Abstract
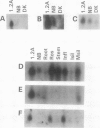
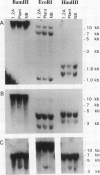
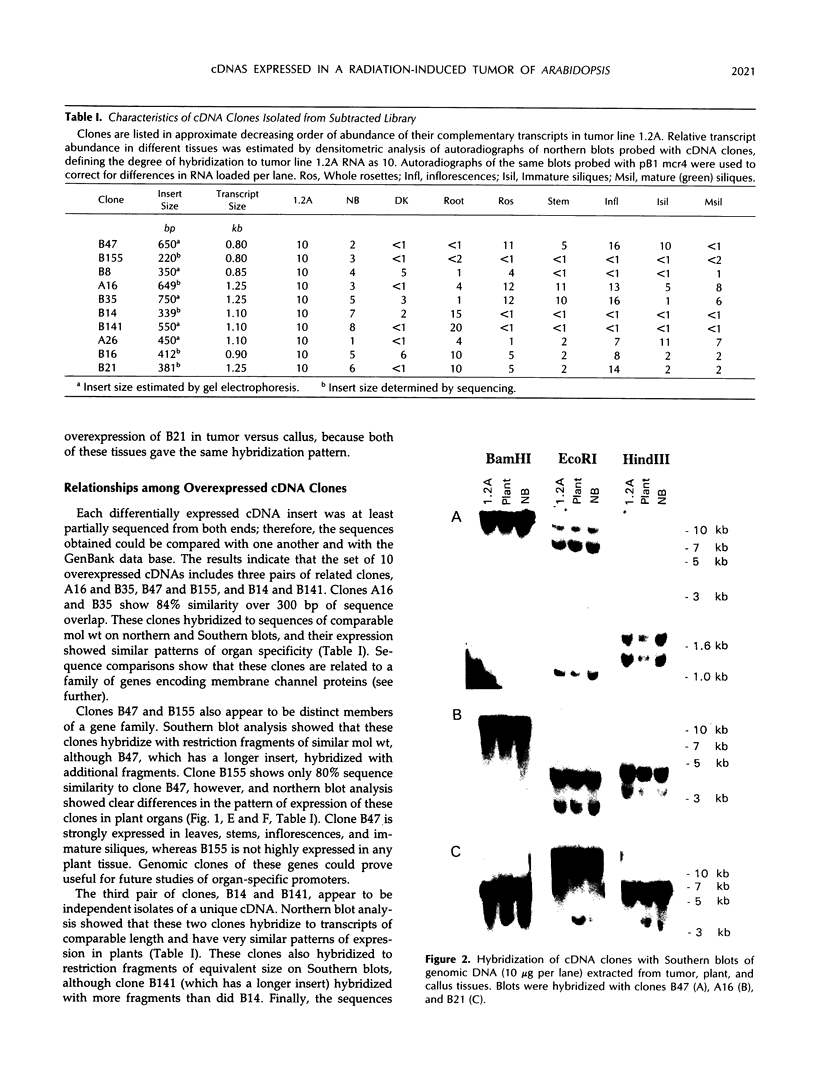
To investigate the molecular mechanisms of hormonal control of growth, we constructed a subtracted cDNA library enriched for sequences expressed more in a hormone-autonomous, radiation-induced tumor tissue line of Arabidopsis thaliana than in normal, hormone-dependent callus. Ten cDNA clones, which are expressed 1.3- to 10-fold more in the tumor line, were isolated and partially characterized. The clones differ greatly in their level of expression in tumor tissue and in their pattern of expression in plant organs. Southern blot hybridization and sequence analysis showed that this group contains three pairs of closely related clones. Northern blot analysis indicates that one pair of clones represents two members of a gene family that are expressed in different plant organs. One of the isolated sequences shows strong sequence similarity to a cDNA encoding a lipid transfer protein. Two sequences are highly similar to those of previously described membrane channel proteins but have different organ specificities. Two other cDNAs have significant sequence similarity to glycine-rich proteins and hydroxy-proline-rich glycoproteins. When used to probe Southern blots, none of the cDNAs identified polymorphisms between tumor and callus DNA, which might be expected if their overexpression were due to local genome rearrangements induced by radiation. The diversity observed among these 10 clones suggests that some are likely to be involved in tumorous growth and not simply specific to a certain cell or tissue type present in the tumor.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Campell B. R., Town C. D. Physiology of Hormone Autonomous Tissue Lines Derived From Radiation-Induced Tumors of Arabidopsis thaliana. Plant Physiol. 1991 Nov;97(3):1166–1173. doi: 10.1104/pp.97.3.1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullis C. A. DNA sequence organization in the flax genome. Biochim Biophys Acta. 1981 Jan 29;652(1):1–15. doi: 10.1016/0005-2787(81)90203-3. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Hanahan D., Meselson M. Plasmid screening at high colony density. Methods Enzymol. 1983;100:333–342. doi: 10.1016/0076-6879(83)00066-x. [DOI] [PubMed] [Google Scholar]
- Hervagault J. F., Ortoleva P. J., Ross J. A plausible model for reversal of neoplastic transformations in plants based on multiple steady states. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10797–10800. doi: 10.1073/pnas.88.23.10797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höfte H., Hubbard L., Reizer J., Ludevid D., Herman E. M., Chrispeels M. J. Vegetative and Seed-Specific Forms of Tonoplast Intrinsic Protein in the Vacuolar Membrane of Arabidopsis thaliana. Plant Physiol. 1992 Jun;99(2):561–570. doi: 10.1104/pp.99.2.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheimer D. G., Haas N., Silflow C. D., Snustad D. P. The beta-tubulin gene family of Arabidopsis thaliana: preferential accumulation of the beta 1 transcript in roots. Gene. 1988;63(1):87–102. doi: 10.1016/0378-1119(88)90548-3. [DOI] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochester D. E., Winer J. A., Shah D. M. The structure and expression of maize genes encoding the major heat shock protein, hsp70. EMBO J. 1986 Mar;5(3):451–458. doi: 10.1002/j.1460-2075.1986.tb04233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Y. T., Cheng C. L., Conkling M. A. Root-specific genes from tobacco and Arabidopsis homologous to an evolutionarily conserved gene family of membrane channel proteins. Nucleic Acids Res. 1990 Dec 25;18(24):7449–7449. doi: 10.1093/nar/18.24.7449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Oliveira D. E., Seurinck J., Inzé D., Van Montagu M., Botterman J. Differential expression of five Arabidopsis genes encoding glycine-rich proteins. Plant Cell. 1990 May;2(5):427–436. doi: 10.1105/tpc.2.5.427. [DOI] [PMC free article] [PubMed] [Google Scholar]