mean = 7) Background exposure to other fluoride: water (0.07 ppm F in the drinking water), data not obtained for toothpaste |
|
| Interventions |
Comparison: FV versus NT
Group 1 (n = 98): NaF varnish group (Duraphat® 22,600 ppm F), applied twice a year, with Q‐tip, about 0.1 ml applied per tooth (1stm) or 0.4 ml per child, left to dry for 15 seconds Group 2 (n = 116): No treatment Post‐op instructions: No hard food for 4 hours, no teeth cleaning until following day |
|
| Outcomes | *2‐year Net1stm DMFS increment ‐ (CA) (E+U)
Reported at 2 and 4* years follow‐ups: 1stm PF‐DMFS; 1stm MD‐BL‐DMFS; 1st molar occlusal CIR, molar failures over time (for molars healthy and fully erupted); costs; drop‐outs (no data by group) |
|
| Notes | School‐classes randomised (15) and children taken as units of analysis for caries increment analyses, molars as units for caries incidence and survival analyses; number of children by group NR Baseline characteristics (age, gender, SES, dft, 1stmF/DM, 1stmM; 1stmDMFS) described as 'balanced' (results NR) Clinical (VT) caries assessment by 1 examiner; diagnostic threshold = CA; state of tooth eruption included = E+U; examiner reproducibility checks (Kappa coefficient) in 10% sample greater than 0.71 in all 1stm DFT measurements *Only survival analysis results (molar failures over time) reported at 4 years, when results were not available for DMFS increment | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "First and second year classes were assigned at random into groups" Quote from correspondence: "The school classes allocation was not completely random since it had some restrictions: No more than 3 classes from same treatment group in the same school, and the total number of children should be at least more or less equilibrated between the groups. Thus, after the first random assignment, they were conditional" Comment: Probably was not a randomly generated sequence |
| Allocation concealment (selection bias) | High risk | Quote: "..clusters (n = 11) not randomised at once" Comment: The likely non‐random method used for sequence generation would not allow for allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: ".......and a control group which had no intervention" Comment: No placebo described |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "All children received biannual exams by a dentist using standardized criteria, and who was unaware of group assignments" Comment: Blind outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Overall drop‐out for length of follow‐up (reported for individuals within clusters only): 14.4% in 2 years. Drop‐outs by group (for the 2 relevant groups of the 3 in the trial) : 17/115 FV, 19/135 NT (14.8%, 14.1%). Reason for losses: 5 children were excluded from FV group only due to unerupted teeth; moved to other schools (numbers not reported by group) Comment: Recruitment of children was correctly done before clusters (school classes) had been randomised. Numbers lost were not unduly high for the length of follow‐up, and showed no differential losses between groups. However, the exclusions after allocation done in the treatment group only have the potential to introduce bias. Caries data used in analysis pertain to participants followed up for the entire study duration (and analysis done at individual level within clusters does not take clustering into account) |
| Selective reporting (reporting bias) | Low risk | Outcomes reported: 1stm DMFS increment ‐ (CA) (E+U), at 2 and 9 years follow‐ups; 1stm PF‐DMFS, 1stm MD‐BL‐DMFS,1st molar occlusal CIR, molar failures over time (for molars healthy and fully erupted), drop‐outs, costs Comment: Trial protocol not available. All pre‐specified outcomes (in Methods) were reported and were reported in the pre‐specified way |
| Baseline characteristics balanced? | Low risk | Prognostic factors not reported by group: 1stm DMFS: 0.45 (0.99) FV, 0.74 (1.43) NT Mean age: 7.28; gender (51% M 49% F); mean SES (class IV), mean dft 2.52 (2.90), 1stm F/DMF: 4.3%, 1stmM: 0 (not reported by group) Comment: Initial caries appears slightly imbalanced between groups (for individual within clusters). Adjustments in analysis for this and other factors are reported though |
| Free of contamination/co‐intervention? | Unclear risk | No information provided |
Chu 2002.
| Methods | Design:5‐arm quasi‐RCT (3 arms are not eligible for inclusion in this review) Location: Hong Kong, China Study started: Not reported |
|
| Participants | Number randomised: 146 (73, 73) Number analysed: 123 (61, 62) at 30 months Age range: 3‐5 years with caries in upper primary anteriors, mean age at baseline 4 years Background exposure to other fluoride: water (below 0.2 ppm), toothpaste Other background exposures: Oral health education was provided to all participants |
|
| Interventions |
Comparison: FV* versus 'PL'
Group 1 (n = 73): 5% NaF varnish group (Duraphat® 22,600 ppm F), applied 4 times a year, at schools (kindergartens), to carious surfaces, with small brush, left to dry (duration NR) Group 2 (n = 73): Water painted onto carious teeth Post‐op instructions: Not reported |
|
| Outcomes | Reported at 18 and 30 months follow‐up: Number of new carious tooth surfaces (upper anterior teeth); number of arrested carious tooth surfaces; percentage of arrested caries that were black; increment of non‐vital teeth; drop‐outs |
|
| Notes | Baseline characteristics (ds, dmfs, age) balanced
Clinical (VT + fibre‐optic light) caries assessment by 1 examiner, diagnostic threshold = CA "caries diagnosed at the cavitation level and explored with a sickle shaped probe". Radiographic assessment NR; state of tooth eruption NR. Intra‐examiner reliability calculated. Kappa (0.95‐0.98) * A FV study group receiving 5% sodium fluoride + prior caries removal was not considered **Additional analysis of multilevel grouped survival data with time‐varying regression coefficients and bayesian analysis of clustered multiple interval‐censored data (failure times) also reported for arrested caries, but not considered |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "Children were sequentially allocated...first child who came for examination to the first group, second child to the second group..." Comment: Alternation used to generate sequence |
| Allocation concealment (selection bias) | High risk | No information provided. However, the non‐random method used for allocation would not allow for allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Children in the fourth group had fluoride varnish applied....Only water was painted onto the carious teeth in the last group of children" Comment: Use of 'placebo' described |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quotes: "Examinations were carried out every 6 months after baseline by the same examiner without knowing the subjects treatment group assignments"; "Dentists who were not involved in the examination of the children performed the treatments" Comment: Blind outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Overall drop‐out for length of follow‐up: 15.8% in 30 months (for the 2 relevant groups of the 5 in the trial) Drop‐outs by group: 12/73 FV, 11/73 'PL'. Reasons for losses: Not reported Comment: Numbers lost were not unduly high for the length of follow‐up, and showed no differential loss between groups. It is unclear if reasons for the missing outcome data are acceptable and balanced. Caries data used in the analysis pertain to participants present at final examination |
| Selective reporting (reporting bias) | Low risk | Outcomes reported at 18 and 30 months follow‐ups; dmfs, arrested caries surfaces, percentage of arrested caries that were black, non‐vital teeth Drop‐outs Comment: Trial protocol not available |
| Baseline characteristics balanced? | Low risk | Prognostic factors reported: ds: 3.54 (2.34) FV, 3.76 (2.68) 'PL' dmfs: 4.33 (3.84) FV, 4.24 (2.84) PL; mean age: 4.0 (0.8) years (all groups) Comment: Initial caries appears balanced between groups |
| Free of contamination/co‐intervention? | Low risk | Comment: No apparent unbalanced provision of additional interventions/no difference in co‐interventions No apparent contamination |
Clark 1985.
| Methods | Design: 3‐arm parallel RCT Location: Quebec, Canada Study started: 1981 |
|
| Participants | Number randomised: 787 Number analysed: 676 analysed at 2.5* years (available at 2nd examination, present in at least 5 of 6 treatments) Age range: 6‐7 years Background exposure to other fluoride: toothpaste + others |
|
| Interventions |
Comparison: FV (2 groups) + ptc versus 'PL' + ptc
Group 1 (n = 232): FV group: Fluor Protector® Difluorsilane (7000 ppm F), applied twice a year, about 0.5 ml applied per child
Group 2 (n = 280): FV group: Duraphat® NaF (22,600 ppm F), applied twice a year, about 0.5 ml applied per child Group 3 (n = 275): Water, applied in the same manner as test groups |
|
| Outcomes | 2.5‐year* DMFS increment ‐ (CA) (E+U) dfs increments Reported at 1.5, 2.5* and 4.5 years follow‐ups**: O‐DMFS; MD‐DMFS; BL‐DMFS | |
| Notes | Baseline characteristics (dental age, DMFS) 'balanced'
Clinical (VT) caries assessment by 2 examiners; diagnostic threshold = CA; state of tooth eruption included = E+U/E; duplicate examination of 10% sample between examiners done (mean difference of 0.86 DMFS), "results of integrated analysis of treatment and examiner effects remained the same (significant)"
* Results closest to 3 years chosen
**Results presented separately by examiner and combined (integrated results chosen) Prior prophylaxis with non‐fluoride paste carried out in both groups nothing to eat and no brushing for 3‐4 hours |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "All children examined at baseline were stratified by dental age... and group assignments were made randomly from within each of the resulting partitions" Comment: Not enough information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quotes: "The study was also double blind; neither the examiners nor the participants were aware of group assignments" "Children in group 1 were treated with Fluor‐Protector, children in group 2 with Durafluor and children in group 3 were treated with water" "Clinical procedures were performed by dental hygienists" Comment: Use of 'placebo' described |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quotes: "The study was also double blind; neither the examiners nor the participants were aware of group assignments" "Clinical procedures were performed by dental hygienists" Comment: Blind outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Overall drop‐out for length of follow‐up: 14% in 2.5 years. Drop‐outs by group: 35/232 FV1, 35/280 FV2, 41/275 'PL' (15%, 12.5%, 15%). Reasons for attrition NR fully, but exclusions based on compliance with at least 5 of the 6 treatments Comment: Numbers lost were not unduly high for the length of follow‐up, and showed no differential loss between groups. It is unclear if reasons for the missing outcome data are acceptable and balanced. Caries data used in analysis pertain to participants present at all examinations, who had received at least 5 of the 6 treatments |
| Selective reporting (reporting bias) | Low risk | Outcomes reported: DMFS increment (CA) (E+U) and 1st & 2nd molars dfs increment (CA) (E), at 1.5, 2.5 and 4.5 years follow‐ups; O‐DMFS, MD‐DMFS, BL‐DMFS, FS/DMFS and DS/DMFS ratios, drop‐outs Comment: Trial protocol not available. All pre‐specified outcomes (in Methods) were reported and were reported in the pre‐specified way |
| Baseline characteristics balanced? | Low risk | Prognostic factors reported: DMFS: 0.44 (FV1), 0.45 (FV2), 0.36 ('PL'); dental age: primary teeth number 16.5 permanent 4.4 (FV1); primary 16.7, permanent 5.1 (FV2); primary 17.1, permanent 4.9 ('PL') Comment: Initial caries appears balanced between groups |
| Free of contamination/co‐intervention? | Unclear risk | Quote: "...most of the children probably used a fluoride dentifrice at home, and some probably received daily fluoride supplements" Comment: Unclear risk of co‐intervention |
Frostell 1991.
| Methods | Design: 6‐arm RCT, 2 arms included in this review Location: Malmö, Sweden Study started: 1977 6‐arm study. Parents who consented had children randomly allocated to 1 of 4 sugar ± varnish arms. Children whose parents did NOT consent to the sugar study were quasi‐randomised to either varnish (D) or no treatment (C) by alternation. We have included only arms D and C in this review. Reasons for losses not fully reported; exclusions based on compliance with study protocol, any differential group losses not assessable |
|
| Participants | Number randomised: Unclear Number analysed: 206 (113 (D) and 93 (C)) present for all examinations Age range: 4 years at baseline Background exposure to other fluoride: "There were no statistically significant differences in the use of F toothpaste, tablets and mouthrinse solutions between the 6 groups" Fluoride in water supply 0.2 ppm |
|
| Interventions |
Comparison: FV + ptc versus NT
Group D (n = 113): NaF group (Duraphat®) = 22,600 ppm F) Group C (n = 93): No treatment In D group all tooth surfaces were polished with pumice and rubber cap, and approximal surfaces were flossed, followed by a "thorough mouthrinse with water". Varnish was applied twice a year, with small brush, left to dry for 2 minutes, teeth were rinsed and any surfaces not coated were re‐coated. Teeth gently sprayed with water, no hard foods or toothbrushing till following day |
|
| Outcomes | Caries (CA) incidence and prevalence at 2 years (dmfs2 all caries, dmfs1 macroscopic caries only, dmft1 macroscopic caries only) | |
| Notes | Baseline characteristics: Only caries data at baseline reported ‐ no difference between groups
Clinical (VT) caries assessment by 2 examiners; diagnostic threshold = CA and NCA; state of tooth eruption included = E. Radiographic assessment (4 postBW) by 1 examiner; diagnostic threshold = DR and ER. Diagnostic errors NR Manufacturer thanked but unclear what for |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "The children whose parents did not want to participate in the sugar groups were assigned randomly to one of two groups, one with and one without Duraphat" Quote from correspondence: "Yes, every second child was treated with Duraphat" Comment: Alternation used to generate sequence |
| Allocation concealment (selection bias) | High risk | No information provided. However, the non‐random method used for allocation would not allow for allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quotes: "The children were assigned to one of two groups, one with and one without Duraphat" Comment: No placebo described. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quotes: "They were read by one and the same examiner (MP) who did not know which year the films were taken or to which group the child belonged" Comment: Blind outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Overall drop‐out for length of follow‐up: Not reported. Drop‐outs by group: Not reported. Participants present at final examination: 93 FV, 113 NT Reasons for losses: Not fully reported, but quote from correspondence: "a number of the children in the FV‐group (as well as a few in the NT‐group) did not follow the whole procedure and were excluded" Comment: Data on numbers randomised (at start) not available and drop‐out data not obtainable ‐ any differential group losses not assessable Caries data used in analysis pertain to participants present at all examinations, "who followed the study from the beginning to the end" |
| Selective reporting (reporting bias) | Low risk | Outcomes reported: dmfs increment ‐ (E) (CA/NCA)cl + (DR/ER) xr and dmft, at 2 years follow‐up Comment: Trial protocol not available. All pre‐specified outcomes (in Methods) were reported and were reported in the pre‐specified way |
| Baseline characteristics balanced? | Low risk | Prognostic factors reported: dmfs: 4.36 (FV), 5.14 (NT); dmft: 3.63 (FV), 4.43 (NT) Comment: Initial caries appears balanced between groups |
| Free of contamination/co‐intervention? | Unclear risk | No information provided |
Gugwad 2011.
| Methods | Design: 2‐arm RCT Location: India Study started: 2008 |
|
| Participants | Number randomised: 250 Number analysed: 211 Age range: 6‐7 years at baseline Backgound exposure to fluoride: No water fluoridation, exposure to other sources of fluoride assessed and found similar in each group Other background exposures: Oral hygiene instruction at baseline |
|
| Interventions |
Comparison: FV versus NT
Group 1 (n = 106): 5% NaF (Cavity Shield = 22,600 ppm F), unclear which dose used (0.25, 0.40 ml). Unclear where applied, 3 times in 1 week with small brush, left to dry for few seconds Group 2 (n = 105): No treatment Post‐op instructions: Abstain brushing and flossing and avoid chewing on hard food, no hot drinks, no alcohol for entire day |
|
| Outcomes | Reported at 1 year follow‐up: deft, deftp (posterior teeth), defs, defsp (posterior teeth), DMFT, DMFS (CA and NCA + xr) | |
| Notes | Participants randomised (numbers NR)
Baseline characteristics 'balanced' Oral hygiene instruction to both groups Clinical (VT) caries assessment (ADA type iii) using mouth mirror and probe; diagnostic threshold = CA and NCA; = E. Radiographic assessment (2 postBW) baseline and follow‐up; diagnostic threshold = DR and ER. Diagnostic errors NR Prior prophylaxis with non‐fluoride paste carried out in both groups |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote "Two hundred fifty children (6‐7 years) randomized into varnish and control groups" Quote from correspondence: "Children selected were randomly allocated to the groups (by lottery method)" Comment: Probably random sequence |
| Allocation concealment (selection bias) | Low risk | Quote from correspondence: "Allocation concealment and triple blinding (Examiner, Subject and Interpreter) were done" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote from correspondence: "...and triple Blinding (examiner, subject and interpreter) were done" Comment: But no placebo varnish described |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote from correspondence: "...and triple Blinding (examiner, subject and interpreter) were done" Comment: Blind outcome assessment indicated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Overall drop‐out for length of follow‐up: 39/250. Drop‐outs by group: 21/125 FV; 18/125 Control group. Participants present at final examination: 106 FV, 105 NT. Reasons for losses: Not reported Comment: Caries data used in analysis pertain to participants present at all examinations, "who followed the study from the beginning to the end" |
| Selective reporting (reporting bias) | Unclear risk | Outcomes reported: defs, defsp, deft, deftp, DMFS at baseline and 1 year follow‐up, but no increment reported Comment: Trial protocol not available. Increment calculated in methods but not reported |
| Baseline characteristics balanced? | Low risk | Prognostic factors reported: dmfs Comment: Initial caries appears balanced between groups |
| Free of contamination/co‐intervention? | Low risk | Exposure to other sources of fluoride assessed and found similar in each group |
Hardman 2007.
| Methods | Design: Cluster RCT Location: Manchester, UK Study started: Not reported |
|
| Participants | Number randomised: 2091 (1025, 1066) Number analysed: 664 (334, 330) in 24 schools present at baseline and final 24 months follow‐up Age range: 6‐8 years, mean age at baseline 7 years Background exposure to other fluoride: 1450 ppm F toothpaste supplied prior to baseline and final, milk |
|
| Interventions |
Comparison: FV versus NT
Group 1 (n = 1025): NaF varnish group (Duraphat® 22,600 ppm F), applied 6 monthly, at schools, to all surfaces of the primary and first permanent molars, with small brush, and left to dry (duration NR) Group 2 (n = 1066): No treatment Post‐op instructions: No control over drinking or eating after application |
|
| Outcomes | 2‐year mdfs increment ‐ (CA/NCA)cl + FOTI (E) Reported at 2 years follow‐up: Proportion of children with new 1stm DFS(CA/NCA); 3 levels of caries diagnosis small + large enamel lesions + dentine lesions |
|
| Notes | Baseline characteristics (dft/DFT/age/dmft > 0 / DMFT > 0) balanced Clinical (V/FOTI) caries assessment by 1 examiner; diagnostic threshold = CA and NCA; state of tooth eruption included = E/U; 15% sample re‐examined; K statistics 0.94 and 0.89 for intra‐examiner reliability at the first and last examinations | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The children were clustered within the unit of randomisation, the school. In half the schools year 2 children were allocated to the test group and year 3 children served as the control. In the other schools year 3 were the test group and year 2 the control. Randomisation, by a statistician, was used to allocate the combination of test and control year groups using a computer generated randomisation sequence" Comment: However recruitment was done after clusters (the schools) had been randomised (pre‐recruitment drop‐outs: 1177/2091 (56.1%), and might have led to the low numbers recruited, recruitment of participants with lower caries levels, and (selection bias ‐ the knowledge of whether each cluster is an intervention or control cluster could affect the types of participants recruited) |
| Allocation concealment (selection bias) | Low risk | Quote: "Clusters (n = 24) randomised at once" Comment: When clusters are large in numbers and are randomised at once as in this case, allocation concealment should not be an issue |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quotes: "The children and the therapists applying the varnish were not blinded" Comment: No placebo described |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quotes: "This was a single blind study as the examiner was unaware of the test or control status of the children"; "At baseline and after 26 months children were examined by one trained and calibrated examiner, who was blind to the children's allocation" Comment: Blind outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Overall drop‐out for length of follow‐up (reported for individuals within clusters only): 68% in 2 years. Drop‐outs by group: 691/1025 FV, 734/1066 NT (67%, 69%). Reasons for drop‐outs (FV, NT): Left during study (47, 16), left at final exam (28, 55), absent (33, 22), refused exam (2, 1), withdrawn (0, 1) "...an intention‐to‐treat approach applied in which data from each child present at the follow‐up examination was analysed according to their randomised group" Comment: Although no differential losses between groups were apparent and reasons for missing data are acceptable and balanced between groups, final numbers lost unduly high for the length of follow‐up. Caries data used in the analysis pertain to participants present at baseline and final examinations (and analysis done at individual level within clusters does not take clustering into account) |
| Selective reporting (reporting bias) | Low risk | Outcomes reported: dfs/DFS increment (CA)cl + FOTI at 2 years follow‐up; proportion of children with new 1stm DMFS Comment: Trial protocol not available. Pre‐specified outcomes reported |
| Baseline characteristics balanced? | Low risk | Prognostic factors reported: DFT: 0.15 (0.45) FV, 0.15 (0.54) NT; dft: 2.53 (2.43) FV, 2.26 (2.46) NT; Townsend score range (SES): ‐0.53 to 10.77 (for both groups); primary teeth caries prevalence: 67.7% FV, 60.9% NT, permanent teeth caries prevalence: 11.4% FV, 8.8% NT Comment: Initial caries appears balanced between groups (for individuals within clusters) |
| Free of contamination/co‐intervention? | Low risk | Quote: "During the study period a fluoride milk scheme was introduced. At the time of the final examination 18 of the 24 schools were offering fluoridated milk to those who consented and paid for it. This had been available for 15 months for 3 schools, between 3 and 12 months for 11 schools and the remaining 4 had just started on the scheme. Logistic regression analyses revealed no evidence of association with fluoride milk availability" Comment: Possibility of co‐intervention with fluoridated milk, but analysis for caries showed no association with the milk availability |
Holm 1979.
| Methods | Design: 2‐arm quasi‐RCT Location: Sweden Study started: Not reported |
|
| Participants | Number randomised: 250 (125, 125) Number analysed: 225 (112, 113) analysed at 2 years (available at final examination) Age range: Mean and median 3 years Background exposure to other fluoride: 0.3 ppm water fluoridation. At 5 years of age "no differences in toothbrushing frequency, regular use of fluoride tablets or use of fluoridated toothpaste" |
|
| Interventions |
Comparison: FV + ptc versus NT + ptc
Group 1 (n = 125): NaF varnish group (Duraphat® 22,600 ppm F), applied twice a year, with thin brush, left to dry (duration NR) Group 2 (n = 125): No treatment Post‐op instructions: No hard food or toothbrushing until following day |
|
| Outcomes | 2‐year defs increment ‐ (E) (CA)cl + (DR)xr Reported at 1 and 2 years follow‐ups: O‐defs; MD‐defs; BL‐defs; ds (NCA); proportion of children with 1 or more new defs (at CA level); drop‐outs | |
| Notes | Baseline characteristics (defs) unbalanced, 1.05 in FV, 0.71 in NT Clinical (VT) caries assessment by 1 examiner; diagnostic threshold = CA and NCA; state of tooth eruption included = E. Radiographic assessment (if required) by 1 examiner; diagnostic threshold = DR. Diagnostic errors NR | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "At baseline examination, every other child was assigned to the test group and the remainder to the control group" Comment: Not randomised. Alternation used to allocate into groups |
| Allocation concealment (selection bias) | High risk | No information provided. However, the non‐random method used for allocation would not allow for allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Fluoride varnish was applied to the teeth of the children in the test group......No placebo treatment was performed in the control group" Comment: No placebo described. Parents were not aware, however, that their children were taking part in any experiment and regarded the treatment as a routine part of the Public Dental Health Service given to all children |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Annual caries exam was performed by the same examiner and was single blind" Comment: Blind outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Overall drop‐out for length of follow‐up: 10% in 2 years. Drop‐outs by group: 13/125 FV, 12/125 NT. Reason for losses: Moving out of town 13 FV, 12 NT Comment: Numbers lost were not unduly high for the length of follow‐up, were reported by group and showed no differential losses between groups. The only reason reported for missing data is acceptable and balanced between groups. Caries data pertain to participants present at final examinations |
| Selective reporting (reporting bias) | Low risk | Outcomes reported: defs increment ‐ (E) (CA)cl + (DR)xr, at 1 and 2 years follow‐ups; O‐defs, MD‐defs, BL‐defs, ds (NCA); proportion of children with 1 or more new defs (at CA level); drop‐outs Comment: Trial protocol not available. All pre‐specified outcomes (in Methods) were reported and were reported in the pre‐specified way |
| Baseline characteristics balanced? | High risk | Prognostic factor reported: ds (CA): 1.05 (2.34) FV, 0.71 (1.62) NT; ds (NCA) 1.16 (3.11) FV, 0.59 (1.81) NT Mean age: 3 years (both groups) Comment: Initial caries appears unbalanced between groups |
| Free of contamination/co‐intervention? | Unclear risk | Quotes: "After each annual examination the child was given dental treatment by the Public Dental Health Service if necessary" and "...children in the test group had two more appointments with the dentist during these 2 years......." Comment: These more frequent visits might have made both children and dentists rather more concerned about dental health cannot be totally excluded |
Holm 1984.
| Methods | Design: 2‐arm RCT Location: Eslöv, Sweden Study started: 1977 |
|
| Participants | Number randomised: 120 (numbers by group NR) Number analysed:109 Age range: Every child aged 5 years and 9 months at baseline registration Background exposure to other fluoride: Water (Eslöv drinking water contained 0.4‐0.9 ppm F), "From the age of 6 years the children received organized dental care and took part in a weekly fluoride rinsing program (0.025% NaF for the 1st year, thereafter 0.2% NaF)" |
|
| Interventions |
Comparison: FV + ptc** versus NT
Group 1 (n = NR): NaF varnish group (Duraphat® 22,600 ppm F), applied twice a year, with a pencil (probe used to press the varnish into fissure) Group 2 (n = NR): No treatment Post‐op instructions: No hard food or toothbrushing of treated surfaces until following day **Prior prophylaxis with non‐fluoride paste carried out in FV group only |
|
| Outcomes | 2‐year 1stm DFS (fissures only) increment ‐ (CA) (U) Reported at 2 years follow‐up: 1stm DFT increment; proportion of children with 1 or more new 1stm DFS (at CA level), proportion of carious 1st molars | |
| Notes | Baseline characteristics (dmfs) 'balanced' Clinical (VT) caries assessment by 1 examiner (probe had to stick into cavity); diagnostic threshold = CA; state of tooth eruption included = U; intra‐examiner reproducibility checks for 1st molars (icc 0.98) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "...the children were randomly divided into a test and a control group" Comment: Not enough information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote from correspondence: "Were the treatments conducted blind? In the proper sense, no, since we did not use a placebo varnish" Comment: No placebo described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote from correspondence: "Were the treatments conducted blind? In the proper sense, no, since we did not use a placebo varnish" Comment: No mention of blinding of assessors in the report, radiographic examinations performed independently of clinical examinations though, and additional information is ambiguous about blinding Blind outcome assessment deemed likely but it remains unclear |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Overall drop‐out: 25/120 (20.8%) in 2 years. Drop‐outs not reported by group. Reasons for losses (numbers not reported by group): Excluded because molars had erupted at baseline examination (based on not meeting inclusion criteria, but probably after randomisation) (7), moved away (2), unwilling to participate (2) Comment: Numbers lost were not unduly high for the length of follow‐up. Differential losses are not assessable and it is unclear whether reasons for missing outcome data are balanced. Caries data used in analysis pertain to participants present after all reported losses (as above) |
| Selective reporting (reporting bias) | Low risk | Outcomes reported: 1stm DFS (fissures only) increment ‐ (CA) (U), at 2 years follow‐up; 1stm DFT increment; proportion of children with 1 or more new 1stm DFS (at CA level); proportion of carious 1st molars Comment: Trial protocol not available. All pre‐specified outcomes (in Methods) were reported and were reported in the pre‐specified way |
| Baseline characteristics balanced? | Low risk | Prognostic factor reported: Initial DMFT/S: 0; mean age: 5.75 years (all participants); dmfs (total): 8.32 (8.32) FV, 10.8 (8.27) NT; dmfs (proximal): 3.50 (3.50) FV, 4.86 (3.67) NT Comment: Initial caries appears balanced between groups |
| Free of contamination/co‐intervention? | Low risk | Quote: "From the age of 6 years the children received organized dental care and took part in a weekly fluoride rinsing program (0.025% NaF for the 1st year, thereafter 0.2% NaF" Comment: Exposure assumed to be similar in each group |
Koch 1975.
| Methods | Design: 2‐arm RCT Location: Jönköping, Sweden Study started: 1973 |
|
| Participants | Number randomised: 135 (numbers by group NR) Number analysed: 121 (60, 61) analysed at 1 year (available at final examination) Age range: 15 years at start of study Background exposure to other fluoride: Children in both groups exposed to local dental health programme involving mouthrinsing with 0.2% NaF solution every 2 weeks |
|
| Interventions |
Comparison: FV + ptc** versus NT
Group 1 (n = 60): NaF varnish group (Duraphat® 22,600 ppm F), applied twice a year, with a cotton swab, about 0.7 ml applied per child (full mouth treatment), left to dry for 2 minutes Group 2 (n = 61): No treatment Post‐op instructions: No hard food or toothbrushing until following day **Prior prophylaxis with non‐fluoride paste carried out in FV group only |
|
| Outcomes | 1 year DMFS increment ‐ (E) (CA/NCA)cl + (DR/ER)xr
Reported at 1 year follow‐up: O‐DMFS; MD‐DMFS; BL‐DMFS Data for cavitated dentine lesions separate to initial enamel caries ‐ clinical and radiographs combined |
|
| Notes | Baseline characteristics (DMFS) balanced Clinical (VT) caries assessment by 1 examiner; diagnostic threshold = CA and NCA; state of tooth eruption included = E. Radiographic assessment (full‐mouth BW) by 1 examiner; diagnostic threshold = DR and ER. Intra‐examiner reproducibility checked for DMFS cl + xr examinations in 20% sample (mean difference of 0.2 DS) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The children were randomly divided into a test and a control group" Comment: Not enough information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: No placebo described |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quotes: "The examiner did not know whether the child belonged to the test or the control group"; "All children were examined clinically and radiographically" Comment: Blind outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Overall drop‐out for length of follow‐up: 10% (14/135) in 1 year. Drop‐outs by group: Not reported. Reasons for losses: Not reported Comment: Numbers lost were not unduly high for the length of follow‐up. It is unclear if there were differential losses, and if reasons for missing outcome data are acceptable and balanced. Caries data used in analysis pertain to participants present at final examinations |
| Selective reporting (reporting bias) | Low risk | Outcomes reported: DMFS increment ‐ (E) (CA/NCA)cl + (DR/ER)xr, at 1 year follow‐up; O‐DMFS, MD‐DMFS, BL‐DMFS Comment: Trial protocol not available. All pre‐specified outcomes (in Methods) were reported and were reported in the pre‐specified way |
| Baseline characteristics balanced? | Low risk | Prognostic factor reported: DMFS 31.0 (10) FV, 27.4 (11) NT Comment: Initial caries appears balanced between groups |
| Free of contamination/co‐intervention? | Low risk | Quote: "During the experimental year, all the children in both the test and control groups were exposed to the local dental health program consisting of mouthrinsing with 0.2% NaF solution every fortnight" Comment: Assumed that exposure to fluoride mouthrinse similar in both groups |
Lawrence 2008.
| Methods | Design: Cluster RCT‐ 2 arms Location: Sioux Lookout Zone (SLZ), Northwest Ontario, Canada Study started: 2003 |
|
| Participants | Number randomised: 1275 (915, 360) Number analysed: 1160 (832, 328) (ITT numbers used) in 20 communities analysed at 2 years (present for at least 1 follow‐up examination) Age range: 5 months‐5 years (mean = 2.5) Background exposure to other fluoride: None reported Other background exposures: OH counselling of caregivers (promoting good oral health habits/awareness) |
|
| Interventions |
Comparison: FV versus NT
Group 1 (n = 915): 5% NaF varnish group (Duraflor® 22,600 ppm F), applied 2 to 3 times/year, to all surfaces of the primary dentition, with small brush, and left to dry (duration NR) Group 2 (n = 360): NT Post‐op instructions: Pamphlet distributed with post‐fluoride application instructions |
|
| Outcomes | 2‐year Net dmfs increment ‐ (CA)cl (E/U) Reported at 2 years follow‐up: Caries incidence; net caries increment calculated form change from sound, white spot or filled at baseline to 'clinical caries' missing due to carious extraction or stainless steel crown at follow‐up. Caries reversals (white spots/early demineralised to sound) were subtracted from the caries increment creating 'net' caries increment |
|
| Notes | Baseline characteristics balanced
Clinical caries assessment by 6 examiners, diagnostic threshold = CA Kappa values for inter‐examiner agreement ranged from 0.61 to 0.8 in all survey years |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A randomisation master list based on computer generated random numbers assigned each community to a group" |
| Allocation concealment (selection bias) | Low risk | Quote: "..clusters (n = 20) randomised at once" Comment: When clusters are large in numbers and are randomised at once as in this case, allocation concealment should not be an issue |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quotes: "The treatment consisted of FV 2 times per year with caregiver counselling while the no‐treatment controls received counselling alone" Comment: No placebo described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quotes: "Six teams of dental hygienists and recorders were flown into the participating communities ... to carry out the oral examinations and interviews" "Different examiners were sent to different communities each year to keep them masked to the community's treatment assignment" "Dental hygienists applied the varnish using a standard method of application" Comment: Blind outcome assessment is mentioned but there is apparently conflicting information about blinding since examiners appear to have been involved in giving treatment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Overall drop‐out for length of follow‐up (reported for individuals within clusters only): 9% in 2 years. Drop‐outs by group: 97/915 FV, 32/328 NT (10.6%, 9.8%). Reasons for losses (FV/NT): Relocated (38/7), lost to contact (10/14), did not attend appointment (34/7), sick (0/1), in foster care (8/0), parents unable to bring in (1/2), deceased (3/0), discontinued intervention (1/0), unco‐operative (1/0). "ITT analysis was carried out for 1146 children who completed either the 12 or 24 month follow‐up" Comment: Recruitment of children was correctly done before clusters (the communities) had been randomised. Numbers lost were not unduly high for the length of follow‐up, and showed no differential losses between groups. Reasons for losses are acceptable and balanced between groups. Caries data used in the analysis pertain to participants present for at least 1 follow‐up exam (and analysis done at individual level within clusters takes clustering into account) |
| Selective reporting (reporting bias) | Low risk | Outcomes reported: dmfs increment (CA)cl at 2 years follow‐up; caries incidence; drop‐outs Comment: Trial protocol not available. The primary outcome was reported but secondary outcomes (cost, quality of life, side effects, acceptability) will be reported subsequently |
| Baseline characteristics balanced? | Low risk | Prognostic factors reported: dmft: 7.19 (6.29) FV, 6.52 (6.16) NT Mean age: 2.54 (1.23) FV, 2.51 (1.18) NT dfs: 12.89 (16.02) FV, 11.80 (16.30) NT Percentage caries‐free: 27.3% FV, 31.1% NT dt/dmft: 73.9% FV, 73.3% NT Comment: Initial caries appears balanced between groups (for individuals within clusters) |
| Free of contamination/co‐intervention? | Low risk | Comment: No apparent unbalanced provision of additional interventions/no difference in co‐interventions. No apparent risk of contamination |
Liu 2012.
| Methods | Design: RCT (4 arms ‐ sealant, varnish, fluoride solution, placebo)‐ 2 included in this review Location: Guangzhou, southern China Study started: April 2008 |
|
| Participants | Number randomised: 252 children (778 first molars) (molars with ICDAS 2 included carious by Diagnodent excluded) Number analysed: 240 children (737 first molars) Age range: Grade 2 or 3 (mean age 9.1 years) Background exposure to other fluoride: 90% of toothpastes on sale in area were fluoridated |
|
| Interventions |
Comparison: FV + OH education versus PL + OH education
Group 1 (n =124 children, 385 teeth): 5% varnish group (Duraphat® = 22,600 ppm F Colgate Palmolive Ltd, Waltrop, Germany), applied every 6 months, plus oral health education (no details reported), tooth was isolated using cotton balls and varnish applied to pit/fissures with small disposable brush, and left to dry (child instructed not to eat or drink for 30 minutes) Group 2 (n = 128 children, 393 teeth): Placebo (water) applied annually and oral health education |
|
| Outcomes | 2‐year new dentine caries, prevented fraction Reported at 2 years follow‐up |
|
| Notes | Sample size calculation reported: To detect a 10% difference in new caries it was estimated that 1478 teeth and 493 children were required across the 4 arms of the study (power 80%, α = 0.05, ICC 0.2) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "..an assistant, using computer generated random numbers, allocated the children individually among 4 groups" |
| Allocation concealment (selection bias) | Unclear risk | Allocation was done by an assistant, and treatments were applied by a dentist. Unclear how allocation was communicated to treating dentist |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of patients not done. Sealant application process different from varnish application process. Varnish applied every 6 months, placebo applied annually |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Status of the molars, ...was assessed every 6 months by the same blinded examiner" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 12/252 (5%) children excluded from analysis at 2 years (8/124 in varnish ‐ 7 children moved from area and 1 excluded due to orthodontic treatment and 4/128 in placebo group left the area) |
| Selective reporting (reporting bias) | Low risk | Planned outcomes of new dentinal caries and preventive fraction reported |
| Baseline characteristics balanced? | Low risk | Varnish and placebo groups similar at baseline |
| Free of contamination/co‐intervention? | Low risk | No co‐intervention identified |
Milsom 2011.
| Methods | Design: 2‐arm cluster RCT Location: Lancashire, UK Study started: 2006 |
|
| Participants | Number randomised and eligible: 2967 (1473, 1494) Number analysed: 2604 at 3 years Age range: 7‐8 average age 8.1 years Backgound exposure to fluoride: Toothpaste + rinse |
|
| Interventions |
Comparison: FV versus NT
Group 1 (n = 1473): 5% NaF varnish (Duraphat® 22,600 ppm F), applied 3 times a year over 3 years in school, with small brush, 0.1 ml applied per child Group 2 (n = 1494): No treatment Post‐op instructions: No other fluoride treatments for 2 days |
|
| Outcomes | 3‐year DFS, DFT increment (1stm), number with caries (DFS, DFT) CA | |
| Notes | Baseline characteristics (DFS) 'balanced' Clinical caries assessment by 8 examiners | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...using computer‐generated random numbers, stratified by the locality of the school and the size of the school" |
| Allocation concealment (selection bias) | Low risk | Quote: "An ordered list of random group codes for all schools was produced, and only the study statistician and the trial manager had access to these codes" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of participants mentioned and no placebo used |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Examiners and their assistants were given a sealed envelope containing the allocation code for the school; this was opened after all the baseline examinations had been completed and the dentist made another appointment for application of the fluoride varnish in the test schools. This system ensured allocation concealment and facilitated efficient delivery of the intervention" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Overall drop‐out for length of follow‐up (reported for individuals within clusters only): 12% in 3 years. Drop‐outs by group: 197/1473 FV, 166/1494 NT (13%, 11%). Reasons for losses (FV/NT): Not explained Comment: Numbers lost were not unduly high for the length of follow ‐up, and showed no differential losses between groups. Losses are acceptable and balanced between groups. Caries data used in the analysis pertain to participants present for at follow‐up exam (and analysis done taking clustering into account) |
| Selective reporting (reporting bias) | Low risk | Outcomes reported: DMFS/T increment (CA)cl at 3 years follow‐up; caries progression/prevalence. Drop‐outs |
| Baseline characteristics balanced? | Low risk | Prognostic factors reported: initial DFS: 3 FV, 3 NT Toothbrushing frequency, toothpaste use, participation in rinsing programme, SES are not tabulated but reported as balanced between groups |
| Free of contamination/co‐intervention? | Low risk | Quote: "Participants were advised not to have fluoride treatment administered by their dentist for 2 days after application of the varnish" |
Modeer 1984.
| Methods | Design: 2‐arm RCT Location: Stockholm, Sweden Study started: Not reported |
|
| Participants | Number randomised: 236 (118, 118) Number analysed: 194 (87, 107) analysed at 3 years (available at final examination) Age range: 14 years at start of study Background exposure to other fluoride: Water (local drinking water contained 0.24 ppm F), children in both groups exposed to dental health programme involving mouthrinsing with 0.2% NaF solution every 2 weeks (for the whole duration of the study) |
|
| Interventions |
Comparison: FV + ptc** versus NT
Group 1 (n = 87): 5% NaF varnish group (Duraphat® 22,600 ppm F), applied 4 times a year, with small brush, 0.3 to 0.5 ml applied per child Group 2 (n = 107): No treatment Post‐op instructions: No eating for 4 hours after application, no toothbrushing until following day **Prior prophylaxis with non‐fluoride paste carried out in FV group only |
|
| Outcomes | 3‐year MD‐DFS increment ‐ (E) (ER/DR)xr Reported at 3 years follow‐up: Caries progression rate | |
| Notes | Baseline characteristics (toothbrushing frequency, toothpaste use, participation in rinsing programme, SES) described as 'balanced' (values NR); initial DFS unbalanced No clinical assessment of caries Radiographic assessment (4 postBW) by 1 examiner; intra‐examiner reproducibility checks (icc = 0.89) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The children who participated during the 3‐year period were randomly divided into a fluoride varnish group and a control group" Quote from correspondence: "I do not exactly remember. It was either by lot or using special random numbers" Comment: Probably adequate |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quotes: "The fluoride varnish application was carried out by a specially trained dental nurse" Comment: No placebo described |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quotes: "Radiographic registrations were made without knowing the group to which the child belonged"; "The fluoride varnish application was carried out by a specially trained dental nurse" Comment: Blind outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Overall drop‐out for length of follow‐up: 18% in 3 years. Drop‐outs by group: 31/118 FV, 11/118 NT. Reasons for losses (FV, NT): No co‐operation (20, 0), moved out (5, 3), orthodontic treatment (6, 8) Comment: Numbers lost were not unduly high for the length of follow‐up, but there is a differential loss between groups (26.3% FV, 9.3% NT) and 1 of the reasons for missing data (no co‐operation) is unbalanced (unacceptable since differential drop‐out might be due to treatment). Caries data used in the analysis pertain to participants present at final examinations |
| Selective reporting (reporting bias) | Low risk | Outcomes reported: 1st & 2nd mpmMD‐DFS increment ‐ (E) (ER/DR) xr, at 3 years follow‐up; caries progression rate; drop‐outs Comment: Trial protocol not available. All pre‐specified outcomes were reported and were reported in the pre‐specified way |
| Baseline characteristics balanced? | High risk | Prognostic factors reported: Initial DFS: 1.1 FV, 1.7 NT Toothbrushing frequency, toothpaste use, participation in rinsing programme, SES are not tabulated but reported as balanced between groups Comment: Initial DFS levels unbalanced |
| Free of contamination/co‐intervention? | Low risk | Quote: "The children in both the fluoride varnish group and the control group participated in the routine fluoride rinsing programme which consisted of mouthrinses every 14 days with a 0.2% NaF solution during the entire experimental period" Comment: No apparent co‐intervention, exposure assumed to be similar in both groups |
Salazar 2008.
| Methods | Design: 2‐arm parallel group RCT Location: Rio de Janeiro, Brazil Study started: June 2006 to July 2007 |
|
| Participants | Number randomised: 200 Number analysed: 148 Age range: 12‐48 months Background exposure to other fluoride: Majority of participants exposed to fluoridated water but concentration varied depending on area of residence. 84% in varnish and 75% of control group used fluoride toothpaste but not all children brushed daily and some children brushed unsupervised which may have compromised effectiveness |
|
| Interventions |
Comparison: FV versus PL
Group 1 (n = 71): 5% NaF varnish group (Duraphat® 22,600 ppm F), applied every 6 months (2 applications) Group 2 (n = 77 ): Placebo applied every 6 months (2 applications) All children had their teeth cleaned with water, then isolated with cotton rolls and dried with air prior to the application of either varnish or placebo with a microbrush Post‐op instructions: Children were instructed no to eat hard food or brush their teeth on the day of application |
|
| Outcomes | 1 year follow‐up: New dentinal caries lesions, mean caries increment, adverse effects | |
| Notes | Visual caries diagnosis ICDAS (CA + NCA) High caries prevalence population. Approximately 1/2 children had caries in the primary teeth and approximately 1/4 had dentinal caries Sample size calculation reported that 85 children per group were required to show a reduction in caries from 33% to 15%, and additional 15 recruited per group to allow for anticipated loss to follow‐up |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...computerised randomisation using Excel software" |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes used to conceal the allocation from the researchers |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Children/caregivers/operators and outcome assessors blinded to allocated treatment |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Children/caregivers/operators and outcome assessors blinded to allocated treatment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 200 children randomised and 29 and 23 excluded from the analysis because they did not attend the final examination. Overall loss is high but reasons given are loss of contact with family due to change of address or telephone number for both groups |
| Selective reporting (reporting bias) | Low risk | Caries outcome and adverse effects reported |
| Baseline characteristics balanced? | Low risk | Both caries prevalence and demographic factors appear to be balanced at baseline |
| Free of contamination/co‐intervention? | Unclear risk | Unclear what the actual exposure to fluoride toothpaste and fluoridated water was |
Sköld 2005.
| Methods | Design: 4‐arm RCT Location: Sweden (West coast) Study started: 1998 |
|
| Participants | Number randomised: 854 Number analysed: 758 analysed at 3 years (present for all examinations) Age range: 13‐16 years (all subjects 13 years at start of 3‐year study) Background exposure to other fluoride: Water (in 1 of 3 trial sites only), toothpaste |
|
| Interventions |
Comparison: FV (3 groups) + ptc** versus NT Group 1 (n = 190): NaF varnish (Duraphat® 22,600 ppm F), twice a year Group 2 (n = 186): NaF varnish (Duraphat® 22,600 ppm F), 3 times a year Group 3 (n = 201): NaF varnish (Duraphat® 22,600 ppm F), 8 times a year Group 4 (n = 181): No treatment Application in mobile units in schools, to all posterior approximal surfaces, with syringe, 0.3 mL (1 drop) applied, left to dry (duration NR) Post‐op instructions: Refrain from eating hard foods on that day; no brushing until next day **Toothbrushing with non‐fluoride paste carried out in FV groups only |
|
| Outcomes | 3‐year DFS incidence ‐ (E) (DR/ER)xr (only) Reported at 3 years follow‐up: DS; FS; caries progression | |
| Notes | Baseline characteristics (DS/FS) balanced X‐Ray caries assessment by 1 examiner; diagnostic threshold = DR and DE; state of tooth eruption included = E; for intra‐examiner reliability, 10% of the radiographs read twice with an interval of 2 months (Kappa 0.90 for all scores and 0.82 for carious surfaces) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "...the adolescents were randomly allocated within each school class into 4 groups" Comment: Not enough information given |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quotes: "Three trained dental nurses and one dental hygienist performed all the treatments..."; "...treated adolescents with fluoride....and no treatment (control)" Comment: No placebo described |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quotes: "...films were scored and analysed blindly by one of the authors"; "Three trained dental nurses and one dental hygienist performed all the treatments..." Comment: Blind outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Overall drop‐out for length of follow‐up: 11% in 3 years. Drop‐outs by group: Not reported. Reasons for losses: Moving away from the area, not attending all treatment sessions Comment: Numbers lost overall were reported, and not unduly high for length of follow‐up. Numbers randomised (at start) were not reported by group, thus drop‐outs by group not obtainable. It is unclear if reasons for the missing outcome data are acceptable and balanced. Caries data used in the analysis pertain to participants followed up for the entire study duration and attending all treatment sessions |
| Selective reporting (reporting bias) | Low risk | Outcomes reported: 1st and 2nd mpmDS/FS final prev and DFS incidence (CA/NCA) xr at 3 years follow‐up; drop‐outs Comment: Trial protocol not available. All pre‐specified outcomes (in Methods) were reported and were reported in the pre‐specified way |
| Baseline characteristics balanced? | Low risk | Prognostic factors reported: DS (approximal dentine): 0.15 (0.51) FV1, 0.12 (0.36) FV2, 0.13 (0.49) FV3, 0.07 (0.28) NT FS: 0.13 (0.48) FV1, 0.10 (0.43) FV2, 0.08 (0.46) FV3, 0.13 (0.45) NT DS (approximal enamel): 2.15 (3.37)FV1, 2.13 (3.30) FV2, 2.36 (3.86) FV3, 1.75 (2.43) NT Comment: Initial caries appears balanced between groups |
| Free of contamination/co‐intervention? | High risk | Quotes: "All participants in this study attended the dental clinics for regular check‐ups and were given prophylactic treatment according to their actual caries risk. The dentists who treated them had no knowledge to which group they belonged" "... 95% of the adolescents in all areas were treated with one F varnish at the yearly check‐up, ... all of them, independent of area and caries risk revealed they brushed their teeth twice a day using an F toothpaste" Comment: Although dentists treating the children in the yearly check‐ups were unaware of group assignment, 95% of all children were treated with 1 application of fluoride varnish, an apparent contamination |
Tagliaferro 2011.
| Methods | Design: Quasi‐randomised CCT Location: Piracicaba, Brazil Study started: Unclear, protocol registered in 2004, before 2008 |
|
| Participants | Number randomised: 219 Number analysed: 181 (109, 110) Age range: 6‐8 years Background exposure to other fluoride: Water 0.7 ppm, toothpaste Other background exposures: OH education |
|
| Interventions |
Comparison: FV versus NT
Group 1(n = 109): 5% NaF varnish (Duraphat® 22,600 ppm F), applied 6 monthly, at schools, to all surfaces of first permanent molars, with small brush, and left to dry Group 2 (n = 110): No treatment All participants received oral health education, and a toothbrush, floss, and fluoride toothpaste for toothbrushing prior to examinations (5 during the study) Post‐op instructions: No chewing or brushing after application, no eating of hard food till next day |
|
| Outcomes | Reported at 2 years follow‐up: DMFS (all), DMFS (1stm), increment (CA/CA+NCA)cl (E/U) | |
| Notes | Baseline characteristics (dmft/DMFT/age) balanced. Surfaces affected: NR (dmft = 4.4/ DMFT = 0.3) Clinical (VT) caries assessment by 1 examiner; diagnostic threshold = CA and NCA; state of tooth eruption included = E/U; 10% sample re‐examined at each examination; examiner calibration (Kappa 0.90 CA/ 0.95 CA+NCA) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "This study was a systematically randomized trial ...Then they were systematically allocated in each treatment group by the main researcher..." Quote from correspondence: "At baseline examination, children were systematically allocated in each treatment group, as follows: approximately 10 children of each classroom were taken to the examiner by the dental hygienist (she did not know the risk of caries level of the children before each examination) who organized them in a queue at random. The examiner (Pardi V) performed the examination of the first child, the main research (Tagliaferro EP) recorded the data in a specific form and classified the child in high or low caries risk, according to pre‐established criteria. After that, each classified children were allocated in the Control, Varnish or Sealant group in this sequence" Comment: Alternation used to generate sequence |
| Allocation concealment (selection bias) | High risk | Quote from correspondence: "The same researcher did the allocation and applied the sealants. Also, the non‐random method used for allocation would not allow for allocation concealment" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No placebo varnish described |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "...the calibrated dentist was not aware of group assignments during evaluations" Quote from correspondence: "The examiner did not see the records/documents used for recording the interventions in each child" Comment: Blind outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Overall drop‐out for length of follow‐up: 17% in 2 years. Drop‐outs by group: 19/109 2FV, 19/110 2NT (17.4%, 17.3%). Reason for losses: Moving out and refusing final examination (NR by group) Comment: Numbers lost were not unduly high for the length of follow‐up, were reported by group, and showed no differential losses between groups. The reasons reported for missing data are acceptable (although unclear if balanced) between groups. Caries data seem to pertain to participants present at final examinations |
| Selective reporting (reporting bias) | Low risk | Outcomes reported: 1stm ODMFS increment ‐ (CA/NCA) (E+U), at 2 years follow‐up; drop‐outs Comment: Trial protocol not available. All pre‐specified outcomes (in Methods) were reported and were reported in the pre‐specified way |
| Baseline characteristics balanced? | Low risk | Prognostic factors reported: DMFT: 0.26 (0.58) FV, 0.35 (0.67) NT. dmft: 4.28 (2.54) FV, 4.53 (3.0) NT; mean age: 7.0 (0.7) years (all groups) Comment: Initial caries appears balanced between groups |
| Free of contamination/co‐intervention? | Low risk | Comment: No apparent co‐intervention or contamination |
Tewari 1990.
| Methods | Design: 4‐arm RCT (2 arms included in this review) Location: Chandigarh, India Study started: In/before 1982 |
|
| Participants | Number randomised: 657 Number analysed: 618 children analysed at 2.5* years (available at 2nd examination)) Age range: 6‐12 years (mean = 8.5) Background exposure to other fluoride: Water (drinking water contained 0.3 ppm F) |
|
| Interventions |
Comparison: FV + ptc versus 'PL' + ptc
Group 1 (n = 331): NaF varnish group (Duraphat® 22,600 ppm F), applied twice a year, with single tufted brush, about 0.5 ml applied per child, left to dry for 4 minutes Group 2 (n = 326): Double distilled water Post‐op instructions: No rinsing or drinking for 1 hour after application, no solids (only liquids and semisolids) until following morning |
|
| Outcomes | 2.5‐year NetDMFS increment ‐ (CA/NCA) (E/U) Reported at 1.5 and 2.5 year follow‐ups: ODMFS (CA/NCA) (E/U); MDDMFS (CA/NCA) (E/U); BLDMFS (CA/NCA) (E/U); DMFT (CA/NCA) (E/U) | |
| Notes | Baseline characteristics (age, DMFS, DMFT) balanced
Clinical (VT) caries assessment by 2 examiners; diagnostic threshold = NCA/CA; state of tooth eruption included = E/U; constant duplicate examination of 10% sample between same and both examiners (results NR)
*Final 4.5 years results not available (but results closest to 3 years were chosen) Prior prophylaxis with non‐fluoride paste carried out in both groups |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...children were randomly allocated into 4 groups with the help of a computer, but were stratified according to age, sex, number of erupted permanent teeth, socio‐economic status and previous caries experience" Comment: Most likely a computer generated sequence used |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "...teeth of children in the control group was painted with double distilled water" Comment: Most likely participants blinded. Blind outcome assessment and use of 'placebo' described |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quotes: "The study was single blind as the recorder did not know the fluoride group to which the child belonged nor the previous recording"; "...teeth of children in the control group was painted with double distilled water" Comment: Most likely assessors and participants blinded. Blind outcome assessment and use of 'placebo' described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Overall drop‐out for length of follow‐up: 6% in 2.5 years. Drop‐outs by group: 20/331 FV, 19/326 'PL'. Reasons for losses: NR Comment: Numbers lost were not unduly high for the length of follow‐up, and showed no differential loss between groups. "Balancing of DMFT and DMFS as well as the mean age was not disturbed between the various experimental groups due to the attrition of the trial population." Caries data used in the analysis pertain to participants present at final examination |
| Selective reporting (reporting bias) | Low risk | Outcomes reported: DMFS increment ‐ (CA/NCA) (E/U), at 1.5 and 2.5 year follow‐ups; ODMFS (CA/NCA) (E/U); MDDMFS (CA/NCA) (E/U), BLDMFS (CA/NCA) (E/U), DMFT (CA/NCA) (E/U); drop‐outs Comment: Trial protocol not available. All pre‐specified outcomes (in Methods) were reported and were reported in the pre‐specified way |
| Baseline characteristics balanced? | Low risk | Prognostic factors reported: DMFT: 2.0 (1.88) FV, 1.87 (1.87) PL; DMFS: 2.60 (2.43) FV, 2.38 (2.29) PL; mean age: 8.43 FV, 8.33 PL Comment: Initial caries appears balanced between groups, because these were stratified in the randomisation process |
| Free of contamination/co‐intervention? | Low risk | No information provided |
Weintraub 2006.
| Methods | Design: 3‐arm RCT Location: San Francisco, USA Study started: 2000 |
|
| Participants | Number randomised: 376 Number analysed: 280 analysed at 2 years (available at any examination) Age range: 6‐44 months (0.5‐3.7 years; mean = 1.8 years) Background exposure to other fluoride: Water, toothpaste Other background exposures: OH counselling |
|
| Interventions |
FV (2 groups) versus 'PL' Group 1 (n = 124): NaF varnish (Duraphat® 22,600 ppm F), twice a year Group 2 (n = 126): NaF varnish (Duraphat® 22,600 ppm F), once a year Application in health centres, to all teeth surfaces, teeth dried with gauze, varnish applied with brush, 0.1 mL (1 drop) applied per arch, left to dry (duration NR) Group 3 (n = 126): NaF varnish applied to gauze, which was then folded and the dry area used to wipe the child's teeth ensuring that no NaF varnish was applied Pre/post‐op instructions: Refrain from brushing children's teeth with F dentifrice the day of varnish treatment |
|
| Outcomes | 2‐year dfs increment ‐ (CA/NCA)cl (E) Reported at 1 and 2 years follow‐ups: Any caries incidence/no caries incidence(CA/NCA) Adverse events reported |
|
| Notes | Baseline characteristics (initial caries = 0, age) balanced
Clinical caries assessment by 1 examiner, diagnostic threshold = CA/NCA Intra‐examiner reliability, from repeat examinations of 21 children, yielded Kappa statistic of 0.96 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The team's biostatisticians conducted the computer generated random assignment of participants" |
| Allocation concealment (selection bias) | Low risk | Quote: "Assignment was concealed in sealed, opaque, labelled envelopes, unopened until time for treatment by the clinician" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Masking accompanying caregivers to the control group assignment was attempted. The control group's tray set‐up was the same. For children in this group, fluoride varnish was placed on gauze, which was then folded. The dry area was used to wipe the child's teeth, and no fluoride varnish was applied" Comment: Use of 'placebo' described |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quotes: "One paediatric dentist (FRG) masked to treatment groups, conducted all dental examinations"; "One dentist (BJ) who spoke English, Spanish, and Cantonese provided clinical interventions at both sites" Comment: Blind outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Overall drop‐out for length of follow‐up: 26% in 2 years. Drop‐outs by group (based on data from all children with any follow‐up exam): 31/124 FV1, 39/126 FV2, 26/126 'PL'. Reasons for losses: Not reported. "For primary analysis, we used the intention‐to‐treat approach.... Analysis used data from all children with a 12‐ or 24‐month follow‐up exam." "Markov Chain Monte Carlo estimation was used in multiple imputation of missing data." Comment: Numbers lost were not unduly high given the length of follow‐up, and losses between FV and PL not statistically significantly different. It is unclear if reasons for the missing outcome data are acceptable and balanced. Caries data used in the analysis pertain to participants at a follow‐up examination |
| Selective reporting (reporting bias) | Low risk | Outcomes reported: dfs (CA)cl increment at 1 and 2 years follow‐ups; dfs(NCA/CA)cl; caries incidence; drop‐outs Comment: The pre‐specified primary outcome was reported in the pre‐specified way but the secondary outcomes (diet, bottle use, dental utilisation) will be reported subsequently |
| Baseline characteristics balanced? | Low risk | Prognostic factors reported for all groups: Mean age 1.8 (0.6) Comment: As regards initial caries (dfs), eligibility criteria for the trial was that all primary teeth should be caries‐free without demineralisation, therefore this characteristic was balanced |
| Free of contamination/co‐intervention? | Low risk | The protocol violation caused by the provision of placebo varnish to the intervention group was accounted for in the ITT analysis |
Yang 2008.
| Methods | Design: Double blind 4‐arm RCT ‐ 3 arms included in this review Location: Chongqing City, China Study started: December 2004 |
|
| Participants | Number randomised: 150 Number analysed: 148 Age range: 3 years old at baseline Gender: M 79 / F 71 |
|
| Interventions |
Comparison: FV (2 groups) versus PL Group 1 (n = 37): 0.5% FV (Fluor Protector = 5000 ppm) applied with cotton swab twice after teeth were dried. Children told not to eat or drink for 30 minutes. Treatment was applied every 6 months Group 2 (n = 38): 0.1% FV (Fluor Protector = 1000 ppm) applied with cotton swab twice after teeth were dried. Children told not to eat or drink for 30 minutes. Treatment was applied every 6 months Group 4 (n = 36): Placebo (water) applied with cotton swab twice after teeth were dried. Children told not to eat or drink for 30 minutes. Treatment was applied every 6 months Study duration: 2 years Unclear who applied the interventions, or where the applications took place. There was also a third intervention group (Group 3) 0.5% sodium fluoride which was excluded from the review |
|
| Outcomes | Prevalence of caries (CA), dmft, dmfs, number of missing teeth | |
| Notes | Translated by Chunjie Li (September 2012) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The authors only described that the participants were allocated randomly without mentioning the methods of randomisation |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blinded trial |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The outcome assessors were blinded to allocated treatment but the details of how this was done are not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 lost to follow‐up: 1 in 0.1% fluoride varnish group and 1 in 0.5% sodium fluoride group. Both of these participants were not included in the analysis. Reason was unclear. Unlikely to have introduced a bias |
| Selective reporting (reporting bias) | Low risk | Planned outcomes prevalence of caries, dmft, dmfs reported |
| Baseline characteristics balanced? | Low risk | Prevalence of caries, dmft, dmfs were comparable at baseline |
| Free of contamination/co‐intervention? | Unclear risk | It was unclear whether participants were exposed to other treatments during the trial |
Drop‐out rates based only on groups relevant to review, on relevant follow‐ups, unless otherwise stated. Baseline caries experience averaged among relevant study arms, and based on the study sample analysed at the end of treatment period (final sample), unless otherwise stated. Age range (average age when reported) at the time the study started based on all study participants (or on groups relevant to the review when data were available) 1stm = first permanent molar; 'A' = classified as double‐blind but participants may not be blind (as a 'PL' was used); ADA = American Dental Association; CaF = calcium fluoride; CA = lesions showing loss of enamel continuity that can be recorded clinically (undermined enamel, softened floor/walls) or showing frank cavitation; CCT = controlled clinical trial; CIR = caries incidence rate; cl = clinical examination; deft/s = decayed, extracted and filled primary teeth or surface; dmft/s = decayed, missing (or extracted) and filled primary teeth or surface; D(M)FS/T = decayed, (missing) and filled permanent surfaces or teeth; DR = radiolucency into dentine; E = teeth erupted at baseline; ER = any radiolucency in enamel/enamel‐dentine junction; F = fluoride; FOTI = fibre‐optic transillumination; FV = fluoride varnish treatment; icc = intra‐class correlation coefficient (for inter‐rater reliability); ICC= intra‐cluster correlation coefficient; ICDAS = International Caries Detection and Assessment System; ITT = intention‐to‐treat; M = missing permanent teeth; MD = mesio and distal surfaces; N = numbers; NaF = sodium fluoride; NCA = non‐cavitated enamel lesions visible as white spots or discoloured fissures; NR = not reported; NS = not significant; NT = no treatment; O = occlusal surfaces; OH = oral health; PF = pit and fissure surfaces; PL = placebo varnish; 'PL' = not a true placebo (inactive treatment other than varnish used); postBW = posterior bite‐wing x‐ray assessment; ppm F = parts per million of fluoride; ptc = prior tooth‐cleaning performed with or without a non‐fluoride paste; RCT = randomised controlled trial; SES = socio‐economic status; U = teeth unerupted at baseline; VT = visual‐tactile assessment; xr = radiographic examination.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Alves 1997 | Length of follow‐up of less than 1 year/school year (6 months) |
| Autio‐Gold 2001 | Follow‐up is less than 1 year/school year |
| Billy‐Pryga 1983 | Split‐mouth design ‐ sites within a participant's mouth were allocated to treatment and control groups |
| Bodnar 1984 | Non‐randomised split‐mouth study where only 1 first molar per child was treated and another was used as control |
| Demito 2011 | Study on patients undergoing treatment with fixed orthodontic appliances |
| Dülgergil 2005 | Additional non‐fluoride‐based interventions associated to fluoride varnish |
| Grodzka 1982 | No random or quasi‐random allocation used. Open outcome assessment reported after contacting author |
| Hetzer 1973 | Additional non‐fluoride‐based intervention associated to fluoride varnish. Random or quasi‐random allocation not stated or indicated. Blind outcome assessment not stated and unlikely |
| Heuser 1968 | No random or quasi‐random allocation used (non‐random concurrent control). Blind outcome assessment not stated and unlikely. Varnish applied once in 15 months |
| Hochstein 1975 | Medically compromised group of children selected. No random or quasi‐random allocation used (non‐random concurrent control). Open outcome assessment |
| Ivanova 1990 | Random or quasi‐random allocation not stated or indicated. Blind outcome assessment not stated and unlikely |
| Ji 2007 | Communication between Chunjie Li and the review authors confirmed that allocation was based on the preference of clinicians and was not randomly allocated |
| Kolehmainen 1979 | Split‐mouth design ‐ sites within a participant's mouth were allocated to treatment and placebo groups |
| Kolehmainen 1981 | Split‐mouth design ‐ sites within a participant's mouth were allocated to treatment and placebo groups |
| Kunin 1991 | Random or quasi‐random allocation not stated or indicated. Blind outcome assessment not stated and unlikely |
| Lagutina 1978 | Random or quasi‐random allocation not stated or indicated. Blind outcome assessment not stated and unlikely |
| Lieser 1978 | No random or quasi‐random allocation used (non‐random concurrent control ‐ by matching procedure). Blind outcome assessment not stated and unlikely |
| Lindquist 1989 | Fluoride‐based intervention associated to control group |
| Maiwald 1974 | Random or quasi‐random allocation not stated or indicated |
| Maiwald 1978 | Random or quasi‐random allocation not stated or indicated. Blind outcome assessment not stated and unlikely |
| Mari 1988 | Random or quasi‐random allocation not stated or indicated. Blind outcome assessment not stated and unlikely |
| Mari 1988a | Random or quasi‐random allocation not stated or indicated (Note ‐ 2 clusters, each assigned to 1 of the 2 groups) |
| Murray 1977 | Split‐mouth design ‐ sites within a participant's mouth were allocated to treatment and placebo groups |
| Pashaev 1977 | Split‐mouth design ‐ sites within a participant's mouth were allocated to treatment and control groups. Random or quasi‐random allocation not stated. Blind outcome assessment not stated and unlikely |
| Petersson 1998 | No random or quasi‐random allocation used (non‐random concurrent controls ‐ by matching procedure). Blind outcome assessment not stated and unlikely |
| Ramos 1995 | Open outcome assessment |
| Ramos‐Gomez 2012 | Control group of children received varnish as required if lesions developed |
| Riethe 1977 | Split‐mouth design ‐ sites within a participant's mouth were allocated to treatment and control groups |
| Rodríguez Miró 1988 | Additional non‐fluoride‐based intervention associated to fluoride varnish |
| Ruszynska 1978 | Split‐mouth design ‐ sites within a participant's mouth were allocated to treatment and control groups |
| Salem 1979 | Split‐mouth design ‐ sites within a participant's mouth were allocated to treatment and control groups |
| Schioth 1981 | 5 tooth cleaning treatments given to varnish group only. Unclear |
| Schmidt 1970 | Split‐mouth design ‐ sites within a participant's mouth were allocated to treatment and control groups |
| Seppä 1982 | Split‐mouth design ‐ sites within a participant's mouth were allocated to treatment and control groups |
| Shobha 1987 | Random or quasi‐random allocation not stated or indicated. Blind outcome assessment not stated and unlikely (Note ‐ Main outcome data not reported in control group (and not obtainable)) |
| Slade 2011 | Additional non‐fluoride and fluoride‐based interventions associated to fluoride varnish |
| Splieth 2000 | No random or quasi‐random allocation used (non‐random concurrent control). Split‐mouth study |
| Suntsov 1991 | Random or quasi‐random allocation not stated or indicated. Blind outcome assessment not stated and unlikely (Note ‐ Only post‐treatment effects reported) |
| Suwansingha 2011 | Non‐randomised split‐mouth study. Length of follow‐up of less than 1 year (6 months) |
| Todorashko 1983 | Additional fluoride‐based intervention associated to fluoride varnish. Random or quasi‐random allocation not stated or indicated. Blind outcome assessment not stated and unlikely |
| Tranaeus 2001 | Additional fluoride‐based interventions associated to fluoride varnish. Length of follow‐up of less than 1 year/school year (6 months) (Note ‐ Main outcome data not reported) |
| Treide 1980 | No mention of randomisation (German translation) |
| van Eck 1984 | No random or quasi‐random allocation used (non‐random concurrent control ‐ by matching procedure) |
| Wacińska‐Drabińska 1987 | Children were randomly selected for participation but not randomly allocated to treatment groups |
| Wegner 1976 | Medically compromised group of children selected. No random or quasi‐random allocation used (non‐random concurrent control). Blind outcome assessment not stated and unlikely |
| Winter 1975 | No random or quasi‐random allocation used (non‐random concurrent control). Blind outcome assessment not stated and unlikely |
| Wojtowicz 1986 | No blind outcome assessment |
| Xhemnica 2008 | Length of follow‐up of less than 1 year/school year (7 months) |
| Zimmer 1999 | No random or quasi‐random allocation used (non‐random concurrent control). Blind outcome assessment not used |
Characteristics of ongoing studies [ordered by study ID]
Macpherson 2012.
| Trial name or title | Comparison of the caries‐protective effect of fluoride varnish (Duraphat®) with treatment as usual in nursery school attendees receiving preventive oral health support through the Childsmile Oral Health Improvement Programme: an RCT |
| Methods | Randomised controlled trial, parallel assignment, double blind (subject, caregiver, investigator, outcomes assessor) |
| Participants | 3‐ to 4‐year old children in nursery schools |
| Interventions | ‐ Treatment as usual (i.e. any treatment from the family dentist, plus the preventive intervention programme offered to nursery school children, including daily supervised toothbrushing, distribution of toothbrushes and toothpaste and oral health advice given at nursery school) ‐ Duraphat® fluoride varnish (0.25 ml per application painted on tooth surfaces up to 4 6‐monthly applications) in the nursery school setting |
| Outcomes | Dental caries, 2‐year follow‐up |
| Starting date | October 2012 |
| Contact information | Styephen Turner, s.turner@dundee.ac.uk |
| Notes |
Differences between protocol and review
We have removed mention of school year from the 'Types of studies' section and now require all included studies to have at least a 12‐month follow‐up. We have removed 'unacceptability of drop‐outs during the trial/post‐randomisation exclusions (in non‐placebo trials)' as we now do not feel that this can be looked at in this way due to the large numbers of drop‐outs in both groups, for other reasons.
Contributions of authors
Valeria Marinho (VM) has conceived, designed, written and co‐ordinated the original review, and the updated review. VM performed extensive work in the original review that was the foundation of the updated review, including all data analysis. For both the original and current versions, VM has designed and undertaken search strategies, screened search results, organised retrieval of papers, screened papers against eligibility criteria, selected studies, extracted data, undertaken risk of bias assessments, entered data into Excel and RevMan, written to authors of trial reports, obtained and provided additional data about reports, provided advice on multiple aspects of the review, including analysis, written and revised the update.
Helen Worthington (HW) contributed to the development of the update, undertook screening of full search, extracted data for the 'Characteristics of included studies' tables, undertook risk of bias, extracted outcome data, undertook analysis including meta‐regression, crafted PRISMA flow chart and 'Summary of findings' table, and wrote the update.
Tanya Walsh (TW) undertook screening of full search, extracted data for the 'Characteristics of included studies' tables, undertook risk of bias, extracted outcome data, undertook analysis including meta‐regression and prediction intervals, wrote the results section.
Jan Clarkson (JC) contributed to the development of the update, assisted with screening, extracted data for the 'Characteristics of included studies' tables, checked risk of bias and wrote the update.
Sources of support
Internal sources
Queen Mary University of London, UK.
Department of Epidemiology and Public Health (UCL), UK.
Systematic Reviews Training Unit, Institute of Child Health (UCL), UK.
Medical Research Council, UK.
The University of Manchester, UK.
-
Manchester Academic Health Sciences Centre (MAHSC), UK.
The Cochrane Oral Health Group is supported by MAHSC and the NIHR Manchester Biomedical Research Centre
External sources
CAPES ‐ Ministry of Education, Brazil.
-
Cochrane Oral Health Group Global Alliance, UK.
All reviews in the Cochrane Oral Health Group are supported by Global Alliance member organisations (British Orthodontic Society, UK; British Society of Paediatric Dentistry, UK; Canadian Dental Hygienists Association, Canada; National Center for Dental Hygiene Research & Practice, USA and New York University College of Dentistry, USA) providing funding for the editorial process (http://ohg.cochrane.org/)
-
National Institute for Health Research (NIHR), UK.
CRG funding acknowledgement: The NIHR is the largest single funder of the Cochrane Oral Health Group
Disclaimer: The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the NIHR, NHS or the Department of Health
Declarations of interest
Tanya Walsh and Helen Worthington were authors of the report of the Milsom 2011 trial but had no involvement with the risk of bias assessment for this study.
Edited (no change to conclusions)
References
References to studies included in this review
Arruda 2012 {published data only}
- Arruda AO, Senthamarai Kannan R, Inglehart MR, Rezende CT, Sohn W. Effect of 5% fluoride varnish application on caries among school children in rural Brazil: a randomized controlled trial. Community Dentistry and Oral Epidemiology 2012;40(3):267‐76. [DOI] [PubMed] [Google Scholar]
Borutta 1991 {published and unpublished data}
- Borutta A, Kunzel W, Rubsam F. The caries‐protective efficacy of 2 fluoride varnishes in a 2‐year controlled clinical trial [Kariesprotektive Wirksamkeit zweier Fluoridlacke in einer klinisch kontrollierten Zweijahresstudie]. Deutsche Zahn Mund und Kieferheilkunde Zentralblatt 1991;79:543‐9. [PubMed] [Google Scholar]
Borutta 2006 {published and unpublished data}
- Borutta A, Hufnagl S, Möbius S, Reuscher G. Caries inhibition of fluoride varnishes among pre‐school children: results after one‐year. Oralprophylaxe 2006;28(1):8‐14. [Google Scholar]
- Borutta A, Reuscher G, Hufnagl S, Möbius S. Caries prevention with fluoride varnishes among preschool children [Kariesprophylaxe mit Fluoridlacken bei Vorschulkindern]. Gesundheitswesen 2006;68(11):731‐4. [DOI] [PubMed] [Google Scholar]
- Terekhova TN, Borutta A, Shakovets NV, Klenovskaia MI, Minchenia OV. Dependence of caries protective effect of fluoride varnishes applications on first permanent molars in schoolchildren on the intensity of caries of temporary teeth. Stomatologiia 2011;90(6):61‐5. [PubMed] [Google Scholar]
Bravo 1997 {published and unpublished data}
- Bravo M, Baca P, Llodra JC, Osorio E. A 24‐month study comparing sealant and fluoride varnish in caries reduction on different permanent first molar surfaces. Journal of Public Health Dentistry 1997;57:184‐6. [DOI] [PubMed] [Google Scholar]
- Bravo M, García Anllo I, Baca P, Llodra JC. A 48‐month survival analysis comparing sealant (Delton) with fluoride varnish (Duraphat) in 6‐ to 8‐year‐old children. Community Dentistry and Oral Epidemiology 1997;25:247‐50. [DOI] [PubMed] [Google Scholar]
- Bravo M, Llodra JC, Baca P, Osorio E. Effectiveness of visible light fissure sealant (Delton) versus fluoride varnish (Duraphat): 24‐month clinical trial. Community Dentistry and Oral Epidemiology 1996;24:42‐6. [DOI] [PubMed] [Google Scholar]
- Bravo M, Montero J. Effectiveness of visible light fissure sealant (Delton) versus fluoride varnish (Duraphat): a 9‐year clinical trial. Journal of Dental Research 2002;81(Spec Iss A):A‐185 (Abs No 1347). [Google Scholar]
- Bravo M, Montero J, Bravo JJ, Baca P, Llodra JC. Sealant and fluoride varnish in caries: a randomized trial. Journal of Dental Research 2005;84(12):1138‐43. [DOI] [PubMed] [Google Scholar]
- Bravo Pérez M, Llodra Calvo JC, Baca García P, Osorio Ruiz E, Junco Lafuente P. Fissure sealants versus fluorine varnish on the first permanent molars: economic assessment [Selladores de fisuras frente a barniz de flúor en primeros molares permanentes: evaluación económica]. Atención Primaria 1995;15(3):143‐7. [PubMed] [Google Scholar]
Chu 2002 {published data only}
- Chu CH, Lo EC, Lin HC. Effectiveness of silver diamine fluoride and sodium fluoride varnish in arresting dentin caries in Chinese pre‐school children. Journal of Dental Research 2002;81:767‐70. [DOI] [PubMed] [Google Scholar]
- Lo EC, Chu CH, Lin HC. A community‐based caries control program for pre‐school children using topical fluorides: 18‐month results. Journal of Dental Research 2001;80(12):2071‐4. [DOI] [PubMed] [Google Scholar]
- Wong MC, Lam KF, Lo EC. Analysis of multilevel grouped survival data with time‐varying regression coefficients. Statistics in Medicine 2011;30(3):250‐9. [DOI] [PubMed] [Google Scholar]
- Wong MC, Lam KF, Lo EC. Bayesian analysis of clustered interval‐censored data. Journal of Dental Research 2005;84(9):817‐21. [DOI] [PubMed] [Google Scholar]
Clark 1985 {published data only (unpublished sought but not used)}
- Clark DC, Stamm JW, Quee TC, Robert G. Results of the Sherbrooke‐Lac Megantic fluoride varnish study after 20 months. Community Dentistry and Oral Epidemiology 1985;13:61‐4. [DOI] [PubMed] [Google Scholar]
- Clark DC, Stamm JW, Robert G, Tessier C. Results of a 32‐month fluoride varnish study in Sherbrooke and Lac‐ Megantic, Canada. Journal of the American Dental Association 1985;111:949‐53. [DOI] [PubMed] [Google Scholar]
- Clark DC, Stamm JW, Tessier C, Robert G. The final results of the Sherbrooke‐Lac Megantic fluoride varnish study. Journal of the Canadian Dental Association 1987;53:919‐22. [PubMed] [Google Scholar]
Frostell 1991 {published and unpublished data}
- Frostell G, Birkhed D, Edwardsson S, Goldberg P, Petersson LG, Priwe C, et al. Effect of partial substitution of invert sugar for sucrose in combination with Duraphat treatment on caries development in preschool children: the Malmö Study. Caries Research 1991;25(4):304‐10. [DOI] [PubMed] [Google Scholar]
- Peyron M, Matsson L, Birkhed D. Progression of approximal caries in primary molars and the effect of Duraphat treatment. Scandinavian Journal of Dental Research 1992;100:314‐8. [DOI] [PubMed] [Google Scholar]
Gugwad 2011 {published and unpublished data}
- Gugwad SC, Shah P, Lodaya R, Bhat C, Tandon P, Choudhari S, et al. Caries prevention effect of intensive application of sodium fluoride varnish in molars in children between age 6 and 7 years. Journal of Contemporary Dental Practice 2011;12:408‐13. [DOI] [PubMed] [Google Scholar]
Hardman 2007 {published and unpublished data}
- Hardman MC, Davies GM, Duxbury JT, Davies RM. A cluster randomised controlled trial to evaluate the effectiveness of fluoride varnish as a public health measure to reduce caries in children. Caries Research 2007;41:371‐6. [DOI] [PubMed] [Google Scholar]
Holm 1979 {published data only}
- Holm AK. Effect of a fluoride‐containing varnish (Duraphat) in preschool children. Journal of Dental Research 1978;57:275 (Abs No 804). [Google Scholar]
- Holm AK. Effect of fluoride varnish (Duraphat) in preschool children. Community Dentistry and Oral Epidemiology 1979;7:241‐5. [DOI] [PubMed] [Google Scholar]
Holm 1984 {published and unpublished data}
- Holm GB, Holst K, Mejare I. The caries‐preventive effect of a fluoride varnish in the fissures of the first permanent molar. Acta Odontologica Scandinavica 1984;42:193‐7. [DOI] [PubMed] [Google Scholar]
Koch 1975 {published data only}
- Koch G, Petersson LG. Caries preventive effect of a fluoride‐containing varnish (Duraphat) after 1 year's study. Community Dentistry and Oral Epidemiology 1975;3:262‐6. [DOI] [PubMed] [Google Scholar]
Lawrence 2008 {published data only}
- Lawrence HP, Binguis D, Douglas J, McKeown L, Switzer B, Figueiredo R, et al. A 2‐year community‐randomized controlled trial of fluoride varnish to prevent early childhood caries in Aboriginal children. Community Dentistry and Oral Epidemiology 2008;36:503‐16. [DOI] [PubMed] [Google Scholar]
- Lawrence HP, Binguis D, Switzer B, McKeown L, Figueiredo R, Laporte A, et al. Randomized fluoride varnish trial for preventing ECC in Aboriginal communities. Canadian Journal of Dental Hygiene 2007;41(2):113. [Google Scholar]
Liu 2012 {published data only}
- Liu BY, Lo EC, Chu CH, Lin HC. Randomized trial on fluorides and sealants for fissure caries prevention. Journal of Dental Research 2012;91(8):753‐8. [DOI] [PubMed] [Google Scholar]
Milsom 2011 {published data only}
- Milsom KM, Blinkhorn AS, Walsh T, Worthington HV, Kearney‐Mitchell P, Whitehead H, et al. A cluster‐randomized controlled trial: fluoride varnish in school children. Journal of Dental Research 2011;90:1306‐11. [DOI] [PubMed] [Google Scholar]
Modeer 1984 {published and unpublished data}
- Modeer T, Twetman S, Bergstrand F. Three‐year study of the effect of fluoride varnish (Duraphat) on proximal caries progression in teenagers. Scandinavian Journal of Dental Research 1984;92:400‐7. [DOI] [PubMed] [Google Scholar]
Salazar 2008 {published data only}
- Salazar M. [Efetividade da aplicação semestral de verniz fluoretado no controle da cárie dentária em pré‐escolares: resultados após 12 meses de acompanhamento]. Effectiveness of Bi‐Annual Fluoride Varnish Application in the Control of Dental Caries in Preschool Children: Results after 12 Months of Follow‐Up [Thesis]. Rio de Janeiro, Brazil: Universidade do Estado do Rio de Janeiro, 2008. [Google Scholar]
Sköld 2005 {published data only}
- Sköld U, Petersson LG, Lith A, Birkhed D. Effect of school‐based fluoride varnish programmes on approximal caries in adolescents from different caries risk areas. Caries Research 2005;39(4):273‐9. [DOI] [PubMed] [Google Scholar]
- Sköld UM. On caries prevalence and school‐based fluoride programmes in Swedish adolescents. Swedish Dental Journal. Supplement 2005;(178):11‐75. [PubMed] [Google Scholar]
Tagliaferro 2011 {published and unpublished data}
- Tagliaferro EP, Pardi V, Ambrosano GM, Meneghim Mde C, Silva SR, Pereira AC. Occlusal caries prevention in high and low risk schoolchildren. A clinical trial. American Journal of Dentistry 2011;24:109‐14. [PubMed] [Google Scholar]
Tewari 1990 {published data only (unpublished sought but not used)}
- Tewari A, Chawla HS, Utreja A. Caries preventive effect of three topical fluorides (1 1/2 years clinical trial in Chandigarh school children of North India). Journal of the International Association of Dentistry for Children 1984;15:71‐81. [Google Scholar]
- Tewari A, Chawla HS, Utreja A. Comparative evaluation of the role of NaF, APF & Duraphat topical fluoride applications in the prevention of dental caries‐‐a 2 1/2 years study. Journal of the Indian Society of Pedodontics and Preventative Dentistry 1990;8:28‐35. [PubMed] [Google Scholar]
Weintraub 2006 {published and unpublished data}
- Eakle WS, Featherstone JD, Weintraub JA, Shain SG, Gansky SA. Salivary fluoride levels following application of fluoride varnish or fluoride rinse. Community Dentistry and Oral Epidemiology 2004;32(6):462‐9. [DOI] [PubMed] [Google Scholar]
- Weintraub JA, Ramos‐Gomez F, Jue B, Shain S, Hoover CI, Featherstone JD, et al. Fluoride varnish efficacy in preventing early childhood caries. Journal of Dental Research 2006;85(2):172‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yang 2008 {published data only}
- Yang G, Lin JH, Wang JH, Jiang L. Evaluation of the clinical effect of fluoride varnish in preventing caries of primary teeth. West China Journal of Stomatology 2008;26(2):159‐61. [PubMed] [Google Scholar]
References to studies excluded from this review
Alves 1997 {published data only}
- Alves AC, Medeiros UV. Fluoride varnish: Therapeutic effect in children of high caries risk. Journal of Dental Research 1997;76(5):951 (Abs No 6). [Google Scholar]
Autio‐Gold 2001 {published data only}
- Autio J, Courts F. Effect of fluoride varnish on caries progression. Journal of Dental Research 2000;79(Spec Iss 1):210 (Abs No 532). [Google Scholar]
- Autio‐Gold JT. Caries Prevention in High‐Risk Preschool Children in the United States [Thesis]. Oulu, Finland: Oulu University Press, 2005. [Google Scholar]
- Autio‐Gold JT, Courts F. Assessing the effect of fluoride varnish on early enamel carious lesions in the primary dentition. Journal of the American Dental Association 2001;132:1247‐53. [DOI] [PubMed] [Google Scholar]
Billy‐Pryga 1983 {published data only}
- Billy‐Pryga Z, Zakrzewka‐Pysz E, Ilewicz L. [Klinische untersuchung der schutzwirkung des Fluor‐Protector‐Lackes in der kariesprophylaxe]. Dental Revue 1980;10:16‐8. [Google Scholar]
- Billy‐Pryga Z, Zakrzewska‐Pysz E. Clinical evaluation of Duraphat and Fluor‐Protector preparations in caries prevention [Ocena kliniczna preparatow Duraphat i Fluor‐Protector w profilaktyce prochnicowej]. Czasopismo Stomatologiczne 1983;36(2):109‐15. [PubMed] [Google Scholar]
Bodnar 1984 {published data only}
- Bodnar Z, Tarjan I, Keri I. Clinical experience with the use of Duraphat lacquer [A "Duraphat" lakk alkalmazasaval kapcsolatos klinikai tapasztalatok]. Fogorvosi Szenile 1984;77:279‐82. [PubMed] [Google Scholar]
Demito 2011 {published data only}
- Demito CF, Rodrigues GV, Ramos AL, Bowman SJ. Efficacy of a fluoride varnish in preventing white‐spot lesions as measured with laser fluorescence. Journal of Clinical Orthodontics 2011;45(1):25‐9. [PubMed] [Google Scholar]
Dülgergil 2005 {published data only}
- Dülgergil CT, Ercan E, Yildirim I. A combined application of ART‐fluoride varnish for immigrant junior field‐workers: 12‐months follow‐up field trial in rural Anatolia. Oral Health & Preventive Dentistry 2005;3:97‐104. [PubMed] [Google Scholar]
Grodzka 1982 {published and unpublished data}
- Grodzka K, Augustyniak L, Budny J, Czarnocka K, Janicha J, Mlosek K, et al. Caries increment in primary teeth after application of Duraphat fluoride varnish. Community Dentistry and Oral Epidemiology 1982;10:55‐9. [DOI] [PubMed] [Google Scholar]
Hetzer 1973 {published data only}
- Hetzer G, Irmisch B. Caries prevention using fluoride varnish (Duraphat)‐‐clinical results and experiences [Kariesprotektion durch Fluorlack (Duraphat)‐‐Klinische Ergebnisse und Erfahrungen]. Deutsche Stomatologie 1973;23:917‐22. [PubMed] [Google Scholar]
Heuser 1968 {published data only}
- Heuser H, Schmidt HF. Dental caries prophylaxis by deep impregnation of the dental enamel with fluorine lac [Zahnkariesprophylaxe durch Tiefenimpragnierung des Zahnschmelzes mit Fluor‐Lack]. Stoma Heidelb 1968;21:91‐100. [PubMed] [Google Scholar]
- Schmidt H. Caries prevention by means of deep impregnation of the dental enamel with fluoride lacquer. Deutsche Stomatologie 1971;21:139‐42. [PubMed] [Google Scholar]
Hochstein 1975 {published data only}
- Hochstein HJ, Hochstein U, Breitung L. Experience with the fluoride lacker Duraphat [Erfahrungen mit dem Fluorlack Duraphat]. ZWR 1975;84:26‐30. [PubMed] [Google Scholar]
Ivanova 1990 {published data only (unpublished sought but not used)}
- Ivanova EN. The comparative efficacy of local anticaries agents [Sravnitel'naia effektivnost' mestnykh protivokarioznykh sredtsv]. Stomatologiia Mosk 1990;69:60‐1. [PubMed] [Google Scholar]
Ji 2007 {published data only (unpublished sought but not used)}
- Ji PH, Xu QL, Ba Y. Clinical evaluation of fluor protector and glass‐ionomer cement used as pit and fissure sealant for preventing pit and fissure caries in children. Shanghai Kou Qiang Yi Xue 2007;16:374‐6. [PubMed] [Google Scholar]
Kolehmainen 1979 {published data only}
- Kolehmainen L, Kerosuo E. The clinical effect of application of a urethane lacquer containing silane fluorine. A one‐year study. Proceedings of the Finnish Dental Society 1979;75:69‐71. [PubMed] [Google Scholar]
Kolehmainen 1981 {published data only}
- Kolehmainen L. Evaluation of a fluoride‐containing varnish in children with low caries incidence. Scandinavian Journal of Dental Research 1981;89:228‐34. [DOI] [PubMed] [Google Scholar]
Kunin 1991 {published data only}
- Kunin AA, Kharin OV. The use of a fluoride varnish and helium‐neon laser light in preventing caries of the primary teeth [Ispol'zovanie ftoristogo laka i sveta gelii‐neonovogo lazera v profilaktike kariesa vremennykh zubov]. Stomatologiia Mosk 1991;(4):71‐2. [PubMed] [Google Scholar]
Lagutina 1978 {published data only}
- Lagutina NJ, Vorobjev VS, Grabetskij AA, Stepanov AV. Clinical evaluation of home fluoride‐containing varnish. Quintessence International 1978;9:63‐6. [PubMed] [Google Scholar]
Lieser 1978 {published data only}
- Lieser O, Schmidt HF. Caries preventive effect of fluoride lacquer after several year's use in children [Kariesprophylaktische Wirkung von Fluorlack nach mehrjahriger Anwendung in der Jugendzahnpflege]. Deutsche Zahnarztliche Zeitschrift 1978;33:176‐8. [PubMed] [Google Scholar]
Lindquist 1989 {published data only}
- Lindquist B, Edward S, Torell P, Krasse B. Effect of different carriers preventive measures in children highly infected with mutans streptococci. Scandinavian Journal of Dental Research 1989;97:330‐7. [DOI] [PubMed] [Google Scholar]
Maiwald 1974 {published data only (unpublished sought but not used)}
- Maiwald HJ. Topical application of fluoride varnish for dental caries prevention in collectives following a 3‐year testing period [Lokalapplikation von Fluorschulzlack zur Kariespravention in Kollektiven nach dreij''ahriger Kontrollzeit]. Stomatologie der DDR 1974;24:123‐5. [PubMed] [Google Scholar]
- Maiwald HJ, Geiger L. Local application of fluorine protective varnish for caries prevention in collectivities [Lokalapplikation von Fluorschutzlack zur Kariesprophylaxe in Kollektiven]. Deutsche Stomatologie 1973;23:56‐63. [PubMed] [Google Scholar]
Maiwald 1978 {published data only}
- Maiwald HJ, Kunzel W, Weatherell J. The use of a fluoride varnish in caries prevention. Journal of the International Association of Dentistry for Children 1978;9:31‐5. [PubMed] [Google Scholar]
Mari 1988 {published data only}
- Mari A, Toth K, Kiss Z, Szita V. The effect of Fluor Protector lacquer on the primary and permanent teeth in children consuming fluoridated table salt [A Fluor Protector lakk hatasa fluorozott sot fogyaszto gyermekek tej‐ es maradofogazatanak allapotara]. Fogorvosi Szemle 1988;81:225‐30. [PubMed] [Google Scholar]
Mari 1988a {published data only}
- Mari A, Toth K, Kiss Z, Savay G. The effect of Duraphat lacquer on the condition of the permanent teeth in children consuming fluoridated table salt [A Duraphat lakk hatasa fluorozott sot fogyaszto gyermek maradofogazatanak allapotara]. Fogorvosi Szemle 1988;81:169‐74. [PubMed] [Google Scholar]
Murray 1977 {published data only}
- Murray JJ, Winter GB, Hurst CP. Duraphat fluoride varnish. A 2‐year clinical trial in 5‐year‐old children. British Dental Journal 1977;143:11‐7. [DOI] [PubMed] [Google Scholar]
Pashaev 1977 {published data only}
- Pashaev KP. Comparative assessment of the caries prevention effectiveness of different fluorine preparations [Sravnitel'naia otsenka kariesprofilakticheskoi effektivnosti razlichnykh preparatov ftora]. Stomatologiia Mosk 1977;56:82‐4. [PubMed] [Google Scholar]
Petersson 1998 {published data only}
- Petersson LG, Twetman S, Pakhomov GN. The efficiency of semiannual silane fluoride varnish applications: a two‐year clinical study in preschool children. Journal of Public Health Dentistry 1998;58:57‐60. [DOI] [PubMed] [Google Scholar]
Ramos 1995 {published data only}
- Ramos SB. Effect of Fortnightly Rinsing with a 0.2 per cent NaF Solution and a Fluoride Varnish with 5 per cent NaF in Dental Caries Prevention: Comparative Study in Students of the Northern Region of Sao Paulo city, Brazil, 1992‐1993 [Thesis]. Sao Paulo, Brazil: Universidade de Sao Paulo, 1995. [Google Scholar]
Ramos‐Gomez 2012 {published data only}
- Ramos‐Gomez FJ, Gansky SA, Featherstone JD, Jue B, Gonzalez‐Beristain R, Santo W, et al. Mother and youth access (MAYA) maternal chlorhexidine, counselling and paediatric fluoride varnish randomized clinical trial to prevent early childhood caries. International Journal of Paediatric Dentistry 2012;22(3):169‐79. [DOI] [PMC free article] [PubMed] [Google Scholar]
Riethe 1977 {published data only}
- Riethe P, Streib W, Schubring G. Clinical trials of Nuva Seal, Epoxylite 9070 and Fluor‐Protector [Klinische Untersuchungen mit Nuva Seal, Epoxylite 9070 und Fluor‐Protector]. Deutsche Zahnarztliche Zeitschrift 1977;32:853‐5. [PubMed] [Google Scholar]
Rodríguez Miró 1988 {published data only}
- Rodríguez Miró MJ, Vega Valdés D. Evaluation of semiannual combined‐treatment with fluoride‐chlorhexidine varnish and toothbrushing with chlorhexidine toothpaste for 15 days every 3 months in hypercariogenic children [Valoración del tratamiento combinado de aplicaciones semestrales de barniz‐flúor‐clorhexidine y del cepillado dental por 15 días cada tres meses con la crema dental con clorhexidine en niños hipercariogénicos]. Revista Cubana de Estomatología 1988;25(3):28‐35. [PubMed] [Google Scholar]
Ruszynska 1978 {published data only}
- Ruszynska H, George B, Anholcer H, Haber Milewska T. Clinical evaluation of fluor‐protector varnish in dental caries prevention [Badania kliniczne nad skutecznoscia stosowania lakieru fluor‐protektor w zapobieganiu prochnicy]. Czasopismo Stomatologiczne 1978;31:1021‐5. [PubMed] [Google Scholar]
Salem 1979 {published data only}
- Salem LV, Raschio AJ, Montoya VC. [Klinische untersuchung über die karieshemmende langzeitwirkung des Fluor‐Protector‐Lackes]. Kariesprophylaxe 1979;1:145‐8. [Google Scholar]
Schioth 1981 {published data only}
- Schioth JT. Effect of fluoride lacquering on the need of dental care in a group of adolescent school pupils [Effekten av fluorlakkering pa tannbehandlings‐behovet hos en gruppe ungdomsskoleelever]. Norske Tannlaegeforenings Tidende 1981;91:123‐6. [PubMed] [Google Scholar]
Schmidt 1970 {published data only}
- Schmidt HF. Caries prophylaxis by means of local application of sodium fluoride with special reference to long term fluoridation [Untersuchungen zur Prophylaxe der Zahnkaries durch ortliche Applikation von Natriumfluorid unter besonderer Berucksichtigung der Langzeitfluoridierung]. Stoma Heidelb 1970;23:5‐40. [PubMed] [Google Scholar]
Seppä 1982 {published data only}
- Birkeland JM. Trials ‐ results ‐ conclusions. Duraphat ‐ Fluor Protector [Forsok ‐ funn ‐ konklusjon. Duraphat ‐ Fluor Protector]. Den Norske Tannlaegeforenings Tidende 1981;91(10):368‐9. [PubMed] [Google Scholar]
- Seppä L, Hausen H, Luoma H. Relationship between caries and fluoride uptake by enamel from two fluoride varnishes in a community with fluoridated water. Caries Research 1982;16:404‐12. [DOI] [PubMed] [Google Scholar]
- Seppä L, Hausen H, Tuutti H, Luoma H. Effect of a sodium fluoride varnish on the progress of initial caries lesions. Scandinavian Journal of Dental Research 1983;91:96‐8. [DOI] [PubMed] [Google Scholar]
- Seppä L, Tuutti H, Luoma H. A 2‐year report on caries prevention by fluoride varnishes in a community with fluoridated water. Scandinavian Journal of Dental Research 1981;89:143‐8. [DOI] [PubMed] [Google Scholar]
- Seppä L, Tuutti H, Luoma H. Post‐treatment effect of fluoride varnishes in children with a high prevalence of dental caries in a community with fluoridated water. Journal of Dental Research 1984;63:1221‐2. [DOI] [PubMed] [Google Scholar]
- Seppä L, Tuutti H, Luoma H. Three‐year report on caries prevention of using fluoride varnishes for caries risk children in a community with fluoridated water. Scandinavian Journal of Dental Research 1982;90:89‐94. [DOI] [PubMed] [Google Scholar]
Shobha 1987 {published data only (unpublished sought but not used)}
- Shobha T, Nandlal B, Prabhakar AR, Sudha P. Fluoride varnish versus acidulated phosphate fluoride for schoolchildren in Manipal. Journal of the Indian Dental Association 1987;59:157‐60. [PubMed] [Google Scholar]
Slade 2011 {published data only}
- Roberts‐Thomson KF, Slade GD, Bailie RS, Endean C, Simmons B, Leach AJ, et al. A comprehensive approach to health promotion for the reduction of dental caries in remote Indigenous Australian children: a clustered randomised controlled trial. International Dental Journal 2010;60(3 Suppl 2):245‐9. [PubMed] [Google Scholar]
- Slade GD, Bailie RS, Roberts‐Thomson K, Leach AJ, Raye I, Endean C, et al. Effect of health promotion and fluoride varnish on dental caries among Australian Aboriginal children: results from a community‐randomized controlled trial. Community Dentistry and Oral Epidemiology 2011;39(1):29‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Splieth 2000 {published data only}
- Splieth CH, Baekken S, Rosin M. Effectiveness of different topical fluoride applications in schoolchildren. European Journal of Paediatric Dentistry 2000;1:107‐42. [Google Scholar]
Suntsov 1991 {published data only}
- Suntsov VG, Distel' VA, Zhorova TN, Buiankina RG, Toropov VN, Bulanova EL, et al. The trace efficacy of dental caries prevention in children [Sledovaia effektivnost' profilaktiki kariesa zubov u detei]. Stomatologiia Mosk 1991;(2):69‐71. [PubMed] [Google Scholar]
Suwansingha 2011 {published data only}
- Suwansingha O, Rirattanapong P. Effect of fluoride varnish on caries prevention of partially erupted of permanent molar in high caries risk. The Southeast Asian Journal of Tropical Medicine and Public Health 2012;43(3):808‐13. [PubMed] [Google Scholar]
Todorashko 1983 {published data only}
- Todorashko OV. Results of the combined use of remodent and fluorine lacquer for dental caries prevention in preschoolers [Rezul'taty sochetannogo primeneniia remodenta i ftorlaka dlia profilaktiki kariesa zubov u doshkol'nikov]. Stomatologiia Mosk 1983;62:12‐3. [PubMed] [Google Scholar]
Tranaeus 2001 {published data only}
- Tranaeus S, Al‐Khateeb S, Björkman S, Twetman S, Angmar‐Månsson B. Application of quantitative light‐induced fluorescence to monitor incipient lesions in caries‐active children. A comparative study of remineralisation by fluoride varnish and professional cleaning. European Journal of Oral Sciences 2001;109:71‐5. [DOI] [PubMed] [Google Scholar]
Treide 1980 {published and unpublished data}
- Treide A, Hebenstreit W, Gunther A. Collective preschool caries prevention using a fluoride‐containing varnish [Kollektive Kariespravention im Vorschulalter unter Verwendung eines fluoridhaltigen Lackes]. Stomatologie der DDR 1980;30:734‐9. [PubMed] [Google Scholar]
van Eck 1984 {published data only (unpublished sought but not used)}
- Groeneveld A, Theuns HM, Kwant GW. Effect of a fluoride‐containing lacquer on dental caries. Journal of Dental Research 1982;61:569. [Google Scholar]
- Theuns HM, Groeneveld A, Eck AA. Clinical experimental field trials of the caries‐preventive effect of a fluoride lacquer [Een klinisch experimenteel veldonderzoek naar het caries‐ preventieve effect van een fluoridelak]. Netherlands Tijdschrift voor Tandheelkunde 1985;92:66‐9. [PubMed] [Google Scholar]
- Eck AA, Theuns HM, Groeneveld A. Effect of annual application of polyurethane lacquer containing silane‐fluoride. Community Dentistry and Oral Epidemiology 1984;12:230‐2. [DOI] [PubMed] [Google Scholar]
Wacińska‐Drabińska 1987 {published data only}
- Wacińska‐Drabińska M. Effect of Fluor‐Protector varnish on the inhibition of the carious process in milk teeth [Wplyw lakieru Fluor‐Protector na zahamowanie procesu prochnicowego w zebach mlecznych]. Czasopismo Stomatologiczne 1987;40(8):528‐32. [PubMed] [Google Scholar]
Wegner 1976 {published data only}
- Wegner H. The clinical effect of application of fluoride varnish. Caries Research 1976;10:318‐20. [DOI] [PubMed] [Google Scholar]
Winter 1975 {published data only}
- Winter K. Caries prophylaxis by local application of sodium fluoride lacquer [Kariesprophylaxe durch Lokalapplikation von Natriumfluorid‐Lack]. Zahnarztlicher Gesundheitsdienst 1975;(3):1‐2. [PubMed] [Google Scholar]
- Winter K. Caries prophylaxis by means of local application of sodium fluoride lacquer [Kariesprophylaxe durch Lokalapplikation von Natriumfluorid‐Lack]. Zahnarztliche Mitteilungren 1975;65:215‐21. [PubMed] [Google Scholar]
Wojtowicz 1986 {published data only}
- Wojtowicz D, Banach J. Clinical evaluation of Duraphat varnish in the prevention of dental caries in children and in the control of cervical hypersensitivity in adults [Ocena kliniczna laku Duraphat w profilaktyce prochnicy zebow u dzieci oraz w zwalczaniu wrazliwosci szyjek zebow u doroslych]. Czasopismo Stomatologiczne 1986;39:213‐8. [PubMed] [Google Scholar]
Xhemnica 2008 {published data only}
- Xhemnica L, Sulo D, Rroço R, Hysi D. Fluoride varnish application: a new prophylactic method in Albania. Effect on enamel carious lesions in permanent dentition. European Journal of Paediatric Dentistry 2008;9:93‐6. [PubMed] [Google Scholar]
Zimmer 1999 {published data only}
- Zimmer S, Robke FJ, Roulet JF. Caries prevention with fluoride varnish in a socially deprived community. Community Dentistry and Oral Epidemiology 1999;27:103‐8. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
Macpherson 2012 {unpublished data only}
- Macpherson L. Comparison of the caries‐protective effect of fluoride varnish (Duraphat®) with treatment as usual in nursery school attendees receiving preventive oral health support through the Childsmile Oral Health Improvement Programme: an RCT. http://clinicaltrials.gov/show/NCT01674933. [DOI] [PMC free article] [PubMed]
Additional references
Ammari 2003
- Ammari AB, Bloch‐Zupan A, Ashley PF. Systematic review of studies comparing the anti‐caries efficacy of children's toothpaste containing 600 ppm of fluoride or less with high fluoride toothpastes of 1,000 ppm or above. Caries Research 2003;37(2):85‐92. [DOI] [PubMed] [Google Scholar]
Azarpazhooh 2008
- Azarpazhooh A, Main PA. Fluoride varnish in the prevention of dental caries in children and adolescents: a systematic review. Journal of the Canadian Dental Association 2008;74(1):73‐9. [PubMed] [Google Scholar]
Bader 2004
- Bader J, Ismail A. Survey of systematic reviews in dentistry. Journal of the American Dental Association 2004;135:464‐73. [DOI] [PubMed] [Google Scholar]
Bartizek 2001
- Bartizek RD, Gerlach RW, Faller RV, Jacobs SA, Bollmer BW, Biesbrock AR. Reduction in dental caries with four concentrations of sodium fluoride in a dentifrice: a meta‐analysis evaluation. Journal of Clinical Dentisty 2001;12(3):57‐62. [PubMed] [Google Scholar]
Bawden 1998
- Bawden JW. Fluoride varnish: a useful new tool for public health dentistry. Journal of Public Health Dentistry 1998;58:266‐9. [DOI] [PubMed] [Google Scholar]
Beltrán‐Aguilar 2000
- Beltrán‐Aguilar ED, Goldstein JW, Lockwood SA. Fluoride varnishes. A review of their clinical use, cariostatic mechanism, efficacy and safety. Journal of the American Dental Association 2000;131(5):589‐96. [DOI] [PubMed] [Google Scholar]
Bratthall 1996
- Bratthall D, Hansel Petersson G, Sundberg H. Reasons for the caries decline: what do the experts believe?. European Journal of Oral Sciences 1996;104:416‐22. [DOI] [PubMed] [Google Scholar]
Burt 1998
- Burt BA. Prevention policies in the light of the changed distribution of dental caries. Acta Odontologica Scandinavica 1998;56:179‐86. [DOI] [PubMed] [Google Scholar]
Carvalho 2010
- Carvalho DM, Salazar M, Oliveira BH, Coutinho ES. Fluoride varnishes and decrease in caries incidence in preschool children: a systematic review. Revista Brasileira de Epidemiologia 2010;13(1):139‐49. [DOI] [PubMed] [Google Scholar]
Chaves 2002
- Chaves SC, Vieira‐da‐Silva LM. Anticaries effectiveness of fluoride toothpaste: a meta‐analysis. Revista de Saúde Pública 2002;36(5):598‐606. [DOI] [PubMed] [Google Scholar]
Chen 1995
- Chen MS. Oral health of disadvantaged populations. In: Cohen LK, Gift CH editor(s). Disease Prevention and Oral Health Promotion. Copenhagen: Munksgaard, 1995:152‐212. [Google Scholar]
Clark 1982
- Clark DC. A review on fluoride varnishes: an alternative topical fluoride treatment. Community Dentistry and Oral Epidemiology 1982;10:117‐23. [DOI] [PubMed] [Google Scholar]
Clark 1985a
- Clark DC, Hanley JA, Stamm JW, Weinstein PL. An empirically based system to estimate the effectiveness of caries‐ preventive agents. A comparison of the effectiveness estimates of APF gels and solutions, and fluoride varnishes. Caries Research 1985;19:83‐95. [DOI] [PubMed] [Google Scholar]
De Bruyn 1987
- Bruyn H, Arends J. Fluoride varnishes‐a review. Journal de Biologie Buccale 1987;15:71‐82. [PubMed] [Google Scholar]
de Liefde 1998
- Liefde B. The decline of caries in New Zealand over the past 40 years. New Zealand Dental Journal 1998;94:109‐13. [PubMed] [Google Scholar]
Dubey 1965
- Dubey SD, Lehnhoff RW, Radike AW. A statistical confidence interval for true per cent reduction in caries‐incidence trials. Journal of Dental Research 1965;44:921‐3. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey‐Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple graphical test. BMJ 1997;315(7109):629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Featherstone 1988
- Featherstone JDB, Cate JM. Physicochemical aspects of fluoride‐enamel interactions. In: Ekstrand J, Fejerskov O, Silverstone LM editor(s). Fluoride in Dentistry. Copenhagen: Munksgaard, 1988:125‐49. [Google Scholar]
Featherstone 1999
- Featherstone JD. Prevention and reversal of dental caries: role of low level fluoride. Community Dentistry and Oral Epidemiology 1999;27:31‐40. [DOI] [PubMed] [Google Scholar]
Glass 1982
- Glass RL. The first international conference on the declining prevalence of dental caries. Journal of Dental Research 1982;61:1301‐83. [Google Scholar]
Harbord 2005
- Harbord RM, Egger M, Sterne JA. A modified test for small‐study effects in meta‐analyses of controlled trials with binary endpoints. Statistics in Medicine 2006;25(20):3443‐57. [DOI] [PubMed] [Google Scholar]
Helfenstein 1994
- Helfenstein U, Steiner M. Fluoride varnishes (Duraphat): a meta‐analysis. Community Dentistry and Oral Epidemiology 1994;22:1‐5. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Horowitz 1996
- Horowitz HS, Ismail AI. Topical fluorides in caries prevention. In: Fejerskov O, Ekstrand J, Burt BA editor(s). Fluoride in Dentistry. Copenhagen: Munksgaard, 1996:311‐27. [Google Scholar]
Johnson 1993
- Johnson MF. Comparative efficacy of NaF and SMFP dentifrices in caries prevention: a meta‐analytic overview. Caries Research 1993;27:328‐36. [DOI] [PubMed] [Google Scholar]
Johnston 1994
- Johnston DW. Current status of professionally applied topical fluorides. Community Dentistry and Oral Epidemiology 1994;22:159‐63. [DOI] [PubMed] [Google Scholar]
Kallestal 1999
- Kallestal C, Wang NJ, Petersen PE, Arnadottir IB. Caries‐preventive methods used for children and adolescents in Denmark, Iceland, Norway and Sweden. Community Dentistry and Oral Epidemiology 1999;27:144‐51. [DOI] [PubMed] [Google Scholar]
Krasse 1996
- Krasse B. The caries decline: is the effect of fluoride toothpaste overrated?. European Journal of Oral Sciences 1996;104:426‐9. [DOI] [PubMed] [Google Scholar]
Marthaler 1994
- Marthaler TM, Steiner M, Menghini G, Bandi A. Caries prevalence in Switzerland. International Dental Journal 1994;44:393‐401. [PubMed] [Google Scholar]
Marthaler 1996
- Marthaler TM, O'Mullane DM, Vrbic V. The prevalence of dental caries in Europe 1990‐1995. ORCA Saturday afternoon symposium 1995. Caries Research 1996;30:237‐55. [DOI] [PubMed] [Google Scholar]
Marthaler 2004
- Marthaler TM. Changes in dental caries 1953‐2003. Caries Research 2004;38(3):173‐81. [DOI] [PubMed] [Google Scholar]
Murray 1991
- Murray JJ, Rugg‐Gunn AJ, Jenkins GN (editors). A history of water fluoridation. Fluorides in Caries Prevention. Oxford: Wright, 1991:7‐37. [Google Scholar]
Murray 1991a
- Murray JJ, Rugg‐Gunn AJ, Jenkins GN (editors). Fluoride toothpastes and dental caries. Fluorides in Caries Prevention. Oxford: Wright, 1991:127‐60. [Google Scholar]
Nadanovsky 1995
- Nadanovsky P, Sheiham A. Relative contribution of dental services to the changes in caries levels of 12‐year‐old children in 18 industrialized countries in the 1970s and early 1980s. Community Dentistry and Oral Epidemiology 1995;23:331‐9. [DOI] [PubMed] [Google Scholar]
O'Mullane 1994
- O'Mullane DM. Introduction and rationale for the use of fluoride for caries prevention. International Dental Journal 1994;44:257‐61. [PubMed] [Google Scholar]
Ogaard 1994
- Ogaard B, Seppa L, Rolla G. Professional topical fluoride applications‐clinical efficacy and mechanism of action. Advances in Dental Research 1994;8:190‐201. [DOI] [PubMed] [Google Scholar]
Petersen 2004
- Petersen PE, Lennon MA. Effective use of fluorides for the prevention of dental caries in the 21st century: the WHO approach. Community Dentistry and Oral Epidemiology 2004;32:319‐21. [DOI] [PubMed] [Google Scholar]
Petersson 1993
- Petersson LG. Fluoride mouthrinses and fluoride varnishes. Caries Research 1993;27 Suppl 1:35‐42. [DOI] [PubMed] [Google Scholar]
Petersson 1997
- Petersson LG, Twetman S, Pakhomov GN. Fluoride Varnish for Community‐Based Caries Prevention in Children. Geneva: World Health Organization, 1997. [Google Scholar]
Petersson 2004
- Petersson LG, Twetman S, Dahlgren H, Norlund A, Holm AK, Nordenram G, et al. Professional fluoride varnish treatment for caries control: a systematic review of clinical trials. Acta Odontologica Scandinavica 2004;62(3):170‐6. [DOI] [PubMed] [Google Scholar]
Primosch 1985
- Primosch RE. A report on the efficacy of fluoridated varnishes in dental caries prevention. Clinical Preventative Dentistry 1985;7:12‐22. [PubMed] [Google Scholar]
Reisine 2001
- Reisine ST, Psoter W. Socioeconomic status and selected behavioral determinants as risk factors for dental caries. Journal of Dental Education 2001;65(10):1009‐16. [PubMed] [Google Scholar]
Riley 2011
- Riley RD, Higgins JP, Deeks JJ. Interpretation of random effects meta‐analyses. BMJ 2011;342:549. [DOI] [PubMed] [Google Scholar]
Ripa 1989
- Ripa LW. Review of the anticaries effectiveness of professionally applied and self‐applied topical fluoride gels. Journal of Public Health Dentistry 1989;49:297‐309. [DOI] [PubMed] [Google Scholar]
Ripa 1990
- Ripa LW. An evaluation of the use of professional (operator‐applied) topical fluorides. Journal of Dental Research 1990;69 Spec No:786‐96. [DOI] [PubMed] [Google Scholar]
Ripa 1991
- Ripa LW. A critique of topical fluoride methods (dentifrices, mouthrinses, operator‐, and self‐applied gels) in an era of decreased caries and increased fluorosis prevalence. Journal of Public Health Dentistry 1991;51:23‐41. [DOI] [PubMed] [Google Scholar]
Rolla 1991
- Rolla G, Ogaard B, Cruz R‐d‐A. Clinical effect and mechanism of cariostatic action of fluoride‐containing toothpastes: a review. International Dental Journal 1991;41:171‐4. [PubMed] [Google Scholar]
Rozier 2001
- Rozier RCG. Effectiveness of methods used by dental professionals for the primary prevention of dental caries. Journal of Dental Education 2001;65:1063‐72. [PubMed] [Google Scholar]
Seppa 1991
- Seppa L. Studies of fluoride varnishes in Finland. Proceedings of the Finnish Dental Society 1991;87:541‐7. [PubMed] [Google Scholar]
Sharp 1998
- Sharp S. Meta‐analysis regression. Stata Technical Bulletin 1998; Vol. 42:16‐22.
Shen 2002
- Shen C, Autio‐Gold J. Assessing fluoride concentration uniformity and fluoride release from three varnishes. Journal of the American Dental Association 2002;133(2):176‐82. [DOI] [PubMed] [Google Scholar]
Stamm 1984
- Stamm JW, Bohannan HM, Graves RC, Disney JA. The efficiency of caries prevention with weekly fluoride mouthrinses. Journal of Dental Education 1984;48(11):617‐26. [PubMed] [Google Scholar]
Stamm 1995
- Stamm JW. Clinical trials of neutral sodium fluoride and sodium monofluorophosphate dentifrices. In: Bowen WH editor(s). Relative Efficacy of Sodium Fluoride and Sodium Monofluorophosphate as Anti‐Caries Agents in Dentifrices. London: The Royal Society of Medicine Press Limited, 1995:43‐58. [Google Scholar]
Steiner 2004
- Steiner M, Helfenstein U, Menghini G. Effect of 1000 ppm relative to 250 ppm fluoride toothpaste. American Journal of Dentistry 2004;17(2):85‐88. [PubMed] [Google Scholar]
Strohmenger 2001
- Strohmenger L, Brambilla E. The use of fluoride varnishes in the prevention of dental caries:a short review. Oral Diseases 2001;7:71‐80. [PubMed] [Google Scholar]
ten Cate 1999
- Cate JM. Current concepts on the theories of the mechanism of action of fluoride. Acta Odontologica Scandinavica 1999;57(6):325‐9. [DOI] [PubMed] [Google Scholar]
Thompson 1999
- Thompson SG, Sharp SJ. Explaining heterogeneity in meta‐analysis: a comparison of methods. Statistics in Medicine 1999;18(20):2693‐708. [DOI] [PubMed] [Google Scholar]
Twetman 2004
- Twetman S, Petersson L, Axelsson S, Dahlgren H, Holm AK, Källestål C, et al. Caries‐preventive effect of sodium fluoride mouthrinses: a systematic review of controlled clinical trials. Acta Odontologica Scandinavica 2004;62(4):223‐30. [DOI] [PubMed] [Google Scholar]
van Rijkom 1998
- Rijkom HM, Truin GJ, van't Hof MA. A meta‐analysis of clinical trials on the caries‐inhibiting effect of fluoride gel treatment. Caries Research 1998;32:83‐92. [DOI] [PubMed] [Google Scholar]
Whitford 1992
- Whitford GM. Acute and chronic fluoride toxicity. Journal of Dental Research 1992;71:1249‐54. [DOI] [PubMed] [Google Scholar]
WHO 1994
- World Health Organization. Fluorides and oral health. Report of a WHO Expert Committee on Oral Health Status and Fluoride Use. Geneva: World Health Organization, 1994:1‐37. [PubMed] [Google Scholar]
Yanover 1982
- Yanover L. Fluoride varnishes as cariostatic agents: a review. Journal of the Canadian Dental Association 1982;48:401‐4. [PubMed] [Google Scholar]
References to other published versions of this review
Marinho 2002
- Marinho VC, Higgins JP, Logan S, Sheiham A. Fluoride varnishes for preventing dental caries in children and adolescents. Cochrane Database of Systematic Reviews 2002, Issue 3. [DOI: 10.1002/14651858.CD002279] [DOI] [PubMed] [Google Scholar]


