Abstract
Background
Gonadotrophin‐releasing hormone agonists (GnRHa) are commonly used in assisted reproduction technology (ART) cycles to prevent a luteinising hormone surge during controlled ovarian hyperstimulation (COH) prior to planned oocyte retrieval, thus optimising the chances of live birth.
Objectives
To evaluate the effectiveness of the different GnRHa protocols as adjuncts to COH in women undergoing ART cycles.
Search methods
We searched the following databases from inception to April 2015: the Cochrane Menstrual Disorders and Subfertility Group Specialised Register, the Cochrane Central Register of Controlled Trials (CENTRAL) in the Cochrane Library (2015, Issue 3), MEDLINE, EMBASE, CINAHL, PsycINFO, and registries of ongoing trials. Reference lists of relevant articles were also searched.
Selection criteria
We included randomised controlled trials (RCTs) comparing any two protocols of GnRHa used in in vitro fertilisation (IVF) or intracytoplasmic sperm injection (ICSI) cycles in subfertile women.
Data collection and analysis
Two review authors independently selected studies, assessed trial eligibility and risk of bias, and extracted the data. The primary outcome measure was number of live births or ongoing pregnancies per woman/couple randomised. Secondary outcome measures were number of clinical pregnancies, number of oocytes retrieved, dose of gonadotrophins used, adverse effects (pregnancy losses, ovarian hyperstimulation, cycle cancellation, and premature luteinising hormone (LH) surges), and cost and acceptability of the regimens. We combined data to calculate odds ratios (OR) for dichotomous variables and mean differences (MD) for continuous variables, with 95% confidence intervals (CIs). We assessed statistical heterogeneity using the I² statistic. We assessed the overall quality of the evidence for the main comparisons using 'Grading of Recommendations Assessment, Development and Evaluation' (GRADE) methods.
Main results
We included 37 RCTs (3872 women), one ongoing trial, and one trial awaiting classification. These trials made nine different comparisons between protocols. Twenty of the RCTs compared long protocols and short protocols. Only 19/37 RCTs reported live birth or ongoing pregnancy.
There was no conclusive evidence of a difference between a long protocol and a short protocol in live birth and ongoing pregnancy rates (OR 1.30, 95% CI 0.94 to 1.81; 12 RCTs, n = 976 women, I² = 15%, low quality evidence). Our findings suggest that in a population in which 14% of women achieve live birth or ongoing pregnancy using a short protocol, between 13% and 23% will achieve live birth or ongoing pregnancy using a long protocol. There was evidence of an increase in clinical pregnancy rates (OR 1.50, 95% CI 1.18 to 1.92; 20 RCTs, n = 1643 women, I² = 27%, moderate quality evidence) associated with the use of a long protocol.
There was no evidence of a difference between the groups in terms of live birth and ongoing pregnancy rates when the following GnRHa protocols were compared: long versus ultrashort protocol (OR 1.78, 95% CI 0.72 to 4.36; one RCT, n = 150 women, low quality evidence), long luteal versus long follicular phase protocol (OR 1.89, 95% CI 0.87 to 4.10; one RCT, n = 223 women, low quality evidence), when GnRHa was stopped versus when it was continued (OR 0.75, 95% CI 0.42 to 1.33; three RCTs, n = 290 women, I² = 0%, low quality evidence), when the dose of GnRHa was reduced versus when the same dose was continued (OR 1.02, 95% CI 0.68 to 1.52; four RCTs, n = 407 women, I² = 0%, low quality evidence), when GnRHa was discontinued versus continued after human chorionic gonadotrophin (HCG) administration in the long protocol (OR 0.89, 95% CI 0.49 to 1.64; one RCT, n = 181 women, low quality evidence), and when administration of GnRHa lasted for two versus three weeks before stimulation (OR 1.14, 95% CI 0.49 to 2.68; one RCT, n = 85 women, low quality evidence). Our primary outcomes were not reported for any other comparisons.
Regarding adverse events, there were insufficient data to enable us to reach any conclusions except about the cycle cancellation rate. There was no conclusive evidence of a difference in cycle cancellation rate (OR 0.95, 95% CI 0.59 to 1.55; 11 RCTs, n = 1026 women, I² = 42%, low quality evidence) when a long protocol was compared with a short protocol. This suggests that in a population in which 9% of women would have their cycles cancelled using a short protocol, between 5.5% and 14% will have cancelled cycles when using a long protocol.
The quality of the evidence ranged from moderate to low. The main limitations in the evidence were failure to report live birth or ongoing pregnancy, poor reporting of methods in the primary studies, and imprecise findings due to lack of data. Only 10 of the 37 included studies were conducted within the last 10 years.
Authors' conclusions
When long GnRHa protocols and short GnRHa protocols were compared, we found no conclusive evidence of a difference in live birth and ongoing pregnancy rates, but there was moderate quality evidence of higher clinical pregnancy rates in the long protocol group. None of the other analyses showed any evidence of a difference in birth or pregnancy outcomes between the protocols compared. There was insufficient evidence to make any conclusions regarding adverse effects.
Plain language summary
Gonadotrophin‐releasing hormone agonists (GnRHa) used as an adjuvant to gonadotrophins in assisted reproduction treatments
Review question
Researchers from the Cochrane Collaboration reviewed the evidence about the most effective way of using gonadotrophin‐releasing hormone agonists (GnRHa) as part of controlled ovarian stimulation in women undergoing assisted reproduction technology (ART).
Background
GnRHa are given along with hormone injections that stimulate the ovaries, in an attempt to prevent spontaneous release of eggs prior to their planned surgical retrieval. GnRHa have been proven to improve pregnancy rates; however, various regimens are described in the literature. We conducted this review to identify the most effective regimens.
Study characteristics
We found 37 randomised controlled trials (RCTs) of 3872 women comparing the use of GnRHa in various protocols. Twenty of these RCTs (1643 women) compared a long protocol with a short protocol. The evidence is current to April 2015.
Key results
In comparisons of long GnRHa protocols (where GnRHa is given for at least 14 days prior to the start of ovarian stimulation) versus short GnRHa protocols (when the GnRHa is given at the start of stimulation) there was no conclusive evidence of a difference in live birth and ongoing pregnancy rates. However there was moderate quality evidence of higher clinical pregnancy rates in the long protocol groups. Our findings suggest that in a population in which 14% of women achieve live birth or ongoing pregnancy using a short protocol, between 13% and 23% will achieve live birth or ongoing pregnancy using a long protocol.
None of the other analyses showed any evidence of a difference in birth or pregnancy outcomes between the protocols compared. There was insufficient evidence to make any conclusions regarding adverse effects. Further research is needed to determine which long protocol is most cost effective and acceptable to women.
Quality of the evidence
The quality of the evidence ranged from moderate to low. The main limitations in the evidence were failure to report live birth or ongoing pregnancy, poor reporting of methods in the primary studies, and imprecise findings due to lack of data. Only 10 of the 37 included studies were conducted within the last 10 years.
Summary of findings
Summary of findings for the main comparison. Long protocol compared with short protocol for pituitary suppression in assisted reproduction.
| Long protocol compared with short protocol for pituitary suppression in assisted reproduction | ||||||
| Population: women undergoing pituitary suppression in assisted reproduction Intervention: long protocol Comparison: short protocol | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Short protocol | Long protocol | |||||
| Live birth or ongoing pregnancies per woman randomised | 138 per 1000 | 172 per 1000 (131 to 225) | OR 1.3 (0.94 to 1.81) | 976 (12 studies) | ⊕⊕⊝⊝ Low¹,² | No evidence of a difference between the groups |
| Clinical pregnancies per woman randomised | 137 per 1000 | 192 per 1000 (158 to 232) | OR 1.5 (1.18 to 1.9) | 1643 (20 studies) | ⊕⊕⊕⊝ Moderate¹ | Benefit to long protocol group |
| *The basis for the assumed risk is the median control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; OR: odds ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
¹High risk of bias associated with poor reporting of methods in the primary studies. ²Imprecision: the confidence interval is compatible with benefit in one or both groups or with no effect.
Summary of findings 2. Long protocol compared with ultrashort protocol for pituitary suppression in assisted reproduction.
| Long protocol compared with ultrashort protocol for pituitary suppression in assisted reproduction | ||||||
| Population: women undergoing pituitary suppression in assisted reproduction Intervention: long protocol Comparison: ultrashort protocol | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Ultrashort protocol | Long protocol | |||||
| Live birth and ongoing pregnancies per woman randomised | 122 per 1000¹ | 198 per 1000 (91 to 376) | OR 1.78 (0.72 to 4.36) | 150 (1 study) | ⊕⊕⊝⊝ Low²,³ | No evidence of a difference between the groups |
| Clinical pregnancies per woman randomised | 161 per 1000⁴ | 230 per 1000 (133 to 370) | OR 1.56 (0.8 to 3.06) | 230 (2 studies) | ⊕⊕⊝⊝ Low²,³ | No evidence of a difference between the groups |
| *The basis for the assumed risk (e.g., the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; OR: odds ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
¹The assumed risk in the control group was determined as a mean baseline risk from the study included in the comparison. ²High risk of bias associated with poor reporting of methods in the primary study or studies. ³Imprecision: the confidence interval is compatible with benefit in one or both groups or with no effect. ⁴The assumed risk in the control group was determined as the median value across included studies.
Summary of findings 3. Short compared with ultrashort protocol for pituitary suppression in assisted reproduction.
| Short protocol compared with ultrashort protocol for pituitary suppression in assisted reproduction | ||||||
| Population: women undergoing pituitary suppression in assisted reproduction Intervention: short protocol Comparison: ultrashort protocol | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Ultrashort protocol | Short | |||||
| Live birth and ongoing pregnancies per woman randomised | Not reported in the included study | ‐ | ‐ | |||
|
Clinical pregnancies per woman randomised |
195 per 1000¹ |
244 per 1000 (102 to 480) |
OR 1.33 (0.47 to 3.81) |
82 (1 study) | ⊕⊕⊝⊝ Very low²,³ | No evidence of a difference between the groups |
| *The basis for the assumed risk (e.g., the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; OR: odds ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
¹The assumed risk in the control group was determined as a mean baseline risk from the study included in the comparison. ²Applicability uncertain: the population is a selected group of participants (poor responders). ³Imprecision: single underpowered trial with a small number of events; the confidence interval is compatible with benefit in either group or with no effect.
Summary of findings 4. Long luteal phase protocol compared with long follicular phase protocol for pituitary suppression in assisted reproduction.
| Long luteal phase protocol compared with long follicular phase protocol for pituitary suppression in assisted reproduction | ||||||
| Population: women undergoing pituitary suppression in assisted reproduction Intervention: long luteal phase protocol Comparison: long follicular phase protocol | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Long follicular phase protocol | Long luteal phase protocol | |||||
| Live birth and ongoing pregnancies per woman randomised | 102 per 1000¹ | 177 per 1000 (90 to 319) | OR 1.89 (0.87 to 4.1) | 223 (1 study) | ⊕⊕⊝⊝ Low²,³ | No evidence of a difference between the groups |
|
Clinical pregnancies per woman randomised |
269 per 1000⁴ | 281 per 1000 (219 to 351) | OR 1.06 (0.76 to 1.47) | 750 (5 studies) | ⊕⊕⊝⊝ Low²,³ | No evidence of a difference between the groups |
| *The basis for the assumed risk (e.g., the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; OR: odds ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
¹The assumed risk in the control group was determined as a mean baseline risk from the study included in the comparison. ²High risk of bias associated with poor reporting of methods in the primary study or studies. ³Imprecision: the confidence interval is compatible with benefit in either group or with no effect.
⁴The assumed risk in the control group was determined as the median value across included studies.
Summary of findings 5. Long protocol continued GnRH agonist compared with long protocol stop GnRH agonist for pituitary suppression in assisted reproduction.
| Long protocol continued GnRH agonist compared with long protocol stop GnRH agonist for pituitary suppression in assisted reproduction | ||||||
| Population: women undergoing pituitary suppression in assisted reproduction Intervention: long protocol continued GnRH agonist Comparison: long protocol stop GnRH agonist | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Long protocol stop GnRH agonist | Long protocol continued GnRH agonist | |||||
| Live birth and ongoing pregnancies Number of live births or ongoing pregnancies per woman randomised | 276 per 1000¹ | 222 per 1000 (138 to 336) | OR 0.75 (0.42 to 1.33) | 290 (3 studies) | ⊕⊕⊝⊝ Low²,³ | No evidence of a difference between the groups |
| Clinical pregnancies Number of clinical pregnancies per woman randomised | 235 per 1000¹ | 207 per 1000 (135 to 302) | OR 0.85 (0.51 to 1.41) | 360 (4 studies) | ⊕⊕⊝⊝ Low²,³ | No evidence of a difference between the groups |
| *The basis for the assumed risk (e.g., the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; GnRH: gonadotrophin‐releasing hormone; OR: odds ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
¹The assumed risk in the control group was determined as a mean baseline risk from the study included in the comparison. ²High risk of bias associated with poor reporting of methods in one or more of the primary studies. ³Imprecision: the confidence interval is compatible with benefit in either group or with no effect.
Summary of findings 6. Long protocol (continued same versus reduced dose GnRHa) for pituitary suppression in assisted reproduction.
| Long protocol (continued same versus reduced dose GnRHa) for pituitary suppression in assisted reproduction | ||||||
|
Population: women undergoing pituitary suppression in assisted reproduction
Intervention: long protocol continued same Comparison: long protocol reduced dose GnRHa | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Long protocol, reduced dose GnRHa | Long protocol, continued same | |||||
| Live birth and ongoing pregnancies per woman randomised | No studies reported this outcome | ‐ | ‐ | |||
| Clinical pregnancies per woman randomised | 377 per 1000¹ | 382 per 1000 (292 to 479) | OR 1.02 (0.68 to 1.52) | 407 (4 studies) | ⊕⊕⊝⊝ Low²,³ | No evidence of a difference between the groups |
| *The basis for the assumed risk (e.g., the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; GnRHa: gonadotrophin‐releasing hormone agonists; OR: odds ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
¹The assumed risk in the control group was determined as the median value across included studies. ²High risk of bias associated with poor reporting of methods in one or more of the primary studies. ³Imprecision: the confidence interval is compatible with benefit in either group or with no effect.
Summary of findings 7. Long protocol (GnRHa until HCG) compared with long protocol (extend GnRHa 12 days after HCG) for pituitary suppression in assisted reproduction.
| Long protocol (GnRHa until HCG) compared with long protocol (extend GnRHa 12 days after HCG) for pituitary suppression in assisted reproduction | ||||||
| Population: women undergoing pituitary suppression in assisted reproduction Intervention: long protocol (GnRHa until HCG) Comparison: long protocol (extend GnRHa 12 days after HCG) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Long protocol (extend GnRHa 12 days after HCG) | Long protocol (GnRHa until HCG) | |||||
| Live birth and ongoing pregnancies per woman randomised | 378 per 1000¹ | 351 per 1000 (229 to 499) | OR 0.89 (0.49 to 1.64) | 181 (1 study) | ⊕⊕⊝⊝ Low² | No evidence of a difference between the groups |
| Clinical pregnancies per woman randomised | 489 per 1000¹ | 494 per 1000 (353 to 636) | OR 1.02 (0.57 to 1.83) | 181 (1 study) | ⊕⊕⊝⊝ Low² | No evidence of a difference between the groups |
| *The basis for the assumed risk (e.g., the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; GnRHa: gonadotrophin‐releasing hormone agonists; HCG: human chorionic gonadotrophin; OR: odds ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
¹The assumed risk in the control group was determined as a mean baseline risk from the study included in the comparison. ²The level of evidence was downgraded by two levels due to imprecision: only one underpowered trial with relatively small number of events and wide confidence interval compatible with benefit in either group or with no effect.
Summary of findings 8. Long protocol: administration of GnRHa for two versus three weeks before stimulation for pituitary suppression in assisted reproduction.
| long protocol: administration of GnRHa for two versus three weeks before stimulation for pituitary suppression in assisted reproduction | ||||||
| Population: women undergoing pituitary suppression in assisted reproduction Intervention: long protocol: administration of GnRHa for two weeks before stimulation Comparison: long protocol: administration of GnRHa for three weeks before stimulation | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Long protocol: administration of GnRHa for three weeks before stimulation | Long protocol: administration of GnRHa for two weeks before stimulation | |||||
| Live birth and ongoing pregnancies per woman randomised | 488 per 1000¹ |
456 per 1000 (261 to 661) |
OR 0.88 (0.37 to 2.05) |
85 (1 study) | ⊕⊕⊝⊝ Low² | No evidence of a difference between the groups |
| Clinical pregnancies per woman randomised | 585 per 1000¹ |
568 per 1000 (355 to 757) |
OR 0.93 (0.39 to 2.21) |
85 (1 study) | ⊕⊕⊝⊝ Low² | No evidence of a difference between the groups |
| *The basis for the assumed risk (e.g., the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; GnRHa: gonadotrophin‐releasing hormone agonists; OR: odds ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
¹The assumed risk in the control group was determined as a mean baseline risk from the study included in the comparison. ²High risk of bias associated with poor reporting of methods in one or more of the primary studies.
Summary of findings 9. Short protocol compared with stop short protocol for pituitary suppression in assisted reproduction.
| Short protocol compared with stop short protocol for pituitary suppression in assisted reproduction | ||||||
| Population: women undergoing pituitary suppression in assisted reproduction Intervention: short protocol Comparison: stop short protocol | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Stop short protocol | Short protocol | |||||
| Live birth and ongoing pregnancies per woman randomised | This outcome was not reported by the included trial | ‐ | ‐ | |||
| Clinical pregnancies per woman randomised | 226 per 10001 | 147 per 1000 (81 to 255) | OR 0.59 (0.3 to 1.17) | 230 (1 study) | ⊕⊕⊝⊝ Low 1,2 | No evidence of a difference between the groups |
| *The basis for the assumed risk (e.g., the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; OR: odds ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
¹The assumed risk in the control group was determined as a mean baseline risk from the study included in the comparison. ²High risk of bias associated with poor reporting of methods in one or more of the primary studies.
Background
Description of the condition
Subfertility affects one in seven couples; a high proportion of them use assisted reproductive technology (ART) in an attempt to improve their chances of conception (Maheshwari 2008). In a natural cycle, only one oocyte is normally produced. Conversely, an ART cycle usually aims to produce more than one oocyte destined for fertilisation, to improve the chances of having a sufficient number of embryos to choose from. Concurrently, it is crucial to prevent an excessive response from the ovaries resulting in ovarian hyperstimulation. In order to produce more oocytes, the ovaries are stimulated with high doses of gonadotrophins. However, there is a risk of a premature surge of luteinising hormone (LH), which could disrupt both normal follicle and oocyte development, resulting in non‐recovery of oocytes. The incorporation of gonadotrophin‐releasing hormone agonists (GnRHa) in controlled ovarian hyperstimulation (COH) protocols has been used in ART to reversibly block pituitary function and prevent a premature LH surge. Use of GnRHa has resulted in significant improvements in treatment, including decreased cancellation of started treatment cycles prior to oocyte recovery and higher pregnancy rates (Fields 2013).
Description of the intervention
Different GnRHa drugs, routes of administration (nasal or systemic), and GnRHa protocols have been used in ART. There are three main protocols involving GnRHa administration, namely, the long, the short, and the ultrashort protocol.
Long protocol: GnRHa is administered at least two weeks before starting stimulation (to achieve suppression of the ovarian activity) and continued up until human chorionic gonadotrophin (HCG) is given, starting from either the second day of the menstrual cycle (long follicular protocol) or the mid‐luteal phase (21st day) of the previous cycle (long luteal protocol).
Short protocol: GnRHa is administered from day one or two of the cycle (day one being the start of the menstrual bleed) and continued with stimulation until the day of HCG administration.
Ultrashort protocol: GnRHa is given for three days, from day two of the cycle (hence, using only the flare‐up effect).
How the intervention might work
Administration of multiple doses of GnRHa causes a reversible blockade of pituitary function after an initial stimulatory phase, the so‐called flare effect. GnRHa suppresses GnRH receptors and causes inhibition of postreceptor events (Daya 2000). The resulting reduction in bioactive LH levels in the serum (Regan 1990) allows multiple follicular development to continue (until ready for oocyte recovery) avoiding the risk of a LH surge and hence premature ovulation (Barlow 1998).
GnRHa are the most commonly used adjuvants for controlled ovarian stimulation (www.ivf‐worldwide.com/survey/survey). Traditionally, the long protocol involves GnRHa use during the entire stimulation phase until HCG administration. Reports showed that low endogenous LH concentrations persist until 10 to 14 days after discontinuation of the GnRHa (Donderwinkel 1993; Sungurtekin 1995). Earlier studies have argued that continuation of GnRHa during the stimulation phase can also lead to profound suppression of mid‐follicular LH, which might be associated with early pregnancy loss (Westergaard 2000). Therefore, GnRHa could be stopped earlier in the long protocol stimulation cycle (Simons 2005), allowing the pituitary to recover in time for the luteal phase without risking a premature LH surge. This could reduce both cost and inconvenience as fewer injections would be needed.
Why it is important to do this review
The original Cochrane review on the topic, published in 1998 and updated in 2009, showed superiority of the long protocols compared with the short or ultrashort protocols. Of note, long protocols are traditionally used in ART, whereas most of the newer alternatives (e.g., antagonists or mild protocols) have been compared with them (Mancini 2011; Mohsen 2013). The second update of this review aimed to examine whether evidence in the last three years on the relative effectiveness of the different GnRHa protocols used as adjuncts to hormonal ovarian stimulation for ART supports the conclusions of the first update.
Objectives
To evaluate the effectiveness of the different GnRHa protocols as adjuncts to COH in women undergoing ART cycles.
Methods
Criteria for considering studies for this review
Types of studies
Only randomised controlled trials (RCTs) comparing various gonadotrophin‐releasing hormone agonist protocols in assisted reproductive technology (ART). We included in vitro fertilisation (IVF) or intracytoplasmic sperm injection (ICSI) treatment cycles. We excluded trials if we found allocation to be non‐random as they are associated with a high risk of bias. We also excluded cross‐over trials as the design is not suitable for this review. We excluded quasi‐randomised trials even if they had been included in the original review.
Types of participants
Women/couples with all types of infertility were eligible for inclusion, undergoing ART and using GnRHa for pituitary down‐regulation.
Types of interventions
Inclusion criteria
Studies comparing any two protocols using gonadotrophin‐releasing hormone agonists (GnRHa) for pituitary suppression in an ART programme. We included ultrashort, short, and long (follicular or luteal with or without discontinuation during the stimulation phase) protocols.
The definitions used in this review for the various protocols were as follows.
Long protocol: GnRHa commenced at least two weeks before starting stimulation and continued up until human chorionic gonadotrophin (HCG) was given.
Short protocol: GnRHa commenced at the same time as starting stimulation and continued up until the day of HCG administration.
Ultrashort protocol: stimulation was commenced one to two days after starting GnRHa (and given only for three days).
Exclusion criteria
We excluded women receiving donor oocytes.
We also excluded the following study comparisons.
GnRHa versus GnRH‐antagonist protocols.
Different routes of administration of GnRHa.
GnRHa versus placebo protocols (Hughes 1992).
Depot versus daily administration of GnRHa, as this is the topic of another Cochrane review (Albuquerque 2013).
Addition of any drug in GnRHa protocols.
Types of outcome measures
We measured the following primary and secondary outcome measures.
Primary outcomes
Number of live births or ongoing pregnancies per woman/couple randomised.
We defined live birth as the delivery of a live foetus after 20 completed weeks of gestational age. We defined ongoing pregnancy as evidence of a gestational sac with foetal heart motion at 12 weeks or later, confirmed with an ultrasound. We decided to combine the two outcomes, as ongoing pregnancy comprises a more meaningful clinical measure compared with any other and in order to give more power to the results of the current update.
When there were multiple live births (e.g., twins or triplets), we counted these as one live birth event.
Secondary outcomes
Number of clinical pregnancies per woman/couple randomised, defined as evidence of a gestational sac with foetal heart motion at six weeks or later, confirmed with an ultrasound. When there were multiple gestational sacs in one woman, we counted these as one clinical pregnancy (Griffin 2002).
Number of oocytes retrieved per woman randomised.
Amount of gonadotrophins administered per woman randomised.
Adverse outcomes
Number of pregnancy losses, defined as the sum of the number of miscarriages (pregnancy loss before 20 completed weeks of gestation) and the number of stillbirths (pregnancy loss after 20 completed weeks of gestation) (Griffin 2002).
Number of ovarian hyperstimulation syndrome (OHSS) events per woman randomised.
Cycle cancellation (defined as cancelled cycle before oocyte retrieval).
Number of premature luteinising hormone (LH) surges.
Other outcomes
Cost of treatment.
Acceptability of the regimen.
Search methods for identification of studies
We analysed all published and unpublished RCTs comparing the various regiments for pituitary down‐regulation using GnRHa in ART without language restriction and in consultation with the Menstrual Disorders and Subfertility Group (MDSG) Trials Search Co‐ordinator.
Electronic searches
We searched the following databases on 23 April 2015, using the search strategy developed by the Menstrual Disorders and Subfertility Group:
the Cochrane Menstrual Disorders and Subfertility Group (MDSG) Specialised Register;
the Cochrane Central Register of Controlled Trials (CENTRAL) in the Cochrane Library (Issue 3, 2015);
MEDLINE;
EMBASE;
CINAHL (Cumulative Index to Nursing and Allied Health Literature); and
PsycINFO.
The searches were conducted using the search strategies listed in the appendices (Appendix 1; Appendix 2; Appendix 3; Appendix 4; Appendix 5).
Searching other resources
We searched the citation lists of relevant publications, review articles, abstracts of scientific meetings, and included studies. In liaison with the Trials Search Co‐ordinator, we included in the review published articles and conference abstracts that are not covered in the Menstrual Disorders and Subfertility Group Specialised Register. In addition, OpenGrey, a system for grey literature produced in Europe, such as research reports, doctoral dissertations, and conference papers (www.opengrey.eu/), was searched.
We searched the following trials registries for published, ongoing, or registered trials:
The metaRegister of Controlled Trials (www.controlled‐trials.com).
The US National Institutes of Health Ongoing Trials Register, a service of the US National Institutes of Health (clinicaltrials.gov/ct2/home).
The World Health Organization International Clinical Trials Registry platform (www.who.int/trialsearch/Default.aspx).
Data collection and analysis
Selection of studies
Four review authors (AM, CS, AG, and GB), in pairs, independently selected the trials for inclusion using forms designed according to Cochrane guidelines. We sought, via e‐mail, additional information on trial methodology and missing data from the authors of trials that appeared to meet the eligibility criteria but had unclear methodology or data that were in an unsuitable form for meta‐analysis. Discussion with SB resolved differences of opinion.
We documented the selection process with a 'Preferred Reporting Items for Systematic Reviews and Meta‐Analyses' (PRISMA) flow chart (Figure 1).
1.
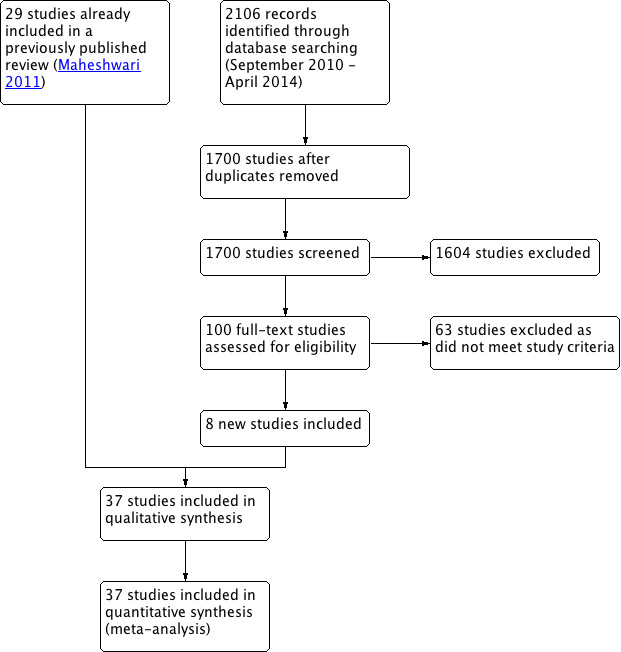
Study flow diagram.
We constructed 'Characteristics of included studies' tables for those trials considered suitable for inclusion (Characteristics of included studies). The 'Characteristics of excluded studies' tables list the excluded studies with reasons for exclusion (Characteristics of excluded studies).
Data extraction and management
Two review authors (AG and GB) independently extracted data from eligible studies using a data extraction form, which we had designed and pilot tested. A third review author (CS) resolved disagreements. Data extracted included study characteristics and outcome data. Where studies had multiple publications, we collated the multiple reports of the same study, so that each study ‐ rather than each report ‐ was the unit of interest in the review, and such studies have a single study identification with multiple references. As required, we corresponded with study investigators for further data on methods, results, or both, via e‐mail.
The data extraction forms included 'Risk of bias' criteria and methodological details, which we have presented in the 'Characteristics of included studies' tables. We managed the data using Review Manager 5.3 software (RevMan 2014).
Appendix 6 shows the information extracted from the studies selected for the review.
Assessment of risk of bias in included studies
Two review authors (AG and GB) independently assessed the included studies for risk of bias using Cochrane's 'Risk of bias' assessment tool (Higgins 2011) to assess selection bias (random sequence generation and allocation concealment), performance bias (blinding of participants and personnel), attrition bias (incomplete outcome data), reporting bias (selective reporting), and other bias. A third review author (CS) resolved disagreements. We described all judgements fully and presented them in the 'Characteristics of included studies' tables, including commentary about each of the domains. This led to an overall assessment of the risk of bias of included studies (Figure 2 and Figure 3).
2.
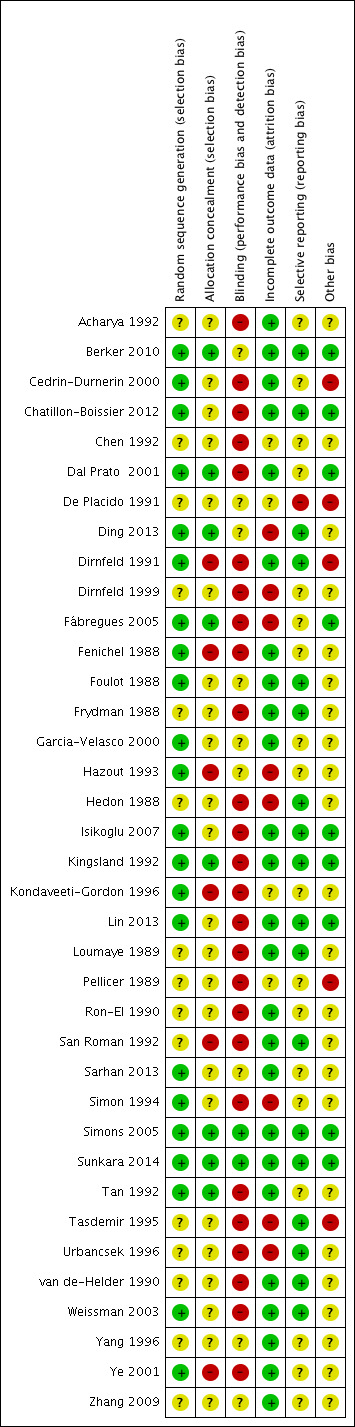
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
3.
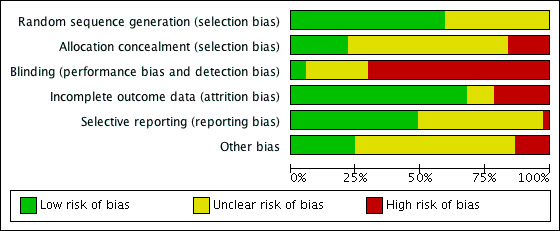
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
We searched for within‐trial selective reporting, such as trials failing to report obvious outcomes or reporting them in insufficient detail to allow inclusion. We sought published protocols and compared the outcomes between the protocol and the final published study.
Measures of treatment effect
For dichotomous data (e.g., live birth and ongoing pregnancy rates), we used the numbers of events in the control and intervention groups of each study to calculate Mantel–Haenszel odds ratios (ORs). For continuous data (e.g., number of oocytes retrieved), we calculated the mean difference (MD) between treatment groups. We presented 95% confidence intervals for all outcomes. Where data to calculate ORs or MDs were not available, our intention was to utilise the most detailed numerical data available that might facilitate similar analyses of included studies (e.g., test statistics, P values). We compared the magnitude and direction of effect reported by studies with how they are presented in the review, taking account of legitimate differences.
Unit of analysis issues
The primary analysis was per woman randomised; we included per‐pregnancy data for some outcomes (e.g., miscarriage). We counted multiple live births (e.g., twins or triplets) as one live birth event.
Dealing with missing data
In the case of missing data in the included studies, we contacted the original investigators by e‐mail or post to request relevant missing information. (We sent a reminder if we had received no reply during the first 20 days.) We reported the data according to intention‐to‐treat principles wherever possible. We assumed that live births had not occurred in participants without a reported outcome. For other outcomes, we analysed only the available data.
If studies reported sufficient detail to calculate MDs but provided no information on the associated standard deviation (SD), we assumed the outcome to have a SD equal to the highest SD from other studies within the same analysis.
Assessment of heterogeneity
Before any meta‐analysis was done, we judged whether there was sufficient similarity between the eligible studies in their design and clinical characteristics to ensure that pooling was valid. We assessed statistical heterogeneity in the results of trials by using the X² test. A low P value (or a large X² statistic relative to its degree of freedom) potentially provides evidence of heterogeneity of intervention effects and shows that results are not influenced by chance alone (Higgins 2011). We used the I² statistic to assess the impact of the heterogeneity on the meta‐analysis and interpreted an I² statistic > 50% as marked heterogeneity (Higgins 2011).
Assessment of reporting biases
In view of the difficulty of detecting and correcting for publication bias and other reporting biases, we aimed to minimise their potential impact by ensuring a comprehensive search for eligible studies and by being alert to duplication of data. In the presence of 10 or more studies in an analysis, we used a funnel plot to explore the possibility of small study effects. This was to guide whether the difference was due to publication or reporting bias. We were aware that there are other sources of asymmetry in funnel plots (Stuck 1998).
Data synthesis
The various comparison groups were as follows:
any long protocol versus any short protocol;
any long protocol versus ultrashort protocol;
any short protocol versus ultrashort protocol;
long luteal protocol versus long follicular phase protocol;
long protocol: continuation versus discontinuation of the GnRHa at start of stimulation;
long protocol: continuation of same‐dose GnRHa versus reduced‐dose GnRHa until HCG administration;
long protocol: discontinuing versus continuing GnRHa after HCG administration;
long protocol: administration of GnRHa for two versus three weeks before stimulation; and
short protocol: continuation of GnRHa versus stopping GnRHa.
We performed analysis using RevMan 5.3 software (RevMan 2014). For binary (or dichotomous) outcomes, we expressed the results for each study as odds ratios (OR) with 95% confidence intervals (CI) and combined them for meta‐analysis, where appropriate. For continuous outcome data, we expressed the results from each study as a difference in means with 95% CI and combined for meta‐analysis using the mean difference (MD).
An increase in the odds of a particular outcome, which may be beneficial (e.g., live birth) or detrimental (e.g., adverse effects), are displayed graphically in the meta‐analyses to the right of the centre‐line and a decrease in the odds of an outcome to the left of the centre‐line.
Subgroup analysis and investigation of heterogeneity
Where data were available, we planned to conduct subgroup analyses to determine the separate evidence within the following subgroups: normal or poor responders, number of embryos transferred, previous failed cycles, maternal age, and duration of treatment. In cases of substantial heterogeneity, our aim was to explore possible explanations in sensitivity analyses. We took any statistical heterogeneity into account when interpreting the results, especially if there was any variation in the direction of effect. We used a fixed‐effect model.
Sensitivity analysis
We performed sensitivity analysis for the primary outcomes to determine whether the conclusions were robust to arbitrary decisions made regarding the eligibility and analysis. These analyses included consideration of whether the review conclusions would have differed in the following ways:
if we had restricted eligibility to studies without high risk of bias (e.g., clear description of sequence generation and allocation concealment methods);
if we had adopted a random‐effects model;
if we had implemented alternative imputation strategies; or
if the summary effect measure we had used was relative risk rather than odds ratio.
We did so by excluding studies with unclear randomisation and studies with incomplete data. There were not enough studies to support meta‐regression or other formal considerations of prognostic factors.
Overall quality of the body of evidence: 'Summary of findings' tables
We prepared 'Summary of findings' tables using GRADEprofiler (GRADEpro). These tables evaluate the overall quality of the body of evidence for the main review outcomes (live birth and clinical pregnancy) using GRADE criteria (study limitations (i.e., risk of bias), consistency of effect, imprecision, indirectness, and publication bias). We justify our judgements about evidence quality (high, moderate, or low) and have documented and incorporated these into the reporting of results for each outcome.
Results
Description of studies
See Characteristics of included studies, Characteristics of excluded studies, Characteristics of ongoing studies, and the 'Preferred Reporting Items for Systematic Reviews and Meta‐Analyses' (PRISMA) flowchart (Figure 1).
Results of the search
After searching the electronic databases, we found a total of 2503 studies: 641 in the Cochrane Menstrual Disorders and Subfertility Group Specialised Register, 722 in the Cochrane Central Register of Controlled Trials (CENTRAL) in the Cochrane Library, 485 in MEDLINE, 369 in EMBASE, 266 in CINAHL (Cumulative Index to Nursing and Allied Health Literature), and 20 studies in PsycINFO. After removing the duplicates and searching other resources, there were approximately 1700 studies left. Of these, 100 seemed eligible for inclusion, and after reading the full text articles, we were able to include 37 studies in the review (eight more than was in the last update). Of note, we considered one study as two different comparisons (De Placido 1991), which were present in the study. One study is ongoing (NCT01006954).
We sent two e‐mails to trial authors (with a reminder); we received responses from nine out of 15 study authors (Chatillon‐Boissier 2012; Corson 1992; Isikoglu 2007; Lin 2013; NCT00436319; Sarhan 2013; Sunkara 2014; Tanaka 2014; Tarin 1990).
Included studies
Design
We included 37 studies (3872 women). All were parallel group randomised controlled trials (RCTs). There were nine different comparison groups.
1. Long versus short protocol
Twenty studies featured this comparison. An a priori power calculation was a feature in one study (Sunkara 2014). Weissman 2003 did a power calculation for pregnancy as the outcome but decided to proceed with number of oocytes as the primary outcome measure because of the large sample size required for determining a significant difference in the pregnancy rate. Only nine studies out of 20 reported adequate randomisation ( Chatillon‐Boissier 2012 ; Dirnfeld 1991 ; Fenichel 1988 ; Foulot 1988 ; Hazout 1993 ; Sunkara 2014 ; Tan 1992; Weissman 2003; Ye 2001). Three studies, Chatillon‐Boissier 2012; Sunkara 2014; Tan 1992, reported concealed allocation. The funnel plot did not suggest any publication bias (Figure 4).
4.
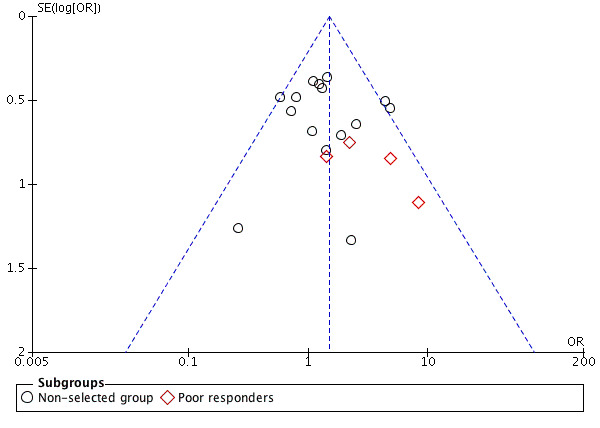
Funnel plot of comparison: 1 Long versus short protocol, outcome: 1.2 Clinical pregnancies.
2. Long versus ultrashort protocol
Two studies featured this comparison. Of the two (Chen 1992; Kingsland 1992), the former reported adequate randomisation and concealed allocation. An a priori power calculation was not a feature of any study.
3. Short versus ultrashort protocol
One study featured this comparison (Berker 2010): the paper described an a priori power calculation, randomisation, and allocation concealment.
4. Long protocol: luteal versus follicular start of gonadotrophin‐releasing hormone agonists (GnRHa)
Five studies featured this comparison. Of them, only Kondaveeti‐Gordon 1996 had an a priori power calculation. Kondaveeti‐Gordon 1996; Urbancsek 1996; and Sarhan 2013 reported clear randomisation and concealment. Although blinding until objective outcome assessment was planned for one study (Kondaveeti‐Gordon 1996), it was revealed after the study was started. Urbancsek 1996 reported more than one cycle per participant.
5. Long protocol: continuation of GnRHa versus stopping GnRHa at start of stimulation
Three studies featured this comparison (Dirnfeld 1999; Garcia‐Velasco 2000; Simons 2005). Of them, only one was double blinded (Simons 2005). All of the three studies reported adequate randomisation and concealment.
6. Long protocol: continuation of same‐dose GnRHa versus reduced‐dose GnRHa until HCG administration
Four studies featured this comparison. All of them reported adequate randomisation, while three reported concealed allocation (Dal Prato 2001; Ding 2013; Fábregues 2005).
7. Long protocol: discontinuing versus continuing GnRHa after HCG administration
One study featured this comparison (Isikoglu 2007). The study reported adequate randomisation (computer‐generated list), blinding, and concealment, but there was no power calculation.
8. Long protocol: administration of GnRHa for two versus three weeks before stimulation
One study featured this comparison (Lin 2013). The study reported adequate randomisation (computer‐generated random numbers two weeks after GnRHa administration), but there was no concealment or blinding.
9. Short protocol: continuation versus stopping GnRHa
One study featured this comparison (Cedrin‐Durnerin 2000). The study reported adequate randomisation, but there was no concealment or blinding.
Participants
1. Long versus short protocol
Inclusion criteria for included studies varied widely. Some studies included women with all causes of infertility, Acharya 1992; Tan 1992; Tasdemir 1995, while others restricted inclusion to women with only tubal factor infertility, Fenichel 1988; Frydman 1988; Loumaye 1989; van de‐Helder 1990; Zhang 2009, or tubal and unexplained infertility (Hazout 1993; Hedon 1988). Some studies excluded women with polycystic ovary syndrome (PCOS) (Foulot 1988; Yang 1996).
The age of the women included was variable in the different studies. Some included only women under 38 years (Fenichel 1988; Hazout 1993; Zhang 2009); others included women up until the age of 40 years (Chatillon‐Boissier 2012; Loumaye 1989; Sunkara 2014; van de‐Helder 1990).
Some studies included women undergoing only the first in vitro fertilisation (IVF) cycle, San Roman 1992; Tasdemir 1995, while others included all IVF cycles (Hazout 1993). Some included only previous low or poor responders, Chatillon‐Boissier 2012; Dirnfeld 1991; Sunkara 2014; Weissman 2003, whereas others excluded previous poor responders (Frydman 1988; van de‐Helder 1990).
2. Long versus ultrashort protocol
Couples with all causes of infertility were included in both studies. Kingsland 1992 only included women with the first cycle.
3. Short versus ultrashort protocol
A total of 82 poor responder participants who underwent intracytoplasmic sperm injection (ICSI) were included in this comparison. Criteria included at least one of the following: day 3 serum follicle‐stimulating hormone (FSH) level > 10 mIU/mL, < 6 total antral follicles, prior cycle cancellation, prior poor response to controlled ovarian hyperstimulation (COH) (either peak E2 < 500 pg/mL, < 6 oocytes retrieved, or both), and aged > 41 (Berker 2010).
4. Long protocol: luteal versus follicular start of GnRHa
Ron‐El 1990 included consecutive women whereas Pellicer 1989 included women with normal ovarian function; Urbancsek 1996 included women with tubal and unexplained infertility, and Sarhan 2013 included women with all types of infertility.
5. Long protocol: continuation of GnRHa versus stopping GnRHa at start of stimulation
Dirnfeld 1999 excluded women with irregular cycles, and Simons 2005 excluded women with PCOS or poor ovarian reserve. Simons 2005 included only women under 39 years of age whereas Dirnfeld 1999 included women up to the age of 42 years. Garcia‐Velasco 2000 had no exclusion criteria for age.
Dirnfeld 1999 included only women with a previous poor response or high FSH; some studies included only previous low responders (Garcia‐Velasco 2000; Simons 2005).
6. Long protocol: continuation of same‐dose GnRHa versus reduced‐dose GnRHa until HCG administration
Inclusion criteria for the included studies varied widely. One, Simon 1994, restricted inclusion to only tubal factor infertility while another included tubal and unexplained infertility (Dal Prato 2001). Dal Prato 2001 excluded women with a risk of hyperstimulation or with poor ovarian reserve while Ding 2013 included women with high response to gonadotrophin stimulation, that is, "women with eight or more subcapsular follicles of 2 to 8 mm in diameter in one plane in either ovary". The age of the women included was variable in the different studies: under 35 (Ding 2013), 38 (Dal Prato 2001), and under 39 years (Simon 1994). Fábregues 2005 and Ding 2013 included women undergoing their first IVF cycle.
7. Long protocol: discontinuing versus continuing GnRHa after HCG administration
One hundred eighty‐one women undergoing 181 consecutive ICSI cycles were included, with a mean age of 30 years.
8. Long protocol: administration of GnRHa for two versus three weeks before stimulation
One hundred participants undergoing IVF/ICSI cycle were included, with a mean age of 29 years. Inclusion criteria: (a) subfertile participants undergoing first IVF/(ICSI) with tubal factor, male factor, or unexplained factor; (b) undertaking a luteal long protocol; (c) basal FSH levels 10 IU/L; and (d) aged 35 years. Exclusion criteria: (a) endometriosis, (b) adenomyosis, and (c) polycystic ovarian syndrome.
9. Short protocol: continuation versus stopping GnRHa
Cedrin‐Durnerin 2000 excluded women older than 43 years and those with anovulation.
Interventions
1. Long versus short protocol
Twenty trials compared a long protocol with a short protocol. In six studies, Acharya 1992; Foulot 1988; Frydman 1988; Hazout 1993; Hedon 1988; Tan 1992, GnRHa was commenced in the follicular phase whereas it was commenced in the luteal phase in the rest of the studies (Chatillon‐Boissier 2012; De Placido 1991; Fenichel 1988; Loumaye 1989; San Roman 1992; Sunkara 2014; Tasdemir 1995; van de‐Helder 1990; Weissman 2003; Ye 2001; Zhang 2009). In two studies, Dirnfeld 1991; Yang 1996, it was not clear whether a follicular or luteal start was used.
There was a wide variation in the dose, type, and route of GnRHa used for down‐regulation in long protocols. Buserelin was used either by nasal spray or subcutaneous injections: 1000 µg twice a day (Dirnfeld 1991); 200 µg five times a day (Acharya 1992); 900 µg/day (Loumaye 1989; Tasdemir 1995; Ye 2001); 300 µg twice a day (De Placido 1991; Frydman 1988; Hedon 1988); 200 µg three times daily (van de‐Helder 1990); 0.3 ml daily (Foulot 1988); 200 µg daily (Tan 1992); and 100 µg/day (Weissman 2003). Decapeptyl was used either as a short‐acting (100 μg/day) (Cedrin‐Durnerin 2000; Chatillon‐Boissier 2012) or long‐acting single intramuscular injection (3.75 mg) (Fenichel 1988) or 1.88 mg of intramuscular Diphereline® (Zhang 2009). Other studies used leuprolide acetate (1 mg/day) (San Roman 1992; Yang 1996). Hazout 1993 repeated the decapeptyl injection twice, which may explain a much higher requirement of gonadotrophins. One study, Sunkara 2014, used nafarelin nasal spray 400 mg twice daily.
In studies comparing a long protocol versus a short protocol, GnRHa was continued at the same dose until HCG administration except in five studies that reduced the dose at confirmation of down‐regulation: reduced from 1000 µg to 600 µg (Dirnfeld 1991), reduced from 1 mg to 0.5 mg/day (San Roman 1992), while Weissman 2003 and Chatillon‐Boissier 2012 halved the agonist dose, and Sunkara 2014 continued with a reduced dose of nafarelin 200 mg twice daily until the administration of HCG injection.
Similarly, the dose of GnRHa for short protocols varied. Weissman 2003 applied a modified short protocol using the flare effect initially (500 μg/day for the initial four days followed by 100 μg until the day of HCG). Yang 1996 used another modification of the short protocol where GnRHa was stopped after seven days.
Dose, regimen, and drugs used for stimulation also varied in all studies as did the inclusion criteria of the population studied (please see the 'Characteristics of included studies' tables).
2. Long versus ultrashort protocol
Of the two studies included in this comparison, Kingsland 1992 used 200 µg daily of buserelin whereas Chen 1992 used 1 mg daily of subcutaneous decapeptyl for the long protocol. Both studies discontinued GnRHa after confirmation of down‐regulation.
The dose of GnRHa for the ultrashort protocol was different as well. Chen 1992 used leuprolide acetate 1 mg daily whereas Kingsland 1992 used 500 μg/day of buserelin on days two, three, and four of the cycle.
Chen 1992 used follicle‐stimulating hormone (FSH) + human menopausal gonadotrophin (HMG) for stimulation whereas Kingsland 1992 used HMG alone
3. Short versus ultrashort protocol
Participants were randomised into two groups.
The participants in the ultrashort gonadotrophin‐releasing hormone (GnRH) agonist/GnRH antagonist group (n = 41) were administered leuprolide acetate at 40 microg subcutaneously/twice daily, started on day two of menses and continued for three consecutive days, followed by gonadotrophins, and GnRH antagonist cetrorelix at 0.25 mg/day when the leading follicle was more than 14 mm, which was continued up to HCG injection.
The participants in the microdose group (n = 41) started to use leuprolide acetate at 40 microg subcutaneously/twice daily on day two of menses, and two days after initiation of GnRHa, gonadotropin stimulation was initiated and continued until HCG day.
The starting dose of recombinant FSH depended on age, body mass index (BMI), and ovarian response to the previous cycle and increased to a maximum of 450 IU/day depending on the ovarian response; it was then individualised after day five (Berker 2010).
4. Long protocol: luteal versus follicular start of GnRHa
Three studies out of five included in this comparison used the same dose of GnRHa for down‐regulation (1200 μg/day), Kondaveeti‐Gordon 1996; Urbancsek 1996, and 0.1 mg of triptorelin subcutaneously daily (Sarhan 2013). Ron‐El 1990 used a long‐acting preparation (3.2 mg decapeptyl) whereas Pellicer 1989 used 600 μg/day buserelin in two divided doses. In Pellicer 1989, the day for luteal start varied, ranging from four to 10 days after ovulation compared with the day 21 to 22 start in the other included studies. This might have had some impact on the outcomes of the luteal phase results. Urbancsek 1996 considered more than one cycle per woman whereas the remaining four studies evaluated only the first cycle. All studies except Pellicer 1989 and Sarhan 2013 used HMG for ovarian stimulation; the former used HMG + FSH, and the latter administered either HMG or FSH.
5. Long protocol: continuation of GnRHa versus stopping GnRHa at start of stimulation
Of the three studies included in this comparison, one used buserelin (1000 μg/day) (Dirnfeld 1999), one used leuprolide acetate (1 mg/day) (Garcia‐Velasco 2000), and the third used triptorelin (0.1 mg/day) (Simons 2005) for down‐regulation. All of the studies stopped GnRHa at confirmation of down‐regulation in the test arm.
Apart from one study (Garcia‐Velasco 2000), which used FSH + HMG, all used HMG alone for stimulation.
6. Long protocol: continuation of same‐dose GnRHa versus reduced‐dose GnRHa until HCG administration
For the four studies in this comparison, there was a variation in the type and dose of GnRHa and the reduction in dose after down‐regulation was confirmed: luteinising hormone‐releasing hormone agonist (LHRHa) commenced at 0.5 mg/day and reduced to 0.1 mg/day (Simon 1994); triptorelin acetate commenced at 0.1 mg/day and reduced to 0.05 mg/day (Fábregues 2005); GnRHa commenced at 100 μg/day and reduced to 50 μg/day (Dal Prato 2001); and triptorelin was initiated during the luteal phase, 0.1 mg/day for 10 days followed by 0.05 mg/day until the concentration of serum oestradiol was </= 40 pg/ml, then the stimulation of the ovaries started and when the diameter of one or more follicles was 14 mm, triptorelin (0.05 mg/day) was withdrawn for two (15/47) or three (32/47) days (Ding 2013).
The stimulation drug varied amongst the studies. Simon 1994 used HMG, Fábregues 2005 and Ding 2013 used recombinant FSH, while Dal Prato 2001 used metrodin.
7. Long protocol: discontinuing versus continuing GnRHa after HCG administration
GnRHa was administered from the 21st day of the preceding cycle. Participants were divided into two groups: (1) (n = 90 participants) participants were continuously administered GnRHa for 12 days after embryo transfer; (2) (n = 91 participants) GnRHa was stopped on the day of HCG administration.
8. Long protocol: administration of GnRHa for two versus three weeks before stimulation
In both groups, a single dose of long‐acting GnRHa (Diphereline®, 1.25 mg) was administered in the mid‐luteal phase. Participants were divided into two groups according to the initiation of gonadotrophins (14 or 21 days after GnRHa administration). Either recombinant follicle‐stimulating hormone (rFSH) or HMG was used for ovarian stimulation.
9. Short protocol: continuation versus stopping GnRHa
There was only one study in this comparison. A short protocol was compared with stopping GnRHa halfway through stimulation rather than continuing until the day of HCG.
Outcomes
Nineteen studies reported either live birth rate or ongoing pregnancy rate (Acharya 1992; Chatillon‐Boissier 2012; Ding 2013; Dirnfeld 1991; Dirnfeld 1999; Foulot 1988; Frydman 1988; Isikoglu 2007; Kingsland 1992; Lin 2013; Loumaye 1989; San Roman 1992; Simons 2005; Sunkara 2014; Urbancsek 1996; van de‐Helder 1990; Yang 1996; Ye 2001; Zhang 2009). With regard to adverse outcomes, 22 studies reported cycle cancellation rate (Acharya 1992; Berker 2010; Cedrin‐Durnerin 2000; Chatillon‐Boissier 2012; Dal Prato 2001; Ding 2013; Dirnfeld 1991; Dirnfeld 1999; Foulot 1988; Frydman 1988; Garcia‐Velasco 2000; Hazout 1993; Isikoglu 2007; Kingsland 1992; Kondaveeti‐Gordon 1996; San Roman 1992; Sarhan 2013; Simons 2005; Sunkara 2014; van de‐Helder 1990; Weissman 2003; Zhang 2009), while two trials reported ovarian hyperstimulation syndrome (OHSS), Ding 2013; Lin 2013, and one study reported miscarriage rate (Lin 2013).
1. Long versus short protocol
The outcomes reported in this comparison group were as follows: live birth/ongoing pregnancies in 12 studies (Acharya 1992; Chatillon‐Boissier 2012; Dirnfeld 1991; Foulot 1988; Frydman 1988; Loumaye 1989; San Roman 1992; Sunkara 2014; van de‐Helder 1990; Yang 1996; Ye 2001; Zhang 2009), clinical pregnancies in 19 studies (Acharya 1992; Chatillon‐Boissier 2012; De Placido 1991; Dirnfeld 1991; Fenichel 1988; Foulot 1988; Frydman 1988; Hazout 1993; Hedon 1988; Loumaye 1989; San Roman 1992; Sunkara 2014; Tan 1992; Tasdemir 1995; van de‐Helder 1990; Weissman 2003; Yang 1996; Ye 2001; Zhang 2009), number of oocytes in 10 studies (Chatillon‐Boissier 2012; Dirnfeld 1991; Hazout 1993; Loumaye 1989; San Roman 1992; Sunkara 2014; Weissman 2003; Yang 1996; Ye 2001; Zhang 2009), dose of gonadotrophins in eight studies (Chatillon‐Boissier 2012; Dirnfeld 1991; Hazout 1993; Sunkara 2014; Weissman 2003; Yang 1996; Ye 2001; Zhang 2009), cycle cancellation in 11 studies (Acharya 1992; Chatillon‐Boissier 2012; Dirnfeld 1991; Foulot 1988; Frydman 1988; Hazout 1993; San Roman 1992; Sunkara 2014; van de‐Helder 1990; Weissman 2003; Zhang 2009), and other outcomes in none of the included studies.
2. Long versus ultrashort protocol
The outcomes reported in this comparison group were as follows: live birth/ongoing pregnancies in one study (Kingsland 1992), clinical pregnancies in two studies (Chen 1992; Kingsland 1992), number of oocytes in two studies (Chen 1992; Kingsland 1992), dose of gonadotrophins in one study (Chen 1992), cycle cancellation in one study (Kingsland 1992), and other outcomes in none of the included studies.
3. Short versus ultrashort protocol
The outcomes reported in this comparison group were as follows: live birth/ongoing pregnancies in none of the included studies, clinical pregnancies in one study (Berker 2010), number of oocytes in one study (Berker 2010), dose of gonadotrophins in one study (Berker 2010), cycle cancellation in one study (Berker 2010), and other outcomes in none of the included studies.
4. Long protocol: luteal versus follicular start of GnRHa
The outcomes reported in this comparison group were as follows: live birth/ongoing pregnancies in one study (Urbancsek 1996), clinical pregnancies in five studies (Kondaveeti‐Gordon 1996; Pellicer 1989; Ron‐El 1990; Sarhan 2013; Urbancsek 1996), number of oocytes in four studies (Kondaveeti‐Gordon 1996; Pellicer 1989; Ron‐El 1990; Sarhan 2013), dose of gonadotrophins in four studies (Kondaveeti‐Gordon 1996; Pellicer 1989; Ron‐El 1990; Sarhan 2013), cycle cancellation in two studies (Kondaveeti‐Gordon 1996; Sarhan 2013), and other outcomes in none of the included studies.
5. Long protocol: continuation of GnRHa versus stopping GnRHa at start of stimulation
The outcomes reported in this comparison group were as follows: live birth/ongoing pregnancies in three studies (Ding 2013; Dirnfeld 1999; Simons 2005), clinical pregnancies in four studies (Ding 2013; Dirnfeld 1999; Garcia‐Velasco 2000; Simons 2005), number of oocytes in four studies (Ding 2013; Dirnfeld 1999; Garcia‐Velasco 2000; Simons 2005), dose of gonadotrophins in four studies (Ding 2013; Dirnfeld 1999; Garcia‐Velasco 2000; Simons 2005), cycle cancellation in three studies (Dirnfeld 1999; Garcia‐Velasco 2000; Simons 2005), and other outcomes (OHSS) in one study (Ding 2013).
6. Long protocol: continuation of same‐dose GnRHa versus reduced‐dose GnRHa until HCG administration
The outcomes reported in this comparison group were as follows: live birth/ongoing pregnancies in none of the included studies, clinical pregnancies in four studies (Dal Prato 2001; Ding 2013; Fábregues 2005; Simon 1994), number of oocytes in three studies (Ding 2013; Fábregues 2005; Simon 1994) dose of gonadotrophins in two studies (Dal Prato 2001; Ding 2013), cycle cancellation in two studies (Dal Prato 2001; Ding 2013), and other outcomes in none of the included studies.
7. Long protocol: discontinuing versus continuing GnRHa after HCG administration
The outcomes reported in this comparison group were as follows: live birth/ongoing pregnancies in one study (Isikoglu 2007), clinical pregnancies in one study (Isikoglu 2007), number of oocytes in one study (Isikoglu 2007), dose of gonadotrophins in one study (Isikoglu 2007), cycle cancellation in one study (Isikoglu 2007), and other outcomes in none of the included studies.
8. Long protocol: administration of GnRHa for two versus three weeks before stimulation
The outcomes reported in this comparison group were as follows: live birth/ongoing pregnancies in one study (Lin 2013), clinical pregnancies in one study (Lin 2013), number of oocytes in one study (Lin 2013) and dose of gonadotrophins in one study (Lin 2013). None of the included studies reported cycle cancellation or other outcomes of interest.
9. Short protocol: continuation versus stopping GnRHa
The outcomes reported in this comparison group were as follows: live birth/ongoing pregnancies rate in none of the included studies, clinical pregnancies in one study (Cedrin‐Durnerin 2000), number of oocytes in none of the included studies, dose of gonadotrophins in one study (Cedrin‐Durnerin 2000), cycle cancellation in one study (Cedrin‐Durnerin 2000), and other outcomes in none of the included studies.
For the characteristics of included studies, see the 'Characteristics of included studies' tables.
Excluded studies
A list of the 63 excluded studies is provided in a table, along with the reasons for exclusion (please see the 'Characteristics of excluded studies' tables).
Risk of bias in included studies
A complete overview of our classification of risk of bias domains can be found in the 'Characteristics of included studies' tables. The following is a summary of methods, participants, and interventions in the included studies for the various comparisons. See Figure 2 and Figure 3.
Allocation
Random sequence generation
Adequate sequence generation was present in 22 out of 37 included studies, which we considered as at low risk of selection bias. For the remaining 15 studies, there was no clear mention of the method of randomisation (Acharya 1992; Chen 1992; De Placido 1991; Dirnfeld 1999; Frydman 1988; Hedon 1988; Loumaye 1989; Pellicer 1989; Ron‐El 1990; San Roman 1992; Tasdemir 1995; Urbancsek 1996; van de‐Helder 1990; Yang 1996; Zhang 2009), so we judged them to be at unclear risk of bias. We rated 22 studies as low risk of this bias, no studies as high risk, and 16 studies as at unclear risk.
Allocation concealment
Eight studies used adequate methods for concealment of the random sequence, using sealed envelopes, and we judged these to be at low risk of selection bias (Berker 2010; Dal Prato 2001; Ding 2013; Fábregues 2005; Kingsland 1992; Simons 2005; Sunkara 2014; Tan 1992). Twenty‐three studies did not report an attempt to conceal the allocation; we judged these to be at unclear risk of bias. We rated six studies as high risk as the authors reported no concealment of allocation (Dirnfeld 1991; Fenichel 1988; Hazout 1993; Kondaveeti‐Gordon 1996; San Roman 1992; Ye 2001) (Figure 2; Figure 3).
Blinding
Although our outcomes of interest were objective, we believe that blinding of clinicians and participants is important in order to avoid performance and detection biases. Blinding the clinician or participants was not a feature in 26 studies included in the review. We judged only two studies as low risk (Simons 2005; Sunkara 2014). We rated nine studies as "unclear" concerning risk of bias, as there were no data regarding blinding (Berker 2010; Dal Prato 2001; De Placido 1991; Ding 2013; Foulot 1988; Garcia‐Velasco 2000; Sarhan 2013; Yang 1996; Zhang 2009).
We rated two studies as at low risk of bias, 26 studies as at high risk, and nine studies as at unclear risk regarding blinding.
Incomplete outcome data
We rated eight out of 37 studies as at high risk of attrition bias (Ding 2013; Dirnfeld 1999; Fábregues 2005; Hazout 1993; Hedon 1988; Simon 1994; Tasdemir 1995; Urbancsek 1996), four out of 37 studies as at unclear risk of attrition bias (Chen 1992; De Placido 1991; Kondaveeti‐Gordon 1996; Pellicer 1989), and the rest of them as at low risk of attrition bias (Acharya 1992; Berker 2010; Cedrin‐Durnerin 2000; Chatillon‐Boissier 2012; Dal Prato 2001; Dirnfeld 1991; Fenichel 1988; Foulot 1988; Frydman 1988; Garcia‐Velasco 2000; Isikoglu 2007; Kingsland 1992; Lin 2013; Loumaye 1989; San Roman 1992; Sarhan 2013; Simons 2005; Sunkara 2014; Tan 1992; van de‐Helder 1990; Weissman 2003; Yang 1996; Ye 2001; Zhang 2009).
We rated 25 studies as at low risk of attrition bias, five studies as at unclear risk, and eight studies as at high risk.
Selective reporting
Eighteen studies reported at least one of the two primary outcomes: live birth or ongoing pregnancy rate. We judged these to be at low risk of reporting bias (Berker 2010; Chatillon‐Boissier 2012; Ding 2013; Dirnfeld 1991; Foulot 1988; Frydman 1988; Hedon 1988; Isikoglu 2007; Kingsland 1992; Lin 2013; Loumaye 1989; San Roman 1992; Simons 2005; Sunkara 2014; Tasdemir 1995; Urbancsek 1996; van de‐Helder 1990; Weissman 2003). Eighteen trials failed to report either of the two primary outcomes for this review, so we judged these to be at unclear risk of reporting bias. We judged one trial to be at high risk because it reported only clinical pregnancy, without reporting any other outcomes (De Placido 1991).
We rated 18 studies as at low risk of bias, one study as at high risk, and 18 studies as at unclear risk regarding selective reporting.
Other potential sources of bias
In the majority of included studies (23 studies), there was insufficient information to assess whether an important risk of bias existed. We judged five trials as high risk for different reasons (Cedrin‐Durnerin 2000; De Placido 1991; Dirnfeld 1991; Pellicer 1989; Tasdemir 1995). In one trial, the median number of embryos transferred was significantly different between the intervention and the control group. Besides, there was no mention of the exact number of participants in each group (Tasdemir 1995). In one study, the intervention and the control group commenced GnRHa on different days (Pellicer 1989). In one study, the long GnRH protocol was commenced in either the luteal or follicular phase (Dirnfeld 1991). In two trials, data regarding the number of participants and other inclusion criteria were lacking (De Placido 1991). One trial excluded an important group of IVF participants (participants with chronic anovulation) from participation and used two variants of short protocol (Cedrin‐Durnerin 2000). We judged the rest of the trials (nine trials) as at low risk for other potential sources of bias.
We rated nine studies as at low risk of bias, five studies as at high risk, and 23 studies as at unclear risk in this domain.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6; Table 7; Table 8; Table 9
1. Long versus short protocol
We included 20 studies in this comparison (Table 1).
Primary outcome measure
1.1 Live birth and ongoing pregnancy rates
There was no evidence of a difference in live birth and ongoing pregnancy rates between the two protocols (odds ratio (OR) 1.30, 95% confidence interval (CI) 0.94 to 1.81; 12 RCTs, n = 976 women, I² = 15%, low quality evidence) (Analysis 1.1; Figure 5; Table 1). Analyses 1.1.1 and 1.1.2 present separately the differences in live and ongoing pregnancy rates. A sensitivity analysis including only studies with adequate randomisation and complete outcome data reporting included five studies (Chatillon‐Boissier 2012; Dirnfeld 1991; Foulot 1988; Sunkara 2014; Ye 2001): there was no evidence of a difference in live birth and ongoing pregnancy rates between the two protocols (OR 1.45, 95% CI 0.83 to 2.52; five RCTs, n = 481 women, I² = 0%, moderate quality evidence).
1.1. Analysis.
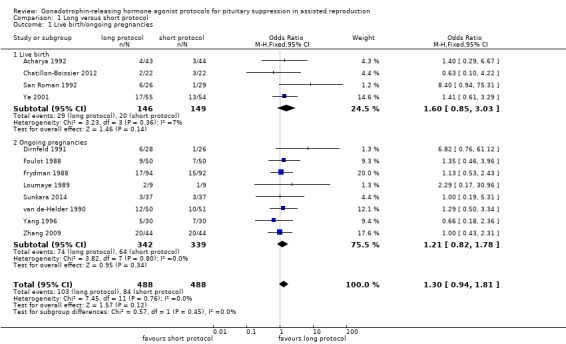
Comparison 1 Long versus short protocol, Outcome 1 Live birth/ongoing pregnancies.
5.
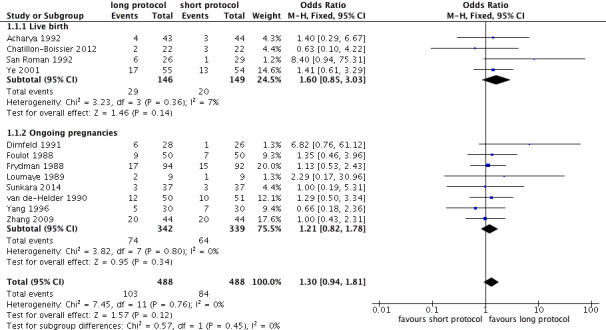
Forest plot of comparison: 1 Long versus short protocol, outcome: 1.1 Live birth/ongoing pregnancies.
Secondary outcomes
1.2 Clinical pregnancy rate
There was evidence of an increase in clinical pregnancy rate (OR 1.50, 95% CI 1.18 to 1.92; 20 RCTs, n = 1643 women, I² = 27%, moderate quality evidence) in the long protocol group when compared with the short protocol group (Analysis 1.2; Figure 6). The subgroup of studies including poor responders only also showed a difference in clinical pregnancy rates (OR 3.12, 95% CI 1.39 to 7.02; four RCTs, n = 232 women, I² = 0%, moderate quality evidence), favouring the long protocol (Analysis 1.2; Figure 6).
1.2. Analysis.
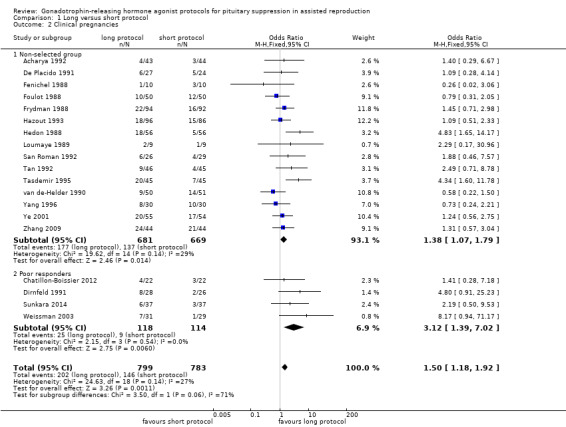
Comparison 1 Long versus short protocol, Outcome 2 Clinical pregnancies.
6.
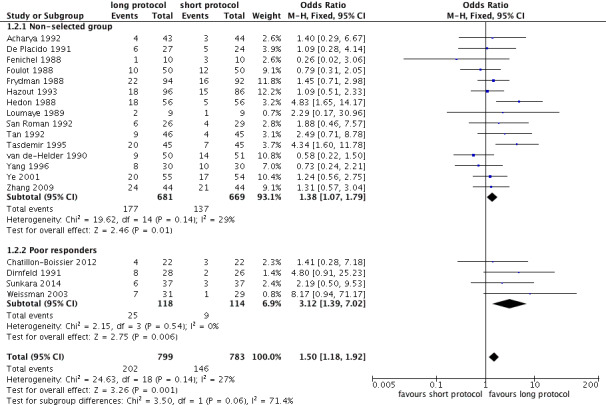
Forest plot of comparison: 1 Long versus short protocol, outcome: 1.2 Clinical pregnancies.
1.3 Number of oocytes
Due to the high heterogeneity of the pooled analysis (10 RCTs, n = 789 women, I² = 91%), we did not pool data. The heterogeneity was among the six studies of unselected women. Of these studies, two showed a significant difference in favour of the long protocol. Subgroup analysis of the four studies including poor responders showed evidence of an increase in the number of oocytes in the long protocol compared with the short protocol (mean difference (MD) 1.40, 95% CI 0.75 to 2.06; four RCTs, n = 227 women, I² = 0%, low quality evidence) (Analysis 1.3).
1.3. Analysis.
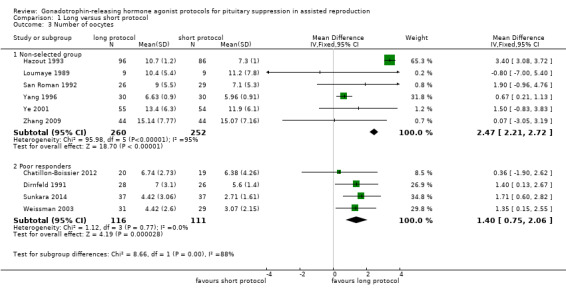
Comparison 1 Long versus short protocol, Outcome 3 Number of oocytes.
1.4 Number of ampoules of gonadotrophins
Due to the high heterogeneity of the pooled analysis (eight RCTs, n = 666 women, I² = 94%), we did not pool data. The heterogeneity was among the four studies of unselected women. All of these studies showed a significant difference in favour of the long protocol. Subgroup analysis of the studies including poor responders showed evidence of a substantial increase in the requirement of gonadotrophins in a long protocol compared with a short protocol (MD 7.07, 95% CI 3.06 to 11.08; four RCTs, n = 227 women, I² = 0%, low quality evidence) (Analysis 1.4).
1.4. Analysis.
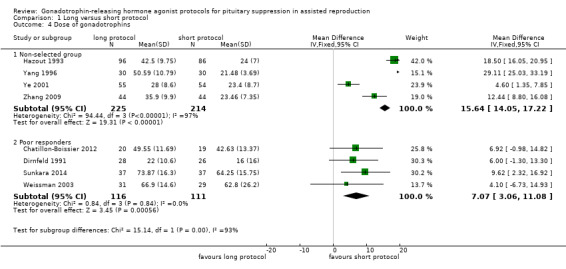
Comparison 1 Long versus short protocol, Outcome 4 Dose of gonadotrophins.
1.5 Cycle cancellation rate
There was no evidence of a difference between the groups in the cycle cancellation rate (OR 0.95, 95% CI 0.59 to 1.55; 11 RCTs, n = 1026 women, I² = 42%, low quality evidence) (Analysis 1.5). Subgroup analysis of the four studies including poor responders showed evidence of fewer cancellations in the long protocol compared with the short protocol (OR 0.31, 95% CI 0.12 to 0.76; four RCTs, n = 227 women, I² = 0%, low quality evidence) (Analysis 1.5).
1.5. Analysis.
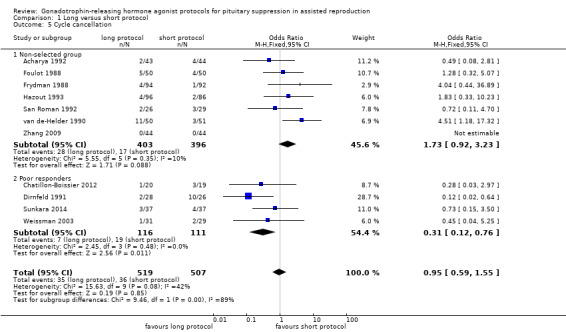
Comparison 1 Long versus short protocol, Outcome 5 Cycle cancellation.
1.6 Other outcomes
There were no studies reporting on other adverse outcomes, cost effectiveness, or acceptability of these drugs.
2. Long versus ultrashort protocol
We included two studies in this comparison (Table 2).
Primary outcome measure
2.1 Live birth and ongoing pregnancy rates
There was no evidence of a difference in live birth and ongoing pregnancy rates when a long protocol was compared with an ultrashort protocol (OR 1.78, 95% CI 0.72 to 4.36; one RCT, n = 150 women, low quality evidence) (Analysis 2.1).
2.1. Analysis.
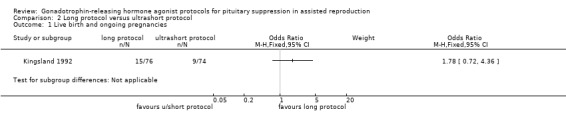
Comparison 2 Long protocol versus ultrashort protocol, Outcome 1 Live birth and ongoing pregnancies.
Secondary outcomes
2.2 Clinical pregnancy rate
There was no evidence of a difference in the clinical pregnancy rate when a long protocol was compared with an ultrashort protocol (OR 1.56, 95% CI 0.80 to 3.06; two RCTs, n = 230 women, I² = 67%, low quality evidence) (Analysis 2.2).
2.2. Analysis.
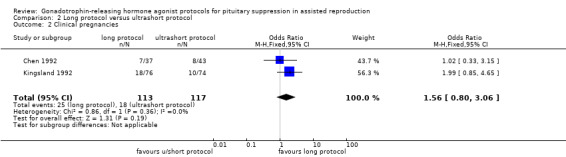
Comparison 2 Long protocol versus ultrashort protocol, Outcome 2 Clinical pregnancies.
2.3 Number of oocytes retrieved
There was no evidence of a difference in the number of oocytes recovered when a long protocol was compared with an ultrashort protocol (MD 0.53, 95% CI ‐0.61 to 1.66; two RCTs, n = 230 women, I² = 67%, low quality evidence) (Analysis 2.3).
2.3. Analysis.
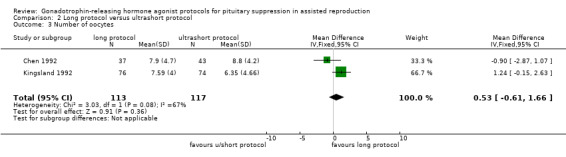
Comparison 2 Long protocol versus ultrashort protocol, Outcome 3 Number of oocytes.
2.4 Number of ampoules of gonadotrophins
There was no evidence of a difference in the ampoules of gonadotrophins used when a long protocol was compared with an ultrashort protocol (MD 1.10, 95% CI ‐1.81 to 4.01; one RCT, n = 80 women, low quality evidence) (Analysis 2.4).
2.4. Analysis.
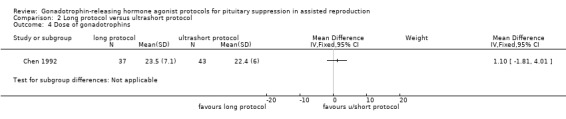
Comparison 2 Long protocol versus ultrashort protocol, Outcome 4 Dose of gonadotrophins.
2.5 Cycle cancellation
There was no evidence of a difference in the cycle cancellation rate when a long protocol was compared with a short protocol (OR 1.11, 95% CI 0.40 to 3.05; one RCT, n = 150 women, low quality evidence) (Analysis 2.5).
2.5. Analysis.
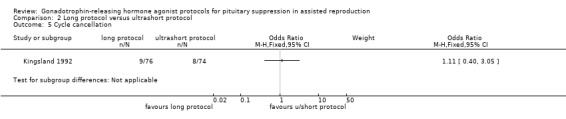
Comparison 2 Long protocol versus ultrashort protocol, Outcome 5 Cycle cancellation.
2.6 Other outcomes
There were no studies reporting on other adverse effects, cost effectiveness, and acceptability of either of these protocols.
Neither sensitivity nor subgroup analysis was done because of the low number of studies reporting on this comparison.
3. Short versus ultrashort protocol
We found only one study for this comparison (Table 3).
Primary outcome measure
3.1 Live birth and ongoing pregnancy rates
There were no studies reporting on this outcome.
Secondary outcome measures
3.2 Clinical pregnancy rate
There was no evidence of a difference in the clinical pregnancy rate when a short protocol was compared with an ultrashort protocol (OR 1.33, 95% CI 0.47 to 3.81; one RCT, n = 82 women, very low quality evidence) (Analysis 3.6).
3.3 Number of oocytes
There was no evidence of a difference in the number of oocytes recovered when a short protocol was compared with an ultrashort protocol (MD 0.70, 95% CI ‐1.83 to 3.23; one RCT, n = 82 women, very low quality evidence) (Analysis 3.7).
3.4 Number of ampoules of gonadotrophins
There was evidence of a difference in the ampoules of gonadotrophins used when a short protocol was compared with an ultrashort protocol (MD ‐13.85, 95% CI ‐21.49 to ‐6.21; one RCT, n = 82 women, very low quality evidence) (Analysis 3.8). Fewer ampoules were used in the short protocol group.
3.5 Cycle cancellation
There was no evidence of a difference in the cycle cancellation rate when a short protocol was used when compared with an ultrashort (OR 1.00, 95% CI 0.13 to 7.46; one RCT, n = 82 women, very low quality evidence) (Analysis 3.9).
3.6 Other outcomes
There were no studies reporting on other adverse effects, cost effectiveness, and acceptability of either of these protocols.
Neither sensitivity nor subgroup analysis was done because of the low number of studies reporting on this comparison.
4. Long lutealversus long follicular phase protocol
We included five studies in this comparison (Table 4).
Primary outcome measure
4.1 Live birth and ongoing pregnancy rates
There was no evidence of a difference in live birth and ongoing pregnancy rates when GnRHa was commenced in the luteal or follicular phase for the long protocol (OR 1.89, 95% CI 0.87 to 4.10; one RCT, n = 223 women, low quality evidence) (Analysis 4.1).
4.1. Analysis.
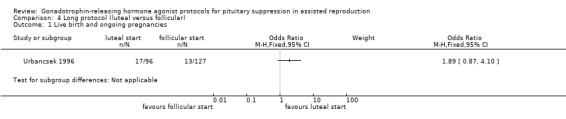
Comparison 4 Long protocol (luteal versus follicular), Outcome 1 Live birth and ongoing pregnancies.
Secondary outcome measures
4.2 Clinical pregnancy rate
There was no evidence of a difference in the pregnancy rate in the luteal start of GnRHa when compared with the follicular start (OR 1.06, 95% CI 0.76 to 1.47; five RCTs, n = 750 women, I² = 52%, low quality evidence) (Analysis 4.2).
4.2. Analysis.
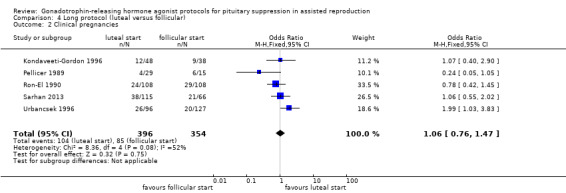
Comparison 4 Long protocol (luteal versus follicular), Outcome 2 Clinical pregnancies.
4.3 Number of oocytes retrieved
There was no evidence of a difference between the groups in the number of oocytes retrieved (MD ‐1.29, 95% CI ‐1.85 to 0.71; four RCTS, n = 527 women, I² = 74%, low quality evidence) (Analysis 4.3).
4.3. Analysis.
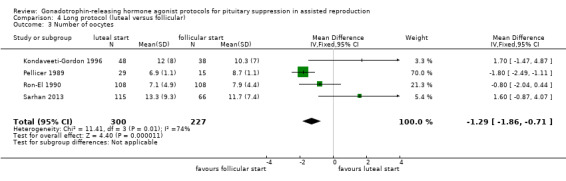
Comparison 4 Long protocol (luteal versus follicular), Outcome 3 Number of oocytes.
4.4 Number of ampoules of gonadotrophins
There was no evidence of a difference in the amounts of gonadotrophins required in luteal start when compared with follicular start in long protocols (MD 1.12, 95% CI ‐0.73 to 2.97; four RCTs, n = 527 women, I² = 51%, low quality evidence) (Analysis 4.4).
4.4. Analysis.
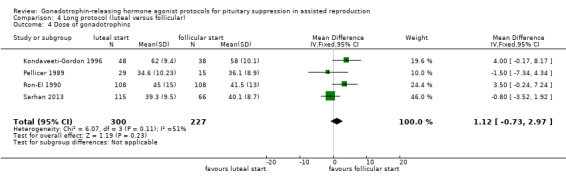
Comparison 4 Long protocol (luteal versus follicular), Outcome 4 Dose of gonadotrophins.
4.5 Cycle cancellation
There was no evidence of a difference in cycle cancellation rates in the luteal or follicular start of GnRHa groups (OR 1.45, 95% CI 0.35 to 6.01; two RCTs, n = 267 women, I² = 0%, low quality evidence) (Analysis 4.5).
4.5. Analysis.
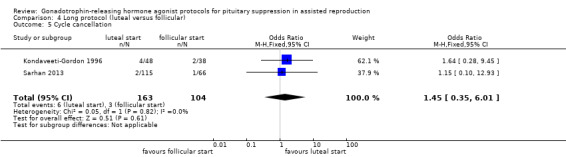
Comparison 4 Long protocol (luteal versus follicular), Outcome 5 Cycle cancellation.
4.6 Other outcomes
There were no studies reporting on other adverse effects, cost effectiveness, and acceptability of either of these protocols.
Neither sensitivity nor subgroup analysis was done because of the low number of studies reporting on this comparison for the primary outcome.
5. Long protocol (continue GnRHa versus stop GnRHa)
We included four studies in this comparison (Table 5).
Primary outcome measure
5.1 Live birth and ongoing pregnancy rates
There was no evidence of a difference in the number of live birth and ongoing pregnancies when GnRHa was stopped compared with when it was continued (OR 0.75, 95% CI 0.42 to 1.33; three RCTs, n = 290 women, I² = 0%, low quality evidence) (Analysis 5.1; Figure 7).
5.1. Analysis.
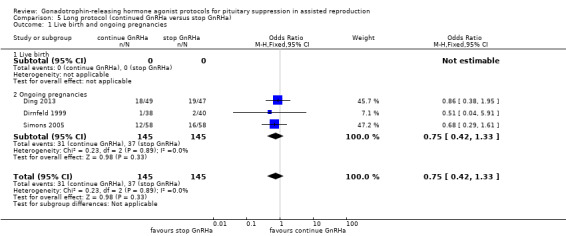
Comparison 5 Long protocol (continued GnRHa versus stop GnRHa), Outcome 1 Live birth and ongoing pregnancies.
7.
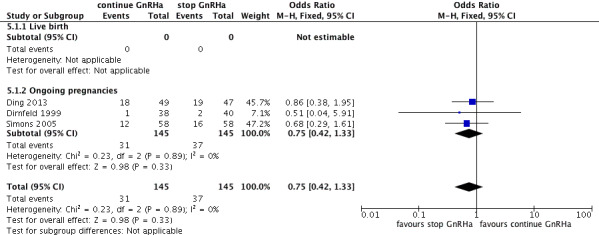
Forest plot of comparison: 5 Long protocol (continued GnRHa versus stop GnRHa), outcome: 5.1 Live birth and ongoing pregnancies.
Secondary outcomes
5.2 Clinical pregnancies
There was no evidence of a difference in the clinical pregnancy rate whether GnRHa was continued or stopped (OR 0.85, 95% CI 0.51 to 1.41; four RCTs, n = 360 women, I² = 0%, low quality evidence) (Analysis 5.2).
5.2. Analysis.
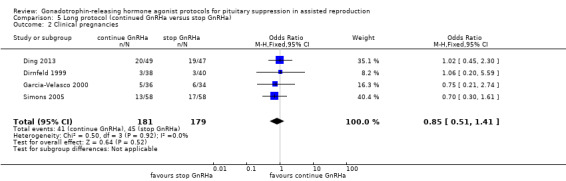
Comparison 5 Long protocol (continued GnRHa versus stop GnRHa), Outcome 2 Clinical pregnancies.
5.3 Number of oocytes
There was no evidence of a difference in the number of oocytes retrieved when GnRHa was continued compared with when it was stopped (MD ‐0.26, 95% CI ‐1.29 to 0.78; four RCTs, n = 360 women, I² = 73%, low quality evidence) (Analysis 5.3).
5.3. Analysis.
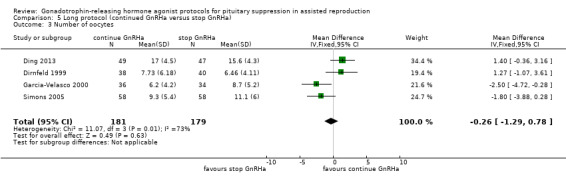
Comparison 5 Long protocol (continued GnRHa versus stop GnRHa), Outcome 3 Number of oocytes.
5.4 Number of ampoules of gonadotrophins
There was no evidence of a difference in the amount of gonadotrophins required in the two groups (MD ‐0.14, 95% CI ‐2.35 to 2.08; four RCTs, n = 360 women, I² = 65%, low quality evidence) (Analysis 5.4).
5.4. Analysis.
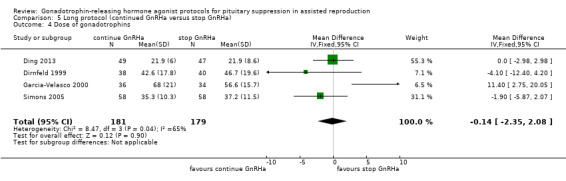
Comparison 5 Long protocol (continued GnRHa versus stop GnRHa), Outcome 4 Dose of gonadotrophins.
5.5 Cycle cancellation rate
There was no evidence of a difference in the cycle cancellation rate when GnRHa was stopped compared with when it was continued (OR 1.47, 95% CI 0.04 to 5.35; three RCTs, n = 264 women, I² = 69%, low quality evidence) (Analysis 5.5).
5.5. Analysis.
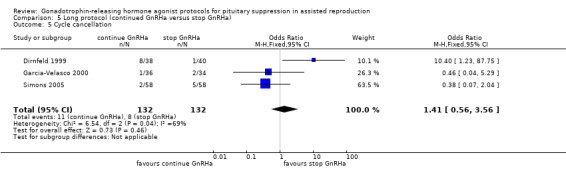
Comparison 5 Long protocol (continued GnRHa versus stop GnRHa), Outcome 5 Cycle cancellation.
5.6 Other outcomes
Neither sensitivity nor subgroup analysis was done because of the low number of studies reporting on this comparison.
There were no studies reporting on other adverse effects, cost effectiveness, and acceptability of either of these protocols, apart from the OHSS rate (Ding 2013).
There was no evidence of a difference in rate of OHSS between the two groups compared (OR 0.47, 95% CI 0.04 to 5.35; one RCT, n = 96 women, low quality evidence) (Analysis 5.6).
5.6. Analysis.
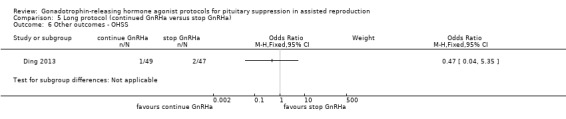
Comparison 5 Long protocol (continued GnRHa versus stop GnRHa), Outcome 6 Other outcomes ‐ OHSS.
6. Long protocol (continued same‐dose GnRHa versus reduced‐dose GnRHa)
We included four RCTs in this comparison (Table 6).
Primary outcome measure
6.1 Live birth and ongoing pregnancy rates
No study reported on this outcome.
6.2 Clinical pregnancy rate
There was no evidence of a difference in the pregnancy rate when the dose of GnRHa was reduced compared with when the same dose was continued (OR 1.02, 95% CI 0.68 to1.52; four RCTs, n = 407 women, I² = 0%, low quality evidence) (Analysis 6.2).
6.2. Analysis.
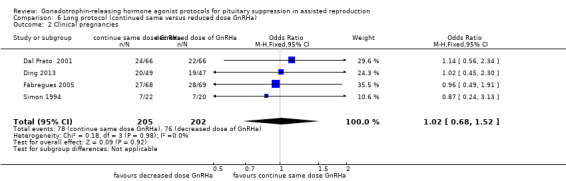
Comparison 6 Long protocol (continued same versus reduced dose GnRHa), Outcome 2 Clinical pregnancies.
6.3 Number of oocytes
There was no evidence of a difference in the number of oocytes retrieved between groups (MD 1.03, 95% CI ‐0.04 to 2.10; three RCTs, n = 275 women, I² = 0%, low quality evidence) (Analysis 6.3).
6.3. Analysis.
Comparison 6 Long protocol (continued same versus reduced dose GnRHa), Outcome 3 Number of oocytes.
6.4 Number of ampoules of gonadotrophins
There was no evidence of a difference in the number of ampoules of gonadotrophins required between the compared groups (MD 0.98, 95% CI ‐1.72 to 3.69; two RCTs, n = 228 women, I² = 58%, low quality evidence) (Analysis 6.4).
6.4. Analysis.
Comparison 6 Long protocol (continued same versus reduced dose GnRHa), Outcome 4 Dose of gonadotrophins.
6.5 Cycle cancellation rate
There was no evidence of a difference in the cycle cancellation rate for the two groups (OR 1.00, 95% CI 0.14 to 7.32; two RCTs, n = 228 women, I² = not applicable, low quality evidence) (Analysis 6.5).
6.5. Analysis.
Comparison 6 Long protocol (continued same versus reduced dose GnRHa), Outcome 5 Cycle cancellation.
6.7 Other outcomes
There were no studies reporting on other adverse effects, cost effectiveness, and acceptability of either of these protocols.
Neither sensitivity nor subgroup analysis was done because of the lack of studies reporting on this comparison and addressing the primary outcome.
7. Long protocol: discontinuing versus continuing GnRHa after HCG administration
We included only one study in this comparison (Table 7).
Primary outcome measure
7.1 Live birth and ongoing pregnancy rates
There was no evidence of a difference in live birth and ongoing pregnancy rates in this comparison (OR 0.89, 95% CI 0.49 to 1.64; one RCT, n = 181 women, low quality evidence) (Analysis 7.1).
7.1. Analysis.
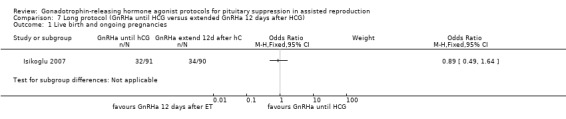
Comparison 7 Long protocol (GnRHa until HCG versus extended GnRHa 12 days after HCG), Outcome 1 Live birth and ongoing pregnancies.
Secondary outcome measures
7.2 Clinical pregnancy rate
There was no evidence of a difference in the clinical pregnancy rate when discontinuing versus continuing GnRHa after HCG administration (OR 1.02, 95% CI 0.57 to 1.83; one RCT, n = 181 women, low quality evidence) (Analysis 7.2).
7.2. Analysis.
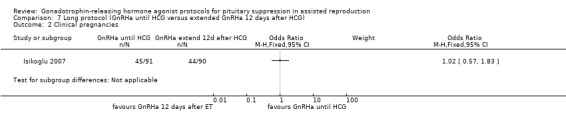
Comparison 7 Long protocol (GnRHa until HCG versus extended GnRHa 12 days after HCG), Outcome 2 Clinical pregnancies.
7.3 Number of oocytes retrieved
There was no evidence of a difference between the two compared groups (MD ‐0.90, ‐3.04 to 1.24; one RCT, n = 181 women, low quality evidence) (Analysis 7.3).
7.3. Analysis.
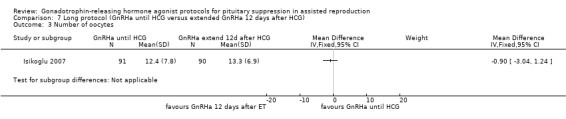
Comparison 7 Long protocol (GnRHa until HCG versus extended GnRHa 12 days after HCG), Outcome 3 Number of oocytes.
7.4 Number of ampoules of gonadotrophins
There was no evidence of a difference in the requirement for gonadotrophins between the two compared groups (MD 2.80, ‐0.55 to 6.15; one RCT, n = 181 women, low quality evidence) (Analysis 7.4).
7.4. Analysis.
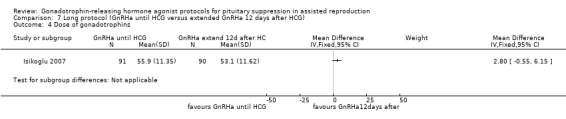
Comparison 7 Long protocol (GnRHa until HCG versus extended GnRHa 12 days after HCG), Outcome 4 Dose of gonadotrophins.
7.5 Cycle cancellation
There was no evidence of a difference in the cycle cancellation rate in either group (OR 1.50, 95% CI 0.24 to 9.20; one RCT, n = 181 women, low quality evidence) (Analysis 7.5).
7.5. Analysis.
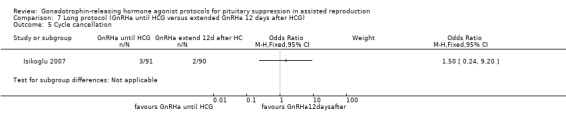
Comparison 7 Long protocol (GnRHa until HCG versus extended GnRHa 12 days after HCG), Outcome 5 Cycle cancellation.
7.6 Other outcomes
There were no studies reporting on other adverse effects, cost effectiveness, and acceptability of either of these protocols.
Neither sensitivity nor subgroup analysis was done because of the low number of studies reporting on this comparison and addressing the primary outcome.
8. Long protocol: administration of GnRHa for two versus three weeks before stimulation
We included only one study in this comparison (Table 8).
Primary outcome measure
8.1 Live birth and ongoing pregnancy rates
There was no evidence of a difference in the live birth and ongoing pregnancy rates when administration of GnRH lasted for three or two weeks, respectively, before stimulation (OR 0.88, 95% CI 0.37 to 2.05; one RCT, n = 85 women, low quality evidence) (Analysis 8.1).
8.1. Analysis.
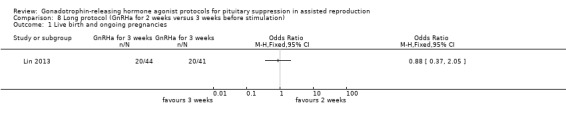
Comparison 8 Long protocol (GnRHa for 2 weeks versus 3 weeks before stimulation), Outcome 1 Live birth and ongoing pregnancies.
Secondary outcome measures
8.2 Clinical pregnancy rate
There was no evidence of a difference in the clinical pregnancy rate when administration of GnRH lasted for three or two weeks, respectively, before stimulation (OR 0.93, 95% CI 0.39 to 2.21; one RCT, n = 85 women, low quality evidence) (Analysis 8.2).
8.2. Analysis.
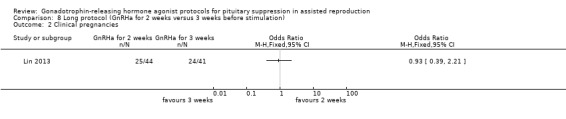
Comparison 8 Long protocol (GnRHa for 2 weeks versus 3 weeks before stimulation), Outcome 2 Clinical pregnancies.
8.3 Total number of oocytes retrieved
There was no evidence of a difference between the groups in the number of oocytes retrieved (MD 12, 95% CI ‐1.90 to 2.14; one RCT, n = 85 women, low quality evidence) (Analysis 8.3).
8.3. Analysis.
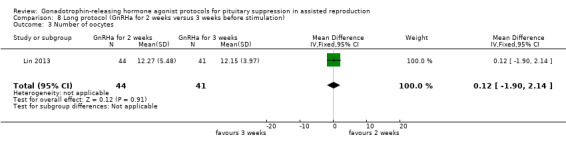
Comparison 8 Long protocol (GnRHa for 2 weeks versus 3 weeks before stimulation), Outcome 3 Number of oocytes.
8.4 Total dose of gonadotrophins
There was no evidence of a difference between the groups in the ampoules of gonadotrophins (MD 207.00, 95% CI ‐44.65 to 458.65; one RCT, n = 85 women, low quality evidence) (Analysis 8.4).
8.4. Analysis.
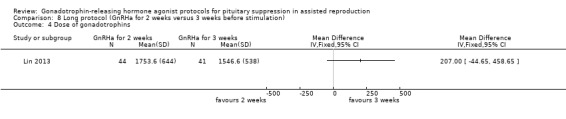
Comparison 8 Long protocol (GnRHa for 2 weeks versus 3 weeks before stimulation), Outcome 4 Dose of gonadotrophins.
8.5 Cycle cancellation rate
There was no study reporting on this outcome.
8.6 Other outcomes
Neither sensitivity nor subgroup analysis was done because of the low number of studies reporting on this comparison and addressing the primary outcome.
There were no studies reporting on other adverse effects, cost effectiveness, and acceptability of either of these protocols, apart from OHSS (Lin 2013) and miscarriage rates (Lin 2013).
a. Miscarriage rate
There was no evidence of a difference in miscarriages between the two groups (OR 0.93, 95% CI 0.18 to 4.87; one RCT, n = 85 women, low quality evidence) (Analysis 8.6)
8.6. Analysis.
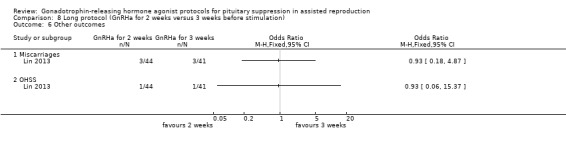
Comparison 8 Long protocol (GnRHa for 2 weeks versus 3 weeks before stimulation), Outcome 6 Other outcomes.
b. OHSS rate
There was no evidence of a difference in OHSS rate between the groups (OR 0.93, 95% CI 0.06 to 15.37; one RCT, n = 85 women, low quality evidence) (Analysis 8.6).
9. Short versus stop short protocol
We included only one study in this comparison (Table 9).
Primary outcome measure
9.1 Live birth and ongoing pregnancy rates
This was not reported for the comparison.
Secondary outcome measures
9.2 Clinical pregnancy rate
There was no evidence of a difference in the clinical pregnancy rate (OR 0.59, 95% CI 0.30 to 1.17; one RCT, n = 230 women, low quality evidence) when a short protocol was compared with a stop short protocol (Analysis 9.2).
9.2. Analysis.
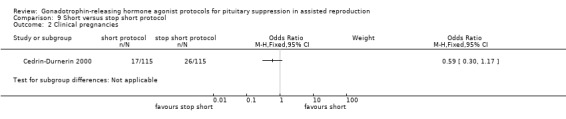
Comparison 9 Short versus stop short protocol, Outcome 2 Clinical pregnancies.
9.3 Total number of oocytes retrieved
This was not reported for the comparison.
9.4 Number of ampoules of gonadotrophins
There was evidence of a difference in the requirement for gonadotrophins with a short stop protocol requiring fewer ampoules of gonadotrophins (MD ‐5.20, ‐8.11 to ‐2.29; one RCT, n = 230 women, low quality evidence) (Analysis 9.4).
9.4. Analysis.
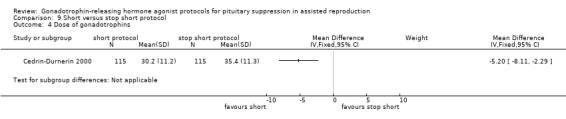
Comparison 9 Short versus stop short protocol, Outcome 4 Dose of gonadotrophins.
9.5 Cycle cancellation
There was no evidence of a difference between the groups in the cycle cancellation rate (OR 0.73, 95% CI 0.34 to 1.59; one RCT, n = 230 women, low quality evidence) (Analysis 9.5).
9.5. Analysis.
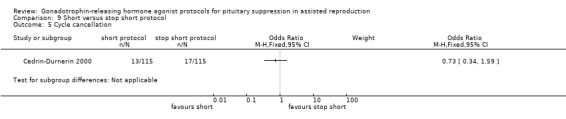
Comparison 9 Short versus stop short protocol, Outcome 5 Cycle cancellation.
9.7 Other outcomes
There were no studies reporting on other adverse effects, cost effectiveness, and acceptability of either of these protocols.
Discussion
Summary of main results
The conclusion from the second update of this systematic review and meta‐analysis is that there was no conclusive evidence that gonadotrophin‐releasing hormone agonist (GnRHa)‐long protocol was associated with an increase in live birth and ongoing clinical pregnancy rates in comparison with the GnRHa‐short protocol, although there was moderate evidence of an increase in clinical pregnancy rates. The finding remained constant after performing sensitivity analysis, removing studies where the method of randomisation and the reporting of outcomes were unclear. Subgroup analysis including four trials studying poor responders only showed a difference in clinical pregnancy rates, number of oocytes retrieved, and cancellation rates, favouring the GnRHa‐long protocol when compared with the GnRHa‐short protocol.
There was no evidence of a difference in live birth and ongoing clinical pregnancy rates in comparisons of other protocols of GnRHa for pituitary down‐regulation in assisted reproduction treatments.
Apart from two studies where there was evidence of a difference in the dose of gonadotrophins used when a GnRHa‐short protocol was compared with a GnRHa‐ultrashort protocol, and when a GnRHa‐short protocol was compared with a GnRHa‐stop short protocol, we found no evidence of any difference for any reproductive outcome (either primary or secondary) when GnRHa was commenced in the follicular phase compared with the luteal phase; stopped, reduced, or continued at the start of stimulation; continued or not after the oocyte triggering; or lasted for two or three weeks before stimulation.
Of note, there was very poor reporting of adverse events among studies in all comparisons, apart from cancellation rates.
Overall completeness and applicability of evidence
In the comparison of GnRH‐long versus GnRH‐short protocol regimens, despite the inclusion of 20 studies, there was no significant statistical heterogeneity (I² = 25%), but, as in many reviews in assisted reproduction, there was evidence of clinical heterogeneity.
The comparison between a luteal versus follicular start of GnRHa was based on five trials. None of them mentioned formation of a cyst, which has been shown to be associated with a follicular phase start of GnRHa (Jenkins 1996). There is controversy over whether cysts are associated with poorer outcomes. On the other hand, there is a risk of inadvertently exposing a pregnancy to GnRHa if administration is commenced in the luteal phase. Four per cent of cases of women undergoing in vitro fertilisation (IVF) have reported such a situation (Ron‐El 1990). None of the studies comparing luteal or follicular phase protocols commented on these outcomes.
Furthermore, the number of studies comparing various ways of GnRHa administration in a long protocol was small: three compared the stopping versus the continuation of GnRHa at start of stimulation, four compared the reduction versus the non‐reduction of the dose during stimulation, one compared the administration for two versus three weeks before stimulation, and another compared the prolongation versus the stopping after the oocyte retrieval 12 days after the embryo transfer.
Similarly, there were few studies for the rest of the comparisons: two compared GnRHa‐long versus ultrashort, one for short versus ultrashort, and one for short versus stop short protocols. Hence, the evidence is insufficient for these comparisons. Also, there were no data on cost effectiveness and acceptability of these protocols to women. Importantly, and as in many systematic reviews and especially Cochrane reviews, we noticed failure of most studies to report on live birth (four out of 20 in the comparison long versus short encompassing the maximum of studies) or adverse events. Moreover, some of the findings only apply to low responders, as this is an issue of applicability.
Quality of the evidence
Although we included 37 studies in the review, most of them were very old. Only 10 were published within the last 10 years; two were published nine years ago (Fábregues 2005; Simons 2005); one, seven years ago (Isikoglu 2007); one, five years ago (Zhang 2009); one, four years (Berker 2010); and the remaining five, within the last two years (Chatillon‐Boissier 2012; Ding 2013; Lin 2013; Sarhan 2013; Sunkara 2014). Because of the length of time elapsed, we were unable to contact most of the authors to get any missing data, such as the method of randomisation. Intention‐to‐treat analysis and an a priori power calculation were not features of any study except for very few in this review.
The general quality evidence for each comparison was low in almost all cases (see the 'Summary of findings' tables). Common limitations were failure to report live birth, risk of bias, and imprecision. Although statistical heterogeneity was not significant in most analyses, there was clinical heterogeneity, with a wide variation in the dose regimens and preparation of the GnRHa used.
For the first comparison 'long versus short protocol', the quality of the evidence was low for the primary outcome (12 studies), low for the secondary outcome 'Clinical pregnancy rate' (20 studies), and low for cancellation rate (11 studies) (Table 1). We observed a significant variation in the outcomes 'Number of oocytes retrieved' and 'Number of ampoules of gonadotrophins', most probably due to the way that these data were presented, such that no pooling of data was performed despite an adequate number of trials (10 and eight studies, respectively). Ideally, we would like to do a subgroup analysis of prognostic factors for where there was significant heterogeneity (Analysis 1.3; Analysis 1.4) based on the number of embryos transferred, previous failed cycles, maternal age, and duration of treatment.
In the rest of the comparisons, the quality of the studies was low for those reporting on the primary and secondary outcomes specified for this review, where reported (in five out of the eight remaining).
Potential biases in the review process
Through the standardised method of identification of studies, we included all relevant studies. We assessed bias according to the Cochrane 'Risk of bias' tool (Higgins 2011) and came to the conclusion that most studies were free of selective reporting. Almost all studies reported pregnancies (clinical). However, most of the studies (even the most recent) did not report live birth, which formed part of the primary outcome measure in this review. There has been considerable debate about what is the best outcome to report in assisted reproduction technology (ART) studies (Min 2004). Although the most reliable effectiveness of an intervention in ART is nowadays considered the reporting of live birth rates, in the current review, the lack of such reporting weakens the robustness of the results obtained.
Agreements and disagreements with other studies or reviews
The results for a GnRHa‐long versus a GnRHa‐short protocol, with pregnancy rate as the outcome, are similar to those in the previous published version of this review despite the fact that we excluded studies analysing gamete intrafallopian transfer (GIFT) cycles, cross‐over trials, and quasi‐randomised trials (included in the last review) in this updated review. This updated review includes further comparisons that were not part of the initial review. These referred to the GnRH‐long protocol: (1) luteal versus follicular start of GnRHa; (2) stopping and reducing the dose of GnRHa versus continuing the same dose; (3) administration of GnRHa for two versus three weeks before stimulation; and (4) discontinuing versus continuing GnRHa after HCG administration, and to the GnRH‐short protocol (short versus stop short).
There are no non‐Cochrane reviews on this topic.
Authors' conclusions
Implications for practice.
When long GnRHa protocols and short GnRHa protocols were compared, we found no conclusive evidence of a difference in live birth and ongoing pregnancy rates, but there was moderate quality evidence of higher clinical pregnancy rates in the long protocol group. None of the other analyses showed any evidence of a difference in birth or pregnancy outcomes between the protocols compared. There was insufficient evidence to make any conclusions regarding adverse effects.
Implications for research.
As adjuvants are almost always used in ART protocols, further research with high quality trials are needed to determine an optimal protocol (when to commence and stop GnRHa and its optimal dose), further identifying the most cost‐effective and acceptable regimen.
We propose comparisons of these protocols using GnRHa in women stratified by type of subfertility and age. Most importantly, for all comparisons included in this review (nine), live birth, ongoing pregnancy rates, or both, should be the primary outcome reported, along with adverse events, as GnRHa protocols have been associated with high incidence of ovarian hyperstimulation syndrome (OHSS) and miscarriage rates. Finally, further parameters should comprise the outcomes of interest, such as the acceptability of the regimens, their cost, and the woman's preference.
What's new
| Date | Event | Description |
|---|---|---|
| 24 July 2015 | New citation required but conclusions have not changed | The addition of 8 new studies did not lead to a change in the conclusions of this review. |
| 24 July 2015 | New search has been performed | 8 studies were added in this update, and 1 co‐author was added. The text was thoroughly changed according to current Menstrual Disorders and Subfertility Group guidelines. New included studies: Berker 2010; Chatillon‐Boissier 2012; Ding 2013; Isikoglu 2007; Lin 2013; Sarhan 2013; Sunkara 2014; and Zhang 2009. |
History
Protocol first published: Issue 1, 2008 Review first published: Issue 8, 2011
| Date | Event | Description |
|---|---|---|
| 11 January 2009 | Amended | Original review has been withdrawn, and a new protocol has been published. Title changed back from 'Long versus short gonadotropin releasing hormone agonist protocols for pituitary desensitization in assisted reproduction cycles' to 'Gonadotrophin‐releasing hormone agonist protocols for pituitary suppression in assisted reproductive treatment'. 11 December 2008: Title changed from 'Gonadotrophin‐releasing hormone agonist protocols for pituitary down regulation in assisted reproductive treatment' to 'Long versus short gonadotropin releasing hormone agonist protocols for pituitary desensitization in assisted reproduction cycles'. |
| 12 November 2007 | New citation required and major changes | Substantive amendment |
Acknowledgements
We would like to thank the previous authors of this review, Salim Daya et al, for allowing us to use their work.
We thank also the authors of Chatillon‐Boissier 2012; Corson 1992; Isikoglu 2007; Lin 2013; NCT00436319; Sarhan 2013; Sunkara 2014; Tanaka 2014 and Tarin 1990 for answering our requests and supplying further information about their trials.
Appendices
Appendix 1. Menstrual Disorders and Subfertility database search
Search strategy for SD265 09.04.14:
Keywords CONTAINS "IVF" or "ICSI" or "in‐vitro fertilisation " or "in‐vitro fertilisation procedure" or "in vitro fertilization" or "intracytoplasmic sperm injection" or "intracytoplasmic morphologically selected sperm injection" or "controlled ovarian hyperstimulation" or "controlled ovarian stimulation" or"COH" or"embryo transfer" or"ovarian hyperstimulation"or "ovarian stimulation" or Title CONTAINS "IVF" or "ICSI" or "in‐vitro fertilisation " or "in‐vitro fertilisation procedure" or "in vitro fertilization" or "intracytoplasmic sperm injection" or "intracytoplasmic morphologically selected sperm injection"or "controlled ovarian hyperstimulation" or "controlled ovarian stimulation" or"COH" or"embryo transfer" or"ovarian hyperstimulation"or "ovarian stimulation"
AND
Keywords CONTAINS "Gonadorelin" or "Gonadotrophin releasing agonist"or "Gonadotrophin releasing hormones"or "gonadotropin releasing hormone agonist" or "Goserelin" or "goserelin acetate" or "goserelin pretreatment" or"Gosereline "or "buserelin"or "busereline"or"leuprolide "or"leuprolin"or"leuprorelin"or"nafarelin"or"triptorelin"or"Lupron"or "Zoladex"or"deslorelin"or "GnRH agonist"or "GnRH a"or"GnRH agonists"or"GnRHa"or"GnRH analog"or"GnRH analogue"or"GnRH analogues"or"Luteinising hormone releasing hormone"or"luteinizing hormone supplementation"or"Lutenising hormone releasing hormone"or"menotropin"or"menotrophin"or"human menopausal gonadotrophin"or"human menopausal gonadotrophins"or "human menopausal gonadotrophins"or Title CONTAINS "Gonadorelin" or "Gonadotrophin releasing agonist"or "Gonadotrophin releasing hormones"or "gonadotropin releasing hormone agonist"
AND
Keywords CONTAINS "desensitisation vs flareup"or"long agonist protocol"or "long‐long protocol"or "long protocol"or"long‐term GnRHa treatment"or"long v short protocol"or"short interval"or"short protocol"or"ultra long protocol"or"ultra‐short protocol"or"reduced dose"or"down regulation"or"follicular phase"or"high dose"or"high dose protocol"or"stop protocol"or"prolonged stimulation"or"day 7"or "continuous"or"early v late"or"early versus late"or"daily"or or"dosage"or"dose"or "long‐term"or"flare‐up"or "flare‐up GnRH agonist"or"flare‐up protocol"or"Protocols"or"dose‐response study"or"dosing regimen
Appendix 2. CENTRAL search
EBM Reviews ‐ Cochrane Central Register of Controlled Trials <March 2015> Search Strategy: 1 exp embryo transfer/ or exp fertilization in vitro/ or exp sperm injections, intracytoplasmic/ or exp gamete intrafallopian transfer/ or exp in vitro oocyte maturation techniques/ (1765) 2 embryo transfer.tw. (1066) 3 in vitro fertili?ation.tw. (1571) 4 intracytoplasmic sperm injection$.tw. (518) 5 (ivf or icsi).tw. (2756) 6 exp Infertility, Female/ (930) 7 exp Primary Ovarian Insufficiency/ (68) 8 exp Infertility/ (1664) 9 (ovar$ adj2 stimulat$).tw. (976) 10 (ovar$ adj2 hyperstimulat$).tw. (675) 11 COH.tw. (162) 12 or/1‐11 (5354) 13 exp gonadotropin‐releasing hormone/ or exp buserelin/ or exp goserelin/ or exp leuprolide/ or exp nafarelin/ or exp triptorelin pamoate/ (1885) 14 gonadotropin‐releasing hormone$.tw. (835) 15 (buserelin or goserelin or leuprolide or nafarelin or triptorelin).tw. (1330) 16 (Lupron or Suprefact or Suprecor).tw. (45) 17 (histrelin or Supprelin).tw. (1) 18 (Zoladex or deslorelin).tw. (236) 19 (Suprelorin or Ovuplant).tw. (0) 20 Synarel.tw. (3) 21 GnRHa.tw. (236) 22 GnRH‐a.tw. (1393) 23 GnRH agonist$.tw. (796) 24 GnRH analog$.tw. (313) 25 luteinizing hormone releasing agonist$.tw. (1) 26 exp Menotropins/ (358) 27 human menopausal gonadotropin$.tw. (239) 28 or/13‐27 (3595) 29 desensiti?ation.tw. (1004) 30 (long adj2 protocol).tw. (335) 31 (short adj2 protocol).tw. (122) 32 (ultra short adj2 protocol).tw. (3) 33 (long adj2 follicular).tw. (10) 34 (ultrashort adj2 protocol).tw. (3) 35 reduced dos$.tw. (840) 36 down regulat$.tw. (923) 37 downregulat$.tw. (587) 38 (follicular adj5 luteal).tw. (293) 39 high dose$.tw. (13920) 40 stop versus non stop.tw. (1) 41 prolonged protocol.tw. (1) 42 7 day.tw. (3291) 43 continu$ versus stop$.tw. (2) 44 short acting.tw. (1380) 45 early cessation.tw. (36) 46 early follicular.tw. (198) 47 different phase$.tw. (173) 48 daily.tw. (78531) 49 long acting.tw. (3713) 50 long luteal.tw. (13) 51 desensiti?e.tw. (31) 52 suppression.tw. (7910) 53 suppress.tw. (1966) 54 (inhibition or inhibit).tw. (16876) 55 (long adj2 protocol$).tw. (375) 56 (short adj2 protocol$).tw. (142) 57 or/29‐56 (119095) 58 12 and 28 and 57 (722)
Appendix 3. MEDLINE search
Database: Ovid MEDLINE(R) In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE(R) Daily and Ovid MEDLINE(R) <1946 to Present>
1 exp embryo transfer/ or exp fertilization in vitro/ or exp sperm injections, intracytoplasmic/ or exp gamete intrafallopian transfer/ or exp in vitro oocyte maturation techniques/ (32710) 2 embryo transfer.tw. (7668) 3 in vitro fertili?ation.tw. (16760) 4 intracytoplasmic sperm injection$.tw. (4912) 5 (ivf or icsi).tw. (18836) 6 exp Infertility, Female/ (23368) 7 exp Primary Ovarian Insufficiency/ (1673) 8 exp Infertility/ (52626) 9 (ovar$ adj2 stimulat$).tw. (4982) 10 (ovar$ adj2 hyperstimulat$).tw. (3842) 11 COH.tw. (1130) 12 or/1‐11 (89231) 13 exp gonadotropin‐releasing hormone/ or exp buserelin/ or exp goserelin/ or exp leuprolide/ or exp nafarelin/ or exp triptorelin pamoate/ (28527) 14 gonadotropin‐releasing hormone$.tw. (11008) 15 (buserelin or goserelin or leuprolide or nafarelin or triptorelin).tw. (4078) 16 (Lupron or Suprefact or Suprecor).tw. (168) 17 (histrelin or Supprelin).tw. (46) 18 (Zoladex or deslorelin).tw. (560) 19 (Suprelorin or Ovuplant).tw. (22) 20 Synarel.tw. (12) 21 GnRHa.tw. (1094) 22 GnRH‐a.tw. (900) 23 GnRH agonist$.tw. (3408) 24 GnRH analog$.tw. (2085) 25 luteinizing hormone releasing agonist$.tw. (5) 26 exp Menotropins/ (3017) 27 human menopausal gonadotropin$.tw. (1344) 28 or/13‐27 (36003) 29 desensiti?ation.tw. (19633) 30 (long adj2 protocol).tw. (843) 31 (short adj2 protocol).tw. (406) 32 (ultra short adj2 protocol).tw. (5) 33 (long adj2 follicular).tw. (47) 34 (ultrashort adj2 protocol).tw. (11) 35 reduced dos$.tw. (3057) 36 down regulat$.tw. (89044) 37 downregulat$.tw. (64084) 38 (follicular adj5 luteal).tw. (2798) 39 high dose$.tw. (101864) 40 stop versus non stop.tw. (1) 41 prolonged protocol.tw. (5) 42 7 day.tw. (15537) 43 continu$ versus stop$.tw. (3) 44 short acting.tw. (5630) 45 early cessation.tw. (285) 46 early follicular.tw. (1652) 47 different phase$.tw. (7846) 48 daily.tw. (348977) 49 long acting.tw. (16507) 50 long luteal.tw. (52) 51 desensiti?e.tw. (1409) 52 suppression.tw. (170539) 53 suppress.tw. (64930) 54 (inhibition or inhibit).tw. (789675) 55 (long adj2 protocol$).tw. (1017) 56 (short adj2 protocol$).tw. (526) 57 or/29‐56 (1536670) 58 12 and 28 and 57 (2202) 59 randomized controlled trial.pt. (370469) 60 controlled clinical trial.pt. (88141) 61 randomized.ab. (290565) 62 randomised.ab. (58371) 63 placebo.tw. (157118) 64 clinical trials as topic.sh. (169329) 65 randomly.ab. (210657) 66 trial.ti. (124866) 67 (crossover or cross‐over or cross over).tw. (60254) 68 or/59‐67 (936052) 69 exp animals/ not humans.sh. (3921813) 70 68 not 69 (863309) 71 58 and 70 (696)
Appendix 4. EMBASE search
Database: Embase <1980 to 2015 Week 16> Search Strategy:
1 exp embryo transfer/ or exp infertility therapy/ (75966) 2 exp female infertility/ or exp fertilization in vitro/ or exp intracytoplasmic sperm injection/ (71995) 3 embryo transfer.tw. (10480) 4 in vitro fertili?ation.tw. (20223) 5 intracytoplasmic sperm injection$.tw. (6184) 6 (ivf or icsi).tw. (28413) 7 exp premature ovarian failure/ (2247) 8 (ovar$ adj2 stimulat$).tw. (6983) 9 (ovar$ adj2 hyperstimulat$).tw. (5230) 10 COH.tw. (1444) 11 or/1‐10 (108706) 12 exp gonadorelin/ or exp gonadorelin agonist/ (36886) 13 exp buserelin acetate/ or exp buserelin/ (4583) 14 exp goserelin/ (5701) 15 exp leuprorelin/ (8556) 16 exp nafarelin acetate/ or exp nafarelin/ (1328) 17 exp triptorelin/ (3972) 18 gonadotrop?in‐releasing hormone$.tw. (14213) 19 (buserelin or goserelin or leuprolide or nafarelin or triptorelin).tw. (5269) 20 (Lupron or Suprefact or Suprecor).tw. (2488) 21 (histrelin or Supprelin).tw. (114) 22 (Zoladex or deslorelin).tw. (2138) 23 (Suprelorin or Ovuplant).tw. (31) 24 Synarel.tw. (319) 25 GnRHa.tw. (1457) 26 GnRH‐a.tw. (1070) 27 GnRH agonist$.tw. (4596) 28 GnRH analog$.tw. (2744) 29 luteinizing hormone releasing agonist$.tw. (7) 30 exp human menopausal gonadotropin/ (7971) 31 human menopausal gonadotrop?in$.tw. (2022) 32 or/12‐31 (57973) 33 11 and 32 (13889) 34 desensiti?ation.tw. (21946) 35 (long adj2 protocol).tw. (1285) 36 (short adj2 protocol).tw. (584) 37 (ultra short adj2 protocol).tw. (8) 38 (long adj2 follicular).tw. (61) 39 (ultrashort adj2 protocol).tw. (13) 40 reduced dos$.tw. (4136) 41 down regulat$.tw. (108195) 42 downregulat$.tw. (79715) 43 (follicular adj5 luteal).tw. (2948) 44 high dose$.tw. (125377) 45 stop versus non stop.tw. (1) 46 prolonged protocol.tw. (5) 47 7 day.tw. (18760) 48 continu$ versus stop$.tw. (3) 49 short acting.tw. (7235) 50 early cessation.tw. (343) 51 early follicular.tw. (1874) 52 different phase$.tw. (8761) 53 daily.tw. (436352) 54 long acting.tw. (20976) 55 long luteal.tw. (76) 56 desensiti?e.tw. (1555) 57 suppression.tw. (186085) 58 suppress.tw. (72208) 59 (inhibition or inhibit).tw. (859108) 60 (long adj2 protocol$).tw. (1526) 61 (short adj2 protocol$).tw. (742) 62 or/34‐61 (1754598) 63 33 and 62 (3688) 64 Clinical Trial/ (829568) 65 Randomized Controlled Trial/ (338773) 66 exp randomization/ (61524) 67 Single Blind Procedure/ (18032) 68 Double Blind Procedure/ (112415) 69 Crossover Procedure/ (38335) 70 Placebo/ (236318) 71 Randomi?ed controlled trial$.tw. (95890) 72 Rct.tw. (13384) 73 random allocation.tw. (1288) 74 randomly allocated.tw. (19790) 75 allocated randomly.tw. (1896) 76 (allocated adj2 random).tw. (707) 77 Single blind$.tw. (13937) 78 Double blind$.tw. (138097) 79 ((treble or triple) adj blind$).tw. (351) 80 placebo$.tw. (193550) 81 prospective study/ (245030) 82 or/64‐81 (1339414) 83 case study/ (25067) 84 case report.tw. (253821) 85 abstract report/ or letter/ (883698) 86 or/83‐85 (1157110) 87 82 not 86 (1302183) 88 63 and 87 (1219)
Appendix 5. CINAHL search
EBSCO: 01.01.08 to 23.04.14.
| S33 | S18 AND S32 | 70 |
| S32 | S19 OR S20 or S21 or S22 OR S23 OR S24 OR S25 OR S26 OR S27 OR S28 OR S29 OR S30 OR S31 | 955,673 |
| S31 | TX allocat* random* | 4,250 |
| S30 | (MH "Quantitative Studies") | 13,306 |
| S29 | (MH "Placebos") | 9,184 |
| S28 | TX placebo* | 33,672 |
| S27 | TX random* allocat* | 4,250 |
| S26 | (MH "Random Assignment") | 39,015 |
| S25 | TX randomi* control* trial* | 86,166 |
| S24 | TX ( (singl* n1 blind*) or (singl* n1 mask*) ) or TX ( (doubl* n1 blind*) or (doubl* n1 mask*) ) or TX ( (tripl* n1 blind*) or (tripl* n1 mask*) ) or TX ( (trebl* n1 blind*) or (trebl* n1 mask*) ) | 764,433 |
| S23 | TX ( (trebl* n1 blind*) or (trebl* n1 mask*) ) | 114 |
| S22 | TX ( (trebl* n1 blind*) or (trebl* n1 mask*) ) | 0 |
| S21 | TX clinic* n1 trial* | 171,126 |
| S20 | PT Clinical trial | 77,731 |
| S19 | (MH "Clinical Trials+") | 186,401 |
| S18 | S8 AND S17 | 157 |
| S17 | S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 | 1,371 |
| S16 | TX (gonadotropin releasing hormone agonist*) | 190 |
| S15 | TX (Luteinising hormone releasing hormone) | 34 |
| S14 | TX GnRH a | 129 |
| S13 | TX buserelin or TX leuprolin or TX leuprorelin or TX nafarelin or TX triptorelin or TX Lupron or TX Zoladex or TX deslorelin | 124 |
| S12 | TX (GnRH agonist*) | 159 |
| S11 | TX Gonadorelin OR TX Leuprolide | 1,015 |
| S10 | TX Goserelin | 237 |
| S9 | (MM "Gonadorelin") OR (MM "Leuprolide") OR (MM "Goserelin") | 590 |
| S8 | S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 | 3,731 |
| S7 | TX embryo* N3 transfer* | 771 |
| S6 | TX ovar* N3 hyperstimulat* | 336 |
| S5 | TX ovari* N3 stimulat* | 246 |
| S4 | TX IVF or TX ICSI | 1,249 |
| S3 | (MM "Fertilization in Vitro") | 1,446 |
| S2 | TX vitro fertilization | 2,852 |
| S1 | TX vitro fertilisation | 266 |
Appendix 6. Information from the studies selected for the review
Trial characteristics
(1) Method and timing of randomisation:
randomisation was adequate (e.g., by computer, random number tables, or drawing lots); or
not clear (e.g., stated but not further described, or did not fall into one of the randomisation categories).
(2) Allocation concealment.
(3) Duration, timing, and location of the trial (single centre or multicentre trial), duration of follow up, and:
outcome data used for primary analysis were complete (follow up to live birth), all randomised women were accounted for with an intention‐to‐treat analysis;
completeness of data uncertain; or
outcome data incomplete, with 5% of the cycles commenced missing some outcome data.
(4) Co‐intervention:
other care provided with the intervention under study was equivalent in the treatment and control groups;
issue of co‐intervention was not considered; or
co‐intervention variations definitely existed.
(5) The presence of a power calculation:
(a) yes (prospective and valid or not valid); or (b) no.
Baseline characteristics of the studied groups
(a) Cause and duration of pre‐existing subfertility (b) Age of the women and parity (c) Investigative work‐up prior to in vitro fertilisation (IVF) (d) Previously administered treatment(s)
Intervention
(a) Type of intervention and control comparator (b) Dose and type of regime
(c) We differentiated between whether the studied population included all women undergoing assisted reproduction technology (ART) or was limited to women who had responded poorly in a previous attempt or were expected to have a diminished response. As different drug regimes of ovarian stimulation can lead to a variable ovarian response, data on the drugs employed was also extracted.
Outcomes
(a) Outcomes reported (b) How outcomes were defined (c) Timing of outcome measurement
Data and analyses
Comparison 1. Long versus short protocol.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Live birth/ongoing pregnancies | 12 | 976 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.30 [0.94, 1.81] |
| 1.1 Live birth | 4 | 295 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.60 [0.85, 3.03] |
| 1.2 Ongoing pregnancies | 8 | 681 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.82, 1.78] |
| 2 Clinical pregnancies | 19 | 1582 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.50 [1.18, 1.92] |
| 2.1 Non‐selected group | 15 | 1350 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.38 [1.07, 1.79] |
| 2.2 Poor responders | 4 | 232 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.12 [1.39, 7.02] |
| 3 Number of oocytes | 10 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 Non‐selected group | 6 | 512 | Mean Difference (IV, Fixed, 95% CI) | 2.47 [2.21, 2.72] |
| 3.2 Poor responders | 4 | 227 | Mean Difference (IV, Fixed, 95% CI) | 1.40 [0.75, 2.06] |
| 4 Dose of gonadotrophins | 8 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 Non‐selected group | 4 | 439 | Mean Difference (IV, Fixed, 95% CI) | 15.64 [14.05, 17.22] |
| 4.2 Poor responders | 4 | 227 | Mean Difference (IV, Fixed, 95% CI) | 7.07 [3.06, 11.08] |
| 5 Cycle cancellation | 11 | 1026 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.59, 1.55] |
| 5.1 Non‐selected group | 7 | 799 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.73 [0.92, 3.23] |
| 5.2 Poor responders | 4 | 227 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.12, 0.76] |
Comparison 2. Long protocol versus ultrashort protocol.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Live birth and ongoing pregnancies | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2 Clinical pregnancies | 2 | 230 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.56 [0.80, 3.06] |
| 3 Number of oocytes | 2 | 230 | Mean Difference (IV, Fixed, 95% CI) | 0.53 [‐0.61, 1.66] |
| 4 Dose of gonadotrophins | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5 Cycle cancellation | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
Comparison 3. Short versus ultrashort protocol.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Live birth and ongoing pregnancies | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Clinical pregnancies | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Number of oocytes | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4 Dose of gonadotrophins | 1 | 82 | Mean Difference (IV, Fixed, 95% CI) | ‐13.85 [‐21.49, ‐6.21] |
| 5 Cycle cancellation | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
3.2. Analysis.
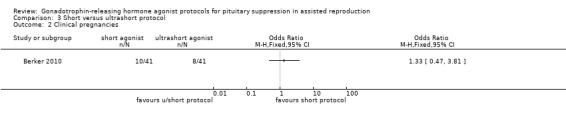
Comparison 3 Short versus ultrashort protocol, Outcome 2 Clinical pregnancies.
3.3. Analysis.
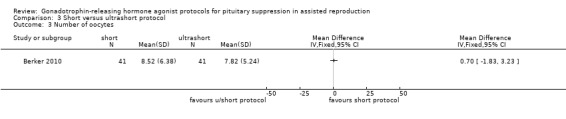
Comparison 3 Short versus ultrashort protocol, Outcome 3 Number of oocytes.
3.4. Analysis.
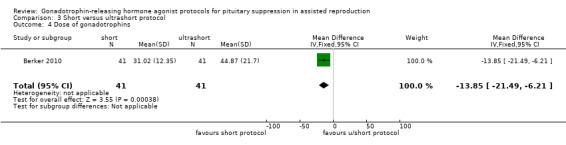
Comparison 3 Short versus ultrashort protocol, Outcome 4 Dose of gonadotrophins.
3.5. Analysis.
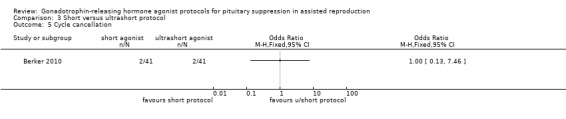
Comparison 3 Short versus ultrashort protocol, Outcome 5 Cycle cancellation.
Comparison 4. Long protocol (luteal versus follicular).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Live birth and ongoing pregnancies | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2 Clinical pregnancies | 5 | 750 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.76, 1.47] |
| 3 Number of oocytes | 4 | 527 | Mean Difference (IV, Fixed, 95% CI) | ‐1.29 [‐1.86, ‐0.71] |
| 4 Dose of gonadotrophins | 4 | 527 | Mean Difference (IV, Fixed, 95% CI) | 1.12 [‐0.73, 2.97] |
| 5 Cycle cancellation | 2 | 267 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.45 [0.35, 6.01] |
Comparison 5. Long protocol (continued GnRHa versus stop GnRHa).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Live birth and ongoing pregnancies | 3 | 290 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.42, 1.33] |
| 1.1 Live birth | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Ongoing pregnancies | 3 | 290 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.42, 1.33] |
| 2 Clinical pregnancies | 4 | 360 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.51, 1.41] |
| 3 Number of oocytes | 4 | 360 | Mean Difference (IV, Fixed, 95% CI) | ‐0.26 [‐1.29, 0.78] |
| 4 Dose of gonadotrophins | 4 | 360 | Mean Difference (IV, Fixed, 95% CI) | ‐0.14 [‐2.35, 2.08] |
| 5 Cycle cancellation | 3 | 264 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.41 [0.56, 3.56] |
| 6 Other outcomes ‐ OHSS | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
Comparison 6. Long protocol (continued same versus reduced dose GnRHa).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Live birth and ongoing pregnancies | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Clinical pregnancies | 4 | 407 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.68, 1.52] |
| 3 Number of oocytes | 3 | 275 | Mean Difference (IV, Fixed, 95% CI) | 1.03 [‐0.04, 2.10] |
| 4 Dose of gonadotrophins | 2 | 228 | Mean Difference (IV, Fixed, 95% CI) | 0.98 [‐1.72, 3.69] |
| 5 Cycle cancellation | 2 | 228 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.14, 7.32] |
Comparison 7. Long protocol (GnRHa until HCG versus extended GnRHa 12 days after HCG).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Live birth and ongoing pregnancies | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2 Clinical pregnancies | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3 Number of oocytes | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4 Dose of gonadotrophins | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5 Cycle cancellation | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
Comparison 8. Long protocol (GnRHa for 2 weeks versus 3 weeks before stimulation).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Live birth and ongoing pregnancies | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Clinical pregnancies | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Number of oocytes | 1 | 85 | Mean Difference (IV, Fixed, 95% CI) | 0.12 [‐1.90, 2.14] |
| 4 Dose of gonadotrophins | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5 Cycle cancellation | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Other outcomes | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6.1 Miscarriages | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 OHSS | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 9. Short versus stop short protocol.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Live birth and ongoing pregnancies | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Clinical pregnancies | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3 Number of oocytes | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Dose of gonadotrophins | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5 Cycle cancellation | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Acharya 1992.
| Methods | Randomised trial The method of allocation was not described The trial was not blinded |
|
| Participants | Couples with all causes of infertility (unexplained: 20%, male factor: 7%, endometriosis: 18%, tubal factor: 55%) | |
| Interventions | Long follicular GnRHa protocol with buserelin acetate 200 μg I/M x 5 daily from day 2 for at least 13 days until ovarian suppression, then 4 ampoules of HMG daily x 3, then 3 ampoules x 1 day, then 2 ampoules daily thereafter and adjusted based on the response versus short GnRHa protocol with buserelin acetate (dose as above) and HMG (dose as above) commencing 1 day later | |
| Outcomes |
|
|
| Notes | Participants in the short protocol group received norethisterone 5 mg twice daily from day 21 of the previous cycle for 7 to 14 days to ensure ovarian suppression and to schedule the cycle start in such a way that the oocyte retrieval was more likely to occur on a weekday 60% of participants had 3 embryo transfers in both groups There was 1 cycle per woman |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomly allocated to 1 or the other protocol using a predetermined schedule |
| Allocation concealment (selection bias) | Unclear risk | The paper did not report allocation concealment |
| Blinding (performance bias and detection bias) All outcomes | High risk | The trial was not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 87 participants were randomised; all participants received treatment and were analysed |
| Selective reporting (reporting bias) | Unclear risk | The published report did not include any of our 2 primary outcomes (live birth/ongoing pregnancy) |
| Other bias | Unclear risk | There was insufficient information to assess whether an important risk of bias existed |
Berker 2010.
| Methods | Randomised trial The trial used computer‐generated block randomisation with sealed envelopes |
|
| Participants | 82 poor responder participants who underwent ICSI Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomised into 2 groups:
The starting dose of recombinant FSH depended on the age, BMI, and ovarian response to the previous cycle and increased to a maximum of 450 IU/day depending on the ovarian response. Dosage of rFSH was individualised after day 5 according to ultrasonographic and hormonal follow‐up Luteal support was initiated on the day of oocyte retrieval and continued until the day of pregnancy testing with vaginal progesterone |
|
| Outcomes |
|
|
| Notes | Cycle cancellation rates were similar in the groups There was 1 cycle per woman The population was selective group (poor responders) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation was computer generated using sealed envelopes |
| Allocation concealment (selection bias) | Low risk | On the day of stimulation initiation, a nurse who assigned participants to their groups opened sealed envelopes with treatment allocation instructions |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | The paper did not mention blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | A total of 82 poor responder participants underwent 78 COH‐ICSI cycles. Of these participants, 41 received the ultrashort GnRH agonist/GnRH antagonist protocol, and 41 received the microdose flare‐up protocol. Cycle cancellation was carried out for 2 participants in ultrashort GnRH agonist/GnRH antagonist protocol group |
| Selective reporting (reporting bias) | Low risk | Most of the outcomes of interest except live birth were reported |
| Other bias | Low risk | We suspected no other bias |
Cedrin‐Durnerin 2000.
| Methods | Randomised trial | |
| Participants | 230 infertile women undergoing new or repeated IVF cycles Exclusion criteria
|
|
| Interventions | Daily subcutaneous injection of Dtrp6‐GnRH (decapeptyl, 100 μg/day) from day 1 of IVF cycle followed by ovarian stimulation with exogenous gonadotrophins 150 IU I/m, with the dose being adjusted according to response Women were randomised into 2 groups:
|
|
| Outcomes |
|
|
| Notes | 2 variants of the short protocol were used There was 1 cycle per woman |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A random number table was used |
| Allocation concealment (selection bias) | Unclear risk | The paper did not report allocation concealment |
| Blinding (performance bias and detection bias) All outcomes | High risk | The trial was not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 230 women were randomised and received therapy. 30 cycles were cancelled, and analysis was presented for 200 women. The paper thoroughly presented reasons for cancellation |
| Selective reporting (reporting bias) | Unclear risk | The published report did not include any of our 2 primary outcomes (live birth/ongoing pregnancy) |
| Other bias | High risk | An important group of IVF participants (participants with chronic anovulation) were excluded from participation. Besides, 2 variants of short protocol were used |
Chatillon‐Boissier 2012.
| Methods | Prospective randomised trial | |
| Participants | 44 "poor responder" participants undergoing an IVF cycle | |
| Interventions | Participants were randomised into 2 groups:
COH with rFSH 300 to 450 UI/d |
|
| Outcomes |
|
|
| Notes | There was 1 cycle per woman There was no pretreatment prior to initiation of GnRHa in both groups This was a special category of participants (poor responders) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised from a computer‐generated list of pseudo‐random permutation of blocks of variable size |
| Allocation concealment (selection bias) | Unclear risk | The paper did not mention allocation concealment |
| Blinding (performance bias and detection bias) All outcomes | High risk | The trial was not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 44 participants were randomised; 39 participants received treatment (reasons specifically mentioned) |
| Selective reporting (reporting bias) | Low risk | The paper reported most outcomes of interest |
| Other bias | Low risk | We suspected no other bias |
Chen 1992.
| Methods | Randomised trial The method of allocation was not described |
|
| Participants | Infertile couples with tubal factor (70%), male factor (10%), endometriosis (18%), and oocyte donation (2%) Average female age: 33 years |
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The random sequence generation was not described |
| Allocation concealment (selection bias) | Unclear risk | The paper did not describe allocation concealment |
| Blinding (performance bias and detection bias) All outcomes | High risk | The trial was not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | There was insufficient reporting of attrition/exclusions to permit a judgement of 'low risk' or 'high risk' |
| Selective reporting (reporting bias) | Unclear risk | The paper did not report ongoing pregnancy and live birth, but they were not the planned outcome measures |
| Other bias | Unclear risk | There was insufficient information to assess whether an important risk of bias existed |
Dal Prato 2001.
| Methods | Prospective randomised | |
| Participants | 132 women undergoing COH for IVF/ICSI, aged between 25 and 38 years with infertility caused by tubal idiopathic and male factor infertility Exclusion criteria
|
|
| Interventions |
Luteal support ‐ natural progesterone in oil |
|
| Outcomes |
|
|
| Notes | Pregnancy was defined as the presence of gestational sac on ultrasound scan performed 4 weeks after embryo transfer There was 1 woman per cycle |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Allocation was done using sealed envelopes containing the name of 1 of the 2 groups |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes were used |
| Blinding (performance bias and detection bias) All outcomes | High risk | Participants were not blinded to the treatment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 132 women were randomised; all women received treatment as allocated |
| Selective reporting (reporting bias) | Unclear risk | The published report did not include our primary outcome |
| Other bias | Low risk | We suspected no other bias |
De Placido 1991.
| Methods | Randomised trial | |
| Participants | Information not provided | |
| Interventions |
|
|
| Outcomes |
|
|
| Notes | This trial was a randomised comparison of depot versus daily GnRHa formulation; it was assumed that allocation to the long or short GnRHa protocol was also randomised. No data were provided on the number of participants undergoing oocyte retrieval and embryo transfer Gonadotrophin administration, method of oocyte retrieval, and luteal phase management was not described Most of the information in the bias table is incomplete as this was an abstract. We wrote to the authors and did not receive any reply |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The method of randomisation was not described |
| Allocation concealment (selection bias) | Unclear risk | The paper did not describe allocation concealment |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | The paper did not describe blinding |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | There was insufficient reporting of attrition/exclusions to permit a judgement of 'low risk' or 'high risk' |
| Selective reporting (reporting bias) | High risk | Only the clinical pregnancy rate per started cycle was reported |
| Other bias | High risk | Data regarding the number of participants and other inclusion criteria were lacking |
Ding 2013.
| Methods | Prospective randomised trial | |
| Participants | 96 participants with high response to gonadotrophin stimulation compared with reference concentrations undergoing IVF/ICSI cycle Inclusion criteria
Exclusion criteria
|
|
| Interventions | 96 participants were allocated to 2 independent groups:
rFSH administration was administered until the triggering of ovulation in both groups |
|
| Outcomes |
|
|
| Notes | Clinical pregnancy was determined by observing a gestational sac by means of echographic screening at 7 weeks of pregnancy Ongoing pregnancy was defined as a conception cycle with at least 1 foetal sac with a positive heartbeat reaching beyond 12 weeks of amenorrhoea There was 1 cycle per participant ET in 29 out of 47 participants in group (1) ET in 26 out of 49 participants in group (2) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was done by computer software |
| Allocation concealment (selection bias) | Low risk | The trial used closed envelopes |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | The paper did not mention blinding |
| Incomplete outcome data (attrition bias) All outcomes | High risk | This study enrolled 96 participants Oocyte retrieval cycles: 47/47 and 49/49. The number of retrieved oocytes was reported, but only 54 out of 96 reached ET because on day 3 (18 cycles in the GnRH agonist withdrawal group and 23 cycles in the control group), all embryos were cryopreserved. The criteria for this choice was not mentioned |
| Selective reporting (reporting bias) | Low risk | ET occurred in 29 out of 47 participants in group (1), and for 26 out of 49 participants in group (2), ET was not reported. However, the ongoing pregnancy rate was reported |
| Other bias | Unclear risk | There was insufficient information to assess whether an important risk of bias existed |
Dirnfeld 1991.
| Methods | Randomised controlled trial | |
| Participants | Infertile couples with a previously cancelled or unsuccessful IVF cycle owing to inadequate response Mean female age: 33.5 (range = 26 to 40) |
|
| Interventions |
Luteal support from day of oocyte retrieval with progesterone oil 100 mg I/M daily |
|
| Outcomes |
|
|
| Notes | We contacted the author. Long GnRH protocol was commenced in either luteal or follicular phase, although no explanation was given regarding how this decision was made | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A random numbered table was used |
| Allocation concealment (selection bias) | High risk | Allocation was not concealed |
| Blinding (performance bias and detection bias) All outcomes | High risk | The trial was not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 54 participants were randomised and received treatment |
| Selective reporting (reporting bias) | Low risk | The paper reported most of our prespecified relevant outcomes |
| Other bias | High risk | We contacted the authors. Long GnRH protocol was commenced in either the luteal or follicular phase |
Dirnfeld 1999.
| Methods | Prospective randomised controlled trial | |
| Participants | 63 participants with previous poor response to COH, high basal FSH (> 8 mIU/ml), or both, undergoing 78 IVF‐ET cycles All causes of infertility were included Exclusion criteria
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | There was more than 1 cycle per participant. Outcomes were described as per cycle Clinical pregnancy was defined as presence of intrauterine gestational sac on first trimester USG, and ongoing pregnancy was defined as 1 that progressed beyond 20 weeks' gestation |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Although the authors mentioned that a random number table was used to generate random sequence, it was not clear how the table was created |
| Allocation concealment (selection bias) | Unclear risk | The paper did not report allocation concealment |
| Blinding (performance bias and detection bias) All outcomes | High risk | The trial was not blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 63 women agreed to participate in the trial, but 78 were included in analysis (78 cycles). It was not clear if 63 or 78 participants were randomised |
| Selective reporting (reporting bias) | Unclear risk | Neither adverse outcomes were mentioned nor live birth rates |
| Other bias | Unclear risk | There was insufficient information to assess whether an important risk of bias existed |
Fenichel 1988.
| Methods | Randomised trial Allocation was done by drawing lots |
|
| Participants |
Inclusion criteria
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | The study also included an arm treated with clomiphene citrate and HMG without GnRHa | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random sequence generation was achieved through drawing lots |
| Allocation concealment (selection bias) | High risk | The paper did not conceal allocation |
| Blinding (performance bias and detection bias) All outcomes | High risk | The trial was not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 30 women were randomised; all received treatment |
| Selective reporting (reporting bias) | Unclear risk | Our primary outcome was not reported |
| Other bias | Unclear risk | There was insufficient information to assess whether an important risk of bias existed |
Foulot 1988.
| Methods | Randomised trial Allocation was done by drawing lots The trial was not blinded |
|
| Participants | Infertile couples Mean female age: 32 years Exclusion criteria
|
|
| Interventions |
Luteal phase support with uterogestan from day of oocyte retrieval |
|
| Outcomes |
|
|
| Notes | A measure of variance was not given for the number of oocytes and ampoules of gonadotrophins | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Allocation was done by drawing lots |
| Allocation concealment (selection bias) | Unclear risk | The paper did not report allocation concealment |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | The paper did not report blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 100 participants were randomised; all received treatment |
| Selective reporting (reporting bias) | Low risk | The ongoing pregnancy rate was reported, but adverse outcomes were not |
| Other bias | Unclear risk | There was insufficient information to assess whether an important risk of bias existed |
Frydman 1988.
| Methods | Randomised controlled trial | |
| Participants | 186 infertile couples with predominantly tubal factor (90%) Exclusion criteria
|
|
| Interventions |
Luteal phase support with dydrogesterone 30 mg daily |
|
| Outcomes |
|
|
| Notes | Participants were randomised to receive HMG and FSH in both protocols (2 interventions) We included the article after internal discussion with SB (as other systematic reviews have shown that FSH and HMG are equivalent) Although outcomes measured the number of oocytes retrieved, we did not include in meta‐analysis as there was a statistically significant difference in the oocytes retrieved in both groups in the HMG and FSH group within the long and short protocol group |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The method of allocation was not described; we wrote to the trial authors but did not receive any reply |
| Allocation concealment (selection bias) | Unclear risk | The paper did not report allocation concealment |
| Blinding (performance bias and detection bias) All outcomes | High risk | The trial was not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 186 participants were randomised; all received treatment. Our outcomes were reported |
| Selective reporting (reporting bias) | Low risk | Most relevant outcomes were reported (ongoing pregnancy rate was reported, but adverse outcomes were not) |
| Other bias | Unclear risk | There was insufficient information to assess whether an important risk of bias existed |
Fábregues 2005.
| Methods | Prospective randomised study | |
| Participants | 150 consecutive infertile women undergoing their first cycle of IVF/ICSI fulfilling Inclusion criteria
All women had normal ovaries and no previous surgery; none of them had occult ovarian failure on the basis of their basal FSH < 12 IU/L |
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Intention‐to‐treat analysis was not done There was 1 cycle per woman A total dose of gonadotropin with variance was given rather than number of ampoules |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was computer generated |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes were used |
| Blinding (performance bias and detection bias) All outcomes | High risk | The trial was not blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 150 women were randomised; all received treatment (13 cycles were cancelled due to low response ‐ there were analyses for 137 women). Although there were 75 women in each group, full data was only reported for 68 and 69 women, respectively |
| Selective reporting (reporting bias) | Unclear risk | There was no mention of our primary outcomes |
| Other bias | Low risk | Although the paper gave an a priori sample size calculation, there was insufficient information to assess whether an important risk of bias existed |
Garcia‐Velasco 2000.
| Methods | Prospective randomised controlled trial | |
| Participants | 70 women who were undergoing stimulation for IVF/ICSI cycles and were previous low responders Inclusion criteria
Exclusion criteria
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | There was 1 cycle per woman This was a special category of participants (poor responders) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A computerised random number list was used |
| Allocation concealment (selection bias) | Unclear risk | The paper did not describe the method of allocation concealment |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | The paper did not report blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 70 women were randomised; all women were included in analysis |
| Selective reporting (reporting bias) | Unclear risk | There was no mention of our primary outcomes |
| Other bias | Unclear risk | There was insufficient information to assess whether an important risk of bias existed |
Hazout 1993.
| Methods | Randomised trial Allocation was done by using permutation blocks of 8 |
|
| Participants |
Inclusion criteria
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | The number of pregnancies in the short protocol group was estimated from the pregnancy rates given | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Allocation was done by using a permutation block of 8 |
| Allocation concealment (selection bias) | High risk | Allocation was not concealed |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | The paper did not report blinding |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 182 women were randomised. 96 received the long protocol. 84 reported in the text versus 86 in the table received the 7‐day protocol. There were no cancellations mentioned for the 7‐day group |
| Selective reporting (reporting bias) | Unclear risk | There was no mention of our primary outcomes |
| Other bias | Unclear risk | There was insufficient information to assess whether an important risk of bias existed |
Hedon 1988.
| Methods | Randomised trial | |
| Participants | Infertile couples (tubal factor: 53%, unexplained: 19%, endometriosis: 7%, combined cause: 22%), excluding those with male factor infertility and ovulation disorders | |
| Interventions |
Luteal support HCG 1500 IU x 2 |
|
| Outcomes |
|
|
| Notes | A measure of variance was not given for the number of oocytes retrieved and number of gonadotropin ampoules | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation was not described |
| Allocation concealment (selection bias) | Unclear risk | The paper did not report allocation concealment |
| Blinding (performance bias and detection bias) All outcomes | High risk | The trial was not blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Although 120 women were randomised, data were available for only 112 women; we wrote to the authors but did not receive any reply. 120 participants were randomised, but 56 participants received treatment in each group = 8 participants were not included due to the reasons mentioned |
| Selective reporting (reporting bias) | Low risk | Most relevant outcomes, including 1 of the primary outcomes in this review, were reported except adverse outcomes |
| Other bias | Unclear risk | There was insufficient information to assess whether an important risk of bias existed |
Isikoglu 2007.
| Methods | Prospective randomised trial | |
| Participants | 181 women undergoing IVF/ICSI | |
| Interventions | GnRHa was administered from the 21st day of the preceding cycle. Participants were divided into 2 groups:
|
|
| Outcomes |
|
|
| Notes | Participants were randomised by a computer‐generated list | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was achieved by a computer‐generated list |
| Allocation concealment (selection bias) | Unclear risk | The paper did not mention allocation concealment |
| Blinding (performance bias and detection bias) All outcomes | High risk | Only embryologists were reported to be blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 181 participants were randomised; all participants were included and mentioned in the analysis |
| Selective reporting (reporting bias) | Low risk | All our outcomes were mentioned |
| Other bias | Low risk | We suspected no other bias |
Kingsland 1992.
| Methods | Randomised trial Allocation was concealed using sealed envelopes |
|
| Participants | Couples with all causes of infertility (tubal factor: 50%, unexplained: 29%, male factor: 14%, endometriosis: 5%) undergoing their first IVF attempt | |
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Women were randomised into 4 groups: A + B without GnRHa and C + D with GnRHa | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A random number table was used |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes were used |
| Blinding (performance bias and detection bias) All outcomes | High risk | The trial was not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 308 women were randomised into 4 groups; all participants received treatment. The number of cancelled cycles was reported |
| Selective reporting (reporting bias) | Low risk | Most of our outcomes were reported |
| Other bias | Low risk | We suspected no other bias |
Kondaveeti‐Gordon 1996.
| Methods | Randomised prospective study | |
| Participants | Women undergoing IVF/ICSI (first cycle only) | |
| Interventions |
|
|
| Outcomes |
|
|
| Notes | There was 1 cycle per woman. Although an a priori power calculation was done, the study was powered only to detect difference in the use of gonadotrophins | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was computer generated with a permuted block |
| Allocation concealment (selection bias) | High risk | Allocation was not concealed |
| Blinding (performance bias and detection bias) All outcomes | High risk | The trial was not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 86 participants were randomised; all participants received treatment and analysed |
| Selective reporting (reporting bias) | Unclear risk | There was no mention of our primary outcomes |
| Other bias | Unclear risk | There was insufficient information to assess whether an important risk of bias existed |
Lin 2013.
| Methods | Prospective randomised controlled study | |
| Participants | 100 participants undergoing IVF/ICSI cycle Inclusion criteria
Exclusion criteria
|
|
| Interventions | In both groups, a single dose of long‐acting GnRHa (Diphereline®, 1.25 mg, 3.75 mg/ampoule) was administered on days 20 to 22 of the mid‐luteal phase. Participants were divided into 2 groups:
Ovarian stimulation was performed with an initial gonadotropin dose of 75 to 300 IU (recombinant FSH (rFSH) or human menopausal gonadotropin (HMG)) |
|
| Outcomes |
|
|
| Notes | Clinical pregnancy was defined as a positive serum HCG result, with US evidence of a gestational sac and foetal heartbeat Μiscarriage rate was defined as the proportion of participants with an initially positive pregnancy test and US evidence of a gestational sac with a foetal pole where pregnancy failed to develop by 12 weeks of gestation Live birth rate was defined as pregnancies over 28 weeks per treatment cycle of ET Luteal phase support was started immediately after oocyte retrieval |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was achieved by computer‐generated random numbers 2 weeks after GnRHa administration |
| Allocation concealment (selection bias) | Unclear risk | The paper did not report allocation concealment |
| Blinding (performance bias and detection bias) All outcomes | High risk | The trial was not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 100 participants from random visits who met the inclusion criteria were recruited. 85 participants were included in analysis. However, all reasons and numbers mentioned for the 15 participants were missing (6 cycles were cancelled due to low response or privacy reasons; ET was cancelled in 6 cycles due to no useable embryos or high risk OHSS ‐ there was no extra justification) |
| Selective reporting (reporting bias) | Low risk | Relevant outcomes were reported |
| Other bias | Low risk | We suspected no other bias |
Loumaye 1989.
| Methods | Randomised trial The method of allocation was not described |
|
| Participants |
Inclusion criteria
|
|
| Interventions |
Luteal phase support with HCG 1500 IU intramuscular on days 6 and 9 after retrieval |
|
| Outcomes |
|
|
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation was not described |
| Allocation concealment (selection bias) | Unclear risk | The paper did not mention allocation concealment |
| Blinding (performance bias and detection bias) All outcomes | High risk | The trial was not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 18 participants were randomised; all received therapy. Cancellation was not mentioned; 17 out of 18 transferred |
| Selective reporting (reporting bias) | Low risk | Our prespecified relevant outcomes were reported, including ongoing pregnancy |
| Other bias | Unclear risk | There was insufficient information to assess whether an important risk of bias existed |
Pellicer 1989.
| Methods | Randomised trial The method of randomisation was not known |
|
| Participants | Women undergoing IVF between 15 January and 31 May 1998 Inclusion criteria
|
|
| Interventions | Pituitary desensitisation was achieved with 300 μgm of buserelin twice a day
HMG + FSH were used for stimulation. Standard dose was used up to day 5, which was then modified according to individual response |
|
| Outcomes |
|
|
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomly allocation into groups, but it was not clear how this was done |
| Allocation concealment (selection bias) | Unclear risk | The paper did not mention allocation concealment |
| Blinding (performance bias and detection bias) All outcomes | High risk | The trial was not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The total number of participants randomised was not mentioned in the methods. In the results section, 44 participants were mentioned as receiving treatment after randomisation |
| Selective reporting (reporting bias) | Unclear risk | There was no mention of our primary outcomes |
| Other bias | High risk | Group 1 and 2 commenced GnRHa on different days, although both were in the luteal phase |
Ron‐El 1990.
| Methods | Random allocation into 2 groups | |
| Participants | 216 consecutive women undergoing IVF/ICSI | |
| Interventions |
HMG was used for stimulation with a standard dose for the first 4 days followed by individual adjustment of doses |
|
| Outcomes |
|
|
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Although the paper reported random allocation, it did not describe the exact method |
| Allocation concealment (selection bias) | Unclear risk | The paper did not describe allocation concealment |
| Blinding (performance bias and detection bias) All outcomes | High risk | The trial was not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 216 women were randomised; all were mentioned to have received treatment |
| Selective reporting (reporting bias) | Unclear risk | There was no mention of our primary outcomes |
| Other bias | Unclear risk | There was insufficient information to assess whether an important risk of bias existed |
San Roman 1992.
| Methods | Randomised trial | |
| Participants | 55 women undergoing IVF‐ET regardless of previous cycle response or number of previous cycles undertaken | |
| Interventions |
|
|
| Outcomes |
|
|
| Notes | A clinical pregnancy was defined as USG visualisation of gestational sac or pathological evidence of trophoblast GnRHa dose was reduced at the start of stimulation |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation was not described |
| Allocation concealment (selection bias) | High risk | Allocation was not concealed |
| Blinding (performance bias and detection bias) All outcomes | High risk | The trial was not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 55 women were recruited and randomised. All women received treatment; outcomes were not reported for 5 of them (low response) |
| Selective reporting (reporting bias) | Low risk | Our prespecified relevant outcomes were reported |
| Other bias | Unclear risk | There was insufficient information to assess whether an important risk of bias existed |
Sarhan 2013.
| Methods | Prospective randomised controlled study | |
| Participants | 181 infertile participants undergoing ICSI cycles | |
| Interventions | All participants started treatment with subcutaneous daily injections of GnRHa (triptorelin). Participants were divided into 2 groups:
In both groups, the agonist treatment was continued until the day of HCG administration |
|
| Outcomes |
|
|
| Notes | Clinical pregnancy was defined by the presence of intrauterine gestational sac(s) with pulsating heart beats on trans‐vaginal ultrasound scan at 5 to 6 weeks' gestation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was achieved using closed envelopes |
| Allocation concealment (selection bias) | Unclear risk | The paper did not report allocation concealment |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | The paper did not report blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 181 participants were randomised. All participants were mentioned as included in analysis |
| Selective reporting (reporting bias) | Unclear risk | There was no mention of our primary outcomes |
| Other bias | Unclear risk | There was insufficient information to assess whether an important risk of bias existed |
Simon 1994.
| Methods | Prospective randomised trial | |
| Participants | 42 women undergoing a fresh cycle of IVF due to tubal obstruction Inclusion criteria
Exclusion criteria
|
|
| Interventions | After pituitary down‐regulation (serum estradiol < 30 pg/ml, serum progesterone < 0.5 ng/ml, and the absence of any ovarian follicle > 10 mm in size), participants were allocated into 2 groups:
Luteal support was provided with intramuscular progesterone injection in oil |
|
| Outcomes |
|
|
| Notes | There was 1 cycle per woman | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random sequence generation was not described |
| Allocation concealment (selection bias) | Unclear risk | The paper did not report allocation concealment |
| Blinding (performance bias and detection bias) All outcomes | High risk | The trial was not blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | In the text, it was reported that 43 women were randomised and received treatment, while in the abstract and tables, it is reported that 42 women received treatment |
| Selective reporting (reporting bias) | Unclear risk | There was no mention of our primary outcomes |
| Other bias | Unclear risk | There was an insufficient rationale or evidence that an identified problem would introduce bias |
Simons 2005.
| Methods | Double‐blind, randomised, multicentre study | |
| Participants | 178 women undergoing IVF/ICSI treatment, history of spontaneous regular cycle between 24 and 35 days Inclusion criteria
Exclusion criteria
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Comparison groups for this review: group L versus group S | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was computer generated |
| Allocation concealment (selection bias) | Low risk | Allocation was concealed in a sealed envelope in a central locker |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Participants and personnel blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 178 participants were randomised; 18 participants were not included in the analysis. (Reasons and numbers were mentioned thoroughly: discontinuation during the stimulation phase or missing LH data) |
| Selective reporting (reporting bias) | Low risk | All our prespecified relevant outcomes were reported |
| Other bias | Low risk | There was insufficient rationale or evidence that an identified problem would introduce bias |
Sunkara 2014.
| Methods | Prospective randomised controlled trial | |
| Participants | 111 women with previous poor ovarian response undergoing IVF Exclusion criteria
|
|
| Interventions | Women were allocated to 3 groups:
|
|
| Outcomes |
|
|
| Notes | Participants were allocated to 1 of the 3 study groups by a third party, distant, internet‐based block randomisation to ensure complete allocation concealment. The clinician performing the OR and the embryologist involved were blinded to the treatment allocation The participants were poor responders |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was computerised |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes were used |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The clinician performing the OR and the embryologist involved were blinded to the treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 111 women were randomised. 19 women did not receive the allocated intervention (reasons mentioned: 3 conceived spontaneously; 16 decided to postpone IVF treatment) |
| Selective reporting (reporting bias) | Low risk | All our planned outcomes were reported |
| Other bias | Low risk | The study appeared to be free of other sources of bias |
Tan 1992.
| Methods | Randomised trial | |
| Participants | Couples with all causes of infertility (unexplained: 25%, male factor: 11%, endometriosis: 5%, tubal factor: 58%) undergoing their first cycle of IVF | |
| Interventions |
Luteal support with HCG 2000 IU x 2 |
|
| Outcomes |
|
|
| Notes | Significantly more cleaved embryos were available for transfer in participants on the long versus the short protocol A measure of variance was not given for the number of oocytes retrieved and number of ampoules of gonadotrophins |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random tables were used |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes were used |
| Blinding (performance bias and detection bias) All outcomes | High risk | The trial was not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 91 women were randomised and received treatment |
| Selective reporting (reporting bias) | Unclear risk | There was no mention of our primary outcomes |
| Other bias | Unclear risk | There was insufficient information to assess whether an important risk of bias existed |
Tasdemir 1995.
| Methods | Randomised trial | |
| Participants | Couples with all causes of infertility (tubal factor: 40%, male factor: 29%, unexplained: 19%, endometriosis: 10%) undergoing their first IVF cycle | |
| Interventions |
Luteal phase support with 2000 IU HCG x 3 |
|
| Outcomes |
|
|
| Notes | The trial author confirmed that the study was randomised | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The exact method of randomisation was not known |
| Allocation concealment (selection bias) | Unclear risk | The paper did not describe allocation concealment |
| Blinding (performance bias and detection bias) All outcomes | High risk | The trial was not blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 90 participants were randomised. The number of participants allocated to each group was not mentioned in the text or tables either. The number of participants receiving treatment and analysed was not mentioned |
| Selective reporting (reporting bias) | Low risk | Live birth rate was reported |
| Other bias | High risk | The median number of embryos transferred was 4 with the long GnRHa protocol and 1 with the short protocol. We obtained confirmation of randomisation in the original review. We did not receive any reply to further queries. There was no power calculation and no mention of the exact number of participants in each group |
Urbancsek 1996.
| Methods | Prospective randomised trial | |
| Participants | 124 women undergoing IVF due to tubal factor or unexplained infertility | |
| Interventions |
|
|
| Outcomes |
|
|
| Notes | There was more than 1 cycle per participant A measure of variance for the number of oocytes was not given Only unexplained infertility and tubal factor were included There was more than 1 cycle per woman; data for only 1 cycle were not available separately |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation was centrally prepared |
| Allocation concealment (selection bias) | Unclear risk | The paper did not report allocation concealment |
| Blinding (performance bias and detection bias) All outcomes | High risk | The trial was not blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Intention‐to‐treat analysis was not done |
| Selective reporting (reporting bias) | Low risk | Live birth and other prespecified outcomes were reported |
| Other bias | Unclear risk | There was insufficient information to assess whether an important risk of bias existed |
van de‐Helder 1990.
| Methods | Randomised trial | |
| Participants |
Inclusion criteria
|
|
| Interventions |
Buserelin was continued until the day of HCG administration |
|
| Outcomes |
|
|
| Notes | The trial included a third group that was randomised not to receive GnRHa. Clinical pregnancy was defined as foetal heart activity seen on ultrasound A measure of variance was not provided for the average number of gonadotrophins ampoules and average number of oocytes retrieved |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation was not described |
| Allocation concealment (selection bias) | Unclear risk | The paper did not mention allocation concealment |
| Blinding (performance bias and detection bias) All outcomes | High risk | The trial was not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 152 participants were randomised; 152 participants received treatment ‐ there were 23 cancellations, all due to low response |
| Selective reporting (reporting bias) | Low risk | Our prespecified outcomes, including ongoing pregnancy rate, were reported |
| Other bias | Unclear risk | There was insufficient information to assess whether an important risk of bias existed |
Weissman 2003.
| Methods | Randomised prospective study | |
| Participants | 60 low responders (from previous cycle) who were undergoing IVF Poor responders were defined as fewer than 5 oocytes retrieved, 3 or fewer follicles 16 mm or larger on the day of cancellation or serum E2 less than 500 pg/ml on the day of HCG administration Only participants with FSH less than 20 IU/L were included |
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Both short and long protocols were modified protocols 1 cycle per woman |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was computer generated |
| Allocation concealment (selection bias) | Unclear risk | The paper did not mention allocation concealment |
| Blinding (performance bias and detection bias) All outcomes | High risk | The trial was not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 60 participants were randomised; treatment was allocated to all of them |
| Selective reporting (reporting bias) | Low risk | Most outcomes of interest were reported |
| Other bias | Unclear risk | There was insufficient information to assess whether an important risk of bias existed |
Yang 1996.
| Methods | Randomised trial | |
| Participants | Couples with all causes of infertility except severe male factor and polycystic ovarian syndrome (tubal factor: 52%, unexplained: 28%, endometriosis: 17%, male factor: 3%) | |
| Interventions |
Luteal support with progesterone vaginal suppositories 200 mg x 2 daily with HCG 1500 IU intramuscular x 4 |
|
| Outcomes |
|
|
| Notes | Long GnRHa was commenced in either the luteal or follicular phase There was 1 cycle per woman |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation was not described; we wrote to the trial authors but received no reply |
| Allocation concealment (selection bias) | Unclear risk | The paper did not mention allocation concealment |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | The paper did not report blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 60 participants were randomised; all received treatment, and no cancellations were reported |
| Selective reporting (reporting bias) | Unclear risk | There was no mention of our primary outcomes |
| Other bias | Unclear risk | There was insufficient information to assess whether an important risk of bias existed |
Ye 2001.
| Methods | Prospective randomised trial | |
| Participants | 109 infertile couples undergoing IVF | |
| Interventions |
|
|
| Outcomes |
|
|
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐based randomisation was used |
| Allocation concealment (selection bias) | High risk | Allocation was not concealed |
| Blinding (performance bias and detection bias) All outcomes | High risk | The trial was not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 109 participants were randomised; all received therapy as shown in the tables |
| Selective reporting (reporting bias) | Unclear risk | There was no mention of our primary outcomes |
| Other bias | Unclear risk | There was insufficient information to assess whether an important risk of bias existed |
Zhang 2009.
| Methods | Prospective randomised trial | |
| Participants | 88 participants with infertility due to tubal factor Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were divided into 2 groups:
|
|
| Outcomes |
|
|
| Notes | The article was in Chinese | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation was not mentioned |
| Allocation concealment (selection bias) | Unclear risk | The paper did not mention allocation concealment |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | The paper did not mention blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 88 participants were randomised; all participants received treatment. Analyses were mentioned for 88 participants (data derived from tables) |
| Selective reporting (reporting bias) | Unclear risk | There was no mention of our primary outcomes |
| Other bias | Unclear risk | There was insufficient information to assess whether an important risk of bias existed |
BMI: body mass index. COH: controlled ovarian hyperstimulation. E2: estradiol. ET: embryo transfer. FSH: follicle‐stimulating hormone. GnRH: gonadotrophin‐releasing hormone. GnRHa: gonadotrophin‐releasing hormone agonists. HCG: human chorionic gonadotrophin. HMG: human menopausal gonadotrophin. I/M: intramuscular. ICSI: intracytoplasmic sperm injection. IGFBP‐4: insulin‐like growth factor binding protein‐4. IGF‐II: insulin‐like growth factor II. IVF: in vitro fertilisation. IVF‐ET: in vitro fertilisation pre‐embryo transfer. LH: luteinising hormone. OHSS: ovarian hyperstimulation syndrome. OR: oocyte retrieval PCOS: polycystic ovary syndrome. rFSH: recombinant follicle‐stimulating hormone. s.c.: subcutaneously. US/USG: ultrasonography.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abd Rabo 2012 | This paper evaluated the effect of using letrozole in improvement of the results of ICSI/ET in women with endometriosis using a long agonist protocol |
| Aflatoonian 2012 | This paper assessed the efficacy of low dose HCG in the late follicular phase in controlled ovarian stimulation using a GnRH agonist protocol |
| Albuquerque 2013 | This was a Cochrane review from the Cochrane Database of Systematic Reviews |
| Antoine 1990 | This paper compared GnRHa with no GnRHa |
| Azem 2010 | There were no data for comparison after repeated attempts to reach the authors. Only the abstract was available |
| Beckers 2000 | Participants were randomised into 3 groups. 2 interventions were compared stopping GnRHa on day 3 of stimulation as well as luteal support |
| Bloch 2011 | This paper used the same study population as in Azem 2010, assessing phycological outcomes |
| Braendle 1989 | Allocation to a short or long protocol was sequential and not random |
| Buvat 1993 | Quasi‐randomisation was used (randomised by year of birth) |
| Cambiaghi 2011 | This paper compared 2 different regimens of long protocols |
| Check 1992 | This was a randomised trial (allocation was based on the last digit of the participant's social security number) comparing long versus ultrashort protocol, but we excluded as it had a cross‐over design |
| Cheon 2008 | This paper compared 2 regiments for the same GnRHa protocol |
| Corson 1992 | This study compared 3 protocols ((a) stopping GnRHa at start of stimulation, (b) reducing GnHa at start of stimulation, (c) no GnRHa at all for both IVF as well as GIFT cycles). We could not extract data on IVF cycles separately. We contacted the authors, but separate data were not available, as the study was very old |
| Dessolle 2011 | This was a prospective non‐randomised study |
| Devroey 1994 | This was a non‐randomised pilot study |
| Dor 1992 | This study compared GnRHa with no GnRHa |
| Eftekhar 2013 | This trial compared daily injection with a single intramuscular dose of GnRHa |
| Elgendy 1998 | This paper reported quasi‐randomisation (alternate IVF numbers) |
| Faber 1998 | This was a non‐randomised study |
| Ferraretti 1996 | This was a retrospective data analysis |
| Fujii 1997 | This paper reported quasi‐randomisation (group allocated based on day of visit to the unit) |
| Garcia 1990 | The method of allocation to short or long luteal GnRHa protocol was stated to be prospective, but no information was provided on whether randomisation was used. We attempted to contact the authors, but received no reply |
| Gersak 1994 | The paper compared GnRHa with no GnRHa |
| Gianaroli 1994 | This study compared 3 different long protocols: (a) buserelin 0.5 mg s.c. twice a day 15 days prior to ovarian stimulation, (b) a single dose of long‐acting triptorelin (3.75 mg) 15 days before ovarian stimulation, (c) long‐acting triptorelin 4 weeks prior to ovarian stimulation followed by daily administration of 0.1 mg agonist until HCG injection. This did not follow any of the defined comparisons in the protocol |
| Gizzo 2014 | Randomisation was according to the luteal phase supplementation |
| Harrison 1994 | This paper compared GnRHa with no GnRHa |
| Huang 2012 | This was a retrospective study |
| Jinno 1996 | This paper was an evaluation of bromocryptine in 1 of 2 groups |
| Jinno 2009 | This paper compared different doses of the same GnRH agonist |
| Ku 2005 | This was a retrospective study |
| Kubik 1990 | The paper compared GnRHa with no GnRHa |
| Kuc 2011 | This was a retrospective study |
| Li 2012 | This paper compared 2 different doses of Lupron Depot in GnRH analogues in a long 21 protocol |
| Liu 2012 | This was a non‐RCT |
| Lorusso 2004 | This was a non‐randomised study |
| Loutradis 1998 | This paper compared 2 regiments in long protocols |
| Marcus 1993 | This was a randomised trial (allocation was by the last digit of the medical file number) comparing long versus ultrashort protocol. We excluded it because of its cross‐over design |
| Maroulis 1991 | 192 women who were referred for IVF. Randomly allocated to group A (protocol with pure FSH‐HMG), group B (received GnRHa in the luteal phase), or group C (received GnRHa in the follicular phase). During the first 9 months, participants were randomly allocated between protocol A and B (in 2:1 ratio) whereas for the last 11 months between protocols A, B, and C |
| McKenna 1989 | Allocation was not random |
| Mochtar 2011 | Allocation depended on the size of leading follicles |
| NCT00436319 | This was a stopped trial (personal communication with authors) |
| NCT02342197 | The primary outcome was the number of oocytes retrieved (not the prespecified outcomes of our review) |
| Neuspiller 1998 | This was a study on oocyte donors |
| Norman 1991 | Allocation to a short or long luteal GnRHa protocol was not random, but based on clinical grounds |
| Padilla 1991 | Participants were allocated to 5 different protocols based on the results of the Lupron screening test. Those with pattern C were randomised into 1 of 3 protocols in phase 1: (1) no GnRHa, (2) double dose GnRHa with flare protocol (not clear whether this was short or ultrashort protocol), or (3) luteal phase GnRHa. In phase 2, they were all given luteal phase GnRHa |
| Pantos 1994 | This study was quasi‐random (alternate) |
| Remorgida 1989 | We excluded as only GIFT cycles were included |
| Rodrigues 2014 | This was a non‐RCT |
| Ron‐El 1992 | Allocation to ultrashort GnRHa protocol was based on the ability of the participant to attend the clinic on day 1 or 2. These participants were matched by age and indication for IVF to participants having the long GnRHa protocol |
| Sarhan 2012 | This paper compared 3 GnRH analogues in long protocols |
| Sathanandan 1989 | This comprised of long luteal GnRHa protocol with leuprolide in participants identified as having poor or abnormal response in a previous stimulation cycle versus short GnRHa protocol with leuprolide in participants undergoing their first cycle of treatment or who had had a satisfactory response in a previous cycle. Allocation was not random, and participant groups were not similar |
| Smitz 1992 | Quasi‐randomisation (allocated to groups according to year of birth) |
| Smitz 1992a | The method of allocation to short or long GnRHa protocol was not stated. Pregnancy was not the outcome in this study because none of the participants had embryo transfer owing to complete failure of fertilisation |
| Stenbæk 2013 | This trial compared a short antagonist versus long agonist protocol |
| Suganuma 1996 | This paper reported pseudo‐randomisation (alternate participants were allocated into the groups). Some participants had cross‐over of groups |
| Tanaka 2014 | The was not an RCT |
| Tarin 1990 | That study was a cytogenetic analysis of human unfertilised oocytes |
| Tarlatzis 1993 | Although the study was designed to have random allocation, in practice the randomisation was incomplete as it was done according to the stimulation protocol, the scheduling convenience, and the cost of the analogue used |
| Tarlatzis 1994 | Although the study was designed to have random allocation, in practice the randomisation was incomplete as it was done according to the stimulation protocol, the scheduling convenience, and the cost of the analogue used |
| Tehraninejad 2010 | The paper compared daily doses versus Lupron Depot of GnRH in a long 21 protocol |
| van de‐Helder 1990b | The paper compared GnRHa with no GnRHa |
| Wu 2012 | Participants were assigned to 4 groups according to serum progesterone and oestradiol concentrations on the day of HCG administration |
| Yang 1991 | The paper compared GnRHa with no GnRHa |
FSH‐HMG: follicle stimulating hormone‐human menopausal gonadotrophin. GIFT: gamete intra‐fallopian transfer. GnRHa: gonadotrophin‐releasing hormone agonists. HCG: human chorionic gonadotrophin. ICSI/ET: intracytoplasmic sperm injection/embryo transfer. IVF: in vitro fertilisation. RCT: randomised controlled trial. s.c.: subcutaneously.
Characteristics of ongoing studies [ordered by study ID]
NCT01006954.
| Trial name or title | Comparison of Micro Dose Gonadotropin‐Releasing Hormone (GnRH) Agonist Flare up & Flare Protocol in Poor Responders in Assisted Reproductive Technology (ART) Cycle |
| Methods | Allocation: randomised Endpoint Classification: efficacy Study Intervention Model: parallel assignment Masking: single blind (participant) Primary purpose: treatment |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions |
|
| Outcomes |
|
| Starting date | September 2008 |
| Contact information | Endocrinology and Female Infertility Department Reproductive Medicine Research Centre Royan Institute Academic Center for Education, Culture and Research (ACECR) Tehran Islamic Republic of Iran 14114 |
| Notes | This study has been completed. No data were published |
Differences between protocol and review
Our protocol mentioned one of the comparison groups as gonadotrophin‐releasing hormone agonists (GnRHa) versus placebo. However, there is a review of randomised controlled trials (RCTs) on this topic (Fields 2013) suggesting that use of GnRHa is associated with a better outcome in assisted reproduction technology (ART). The current review intended to explore which protocol was better.
A short protocol versus a short stop protocol was not listed in the initial comparison groups. However, since we were looking at all protocols for GnRHa for pituitary down‐regulation, we felt it was appropriate to include studies comparing these groups.
The original review was withdrawn, and a new protocol was published.
11 December 2008: The title changed from 'Long versus short gonadotropin releasing hormone agonist protocols for pituitary desensitization in assisted reproduction cycles' to 'Gonadotrophin‐releasing hormone agonist protocols for pituitary suppression in assisted reproductive treatment'.
A further title change from 'Gonadotrophin‐releasing hormone agonist protocols for pituitary down regulation in assisted reproductive treatment' to 'Gonadotropin releasing hormone agonist protocols for pituitary suppression in assisted reproduction' was agreed in 2011.
31 August 2014: we added two comparisons:
long protocol: discontinuing versus continuing GnRHa after HCG administration; and
long protocol: administration of GnRHa for fewer than versus more than 18 days before stimulation.
Contributions of authors
AM: initiated and conceptualised the protocol; undertook data searching, selection of studies, data extraction, drafting of the first update of the review, assessment of studies for inclusion, interpretation and analysis of data, and editing of the second update. CS: co‐drafted the protocol; undertook data searching, selection of studies, and data extraction, and wrote the second update. AG: co‐drafted the protocol; undertook data searching, selection of studies, and data extraction. GB: undertook data searching, selection of studies, and data extraction in the second update. SB: overall supervision and editing of the review.
Timeline
A new search for RCTs will be performed every two years with the review updated accordingly.
Sources of support
Internal sources
Assisted Reproduction Unit, University of Aberdeen, UK.
Assisted Reproduction Unit, 3rd Department of Obstetrics and Gynecology, University of Athens, Greece.
External sources
No sources of support supplied
Declarations of interest
Charalampos S Siristatidis: nothing to declare. Ahmed Gibreel: nothing to declare. George Basios: nothing to declare. Abha Maheshwari: nothing to declare. Siladitya Bhattacharya: nothing to declare.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Acharya 1992 {published data only}
- Acharya U, Small J, Randall J, Hamilton M, Templeton A. Prospective study of short and long regimens of gonadotropin‐releasing hormone agonist in in vitro fertilization program. Fertility and Sterility 1992;57(4):815‐8. [PUBMED: 555693] [DOI] [PubMed] [Google Scholar]
Berker 2010 {published data only}
- Berker B, Duvan Cİ, Kaya C, Aytaç R, Satıroğlu H. Comparison of the ultrashort gonadotropin‐releasing hormone agonist‐antagonist protocol with microdose flare‐up protocol in poor responders: a preliminary study. Journal of the Turkish German Gynecological Association 2010;11(4):187‐93. [PUBMED: 24591934] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cedrin‐Durnerin 2000 {published data only}
- Cedrin‐Durnerin I, Bidart JM, Robert P, Wolf JP, Uzan M, Hugues JN. Consequences on gonadotrophin secretion of an early discontinuation of gonadotrophin‐releasing hormone agonist administration in short term protocol for in vitro fertilization. Human Reproduction (Oxford, England) 2000;15(5):1009‐14. [PUBMED: 10783343] [DOI] [PubMed] [Google Scholar]
Chatillon‐Boissier 2012 {published data only}
- Chatillon‐Boissier K, Genod A, Denis‐Belicard E, Felloni B, Chene G, Seffert P, et al. Prospective randomised study of long versus short agonist protocol with poor responder patients during in vitro fertilization [Étude prospective randomisée protocoles agonistes long versus court chez les patientes mauvaises répondeuses en fécondation in vitro]. Gynécologie Obstétrique & Fertilité 2012;40(11):652‐57. [PUBMED: 22342506] [DOI] [PubMed] [Google Scholar]
Chen 1992 {published data only}
- Chen SU, Yang YS, Ho HN, Hwang JL, Lien YR, Lin HR, et al. Comparison of long‐term versus three‐day leuprolide acetate for ovarian stimulation in human in vitro fertilization program. Journal of Reproduction and Fertility 1992;1:9‐16. [Google Scholar]
Dal Prato 2001 {published data only}
- Dal Prato L, Borini A, Trevisi MR, Bonu MA, Sereni E, Flamigni C. Effect of reduced dose of triptorelin at the start of ovarian stimulation on the outcome of IVF: a randomized study. Human Reproduction (Oxford, England) 2001;16(7):1409‐14. [PUBMED: 11425821] [DOI] [PubMed] [Google Scholar]
De Placido 1991 {published data only}
- Placido G, Zullo F, Colacurcu N, Perrone D, Carravetta C, Montemagno U. Long acting versus daily administration GnRH analogs in IVF (abstract). Human Reproduction. 7th Annual Meeting of the ESHRE and 7th World Congress on IVF and Assisted Procreation 1991;6(Suppl 1):339‐40 (abs # p482). [Google Scholar]
Ding 2013 {published data only}
- Ding LJ, Wang B, Shen XY, Yan GJ, Zhang NY, Hu YL, Sun HX. Withdrawal of GnRH agonist decreases oestradiol and VEGF concentrations in high responders. Reproductive BioMedicine Online 2013;27(2):131‐9. [PUBMED: 23764202] [DOI] [PubMed] [Google Scholar]
Dirnfeld 1991 {published data only}
- Dirnfeld M, Gonen Y, Lissak A, Goldman S, Koifman M, Sorokin Y, Abramovici H. A randomized prospective study on the effect of short and long buserelin treatment in women with repeated unsuccessful in vitro fertilization (IVF) cycles due to inadequate ovarian response. Journal of In Vitro Fertilization and Embryo Transfer: IVF 1991;8(6):339‐43. [PUBMED: 1770275] [DOI] [PubMed] [Google Scholar]
Dirnfeld 1999 {published data only}
- Dirnfeld M, Fruchter O, Yshai D, Lissak A, Ahdut A, Abramovici H. Cessation of gonadotropin‐releasing hormone analogue (GnRH‐a) upon down‐regulation versus conventional long GnRH‐a protocol in poor responders undergoing in vitro fertilization. Fertility and Sterility 1999;72(3):406‐11. [PUBMED: 10519608] [DOI] [PubMed] [Google Scholar]
Fábregues 2005 {published data only}
- Fábregues F, Peñarrubia J, Creus M, Casamitjana R, Vanrell JA, Balasch J. Effect of halving the daily dose of triptorelin at the start of ovarian stimulation on hormone serum levels and the outcome of in vitro fertilization. Fertility and Sterility 2005;83(3):785‐8. [PUBMED: 15749520] [DOI] [PubMed] [Google Scholar]
Fenichel 1988 {published data only}
- Fenichel P, Grimaldi M, Hieronimus S, Olivero JF, Donzeau A, Benoit B, et al. Systematic inhibition of the luteinizing hormone with a gonadoliberin analog, triptorelin, during ovarian stimulation for fertilization in vitro. Choice of protocol [Inhibition systematique de l`hormone luteinisante par un analogue de la gonadoliberine, la triptoreline, au cours de la stimulation ovarienne pour fecondation in vitro: choix du protocole]. Presse Médicale 1988;17(15):719‐22. [PUBMED: 2968548] [PubMed] [Google Scholar]
Foulot 1988 {published data only}
- Foulot H, Dubisson JB, Ranoux C, Aubriot FX, Poirot C. Randomized study comparing the short and long protocols of buserelin in 100 in vitro fertilization cycles [Etude randomisee entre protocole court et protocole long de buserelin concernant 100 cycles de fecondation in vitro]. Contraception, Fertilite, Sexualite 1988;16:628‐9. [Google Scholar]
Frydman 1988 {published data only}
- Frydman R, Belaisch‐Allart J, Parneix I, Forman R, Hazout A, Testart J. Comparison between flare up and down regulation effects of luteinizing hormone‐releasing hormone agonists in an in vitro fertilization program. Fertility and Sterility 1988;50(3):471‐5. [PUBMED: 2970407] [DOI] [PubMed] [Google Scholar]
Garcia‐Velasco 2000 {published data only}
- Garcia‐Velasco JA, Isaza V, Requena A, Martinez‐Salazar FJ, Landazábal A, Remohi J, et al. High doses of gonadotrophins combined with stop versus non‐stop protocol of GnRH analogue administration in low responder IVF patients: a prospective, randomized controlled trial. Human Reproduction (Oxford, England) 2000;15(11):2292‐6. [PUBMED: 11056121] [DOI] [PubMed] [Google Scholar]
Hazout 1993 {published data only}
- Hazout A, Ziegler D, Cornel C, Fernandez H, Lelaidier C, Frydman R. Comparison of short 7 day and prolonged treatment with gonadotropin‐releasing hormone agonist desensitization for controlled ovarian hyperstimulation. Ferility and Sterility 1993;59(3):596‐600. [PUBMED: 8458463] [DOI] [PubMed] [Google Scholar]
Hedon 1988 {published data only}
- Hedon B, Arnal F, Badoc E, Boulot P, Huet JM, Fries N, et al. Randomized comparison of the long and short protocols for ovarian stimulation in association with GnRH agonist for in vitro fertilization [Comparison randomisee protocole long‐protocole court dans les stimulations de l`ovaire en association avec un agoniste de la GnRH en vue de fecondation in vitro]. Contraception, Fertilite, Sexualite 1988;16:624‐7. [Google Scholar]
Isikoglu 2007 {published data only}
- Isikoglu M, Ozgur K, Oehninger S. Extension of GnRH agonist through the luteal phase to improve the outcome of intracytoplasmic sperm injection. The Journal of Reproductive Medicine 2007;52(7):639‐44. [PUBMED: 17847764] [PubMed] [Google Scholar]
Kingsland 1992 {published data only}
- Kingsland C, Tan SL, Bickerton N, Mason B, Campbell S. The routine use of gonadotropin‐releasing hormone agonists for all patients undergoing in vitro fertilization. Is there any medical advantage? A prospective randomized study. Fertility and Sterility 1992;57(4):804‐9. [PUBMED: 1555691] [DOI] [PubMed] [Google Scholar]
Kondaveeti‐Gordon 1996 {published data only}
- Kondaveeti‐Gordon U, Harrison RF, Barry‐Kinsella C, Gordon AC, Drudy L, Cottell E. A randomized prospective study of early follicular or midluteal initiation of long protocol gonadotropin‐releasing hormone in an in vitro fertilization program. Fertility and Sterility 1996;66(4):582‐6. [PUBMED: 8816620] [DOI] [PubMed] [Google Scholar]
Lin 2013 {published data only}
- Lin H, Li Y, Li L, Zhang Q, Wang W, Chen X, et al. Effect of delayed initiation of gonadotropin in luteal long protocol on in vitro fertilization. Gynecological Endocrinology: the Official Journal of the International Society of Gynecological Endocrinology 2013;29(9):846‐50. [PUBMED: 23865696] [DOI] [PubMed] [Google Scholar]
Loumaye 1989 {published data only}
- Loumaye E, Vankrieken L, Depreester S, Psalti I, Cooman S, Thomas K. Hormonal changes induced by short‐term administration of gonadotropin‐releasing hormone agonist during ovarian hyperstimulation for in vitro fertilization and their consequences for embryo development. Fertility and Sterility 1989;51(1):105‐11. [PUBMED: 2491990] [DOI] [PubMed] [Google Scholar]
Pellicer 1989 {published data only}
- Pellicer A, Simón C, Miró F, Castellvi RM, Ruiz A, Ruiz M, et al. Ovarian response and outcome of in‐vitro fertilization in patients treated with gonadotrophin‐releasing hormone analogues in different phases of menstrual cycle. Human Reproduction (Oxford, England) 1989;4(3):285‐9. [PUBMED: 2497135] [DOI] [PubMed] [Google Scholar]
Ron‐El 1990 {published data only}
- Ron‐El R, Herman A, Golan A, Ven H, Caspi E, Diedrich K. The comparison of early follicular and midluteal administration of long acting gonadotropin‐releasing hormone agonist. Fertility and Sterility 1990;54(2):233‐7. [PUBMED: 2143145] [DOI] [PubMed] [Google Scholar]
San Roman 1992 {published data only}
- San Roman GA, Surrey ES, Judd HL, Kerin JF. A prospective randomized comparison of luteal phase versus concurrent follicular phase initiation of gonadotropin‐releasing hormone agonist for in‐vitro fertilization. Fertility and Sterility 1992;58(4):744‐9. [PUBMED: 1426320] [DOI] [PubMed] [Google Scholar]
Sarhan 2013 {published data only}
- Sarhan AM, Harira MM, Alshazly SM. Comparing stimulation requirements and final outcome between early follicular and mid luteal‐starting of pituitary suppression in the long gonadotropin releasing hormone agonist protocol. Fertility and Sterility 2013;100 Suppl (2):S472. [DOI] [PubMed] [Google Scholar]
Simon 1994 {published data only}
- Simon A, Benshushan A, Shushan A, Zajicek G, Dorembus D, Lewin A, et al. A comparison between a standard and reduced dose of D‐Trp‐6‐luteinizing hormone‐releasing hormone administered after pituitary suppression for in‐vitro fertilization. Human Reproduction (Oxford, England) 1994;9(10):1813‐7. [PUBMED: 7844208] [DOI] [PubMed] [Google Scholar]
Simons 2005 {published data only}
- Simons AH, Roelofs HJ, Schmoutziguer AP, Roozenburg BJ, van't Hof‐van den Brink EP, Schoonderwoerd SA. Early cessation of triptorelin in in vitro fertilization: a double blind, randomized study. Fertility and Sterility 2005;83(4):889‐96. [PUBMED: 15820796] [DOI] [PubMed] [Google Scholar]
Sunkara 2014 {published data only}
- Sunkara SK, Coomarasamy A, Faris R, Braude P, Khalaf Y. Long gonadotropin‐releasing hormone agonist versus short agonist versus antagonist regimens in poor responders undergoing in vitro fertilization: a randomized controlled trial. Fertility and Sterility 2014;101(1):147‐53. [PUBMED: 24188873] [DOI] [PubMed] [Google Scholar]
- Sunkara SK, Coomarasamy A, Khalaf Y, Braude P. A three‐arm randomised controlled trial comparing Gonadotrophin Releasing Hormone (GnRH) agonist long regimen versus GnRH agonist short regimen versus GnRH antagonist regimen in women with a history of poor ovarian response undergoing in vitro fertilisation (IVF) treatment: Poor responders intervention trial (PRINT). Reproductive Health 2007; Vol. 4, issue 12. [PUBMED: 18163902] [DOI] [PMC free article] [PubMed]
- Sunkara SK, Coomarasamy AA, Faris RA, Braude PA, Khalaf Y. Effectiveness of the GnRH agonist long, GnRH agonist short and GnRH antagonist regimens in poor responders undergoing IVF treatment: a three arm randomised controlled trial. Human Reproduction 2013;28:311‐356. [Google Scholar]
Tan 1992 {published data only}
- Tan SL, Kingsland C, Campbell S, Mills C, Bradfield J, Alexander N, et al. The long protocol of administration of gonadotropin‐releasing hormone agonist is superior to the short protocol for ovarian stimulation for in‐vitro fertilization. Fertility and Sterility 1992;57(4):810‐4. [PUBMED: 1555692] [DOI] [PubMed] [Google Scholar]
Tasdemir 1995 {published data only}
- Tasdemir M, Tasdemir I, Kodama H, Higuchi M. Is long‐protocol gonadotropin releasing hormone agonist administration superior to the short protocol in ovarian stimulation for in‐vitro fertilization?. International Journal of Fertility and Menopausal Studies 1995;40(1):25‐8. [PUBMED: 7749431] [PubMed] [Google Scholar]
Urbancsek 1996 {published data only}
- Urbancsek J, Witthaus E. Midluteal buserelin is superior to early follicular phase buserelin in combined gonadotropin‐releasing hormone analog and gonadotropin stimulation in in vitro fertilization. Fertility and Sterility 1996;65(5):966‐71. [PUBMED: 8612858] [PubMed] [Google Scholar]
van de‐Helder 1990 {published data only}
- de‐Helder AB, Helmerhorst FM, Blankhart A, Brand R, Waegemaekers C, Naaktgeboren N. Comparison of ovarian stimulation regimens for in vitro fertilization (IVE) with and without a gonadotropin‐releasing hormone (GnRH) agonist: results of a randomized study. Journal of In Vitro Fertilization and Embryo Transfer: IVF 1990;7(6):358‐62. [PUBMED: 2127604] [DOI] [PubMed] [Google Scholar]
Weissman 2003 {published data only}
- Weissman A, Farhi J, Royburt M, Nahum H, Glezerman M, Levran D. Prospective evaluation of two stimulation protocols for low responders who were undergoing in vitro fertilization‐embryo transfer. Fertility and Sterility 2003;79(4):886‐92. [PUBMED: 12749425] [DOI] [PubMed] [Google Scholar]
Yang 1996 {published data only}
- Yang TS, Tsan SH, Wang BC, Chang SP, Ng HT. The evaluation of a new 7‐day gonadotropin‐relasing hormone agonist protocol in the controlled ovarian hyperstimulation for in vitro fertilization. The Journal of Obstetrics and Gynaecology Research 1996;22(2):133‐7. [PUBMED: 8697342] [DOI] [PubMed] [Google Scholar]
Ye 2001 {published data only}
- Ye H, Huang G, Pei L. A prospective, randomized controlled study comparing the effects of gonadotropin‐releasing hormone agonist long and short protocols for in vitro fertilization. Zhonghua Fu Chan Ke Za Zhi 2001;36(4):222‐5. [PUBMED: 11783366] [PubMed] [Google Scholar]
Zhang 2009 {published data only}
- Zhang J, Li Y, Liu J, Liu De, Liu N, Chen X. Effect of 7‐day gonadotropin‐releasing hormone agonist protocol on IGF‐II and IGFBP‐4 levels in the follicular fluid. Zhong Nan Da Xue Xue Bao Yi Xue Ban 2009;34(3):190‐4. [PUBMED: 19349671] [PubMed] [Google Scholar]
References to studies excluded from this review
Abd Rabo 2012 {published data only}
- Abd Rabbo MH, Elmaghraby HA, Mashali NA, Abdel Moneim ME. Effect of aromatase inhibitor (letrozole) with long agonist protocol on the results of ICSI/ET in females with minimal and mild endometriosis. Alexandria Journal of Medicine 2012;48(4):303‐7. [DOI: 10.1016/j.ajme.2012.04.001] [DOI] [Google Scholar]
Aflatoonian 2012 {published data only}
- Aflatoonian A, Yousefnejad F, Eftekhar M, Mohammadian F. Efficacy of low‐dose hCG in late follicular phase in controlled ovarian stimulation usingGnRH agonist protocol. Archives of Gynecology and Obstetrics 2012;286(3):771‐5. [PUBMED: 22619027] [DOI] [PubMed] [Google Scholar]
Albuquerque 2013 {published data only}
- Albuquerque LE, Tso LO, Saconato H, Albuquerque MC, Macedo CR. Depot versus daily administration of gonadotrophin‐releasing hormone agonist protocols for pituitary down regulation in assisted reproduction cycles. Cochrane Database of Systematic Reviews 2013, Issue 1. [DOI: 10.1002/14651858.CD002808.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Antoine 1990 {published data only}
- Antoine JM, Firmin C, Alvarez S, Cornet D, Tibi C, Mandelbaum J, et al. Ovarian stimulation for in vitro fertilization using LHRH agonists: comparison of plasma and intra‐follicular hormone profiles using "short" and "long" protocols [Stimulation ovarienne pour fecondation in vitro utilisant les agonistes de la LHRH: Comparison des profils hormonaux plasmatiques et intra‐folliculaires des protocoles courts et longs]. Journal de Gynecologie, Obstetrique et Biologie de la Reproduction 1990;19(8):1035‐40. [PUBMED: 2150525] [PubMed] [Google Scholar]
Azem 2010 {published data only}
- Azem F, Bloch M, Kuvalsky D, Wagman I, Bibi G, Amit A. Comparison of short and long GnRH agonist protocols using recombinant FSH for IVF/ICSI: a controlled prospective study. Fertility and Sterility 2010;94(4 Suppl):S163. [Google Scholar]
Beckers 2000 {published data only}
- Beckers NG, Laven JS, Eijkemans MJ, Fauser BC. Follicular and luteal phase characteristics following early cessation of gonadotrophin‐releasing hormone agonist during ovarian stimulation for in‐vitro fertilization. Human Reproduction (Oxford, England) 2000;15(1):43‐9. [PUBMED: 10611186] [DOI] [PubMed] [Google Scholar]
Bloch 2011 {published data only}
- Bloch M, Azem F, Aharonov I, Ben Avi I, Yagil Y, Schreiber S, et al. GnRH‐agonist induced depressive and anxiety symptoms during in vitro fertilization‐embryo transfer cycles. Fertility and Sterility 2011;95(1):307‐9. [PUBMED: 20801439] [DOI] [PubMed] [Google Scholar]
Braendle 1989 {published data only}
- Braendle W, Lindner C, Lichtenberg V, Goepel E, Bettendorf G. Endocrine profiles and luteal function during GnRH‐analogue/HMG therapy. Human Reproduction (Oxford, England) 1989;4(8 Suppl):121‐6. [PUBMED: 2533217] [DOI] [PubMed] [Google Scholar]
Buvat 1993 {published data only}
- Buvat J, Marcolin G, Guittard C, Herbaut M, Louvet A, Renouard O. Requiem for the short protocol? [Requiem pour le protocol]. Contraception, Fertilite, Sexualite 1993;21:436. [Google Scholar]
Cambiaghi 2011 {published data only}
- Cambiaghi AS, Leao RBF, Castellotti DS, Nascimento P. Triptorelin every other day can prevent premature LH surge: a strategy to reduce the daily gonadotrophin‐releasing hormone agonists injections. Fertility and Sterility 2011;96(3 Suppl):S175. [Google Scholar]
Check 1992 {published data only}
- Check JH, Nowroozi K, Chase JS. Comparison of short versus long‐term leuprolide acetate‐human menopausal gonadotrophin hyperstimulation in in‐vitro fertilization patients. Human Reproduction (Oxford, England) 1992;7(1):31‐4. [PUBMED: 1551954] [DOI] [PubMed] [Google Scholar]
Cheon 2008 {published data only}
- Cheon KW, Song SJ, Choi BC, Lee SC, Lee HB, Yu SY, et al. Comparison of clinical efficacy between a single administration of long‐acting gonadotrophin‐releasing hormone agonist (GnRHa) and daily administrations of short‐acting GnRHa in in vitro fertilization‐embryo transfer cycles. Journal of Korean Medical Science 2008;23(4):662‐6. [PUBMED: 18756054] [DOI] [PMC free article] [PubMed] [Google Scholar]
Corson 1992 {published data only}
- Corson SL, Batzer FR, Gocial B, Eisenberg E, Huppert LC, Nelson JR. Leuprolide acetate‐prepared in vitro fertilization‐gamete intrafallopian transfer cycles: efficacy versus controls and cost analysis. Fertility and Sterility 1992;57(3):601‐5. [PUBMED: 1740205] [DOI] [PubMed] [Google Scholar]
Dessolle 2011 {published data only}
- Dessolle L, Ferrier D, Colombel A, Fréour T, Jean M, Barrière P. Prolonging GnRH‐agonist to achieve ovarian suppression does not compromise the results of a long protocol. European Journal of Obstetrics, Gynecology and Reproductive Biology 2011;159(1):111‐4. [PUBMED: 21763059] [DOI] [PubMed] [Google Scholar]
Devroey 1994 {published data only}
- Devroey P, Mannaerts B, Smitz J, Coelingh Bennink H, Steirteghem A. Clinical outcome of a pilot efficacy study on recombinant human follicle‐stimulating hormone (Org 32489) combined with various gonadotrophin‐releasing hormone agonist regimens. Human Reproduction (Oxford, England) 1994;9(6):1064‐9. [PUBMED: 7962377] [DOI] [PubMed] [Google Scholar]
Dor 1992 {published data only}
- Dor J, Shulman A, Pariente C, Levran D, Bider D, Menashe Y, et al. The effect of gonadotropin‐releasing hormone agonist on the ovarian response and in vitro fertilization results in polycystic ovarian syndrome: a prospective study. Fertility and Sterility 1992;57(2):366‐71. [PUBMED: 1531200] [DOI] [PubMed] [Google Scholar]
Eftekhar 2013 {published data only}
- Eftekhar M, Rahmani E, Mohammadian F. Comparison of pregnancy outcome in half‐dose Triptorelin and short‐acting Decapeptyl in long protocol in ART cycles: a randomized clinical trial. Iranian Journal of Reproductive Medicine 2013;11(2):133‐8. [PUBMED: 24639738] [PMC free article] [PubMed] [Google Scholar]
Elgendy 1998 {published data only}
- Elgendy M, Afnan M, Holder R, Lashen H, Afifi Y, Lenton W, et al. Reducing the dose of gonadotrophin‐releasing hormone agonist on starting ovarian stimulation: effect on ovarian response and in‐vitro fertilization outcome. Human Reproduction (Oxford, England) 1998;13(9):2382‐5. [PUBMED: 9806253] [DOI] [PubMed] [Google Scholar]
Faber 1998 {published data only}
- Faber BM, Mayer J, Cox B, Jones D, Toner JP, Oehninger S, Muasher SJ. Cessation of gonadotropin‐releasing hormone agonist therapy combined with high‐dose gonadotropin stimulation yields favourable pregnancy results in low responders. Fertility and Sterility 1998;69(5):826‐30. [PUBMED: 9591487] [DOI] [PubMed] [Google Scholar]
Ferraretti 1996 {published data only}
- Ferraretti AP, Magli C, Feliciani E, Montanaro N, Gianaroli L. Relationship of timing of agonist administration in the cycle phase to the ovarian response to gonadotropins in the long down‐regulation protocols for assisted reproductive technologies. Fertility and Sterility 1996;65(1):114‐21. [PUBMED: 8557125] [DOI] [PubMed] [Google Scholar]
Fujii 1997 {published data only}
- Fujii S, Sagara M, Kudo H, Kagiya A, Sato S, Saito Y. A prospective randomized comparison between long and discontinuous‐long protocols of gonadotropin‐releasing hormone agonist for in vitro fertilization. Fertility and Sterility 1997;67(6):1166‐8. [PUBMED: 9176463] [DOI] [PubMed] [Google Scholar]
Garcia 1990 {published data only}
- Garcia JE, Padilla SL, Bayati J, Baramki TA. Follicular phase gonadotropin‐releasing hormone agonist and human gonadotropins: a better alternative for ovulation induction in in vitro fertilization. Fertility and Sterility 1990;53(2):302‐5. [PUBMED: 2105247] [DOI] [PubMed] [Google Scholar]
Gersak 1994 {published data only}
- Gersak K, Meden‐Vrtovec H, Tomazevic T. The effects of gonadotrophin‐releasing hormone agonist on follicular development in patients with polycystic ovary syndrome in an in‐vitro fertilization and embryo transfer programme. Human Reproduction (Oxford, England) 1994;9(9):1596‐9. [PUBMED: 7836506] [DOI] [PubMed] [Google Scholar]
Gianaroli 1994 {published data only}
- Gianaroli L, Ferraretti AP, Feliciani E, Tabanelli C, Magli C, Fortini D. Prospective randomized study of D‐Trp6‐ LHRH versus buserelin in long desensitization protocols for medically assisted conception cycles. Human Reproduction (Oxford, England) 1994;9(2):220‐5. [PUBMED: 8027275] [DOI] [PubMed] [Google Scholar]
Gizzo 2014 {published data only}
- Gizzo S, Andrisani A, Esposito F, Noventa M, Gangi S, Angioni S, et al. Which luteal phase support is better for each IVF stimulation protocol to achieve the highest pregnancy rate? A superiority randomized clinical trial. Gynecological Endocrinology: the official journal of the International Society of Gynecological Endocrinology 2014;30:1‐7. [PUBMED: 25268567] [DOI] [PubMed] [Google Scholar]
Harrison 1994 {published data only}
- Harrison RF, Kondaveeti U, Barry‐Kinsella C, Gordon A, Drudy L, Cottell E, et al. Should gonadotropin‐releasing hormone down‐regulation therapy be routine in in vitro fertilization?. Fertility and Sterility 1994;62(3):568‐73. [PUBMED: 8062954] [PubMed] [Google Scholar]
Huang 2012 {published data only}
- Huang R, Fang C, Xu S, Yi Y, Liang X. Premature progesterone rise negatively correlated with live birth rate in IVF cycles with GnRH agonist: an analysis of 2,566 cycles. Fertility and Sterility 2012;98(3):664‐70.e2. [PUBMED: 22704632] [DOI] [PubMed] [Google Scholar]
Jinno 1996 {published data only}
- Jinno M, Yoshimura Y, Ubukata Y, Nakamura Y. A novel method of ovarian stimulation for in vitro fertilization: bromocriptine‐rebound method. Fertility and Sterility 1996;66(2):271‐4. [PUBMED: 8690115] [DOI] [PubMed] [Google Scholar]
Jinno 2009 {published data only}
- Jinno M, Watanabe A, Hirohama J, Eguchi N, Hatakeyama N. Diminished but significant mid‐cycle LH surge can be induced in the long protocol of GNRH agonist and HMG regimen, resulting in a great increase in the rate of ongoing pregnancy. Fertility and Sterility 2009;92(3 Suppl):S235. [Google Scholar]
Ku 2005 {published data only}
- Ku SY, Choi YS, Jee BC, Suh CS, Choi YM, Kim JG, et al. A preliminary study on reduced dose (33 or 25 g) gonadotropin‐releasing hormone agonist long protocol for multi follicular ovaria stimulation in patients with high basal serum follicular‐stimulating hormone levels undergoing in vitro fertilization‐embryo transfer. Gynaecological Endocrinology: the official journal of the International Society of Gynecological Endocrinology 2005;21:227‐31. [DOI] [PubMed] [Google Scholar]
Kubik 1990 {published data only}
- Kubik CJ, Guzick DS, Berga SL, Zeleznik AJ. Randomized, prospective trial of leuprolide acetate and conventional superovulation in first cycle of in vitro fertilization and gamete intrafallopian transfer. Fertility and Sterility 1990;54(5):836‐41. [PUBMED: 2121551] [DOI] [PubMed] [Google Scholar]
Kuc 2011 {published data only}
- Kuć P, Kuczyńska A, Topczewska M, Tadejko P, Kuczyński W. The dynamics of endometrial growth and the triple layer appearance in three different controlled ovarian hyperstimulation protocols and their influence on IVF outcomes. Gynecological Endocrinology: the official journal of the International Society of Gynecological Endocrinology 2011;27(11):867‐73. [PUBMED: 21231852] [DOI] [PubMed] [Google Scholar]
Li 2012 {published data only}
- Li Y, Yang D, Zhang Q. Clinical outcome of one‐third‐dose depot triptorelin is the same as half‐dose depot triptorelin in the long protocol of controlled ovarian stimulation. Journal of Human Reproductive Sciences 2012;5(1):14‐9. [PUBMED: 22870009] [DOI] [PMC free article] [PubMed] [Google Scholar]
Liu 2012 {published data only}
- Liu N, Ma Y, Li R, Jin H, Li M, Huang X, Feng HL, Qiao J. Comparison of follicular fluid amphiregulin and EGF concentrations in patients undergoing IVF with different stimulation protocols. Endocrine 2012;42(3):708‐16. [PUBMED: 22678853] [DOI] [PubMed] [Google Scholar]
Lorusso 2004 {published data only}
- Lorusso F, Depalo R, Selvaggi L. Relationship between gonadotropin releasing hormone agonist dosage and in vitro fertilization outcome. Gynecological Endocrinology: the official Journal of the International Society of Gynecological Endocrinology 2004;18(2):69‐73. [PUBMED: 15195497] [DOI] [PubMed] [Google Scholar]
Loutradis 1998 {published data only}
- Loutradis D, Drakakis P, Kallianidis K, Bletsa R, Milingos S, Makris N, et al. The effect of the duration of GnRH‐agonist down regulation before ovarian stimulation on the biological and clinical outcome after intracytoplasmic sperm injection. European Journal of Obstetrics Gynecology and Reproductive Biology 1998;80(2):251‐5. [PUBMED: 9846679] [DOI] [PubMed] [Google Scholar]
Marcus 1993 {published data only}
- Marcus SF, Brinsden PR, Macnamee M, Rainsbury PA, Elder KT, Edwards RG. Comparative trial between an ultra‐short and long protocol of luteinizing hormone‐releasing hormone agonist for ovarian stimulation in in‐vitro fertilization. Human Reproduction (Oxford, England) 1993;8(2):238‐43. [PUBMED: 8473427] [DOI] [PubMed] [Google Scholar]
Maroulis 1991 {published data only}
- Maroulis GB, Emery M, Verkauf BS, Saphier A, Bernhisel M, Yeko TR. Prospective randomized study of human menotropin versus a follicular and a luteal phase gonadotropin‐releasing hormone analog‐human menotropin stimulation protocols for in vitro fertilization. Fertility and Sterility 1991;55(6):1157‐64. [PUBMED: 1903732] [DOI] [PubMed] [Google Scholar]
McKenna 1989 {published data only}
- McKenna KM, Foster P, McBain J, Martin M, Johnston WI. Combined treatment with gonadotrophin releasing hormone agonist and gonadotrophins in poor responders to hyperstimulation for in vitro fertilization (IVF): clinical and endocrine results. Australian and New Zealand Journal of Obstetrics and Gynaecology 1989;29(4):428‐32. [PUBMED: 2517193] [DOI] [PubMed] [Google Scholar]
Mochtar 2011 {published data only}
- Mochtar MH, Custers IM, Koks CA, Bernardus RE, Verhoeve HR, Mol BW, et al. Timing oocyte collection in GnRH agonists down‐regulated IVF and ICSI cycles: a randomized clinical trial. Human Reproduction (Oxford, England) 2011;26(5):1091‐6. [PUBMED: 21362684] [DOI] [PubMed] [Google Scholar]
NCT00436319 {published data only}
- NCT00436319. Effect of the duration of GnRH‐analogue downregulation on pregnancy rates in IVF. clinicaltrials.gov/ct2/show/NCT00436319 (accessed 2015).
NCT02342197 {published data only}
- NCT02342197. Comparative study between minidose long protocol and microdose flare protocol in controlled ovarian hyperstimulation for poor responders in ICSI cycles. clinicaltrials.gov/ct2/show/NCT02342197 (accessed 04/2015).
Neuspiller 1998 {published data only}
- Neuspiller F, Levy M, Remohí J, Ruiz A, Simón C, Pellicer A. The use of long‐ and short‐acting forms of gonadotrophin‐releasing hormone analogues in women undergoing oocyte donation. Human Reproduction (Oxford, England) 1998;13(5):1148‐51. [PUBMED: 9647536] [DOI] [PubMed] [Google Scholar]
Norman 1991 {published data only}
- Norman RJ, Warnes GM, Wang X, Kirby CA, Matthews CD. Differential effects of gonadotrophin‐releasing hormone agonists administered as desensitizing or flare protocols on hormonal function in the luteal phase of hyperstimulated cycles. Human Reproduction (Oxford, England) 1991;6(2):206‐13. [PUBMED: 1905309] [DOI] [PubMed] [Google Scholar]
Padilla 1991 {published data only}
- Padilla SL, Smith RD, Garcia JE. The Lupron screening test: tailoring the use of leuprolide acetate in ovarian stimulation for in vitro fertilization. Fertility and Sterility 1991;56(1):79‐83. [PUBMED: 1906020] [DOI] [PubMed] [Google Scholar]
Pantos 1994 {published data only}
- Pantos K, Meimeth‐Damianaki T, Vaxevanoglou T, Kapetanakis E. Prospective study of a modified gonadotropin‐releasing hormone agonist long protocol in an in vitro fertilization program. Fertility and Sterility 1994;61(4):709‐13. [PUBMED: 8150115] [DOI] [PubMed] [Google Scholar]
Remorgida 1989 {published data only}
- Remorgida V, Anserini P, Croce S, Costa M, Ferraiolo A, Centonze A, et al. The duration of pituitary suppression by means of intranasal gonadotropin hormone‐releasing hormone analogue administration does not influence the ovarian response to gonadotropin stimulation and success rate in a gamete intrafallopian transfer (GIFT) program. Journal of In Vitro Fertilization and Embryo Transfer 1989;6(2):76‐80. [PUBMED: 2498446] [DOI] [PubMed] [Google Scholar]
Rodrigues 2014 {published data only}
- Rodrigues RS, Setti AS, Braga D, Valente FM, Iaconelli A, Borges E. Pituitary suppression with a GnRHa short protocol in an alternate day schedule associated with rhCG microdoses. JBRA Assisted Reproduction 2014;18(3):76‐9. [DOI: 10.5935/1518-0557.20140011] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ron‐El 1992 {published data only}
- Ron‐El R, Herman A, Golan A, Soffer Y, Nachum H, Caspi E. Ultrashort gonadotropin‐releasing hormone agonist (GnRH‐a) protocol in comparison with the long‐acting GnRH‐a protocol and menotropin alone. Fertility and Sterility 1992;58(6):1164‐8. [PUBMED: 1459267] [DOI] [PubMed] [Google Scholar]
Sarhan 2012 {published data only}
- Sarhan A, Elsamanoudy A, Harira M. Hormonal responses and clinical outcome are the same with three doses of gonadotropin‐releasing hormone agonist used in short stimulation protocol. Human Reproduction 2012;27 (Suppl 2):ii302‐ii337. [DOI] [PubMed] [Google Scholar]
Sathanandan 1989 {published data only}
- Sathanandan M, Warnes GM, Kirby CA, Petrucco OM, Matthews CD. Adjuvant leuprolide in normal, abnormal, and poor responders to controlled ovarian hyperstimulation for in vitro fertilization/gamete intrafallopian transfer. Fertility and Sterility 1989;51(6):998‐1006. [PUBMED: 2498135] [DOI] [PubMed] [Google Scholar]
Smitz 1992 {published data only}
- Smitz J, Abbeel E, Bollen N, Camus M, Devroy P, Tournaye H, et al. The effect of gonadotrophin‐releasing hormone (GnRH) agonist in the follicular phase on in‐vitro fertilization outcome in normo‐ovulatory women. Human Reproduction (Oxford, England) 1992;7(8):1098‐102. [PUBMED: 1400933] [DOI] [PubMed] [Google Scholar]
Smitz 1992a {published data only}
- Smitz J, Erard P, Camus M, Devroey P, Tournaye H, Wisanto A, Steirteghem AC. Pituitary gonadotrophin secretory capacity during the luteal phase in superovulation using GnRH‐agonists and HMG in a desensitization or flare‐up protocol. Human Reproduction (Oxford, England) 1992;7(9):1225‐9. [PUBMED: 1479002] [DOI] [PubMed] [Google Scholar]
Stenbæk 2013 {published data only}
- Stenbæk DS, Toftager M, Hjordt LV, Jensen PS, Holst K, Holland T, et al. A comparison of changes in mental distress in women undergoing short versus long in vitro fertilization protocol: a clinical prospective randomized trial. Human Reproduction 2013;28(Suppl):i261‐i282. [Google Scholar]
Suganuma 1996 {published data only}
- Suganuma N, Tsukahara SI, Kitagawa T, Furuhashi M, Asada Y, Kondo I. A controlled ovarian hyperstimulation regimen involving intermittent gonadotropin administration with a "short" protocol of gonadotropin releasing hormone agonist for in vitro fertilization. Journal of Assisted Reproduction and Genetics 1996;13(1):43‐8. [PUBMED: 8825166] [DOI] [PubMed] [Google Scholar]
Tanaka 2014 {published data only}
- Tanaka A, Nagayoshi M, Tanaka I. Selection of an optimal controlled ovarian hyperstimulation method in relation to the number of antral follicles in patients less than 40 years old. Fertility and Sterility 2014;102(3):e223. [Google Scholar]
Tarin 1990 {published data only}
- Tarin JJ, Pellicer A. Consequences of high ovarian response to gonadotropins: a cytogenetic analysis of unfertilized human oocytes. Fertility and Sterility 1990;54(4):665‐70. [PUBMED: 2209887] [PubMed] [Google Scholar]
Tarlatzis 1993 {published data only}
- Tarlatzis BC, Pados G, Bontis J, Lagos S, Grimbizis G, Spanos E, et al. Ovarian stimulation with Buserelin/HMG/HCG: prospective randomized study of short versus long protocol. Human Reproduction (Oxford, England) 1993;8(6):807‐12. [PUBMED: 8345067] [DOI] [PubMed] [Google Scholar]
Tarlatzis 1994 {published data only}
- Tarlatzis BC, Grimbizis G, Pournaropoulos F, Bontis J, Lagos S, Pados G, et al. Evaluation of two gonadotropin‐releasing hormone (GnRH) analogues (leuprolide and buserelin) in short and long protocols for assisted reproduction techniques. Journal of Assisted Reproduction and Genetics 1994;11(2):85‐91. [PUBMED: 7819707] [DOI] [PubMed] [Google Scholar]
Tehraninejad 2010 {published data only}
- Tehraninejad ESh, Nekoo EA, Ezabadi Z, Rashidi BH, Amirchaghmaghi E, Matroud EP. Half‐dose, long‐acting gonadotropin‐releasing hormone agonist (Diphereline) is comparable with daily injections of short‐acting gonadotropin‐releasing hormone agonist (Suprefact) in IVF/ICSI cycles. Archives of Medical Science 2010;6(6):945‐9. [PUBMED: 22427771] [DOI] [PMC free article] [PubMed] [Google Scholar]
van de‐Helder 1990b {published data only}
- de‐Helder AB, Helmerhorst FM, Blankhart A, Brand R, Waegemaekers C, Naaktgeboren N. Comparison of ovarian stimulation regimens for in vitro fertilization (IVE) with and without a gonadotropin‐releasing hormone (GnRH) agonist: results of a randomized study. Journal of In Vitro Fertilization and Embryo Transfer 1990;7(6):358‐62. [PUBMED: 2127604] [DOI] [PubMed] [Google Scholar]
Wu 2012 {published data only}
- Wu Z, Li R, Ma Y, Deng B, Zhang X, Meng Y, et al. Effect of HCG‐day serum progesterone and oestradiol concentrations on pregnancy outcomes in GnRH agonist cycles. Reproductive Biomedicine Online 2012;24(5):511‐20. [PUBMED: 22417667] [DOI] [PubMed] [Google Scholar]
Yang 1991 {published data only}
- Yang YS, Ho HN, Lien YR, Hwang JL, Melinda S, Lin HR, et al. The use of a long‐acting gonadotropin‐releasing hormone analog (D‐Trp‐6‐LHRH) for improvement of ovarian stimulation in assisted conception programs. Journal of the Formosan Medical Association 1991;90(11):1081‐5. [PUBMED: 1687055] [PubMed] [Google Scholar]
References to ongoing studies
NCT01006954 {published data only}
- NCT01006954. Comparison of micro dose gonadotropin‐releasing hormone (GnRH) agonist flare up & flare protocol in poor responders in assisted reproductive technology (ART) cycle. clinicaltrials.gov/ct2/show/NCT01006954 (accessed 04/2015).
Additional references
Barlow 1998
- Barlow DH. GnRHagonists and in vitro fertilization. Journal of Reproductive Medicine 1998;43 Suppl:245‐51. [PubMed] [Google Scholar]
Donderwinkel 1993
- Donderwinkel PF, Schoot DC, Pache TD, Jong FH, Hop WC, Fauser BC. Luteal function following ovulation induction in polycystic ovary syndrome patients using exogenous gonadotrophins in combination with a gonadotrophin‐releasing hormone agonist. Human Reproduction (Oxford, England) 1993;8(12):2027‐32. [PUBMED: 8150898] [DOI] [PubMed] [Google Scholar]
Fields 2013
- Fields E, Chard J, James D, Treasure T, Guideline Development Group. Fertility (update): summary of NICE guidance. BMJ 2013;346:f650. [DOI: 10.1136/bmj.f650] [DOI] [PubMed] [Google Scholar]
Griffin 2002
- Griffin PD, Rowe PJ, Vayena E, editors. Current practices and controversies in assisted reproduction: report of a WHO meeting on "Medical, Ethical and Social Aspects of Assisted Reproduction". Geneva, Switzerland: World Health Organization, 2002. [ISBN 92 4 159030 0] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [ updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hughes 1992
- Hughes EG, Fedorkow DM, Daya S, Sagle MA, Koppel P, Collins JA. The routine use of gonadotropin‐releasing hormone agonists prior to in vitro fertilization and gamete intrafallopian transfer: a meta‐analysis of randomized controlled trials. Fertility and Sterility 1992;58(5):888‐96. [PUBMED: 1426372] [DOI] [PubMed] [Google Scholar]
Jenkins 1996
- Jenkins JM. The influence, development and management of functional ovarian cysts during IVF cycles. Journal of the British Fertility Society 1996;1:132‐6. [Google Scholar]
Maheshwari 2008
- Maheshwari A, Hamilton M, Bhattacharya S. Effect of female age on the diagnostic categories of infertility. Human Reproduction (Oxford, England) 2008;23(3):538‐42. [PUBMED: 18308834] [DOI] [PubMed] [Google Scholar]
Mancini 2011
- Mancini F, Tur R, Martinez F, Coroleu B, Rodríguez I, Barri PN. Gonadotrophin‐releasing hormone‐antagonists vs long agonist in in‐vitro fertilization patients with polycystic ovary syndrome: a meta‐analysis. Gynecological Endocrinology: the Official Journal of the International Society of Gynecological Endocrinology 2011;27(3):150‐5. [PUBMED: 21117862] [DOI] [PubMed] [Google Scholar]
Min 2004
- Min JK, Breheny SA, Maclachlan V, Healy DL. What is the most relevant standard of success in assisted reproduction? The singleton, term gestation, live birth rate per cycle initiated: the BESST endpoint for assisted reproduction. Human Reproduction (Oxford, England) 2004;19(1):3‐7. [PUBMED: 14688149] [DOI] [PubMed] [Google Scholar]
Mohsen 2013
- Mohsen IA, Din RE. Minimal stimulation protocol using letrozole versus microdose flare up GnRH agonist protocol in women with poor ovarian response undergoing ICSI. Gynecological Endocrinology: The Official Journal of the International Society of Gynecological Endocrinology 2013;29(2):105‐8. [PUBMED: 23134528] [DOI] [PubMed] [Google Scholar]
Regan 1990
- Regan L, Owen EJ, Jacobs HS. Hypersecretion of luteinising hormone, infertility, and miscarriage. Lancet 1990;336(8724):1141‐4. [PUBMED: 1978024] [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Stuck 1998
- Stuck AE, Rubenstein LZ, Wieland D. Bias in meta‐analysis detected by a simple, graphical test. Asymmetry detected in funnel plot was probably due to true heterogeneity. BMJ 1998;316(7129):469‐70. [PUBMED: 9492685] [PMC free article] [PubMed] [Google Scholar]
Sungurtekin 1995
- Sungurtekin U, Jansen RP. Profound luteinizing hormone suppression after stopping the gonadotropin‐releasing hormone‐agonist leuprolide acetate. Fertility and Sterility 1995;63(3):663‐5. [PUBMED: 7851604] [PubMed] [Google Scholar]
Westergaard 2000
- Westergaard LG, Laursen SB, Andersen CY. Increased risk of early pregnancy loss by profound suppression of luteinizing hormone during ovarian stimulation in normogonadotrophic women undergoing assisted reproduction. Human Reproduction (Oxford, England) 2000;15(5):1003‐8. [PUBMED: 10783342] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Daya 2000
- Daya S. Gonadotropin releasing hormone agonist protocols for pituitary desensitization in in vitro fertilization and gamete intrafallopian transfer cycles. Cochrane Database of Systematic Reviews 2000, Issue 2. [DOI: 10.1002/14651858.CD001299] [DOI] [PubMed] [Google Scholar]
Maheshwari 2011
- Maheshwari A, Gibreel A, Siristatidis CS, Bhattacharya S. Gonadotrophin‐releasing hormone agonist protocols for pituitary suppression in assisted reproduction. Cochrane Database of Systematic Reviews 2011, Issue 8. [DOI: 10.1002/14651858.CD006919.pub3] [DOI] [PubMed] [Google Scholar]


