Abstract
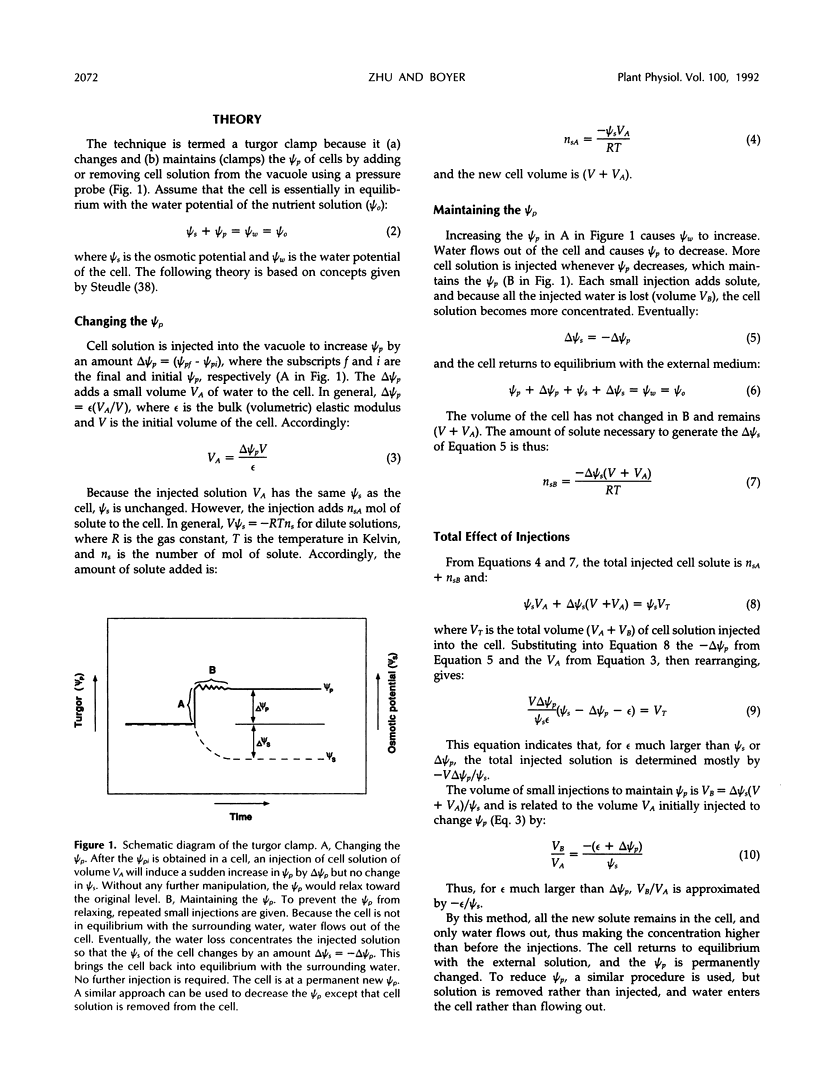
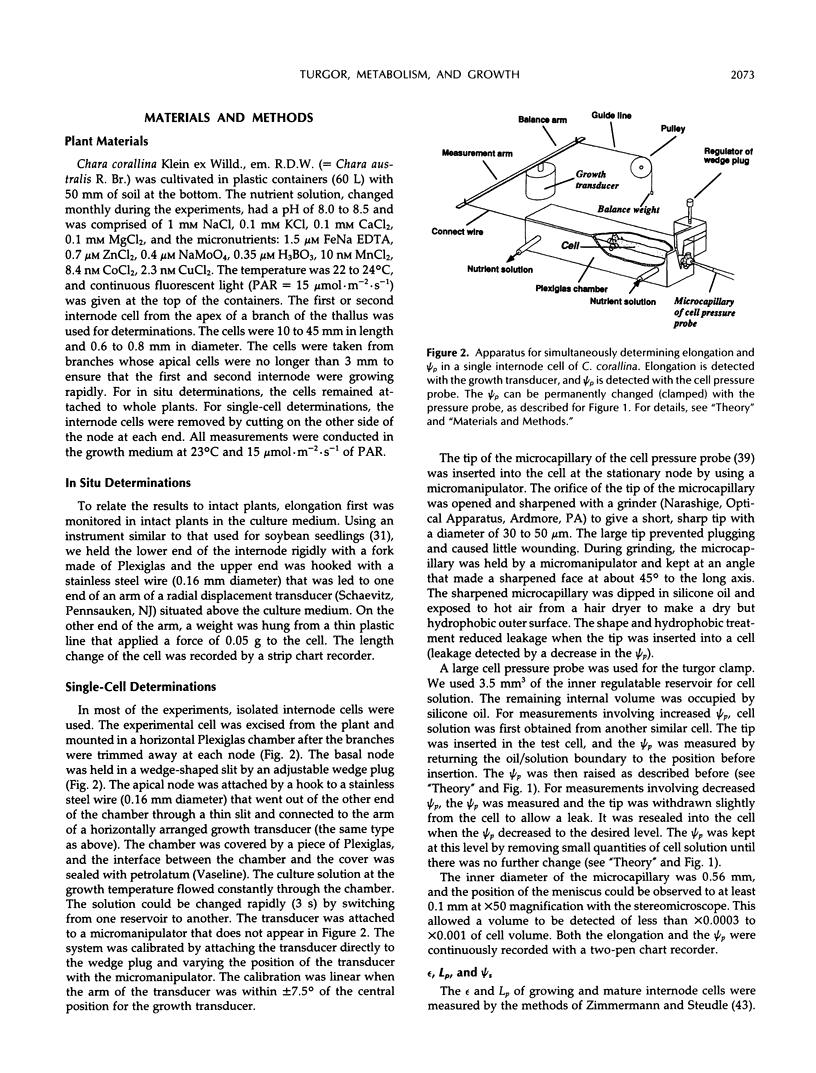
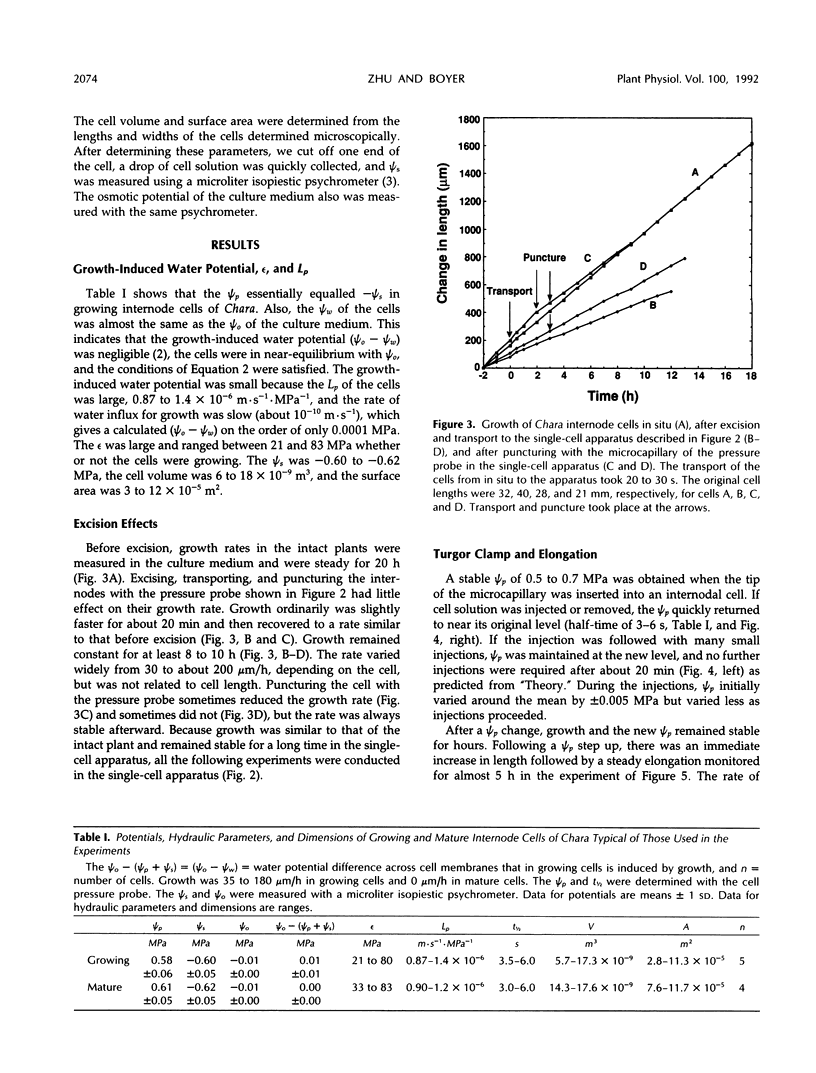
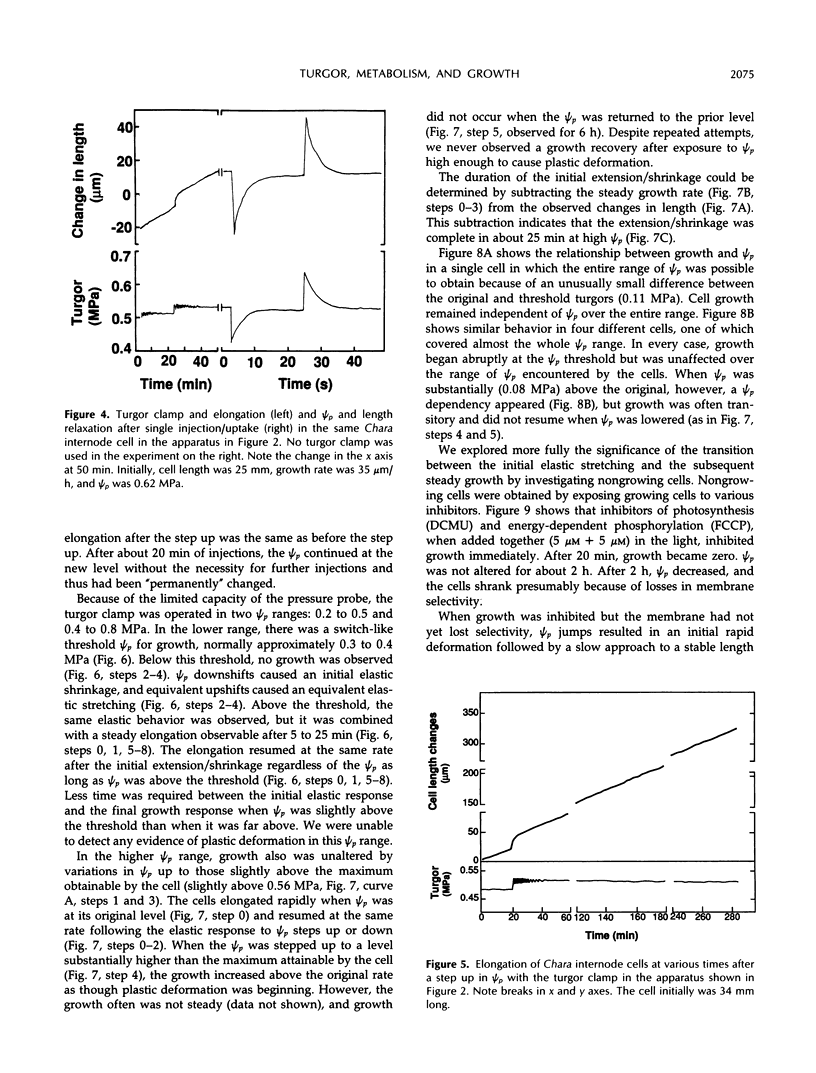
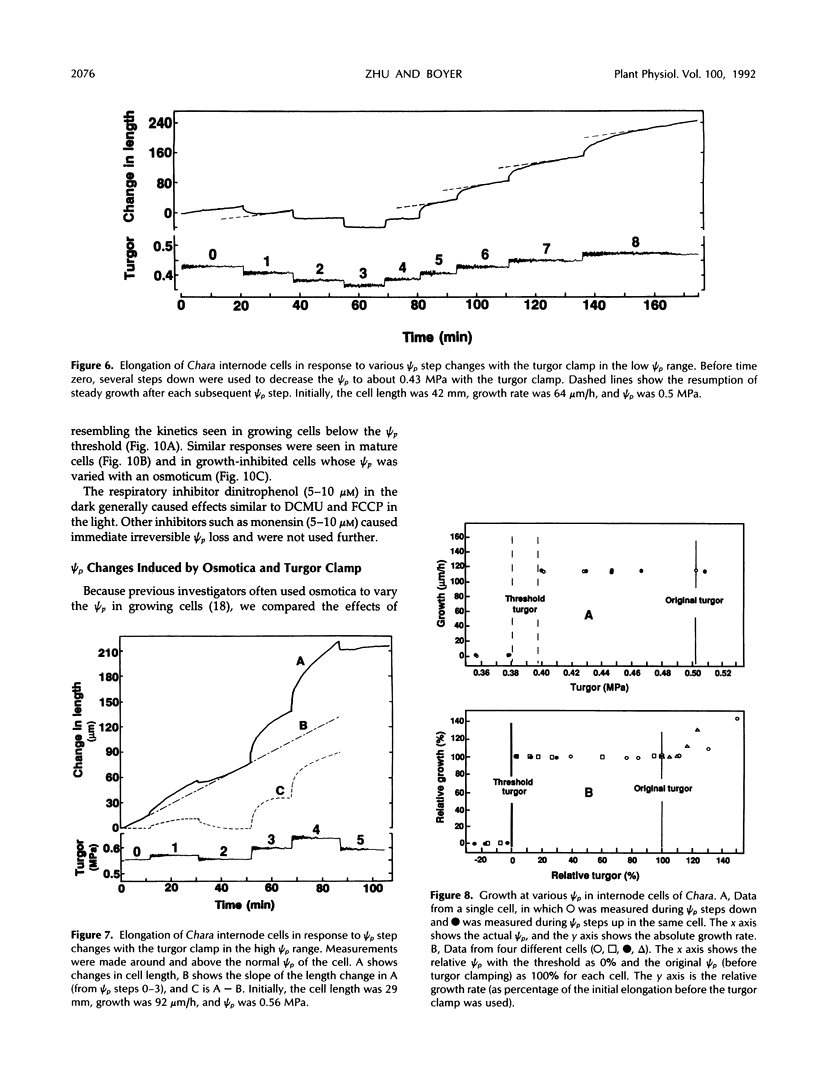
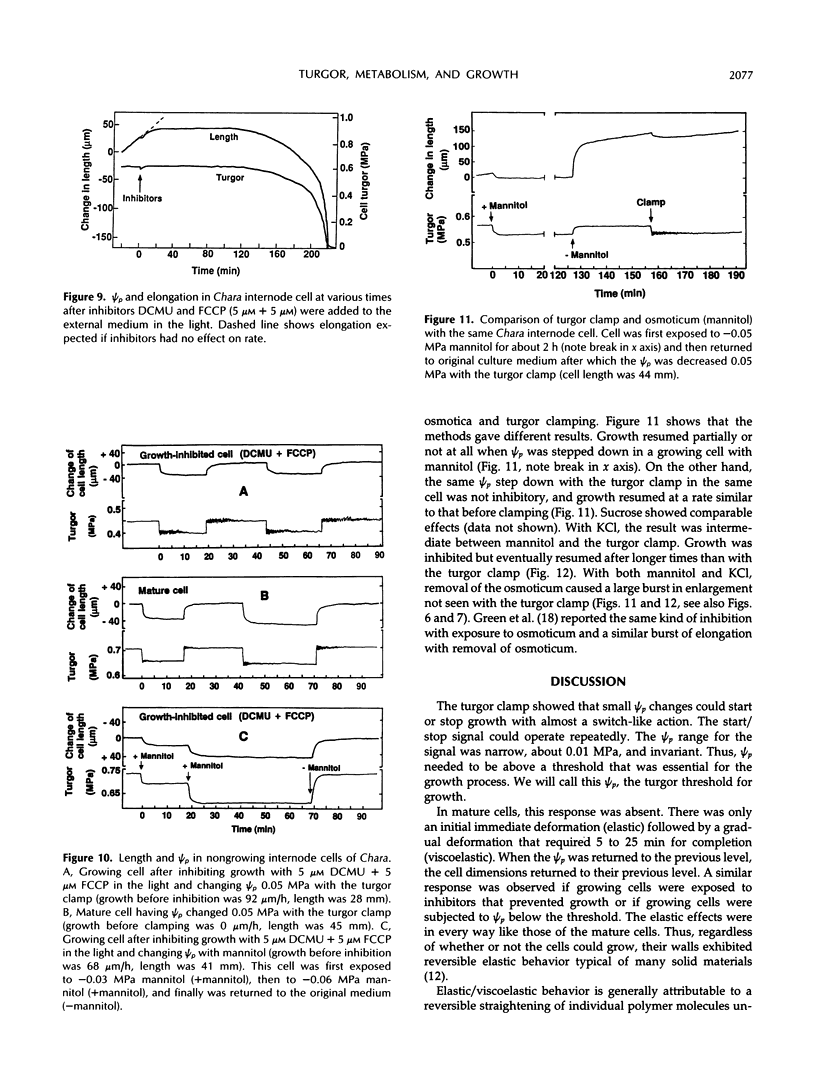
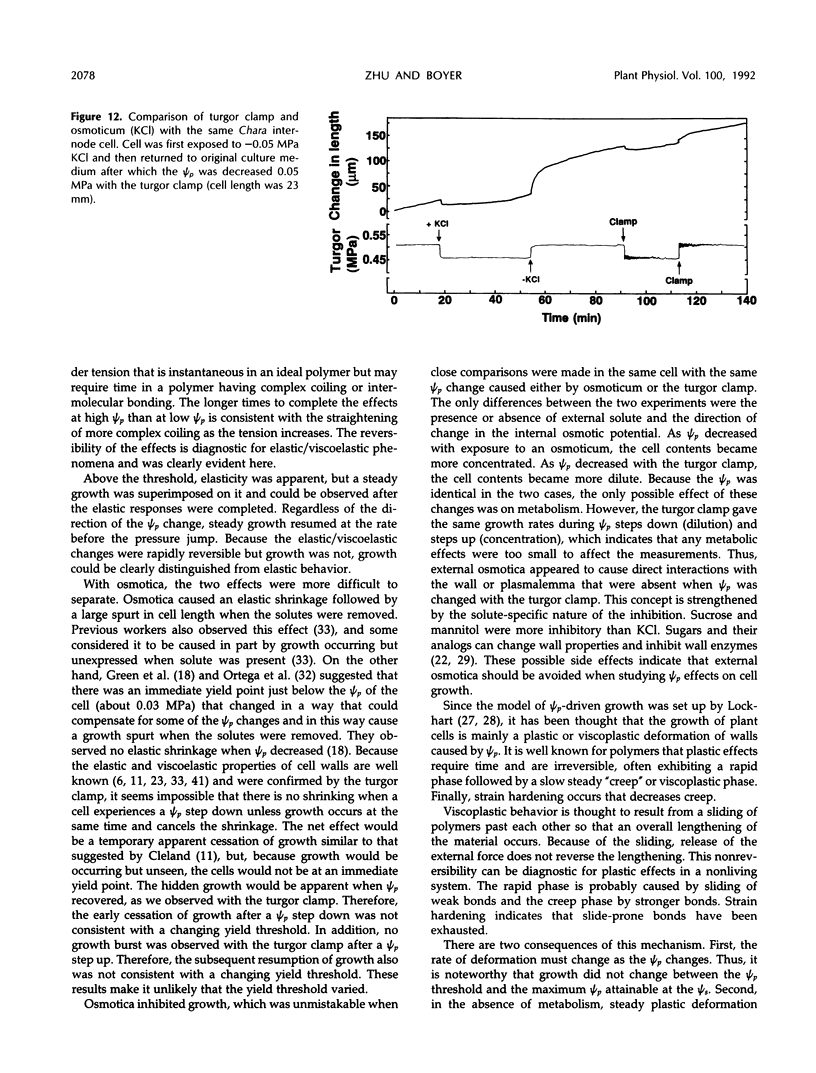
A new method, the turgor clamp, was developed to test the effects of turgor on cell enlargement. The method used a pressure probe to remove or inject cell solution and change the turgor without altering the external environment of the cell walls. After the injections, the cells were permanently at the new turgor and required no further manipulation. Internode cells of Chara corallina grew rapidly with the pressure probe in place when growth was monitored with a position transducer. Growth-induced water potentials were negligible and turgor effects could be studied simply. As turgor was decreased, there was a threshold below which no growth occurred, and only reversible elastic/viscoelastic changes could be seen. Above the threshold, growth was superimposed on the elastic/viscoelastic effects. The rate of growth did not depend on turgor. Instead, the rate was highly dependent on energy metabolism as shown by inhibitors that rapidly abolished growth without changing the turgor. However, turgors could be driven above the maximum normally attainable by the cell, and these caused growth to respond as though plastic deformation of the walls was beginning, but the deformation caused wounding. Growth was inhibited when turgor was changed with osmotica but not inhibited when similar changes were made with the turgor clamp. It was concluded that osmotica caused side effects that could be mistaken for turgor effects. The presence of a turgor threshold indicates that turgor was required for growth. However, because turgor did not control the rate, it appears incorrect to consider the rate to be determined by a turgor-dependent plastic deformation of wall polymers. Instead, above the turgor threshold, the rapid response to energy inhibitors suggests a control by metabolic reactions causing synthesis and/or extension of wall polymers.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bisson M. A., Bartholomew D. Osmoregulation or turgor regulation in chara? Plant Physiol. 1984 Feb;74(2):252–255. doi: 10.1104/pp.74.2.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpita N. C. Cell wall development in maize coleoptiles. Plant Physiol. 1984 Sep;76(1):205–212. doi: 10.1104/pp.76.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green P. B., Erickson R. O., Buggy J. Metabolic and physical control of cell elongation rate: in vivo studies in nitella. Plant Physiol. 1971 Mar;47(3):423–430. doi: 10.1104/pp.47.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoson T., Nevins D. J. beta-d-Glucan Antibodies Inhibit Auxin-Induced Cell Elongation and Changes in the Cell Wall of Zea Coleoptile Segments. Plant Physiol. 1989 Aug;90(4):1353–1358. doi: 10.1104/pp.90.4.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keegstra K., Talmadge K. W., Bauer W. D., Albersheim P. The Structure of Plant Cell Walls: III. A Model of the Walls of Suspension-cultured Sycamore Cells Based on the Interconnections of the Macromolecular Components. Plant Physiol. 1973 Jan;51(1):188–197. doi: 10.1104/pp.51.1.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockhart J. A. An analysis of irreversible plant cell elongation. J Theor Biol. 1965 Mar;8(2):264–275. doi: 10.1016/0022-5193(65)90077-9. [DOI] [PubMed] [Google Scholar]
- Nagahashi G., Tu S. I., Fleet G., Namgoong S. K. Inhibition of cell wall-associated enzymes in vitro and in vivo with sugar analogs. Plant Physiol. 1990 Feb;92(2):413–418. doi: 10.1104/pp.92.2.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonami H., Boyer J. S. Primary events regulating stem growth at low water potentials. Plant Physiol. 1990 Aug;93(4):1601–1609. doi: 10.1104/pp.93.4.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonami H., Boyer J. S. Wall extensibility and cell hydraulic conductivity decrease in enlarging stem tissues at low water potentials. Plant Physiol. 1990 Aug;93(4):1610–1619. doi: 10.1104/pp.93.4.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega J. K., Zehr E. G., Keanini R. G. In vivo creep and stress relaxation experiments to determine the wall extensibility and yield threshold for the sporangiophores of phycomyces. Biophys J. 1989 Sep;56(3):465–475. doi: 10.1016/S0006-3495(89)82694-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray P. M. Radioautographic study of cell wall deposition in growing plant cells. J Cell Biol. 1967 Dec;35(3):659–674. doi: 10.1083/jcb.35.3.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackel K. A., Matthews M. A., Morrison J. C. Dynamic Relation between Expansion and Cellular Turgor in Growing Grape (Vitis vinifera L.) Leaves. Plant Physiol. 1987 Aug;84(4):1166–1171. doi: 10.1104/pp.84.4.1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twente J. W., Twente J. A. Regulation of hibernating periods by temperature. Proc Natl Acad Sci U S A. 1965 Oct;54(4):1044–1051. [PMC free article] [PubMed] [Google Scholar]


