Abstract
Genetic complementation of a sodA sodB Escherichia coli mutant strain was used to clone Rhodobacter capsulatus genes involved in detoxification of superoxide radicals. After sequence analysis, 1 of the 16 identical clones obtained by this selection procedure was shown to contain an open reading frame with sequence similarity to that coding for Fe-containing superoxide dismutases (SodB). The R. capsulatus sodB gene was expressed in E. coli, and the nature of the metal ligand was confirmed by inhibitor sensitivity assays with lysates from both bacterial species. Activity staining of cleared Rhodobacter lysates resolved by polyacrylamide gel electrophoresis indicated that SodB was the only superoxide dismutase present in this phototrophic organism. The sodB gene was expressed at low levels in R. capsulatus cells grown under anaerobic or semiaerobic conditions, but expression was strongly induced upon exposure of the bacteria to air or to methyl viologen. Attempts to construct a sodB mutant in this organism by allelic exchange of the chromosomal copy of the gene with a suicide plasmid containing a mutated sodB gene were unsuccessful, strongly suggesting that the encoded superoxide dismutase is essential for viability of R. capsulatus in aerobic cultures.
Rhodobacter capsulatus is a purple phototrophic bacterium displaying a high metabolic versatility. In the presence of light and under low oxygen tension, Rhodobacter synthesizes ATP through an anoxygenic electron transport around a single photosystem (19). A shift to high oxygen tensions induces the accumulation of respiratory enzymes (12, 14), while the puf and puc operons, encoding the pigment binding proteins of the photosynthetic apparatus, are repressed at the transcriptional level (4). Some of the components of the photosynthetic electron transport system, such as the cytochrome b-cytochrome c1 complex and the ubiquinone pool, also accumulate under aerobic conditions, contributing to the establishment of the respiratory chain after the assembly of terminal oxidase(s) and dehydrogenase(s) (44).
The onset of respiration confronts the growing bacteria with still an additional challenge, as the reduction of molecular oxygen to water proceeds through a series of reactions and intermediates along the respiratory chain. Partial oxygen reduction and/or reoxidation of some of the respiratory components may then occur, leading to the formation of toxic radicals. Active oxygen species (AOS), such as hydrogen peroxide (H2O2) and the superoxide (O2·−) and hydroxyl (OH·) radicals, are unavoidable by-products of aerobic metabolism and also result from exposure of the cells to free radical-generating compounds (xenobiotics and pollutants) and environmental adversity (7, 8, 20, 23). AOS are highly reactive and damage a wide range of biomolecules, most conspicuously DNA (29).
Aerobic organisms have evolved a number of enzymatic and nonenzymatic antioxidant defense mechanisms, which reduce the harmful effects of AOS and maintain the cellular homeostasis between pro-oxidants and antioxidants (51). The basic strategy of the cellular defense system is quite similar in procaryotes and eucaryotes (24, 51). Antioxidant compounds such as glutathione, ascorbate, carotenoids, etc., scavenge AOS directly through chemical reaction (8). Some members of the enzymatic defense barrier are also involved in scavenging (catalases and peroxidases), while others contribute to the reestablishment of viability conditions once the damage has been done (reductases and DNA repair enzymes). The first line of defense in most aerobic organisms is made up of one or more superoxide dismutases (SODs), which eliminate superoxide radicals by catalyzing the following reaction (23, 43): 2 O2·− + 2 H+ → H2O2 + O2. An imbalance of the cellular homeostasis in favor of pro-oxidants is usually defined as oxidative stress (43). Such disruptions often lead to accelerated senescence, disease development, and impaired ability to adapt to changing environments (8, 20, 23). Gene-based defects in antioxidant protection have multiple pleiotropic effects in both eucaryotes and bacteria. For instance, mutations in a Cu/ZnSOD gene are associated with familial amyotrophic lateral sclerosis in humans (39), whereas Escherichia coli mutants deficient in the two major dismutases (FeSOD and MnSOD) are hypersensitive to oxygen and unable to grow on minimal media (10). Conversely, the increase in activity of antioxidant enzymes by genetic engineering has been shown to extend the average life span in Drosophila melanogaster (33) and to improve the stress tolerance of plants and bacteria (1, 6).
The importance of the antioxidant defense mechanisms is mirrored by their complexity, and new components of these systems are continuously described. However, our knowledge of the number and nature of the antioxidant proteins recruited during the transition from anoxygenic photosynthesis to aerobic respiration in phototrophic bacteria lags way behind. Two enzymes involved in H2O2 scavenging have been described in Rhodobacter capsulatus—a peroxidase and a catalase-peroxidase (27). While the former enzyme was inferred to have a protective role during the logarithmic growth phase, the catalase-peroxidase would act during the oxidative conditions prevailing in aging cultures (27), and its expression is regulated by oxygen at the transcriptional level (22). More recently, a gene encoding an oxygen-responsive thioredoxin (trxA) was cloned from the closely related species Rhodobacter sphaeroides (36, 38). Unlike the thioredoxins from other bacterial sources, the R. sphaeroides protein displays glutathione disulfide oxidoreductase activity, which is an absolute requirement for both aerobic and anaerobic growth (37).
In the framework of a systematic effort toward understanding the antioxidant defense systems of phototrophic bacteria, we report here the molecular cloning of the R. capsulatus sodB gene, encoding an iron-containing SOD, by genetic complementation of a sodA sodB E. coli double mutant strain. We also show that expression of this gene in R. capsulatus is strongly induced under oxidative stress conditions and that a SodB-deficient Rhodobacter strain is unable to grow in the presence of air.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
The bacterial strains and plasmids used in this study are listed in Table 1. E. coli cells were grown at 37°C in Luria-Bertani (LB) or M9 medium (41). When required (Table 1), ampicillin, kanamycin, and isopropyl-β-d-thiogalactopyranoside (IPTG) were used at final concentrations of 200 μg/ml, 100 μg/ml, and 0.5 mM, respectively. Plates contained the same medium supplemented with 1.5% (wt/vol) agar.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant genotype | Source or reference |
|---|---|---|
| Strains | ||
| R. capsulatus 37b4 | Wild type | DSM 983a |
| E. coli | ||
| MC1061 | hsdR nctB Δlac | 30 |
| JM109 | supE Δ(lac-proAB) hsdR17 recA1 F′ traD36 proAB+ lacIqlacZΔM15 | 50 |
| DH5α | Φ80d lacZΔM15 recA1 endA1 gyrA96 thi-1 hsdR17 supE44 relA1 deoR Δ(lacZYA-argF)U169 F− | 49 |
| GC4468 | F− Δlac4169 rpsL | 10 |
| QC774 | GC4468 Φ(sodA-lacZ)49 Φ(sodB-kan)1-Δ2 Cmr Kmr | 10 |
| SM10 | recA thi thr leu; chromosomal RP4-2 (Tc::Mu) Tra+ Kmr | 46 |
| Plasmids | ||
| pBSK− | AprlacZ′ f1 | Stratagene |
| pA21 | pBSK− harboring a 3.65-kbp HindIII fragment encoding sodB from R. capsulatus (sodB in the opposite transcription direction to that of the lac promoter). | This study |
| pA22 | pBSK− harboring a 1.70-kbp SmaI fragment containing sodB from R. capsulatus (sodB in the same transcription direction to that of the lac promoter). | This study |
| pUC4KSAC | Apr (source of the Kmr cassette) | 3 |
| pPHU281 | tetA tetR lacZ′ oriT | 28 |
| pHSOD | pHU281 harboring a 1.93-kbp KpnI-SphI fragment from pA21 encoding sodB from R. capsulatus | This study |
| pSODK1 | pHSOD::Kmr cassette ligated into the StuI site of the sodB gene | This study |
DSM, Deutsche Sammlung von Mikroorganismen, Göttingen, Germany.
R. capsulatus 37b4 cells (DSM938) were grown at 32°C in YCC broth (47) or in malate mineral medium (18). When appropriate, tetracycline and kanamycin were used at 1.5 and 20 μg/ml, respectively. Aerobic conditions in liquid media were achieved by incubating 100 ml of culture in 1-liter baffled flasks under vigorous shaking, while semiaerobic growth (oxygen partial pressure of 1 to 2%) was obtained by incubation of 40 ml of culture in 50-ml flasks under gentle agitation. For phototrophic growth, cells were cultured in screw-cap flasks filled to the top with medium and incubated in the light. Anaerobic dark growth on agar plates was achieved with Anaerocults (Merck) by supplementing mineral medium with 0.25% (wt/vol) glucose and with 20 mM dimethyl sulfoxide as the terminal electron acceptor.
Library construction and cloning strategy.
An R. capsulatus genomic library was constructed by isolating total chromosomal DNA as described previously (15). After partial digestion with HindIII, DNA was subjected to agarose gel electrophoresis, and fragments between 3 and 10 kbp were isolated and ligated into compatible sites of pBlueScript SK− (pBSK−). E. coli MC1061 cells were transformed with the ligation mixture, and after being plated onto LB agar containing ampicillin, IPTG, and 50 μg of X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) per ml, 2 × 104 colonies were collected by washing with LB broth and stored at −70°C in the same medium containing 20% (vol/vol) glycerol. Blue colonies represented less than 1% of the colonies found. DNA was introduced into E. coli cells from the QC774 strain (sodA sodB) by electrotransformation with a Bio-Rad Gene Pulser, according to the protocols recommended by the supplier. Transformants were plated onto M9 minimal medium (41), containing ampicillin, IPTG, and 10 nM methyl viologen (MV). After 24 to 48 h, colonies growing in this medium were plated onto LB agar containing ampicillin and finally cultured in liquid LB broth for plasmid isolation. Recombinant DNA techniques were carried out according to established procedures (41). DNA sequencing was performed in an ABI 373A (P/N 402079) DNA sequencer, by using the AmpliTaq protocol after amplification of the recombinant plasmids in E. coli DH5α cells (49).
RNA isolation, blotting, and hybridization.
For oxygen shift experiments, semiaerobic cultures of R. capsulatus 37b4 grown to an optical density at 660 nm (OD660) of 0.4 to 0.5 were transferred to high-oxygen-tension conditions in the same medium with or without 1 mM MV. Samples were taken at the indicated times, and total RNA was isolated with hot phenol (48). RNA (20 μg per lane) was subjected to electrophoresis in 1% (wt/vol) agarose–2.2 M formaldehyde gels. RNA was transferred to nylon membranes (Biodyne B; Pall) by vacuum pressure blotting (Vacuum Blotting, Pharmacia) according to the manufacturer’s recommendations. A 684-bp StuI-NcoI DNA fragment containing part of the sodB gene (see Fig. 1) was labeled with [32P]dATP (Amersham nick translation kit) and used as a hybridization probe for the sodB transcript at 42°C in 50% (vol/vol) formamide. The radioactivity associated with the hybridized RNA bands was quantified with a laser PhosphorImager (Molecular Dynamics) and normalized to the amount of rRNA present in each lane, to correct for differences in sample loading.
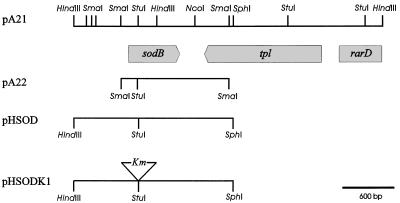
FIG. 1.
Restriction map of the R. capsulatus DNA fragment carrying the sodB gene. Horizontal lines represent Rhodobacter DNA fragments in the sodB region which were cloned into pBSK (pA21 and pA22) or pPHU281 (pHSOD and pHSODK1) to produce the designated plasmids (Table 1). Restriction endonuclease cleavage sites are indicated by vertical bars. The positions of the sodB, tpl, and rarD genes were deduced from homology tests of the sequence data.
Detection of SOD activity in bacterial lysates.
E. coli and R. capsulatus cells were grown to OD of 0.6 (at 600 and 660 nm, respectively), harvested, washed, and disrupted by sonic oscillation (Vibrocell VCX600; Sonics & Materials, Inc.). Supernatants were cleared by centrifugation (E. coli extracts, 10,000 × g, 20 min; R. capsulatus extracts, 50,000 × g, 60 min), and total soluble protein was estimated by using a dye-binding assay (45). Assays measuring SOD activity and sensitivity to H2O2 and KCN were carried out by an in situ staining procedure (5, 17), after electrophoresis of the corresponding cleared lysates in nondenaturing 8% polyacrylamide gels.
E. coli survival tests.
Bacteria were grown to early log phase (4 h at 37°C, OD600 of 0.3 to 0.4) in LB broth supplemented with the corresponding antibiotics (Table 1). Fractions of 1 ml were removed into 10-ml culture tubes containing either sterile distilled water or different amounts of MV added from freshly prepared stock solutions. Tubes were shaken at 37°C for an additional hour. Appropriate dilutions were then spread onto LB plates, which were incubated at 37°C for 14 h to monitor cell viability.
Sequence data analysis.
Protein database searches were performed at the National Center for Biotechnology Information by using the BLAST network service (2). Alignments of 31 bacterial SOD protein sequences (198 aligned positions) were made by using the CLUSTAL W (version 1.5) program. To calculate evolutionary distances, the PAM matrix compiled by Dayhoff et al. (16) was employed. Phylogenetic trees were constructed by using the neighbor-joining distance method (40) or maximum parsimony methods (21). The programs PROTDIST, NEIGHBOR, PROTPARS, SEQBOOT, and CONSENSE, present in the PHYLIP package (version 3.5), were employed in this work (21).
Construction of a SodB-deficient R. capsulatus mutant.
A 1,929-bp DNA fragment containing the entire sodB gene and flanking regions was excised from pA21 by using SphI (Fig. 1) and a KpnI site located in the multiple cloning region of pBSK−. The fragment was isolated and cloned into compatible sites of the suicide vector pPHU281 (28). The Tn903 Kmr cartridge was obtained from plasmid pUC4KSAC (3) as a 1.3-kbp Ecl136II fragment and ligated into the unique StuI site of the sodB coding region cloned in pPHU281. The resulting plasmid (pSODK1) was mobilized into R. capsulatus 37b4 by diparental conjugation, with strain SM10 as the E. coli donor (46). Filter matings were carried out essentially as described by Simon et al. (46). The mating mixtures were removed from the filters, washed in malate mineral broth, and finally spread onto malate or YCC agar plates that were incubated either in air or under anaerobic dark conditions (described above). Kmr exconjugants were first screened by colony hybridization with a DNA probe encoding internal sequences of the Kmr cassette from pUC4KSAC (3). The presence of the wild-type and/or interrupted sodB genes was evaluated by PCR amplification from two primers flanking the StuI site of sodB, corresponding to positions 23 to 40 and 743 to 760 within the coding region (Fig. 1).
Nucleotide sequence accession number.
Nucleotide sequences corresponding to the sodB (SOD) and tpl (tyrosine-phenol lyase) genes from R. capsulatus have been assigned GenBank accession no. AF 022931 and AF 022932, respectively.
RESULTS
Isolation of a Rhodobacter gene conferring superoxide tolerance to SOD-deficient E. coli cells.
To clone R. capsulatus genes whose products could be involved in superoxide detoxification, use was made of the QC774 E. coli strain, from which the sodA and sodB genes were knocked out by insertional mutagenesis (10). This strain displays marginally low levels of SOD activity and is abnormally sensitive to superoxide-propagating compounds, such as MV (10). In addition, the SOD deficiency causes oxygen-dependent auxotrophies, so that the mutant cells do not grow on minimal media (10).
A genome bank of R. capsulatus in pBSK− was used to transform QC774 cells, and transformants were selected by growth on M9 minimal medium in the presence of both ampicillin and MV. Sixteen colonies which grew after 24 to 48 h of selection were reisolated on M9 media. Restriction analysis of rescued plasmid DNA indicated that all 16 clones contained the same 3.65-kbp insert (Fig. 1), highlighting the consistency of the screening procedure employed.
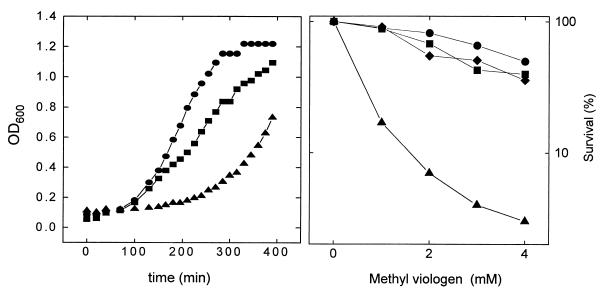
One of the clones (pA21) was tested for oxygen and MV tolerance in liquid LB broth. The results, illustrated in Fig. 2, indicate that the selected sodA sodB transformants were indeed more resistant to these oxidants than the untransformed controls.
FIG. 2.
Sensitivity of SOD-deficient E. coli transformants to oxygen (A) and to MV (B). (A) Cells were grown aerobically in liquid LB broth supplemented with the pertinent antibiotics (Table 1), and the OD600 was recorded at the indicated times. (B) Bacteria were grown (16 h at 37°C), challenged with the indicated concentrations of MV, and tested for viability as described in Materials and Methods. •, GC4468 (pBSK); ▴, QC774 (pBSK); ■, QC774 (pA21); ⧫, QC774 (pA22). Each data point in the survival curves represents the average of two to four independent experiments.
Plasmid pA21 was then transferred to E. coli DH5α for amplification and plasmid isolation. Sequencing on both strands of the insert indicated the presence of three different open reading frames, one of them truncated (Fig. 1). The sequence between nucleotides 673 and 1274 could encode a protein displaying 51% identity with the sodB product from E. coli (10). Homology tests strongly suggest that tpl (Fig. 1) encodes a tyrosine-phenol lyase (26), while the putative polypeptide encoded by the DNA sequence between nucleotides 3148 and 3652 displays 40% similarity with E. coli RarD, a protein involved in DNA repair (13).
To further investigate which regions of the 3.65-kbp fragment allowed by-pass of phenotypes associated with SOD deficiency in QC774 cells, plasmid pA21 was digested with either HindIII or SmaI, and the major R. capsulatus DNA fragments were cloned into compatible sites of pBSK−. When introduced into mutant bacteria, recombinant plasmid pA22 harboring the 1.27-kbp SmaI fragment with the putative sodB region (Fig. 1) provided high levels of protection against MV killing (Fig. 2B), whereas the two cloned HindIII fragments, one of them containing a full-length version of tpl (Fig. 1), were without effect (data not shown). Taken together, the results strongly suggest that the sodB homolog was responsible for the complementation observed.
The sodB gene of R. capsulatus encodes an iron-dependent SOD.
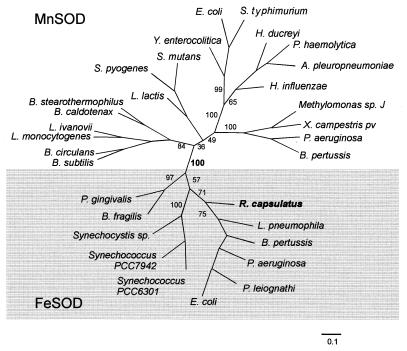
The sodB open reading frame encodes a protein of 200 amino acids with a predicted molecular mass of 22,124 Da and a pI of 5.7. Homology comparisons with other SOD proteins revealed that R. capsulatus SodB is similar to dismutases from various procaryotic organisms (10 representative sequences are shown in Fig. 3). Since the MnSOD and FeSOD polypeptides display extensive sequence similarity, it is often difficult to predict the metal cofactor present in the protein on the sole basis of primary structure comparisons. Some residues, corresponding to positions 79, 80, 82, and 155 in Fig. 3, have been proposed to allow discrimination between FeSOD and MnSOD (34, 35). Rhodobacter SodB contains most of the residues typical of FeSODs and none of those specific for manganese-containing SODs (Fig. 3), suggesting that this enzyme belongs to the class of iron-dependent SOD proteins (34, 35). Moreover, the topology of phylogenetic trees (21, 40) generated by means of both neighbor-joining distance (Fig. 4) and maximum parsimony methods (not shown) included the R. capsulatus SodB protein within the FeSOD clusters.
FIG. 3.
Multiple sequence alignment. Sequence alignment was obtained by using the CLUSTAL W (version 1.5) program. Residues conserved in all 10 sequences are shaded. Asterisks indicate amino acids involved in metal binding which are conserved between the FeSOD and MnSOD outgroups. Residues that help to distinguish the identity of the metal ligand (Fe or Mn) bound to the SOD apoprotein (35, 36) are boxed at positions 79, 80, 82, and 155.
FIG. 4.
Phylogenetic relationships based on SOD sequence comparisons. An unrooted phylogenetic tree was constructed by the neighbor-joining distance method (41). Numerals at branches indicate the number of times that the adjacent two groups it defines occurred, as obtained by the bootstrap procedure from 100 replicated trees (20). The length of each branch is proportional to the calculated evolutionary distance, and the scale (number of substitutions per site) is indicated at the bottom.
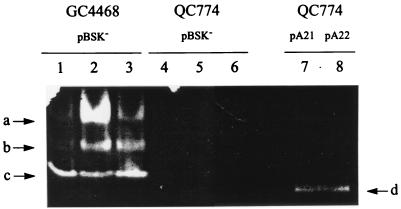
Lysates from transformed bacteria were assayed for SOD activity in nondenaturing polyacrylamide gels. QC774 cells harboring either pA21 or pA22 showed single SOD bands of similar electrophoretic mobility (Fig. 5, lanes 7 and 8). This activity was presumably derived from Rhodobacter DNA, since it was clearly different from the E. coli SOD bands (Fig. 5, lanes 1 to 3), which are lacking in the sodA sodB host (Fig. 5, lanes 4 to 6). Moreover, recombinant plasmids containing the A21 or A22 fragments in opposite directions yielded essentially the same SOD patterns in activity-stained gels (data not shown), suggesting that the plasmid-borne lac promoter is not involved in expression of the cloned sodB gene. Unlike the indigenous SOD species of E. coli (Fig. 5, lanes 1 to 3), expression of the recombinant dismutase in this host was not affected by the presence of oxygen or MV (data not shown).
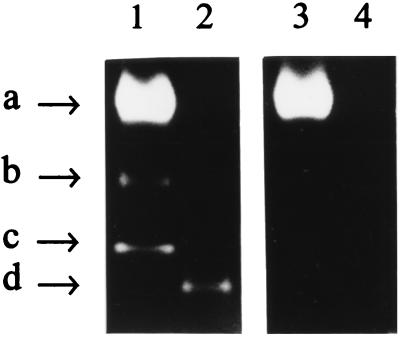
FIG. 5.
Expression of recombinant R. capsulatus FeSOD in E. coli cells. After rupture of E. coli cells harboring the relevant plasmids, supernatants corresponding to 30 μg of total soluble protein were subjected to nondenaturing polyacrylamide gel electrophoresis and activity staining (see Materials and Methods). GC4468 (pBSK) (lanes 1 to 3) and QC774 (pBSK) cells (lanes 4 to 6) were grown semiaerobically (lanes 1 and 4) or aerobically in the absence (lanes 3 and 6) or in the presence (lanes 2 and 5) of 0.1 mM MV. QC774 cells transformed with either pA21 (lane 7) or pA22 (lane 8) were grown aerobically. The arrows on the left side show the positions of the E. coli MnSOD (a), FeSOD (c) and Fe/MnSOD heterooligomer (b) activity bands, whereas arrow d on the right side indicates the activity band corresponding to the recombinant R. capsulatus SOD.
Inhibitor treatments were used to further characterize the SOD metal cofactor. Differential sensitivity against H2O2 and KCN has been reported for MnSOD (H2O2 and KCN resistant) and FeSOD (H2O2 sensitive and KCN resistant), whereas Cu/ZnSODs are sensitive to both reagents (17). The dismutase activity present in soluble extracts of transformed QC774 cells was indeed inhibited by H2O2 (Fig. 6), but remained unaffected by KCN treatment (data not shown), indicating that pA21 encodes an iron-containing SOD.
FIG. 6.
Effect of hydrogen peroxide on SOD activities expressed in E. coli. Lysates of ruptured bacteria (30 μg of soluble protein) were assayed by activity staining after polyacrylamide gel electrophoresis (see Materials and Methods). GC4468 (pBSK) (lanes 1 and 3) and QC774 (pA21) (lanes 2 and 4) E. coli cells were grown aerobically and challenged with 0.1 mM MV as described in the text. Equivalent gels were incubated in phosphate-buffered saline (42) with (lanes 3 and 4) or without (lanes 1 and 2) 5 mM H2O2 for 20 min, washed, and finally stained for SOD activity (4, 16). Arrow labels correspond to the same SOD species indicated in Fig. 5.
Expression of the sodB gene is induced by oxidants in R. capsulatus.
RNA gel blot hybridization experiments were undertaken to determine the expression patterns of the sodB gene in R. capsulatus cells grown under various culture regimes. The results presented in Fig. 7A indicate that a single sodB transcript with a size of approximately 0.8 kb accumulated to moderate levels in cells cultured under semiaerobic conditions. Similar results were obtained with photosynthesizing bacteria grown under strict anaerobiosis (data not shown).
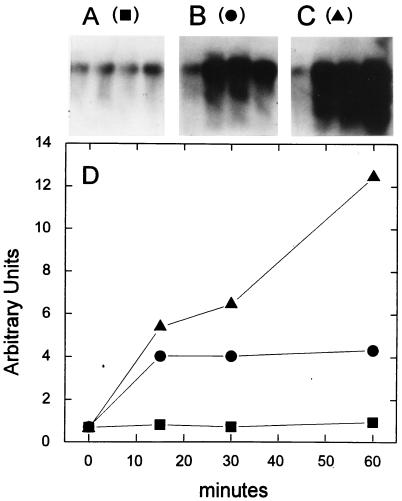
FIG. 7.
Induction of the R. capsulatus sodB gene by oxidative conditions. R. capsulatus cells were grown semiaerobically at 32°C until the cultures reached an OD600 of 0.3 to 0.5. Aliquots were then withdrawn and incubated for an additional hour under semiaerobic (A) or aerobic conditions either in the absence (B) or in the presence (C) of 1 mM MV. At the times indicated (D), the cells were harvested and total RNA was isolated and analyzed by Northern hybridization as described in the text. The amount of radioactivity associated with the hybridized 0.8-kb transcript was plotted as a function of induction time (D).
A shift to respiratory growth caused a rapid, fourfold increase in the steady-state levels of the 0.8-kb species, which reached a maximum in 15 min and remained stable for at least 1 h (Fig. 7B and D). This effect was accompanied by the transient accumulation of shorter RNA species of discrete size (≈0.6 kb) that hybridized with the sodB probe (Fig. 7B). Addition of MV in the presence of oxygen resulted in an even higher induction of sodB, although in this case, the transcript levels increased steadily during the 60 min of incubation (Fig. 7C and D). The presence of the radical propagator also caused the time-dependent accumulation of progressively shorter RNA bands, presumably representing truncated and/or partially degraded sodB transcripts (Fig. 7C).
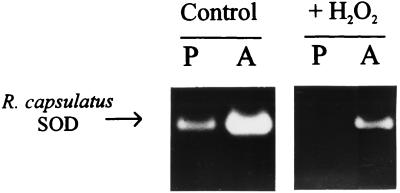
Oxygen-mediated SOD induction was also evident at the protein level. Activity gels indicate a significant increase in the single SOD species detected in R. capsulatus lysates, when the cultures were shifted from anaerobic to respiratory conditions (Fig. 8). Hydrogen peroxide sensitivity confirms that this soluble enzyme is an iron-containing SOD (Fig. 8). Although there is no strict correlation between the amount of protein and the activity displayed in this type of experiments, the results obtained indicate that there is an increase in the amount of functional SOD in the R. capsulatus cytosol upon exposition of the cells to oxidants.
FIG. 8.
Steady-state levels of SOD activity in R. capsulatus cells. R. capsulatus 37b4 cells were grown photosynthetically (P) or aerobically (A) as described in Materials and Methods. Lysates of ruptured bacteria (30 μg of soluble protein) were assayed by activity staining after polyacrylamide gel electrophoresis. Equivalent gels were incubated in phosphate-buffered saline (42) with (left panel) or without (right panel) 5 mM H2O2, washed, and finally stained for SOD activity.
The sodB gene is essential for aerobic viability of R. capsulatus.
To further evaluate the contribution of FeSOD in resistance against oxidative stress, we tried to construct a sodB insertional mutant of R. capsulatus by using the Tn903 kanamycin resistance gene for selection. When mated cells were plated on either malate minimal medium or YCC rich broth and incubated in aerobiosis, no stable sodB mutants could be isolated. Among the 840 Kmr exconjugants isolated under these conditions, about 96% were also Tcr, indicating that they resulted from plasmid addition. The remaining Tcs clones also came from single crossover events, as deduced from in situ hybridization and PCR amplification analyses (data not shown). When Kmr exconjugants were isolated by anaerobic selection on minimal medium (see Materials and Methods), about 8% of the 560 colonies evaluated were Tcs. These Tcs clones failed to grow further when spread on either rich or minimal medium under aerobic growth conditions. The collected results strongly suggest that the FeSOD encoded by sodB is essential for R. capsulatus viability in aerobiosis.
DISCUSSION
Genetic complementation of SOD-deficient derivatives of E. coli was used to clone DNA sequences encoding the first R. capsulatus SOD described to date (Fig. 1). The deduced amino acid sequence showed the highest degree of similarity to iron-containing SODs from several bacterial sources (Fig. 3 and 4). The nature of the ligand cofactor was confirmed by inhibitor sensitivity assays in both E. coli (Fig. 6) and R. capsulatus lysates (Fig. 8). Expression of the recombinant SOD in E. coli cells did not require the presence of an indigenous promoter (Fig. 2, 5, and 6), indicating that the upstream regions of the cloned gene are functional to some extent in the heterologous host. The transcription and translation signals of some genes from phototrophic bacteria (i.e., R. sphaeroides) were reported to be recognized by the E. coli expression machinery, although with a rather low efficiency (32). Indeed, only small amounts of active dismutase were produced by the plasmid-borne R. capsulatus sodB gene in SOD-deficient E. coli mutants, as judged by the results obtained with activity gels (Fig. 5 and 6). Even this limited expression, however, was sufficient to increase the aerobic growth rates and to improve resistance of the host cells against MV killing in the presence of oxygen (Fig. 2). sodB was expressed at low levels in R. capsulatus cells grown under semiaerobic (Fig. 7), or strictly anaerobic, photosynthetic conditions (Fig. 8). However, when bacteria were exposed to oxygen or to MV, expression of this gene was progressively induced more than 10-fold relative to its basal level (Fig. 7), indicating that sodB is up-regulated in response to increased oxidative stress conditions within the cell. The results of the Northern hybridization experiments suggest that this regulation occurs at the mRNA level (Fig. 7).
The sodB gene of E. coli is expressed constitutively under most growth regimes (10) and does not respond to oxidants. In this facultative aerobe, oxygen responsiveness is displayed by sodA, which encodes an Mn-containing SOD. Regulation of sodA expression is complex and affected by several global regulators (11), most conspicuously, by the soxRS regulon, an adaptive regulatory system specifically evolved to cope with superoxide toxicity (25). Oxygen- and MV-dependent induction of sodA expression in E. coli (Fig. 5) closely resembles that of sodB in R. capsulatus (Fig. 7 and 8). However, mechanisms and signals involved in oxidant-dependent sodB regulation are probably different from those operating in E. coli. Indeed, the recombinant SOD was not induced to any significant extent when the host cells were exposed to MV, despite the fact that extensive nontranscribed sequences were present both upstream and downstream of the sodB gene in pA21.
Lysates from R. capsulatus cells grown under various conditions contain a single enzyme staining for SOD activity, which corresponds to the iron-containing dismutase encoded by sodB (Fig. 8). These results indicate that SodB is the only, or at least the major, SOD in R. capsulatus. Although our evidence does not rule out additional mechanisms for MV resistance, the increase in the accumulation of sodB transcripts (Fig. 7) and protein (Fig. 8) likely plays a large role in antioxidant protection in these phototrophic bacteria. Moreover, the inability of SodB-deficient R. capsulatus mutants to grow under aerated conditions indicates that the FeSOD encoded by this gene is essential for aerobic viability of the bacteria and that at least in YCC or malate medium, other enzyme activities cannot substitute for the lost SodB function. Many procaryotes contain at least two SODs, usually with a basic set of constitutive FeSOD and inducible MnSOD (9, 10, 24). Some organisms, however, appear to withstand the hazards of oxygen exposition with the aid of a single SOD. In Lactococcus lactis, an MnSOD provides the cell with a basic level of dismutase activity or with larger amounts when required to cope with the effects of an oxidative challenge (42). A similar behavior is displayed by the unique FeSOD of the obligate anaerobe Porphyromonas gingivalis, which is nevertheless essential for aerotolerance (31).
Finally, our results do not preclude the possibility that additional SOD isoforms might be expressed in R. capsulatus under growth conditions that were not assayed here. The Mn-containing SOD of Bordetella pertussis, for instance, is expressed only under conditions of iron deprivation and remains undetectable in standard growth media supplemented with the transition metal (24). Work is currently in progress to evaluate these possibilities.
The cloning of the R. capsulatus sodB gene provides the basis for further studies of the aerobic life of these photosynthetic organisms. Gene manipulation strategies can now be applied in order to investigate the regulation and sites of action of these scavenging enzymes, as well as their role in the concerted cellular response to oxidative stress in phototrophic bacteria.
ACKNOWLEDGMENTS
This work was supported by the John Simon Guggenheim Foundation, the National Research Council (CONICET, Argentina, PEI no. 0292/97), the Fonds der Chemischen Industrie, and a DAAD fellowship to N. Cortez.
We thank D. Touati (University of Paris 7, Paris, France) who generously provided the E. coli strains GC4468 and QC774 used in this work.
REFERENCES
- 1.Allen R D. Dissection of oxidative stress tolerance using transgenic plants. Plant Physiol. 1995;107:1049–1054. doi: 10.1104/pp.107.4.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altschul S F, Gish W, Miller W, Myers E, Lipman K J. Basic local alignment research tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 3.Barany F. Two-codon insertion mutagenesis of plasmid genes by using single-stranded hexameric oligonucleotides. Proc Natl Acad Sci USA. 1985;82:4202–4206. doi: 10.1073/pnas.82.12.4202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bauer C E. Regulation of photosynthesis gene expression. In: Blankenship R E, Madigan M T, Bauer C E, editors. Anoxygenic photosynthetic bacteria. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1995. pp. 1221–1234. [Google Scholar]
- 5.Beauchamp C, Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem. 1971;44:276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- 6.Bianchi V, Haggård-Ljungquist E, Pontis E, Reichard P. Interruption of the ferredoxin (flavodoxin) NADP+ oxidoreductase gene of Escherichia coli does not affect anaerobic growth but increases sensitivity to paraquat. J Bacteriol. 1995;177:4528–4531. doi: 10.1128/jb.177.15.4528-4531.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burdon R H, O’Kane D, Fadzillah N, Bill V, Boyd P A, Finch R R. Oxidative stress and responses in Arabidopsis thaliana and Oryza sativa subjected to chilling and salinity stress. Biochem Soc Trans. 1996;24:469–472. doi: 10.1042/bst0240469. [DOI] [PubMed] [Google Scholar]
- 8.Cadenas E. Biochemistry of oxygen toxicity. Annu Rev Biochem. 1989;58:79–110. doi: 10.1146/annurev.bi.58.070189.000455. [DOI] [PubMed] [Google Scholar]
- 9.Campbell W S, Laudenbach D E. Characterization of four superoxide dismutase genes from a filamentous cyanobacterium. J Bacteriol. 1995;177:964–972. doi: 10.1128/jb.177.4.964-972.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carlioz A, Touati D. Isolation of superoxide dismutase mutants in E. coli: is superoxide dismutase necessary for aerobic life? EMBO J. 1986;5:623–630. doi: 10.1002/j.1460-2075.1986.tb04256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Compan I, Touati D. Interaction of six global transcription regulators in expression of manganese superoxide dismutase in Escherichia coli K-12. J Bacteriol. 1993;175:1687–1696. doi: 10.1128/jb.175.6.1687-1696.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cox J C, Beatty J T, Favinger J L. Increased activity of respiratory enzymes from photosynthetically grown Rhodopseudomonas capsulata in response to small amounts of oxygen. Arch Microbiol. 1983;134:324–328. [Google Scholar]
- 13.Daniels D L, Plunkett III G, Burland V, Blattner F R. Analysis of the Escherichia coli genome: DNA sequence of the region from 84.5 to 86.5 minutes. Science. 1992;257:771–778. doi: 10.1126/science.1379743. [DOI] [PubMed] [Google Scholar]
- 14.Dastoor F P, Forrest M E, Beatty J T. Cloning, sequencing, and oxygen regulation of the Rhodobacter capsulatus α-ketoglutarate dehydrogenase operon. J Bacteriol. 1997;179:4559–4566. doi: 10.1128/jb.179.14.4559-4566.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davis R W, Botstein D, Roth J R. A manual for genetic engineering. Advanced bacterial genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1980. [Google Scholar]
- 16.Dayhoff M O, Schwartz R M, Orcutt B C. A model of evolutionary change in proteins. In: Dayhoff M O, editor. Atlas of protein sequence and structure. 5, suppl. 3. Washington, D.C: National Biomedical Research Foundation; 1978. pp. 345–452. [Google Scholar]
- 17.Donahue J L, Moses Okpodu C, Cramer C L, Grabau E A, Alscher R G. Responses of antioxidants to paraquat in pea leaves. Plant Physiol. 1997;113:249–257. doi: 10.1104/pp.113.1.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Drews G. Mikrobiologisches Praktikum. 4th ed. Berlin, Germany: Springer-Verlag; 1983. p. 62. [Google Scholar]
- 19.Drews G, Imhoff J F. Phototrophic purple bacteria. In: Shively J M, Barton L L, editors. Variations in autotrophic life. New York, N.Y: Academic Press Ltd.; 1991. pp. 51–97. [Google Scholar]
- 20.Edington S M. As we live and breathe: free radicals and aging. Bio/Technology. 1994;12:37–40. doi: 10.1038/nbt0194-37. [DOI] [PubMed] [Google Scholar]
- 21.Felsenstein J. Phylogenies from molecular sequences: inference and reliability. Annu Rev Genet. 1988;22:521–565. doi: 10.1146/annurev.ge.22.120188.002513. [DOI] [PubMed] [Google Scholar]
- 22.Forkl H, Vandekerckhove J, Drews G, Tadros M H. Molecular cloning, sequence analysis and expression of the gene for catalase-peroxidase (cpeA) from the photosynthetic bacterium Rhodobacter capsulatus B10. Eur J Biochem. 1993;214:251–258. doi: 10.1111/j.1432-1033.1993.tb17918.x. [DOI] [PubMed] [Google Scholar]
- 23.Fridovich I. Superoxide radical and superoxide dismutases. Annu Rev Biochem. 1995;64:97–112. doi: 10.1146/annurev.bi.64.070195.000525. [DOI] [PubMed] [Google Scholar]
- 24.Graeff-Wohlleben H, Killat S, Banemann A, Guiso N, Gross R. Cloning and characterization of an Mn-containing superoxide dismutase (SodA) of Bordetella pertussis. J Bacteriol. 1997;179:2194–2201. doi: 10.1128/jb.179.7.2194-2201.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hidalgo E, Ding H, Demple B. Redox signal transduction via iron-sulfur clusters in the SoxR transcription activator. Trends Biol Sci. 1997;22:207–210. doi: 10.1016/s0968-0004(97)01068-2. [DOI] [PubMed] [Google Scholar]
- 26.Hirahara T, Horinouchi S, Beppu T. Cloning, nucleotide sequence, and overexpression in Escherichia coli of the β-tyrosinase gene from an obligately symbiotic thermophile, Symbiobacterium thermophilum. Appl Microbiol Biotechnol. 1993;39:341–346. doi: 10.1007/BF00192089. [DOI] [PubMed] [Google Scholar]
- 27.Hochman A, Figueredo A, Wall J D. Physiological functions of hydroperoxidases in Rhodobacter capsulatus. J Bacteriol. 1992;174:3386–3391. doi: 10.1128/jb.174.10.3386-3391.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hübner P, Masepohl B, Klipp W, Bickle T A. nif gene expression studies in Rhodobacter capsulatus: ntrC-independent repression by high ammonium concentrations. Mol Microbiol. 1993;10:123–132. doi: 10.1111/j.1365-2958.1993.tb00909.x. [DOI] [PubMed] [Google Scholar]
- 29.Imlay J A, Linn S. DNA damage and oxygen radical toxicity. Science. 1988;240:1302–1309. doi: 10.1126/science.3287616. [DOI] [PubMed] [Google Scholar]
- 30.Meissner P S, Sisk W P, Berman M L. Bacteriophage λ cloning system for the construction of directional cDNA libraries. Proc Natl Acad Sci USA. 1987;84:4171–4175. doi: 10.1073/pnas.84.12.4171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nakayama K. Rapid viability loss on exposure to air in a superoxide dismutase-deficient mutant of Porphyromonas gingivalis. J Bacteriol. 1994;176:1939–1943. doi: 10.1128/jb.176.7.1939-1943.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Neidle E L, Kaplan S. 5-Aminolevulinic acid availability and control of spectral complex formation in HemA and HemT mutants of Rhodobacter sphaeroides. J Bacteriol. 1993;175:2304–2313. doi: 10.1128/jb.175.8.2304-2313.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Orr W C, Sohal R S. Extension of life span by overexpression of superoxide dismutase and catalase in Drosophila melanogaster. Science. 1994;263:1128–1130. doi: 10.1126/science.8108730. [DOI] [PubMed] [Google Scholar]
- 34.Parker M W, Blake C C F. Crystal structure of manganese superoxide dismutase from Bacillus stearothermophilus at 2.4 Å resolution. J Mol Biol. 1988;199:649–661. doi: 10.1016/0022-2836(88)90308-7. [DOI] [PubMed] [Google Scholar]
- 35.Parker M W, Blake C C F. Iron- and manganese-containing superoxide dismutases can be distinguished by analysis of their primary structures. FEBS Lett. 1988;229:377–382. doi: 10.1016/0014-5793(88)81160-8. [DOI] [PubMed] [Google Scholar]
- 36.Pasternak C, Assemat K, Breton A M, Clément-Métral J D, Klug G. Expression of the thioredoxin gene (trxA) in Rhodobacter sphaeroides Y is regulated by oxygen. Mol Gen Genet. 1996;250:189–196. doi: 10.1007/BF02174178. [DOI] [PubMed] [Google Scholar]
- 37.Pasternak C, Assemat K, Clément-Métral J D, Klug G. Thioredoxin is essential for Rhodobacter sphaeroides growth by aerobic and anaerobic respiration. Microbiology. 1997;143:83–91. doi: 10.1099/00221287-143-1-83. [DOI] [PubMed] [Google Scholar]
- 38.Pille S, Chuat J-C, Breton A M, Clément-Métral J D, Galibert F. Cloning, nucleotide sequence, and expression of the Rhodobacter sphaeroides Y thioredoxin gene. J Bacteriol. 1990;172:1556–1561. doi: 10.1128/jb.172.3.1556-1561.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rosen D, Siddique T, Patterson D, Figlewicz D A, Sapp P, Hentati A, Donaldson D, Goto J, O’Regan J P, Deng H X, Rahmani Z, Krizus A, McKenna-Yasek D, Cayabyab A, Gaston S M, Berger R, Tanzi R E, Halperin J J, Herzfeldt B, van den Bergh R, Hung W Y, Bird T, Deng G, Mulder D W, Smyth C, Laing N G, Soriano E, Pericak-Vance M A, Haines J, Rouleau G A, Gusella J S, Horvitz H R, Brown R H., Jr Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature (London) 1993;362:59–62. doi: 10.1038/362059a0. [DOI] [PubMed] [Google Scholar]
- 40.Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- 41.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 42.Sanders J W, Leenhouts K J, Haandrikman A J, Venema G, Kok J. Stress response in Lactococcus lactis: cloning, expression analysis, and mutation of the lactococcal superoxide dismutase gene. J Bacteriol. 1995;177:5254–5260. doi: 10.1128/jb.177.18.5254-5260.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Scandalios J G. Oxygen stress and superoxide dismutases. Plant Physiol. 1993;101:7–12. doi: 10.1104/pp.101.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Scherer S. Do photosynthetic and respiratory electron transport chains share redox proteins? Trends Biol Sci. 1990;15:458–462. doi: 10.1016/0968-0004(90)90296-n. [DOI] [PubMed] [Google Scholar]
- 45.Sedmak J, Grossberg S. A rapid, sensitive and versatile assay for protein using Coomassie brilliant blue G-250. Anal Biochem. 1977;79:544–552. doi: 10.1016/0003-2697(77)90428-6. [DOI] [PubMed] [Google Scholar]
- 46.Simon R, Priefer U, Pühler A. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in gram negative bacteria. Bio/Technology. 1983;1:784–791. [Google Scholar]
- 47.Sistrom W R. Transfer of chromosomal genes mediated by plasmid R68.45 in Rhodopseudomonas sphaeroides. J Bacteriol. 1977;131:526–532. doi: 10.1128/jb.131.2.526-532.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Von Gabain A, Belasco J G, Schottel J L, Chang A C Y, Cohen S N. Decay of mRNA in Escherichia coli: investigation of the fate of specific segments of transcripts. Proc Natl Acad Sci USA. 1983;80:653–657. doi: 10.1073/pnas.80.3.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Woodcock D M, Crowther P J, Doherty J, Jefferson S, De Cruz E, Noyer-Weidner M, Smith S S, Michael M Z, Graham M W. Quantitative evaluation of E. coli host strains for tolerance to cytosine methylation in plasmid and phage recombinants. Nucleic Acids Res. 1989;17:3469–3478. doi: 10.1093/nar/17.9.3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yanisch-Perron C, Vieira J, Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33:103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- 51.Yu B P. Cellular defenses against damage from reactive oxygen species. Physiol Rev. 1994;74:139–162. doi: 10.1152/physrev.1994.74.1.139. [DOI] [PubMed] [Google Scholar]