Abstract
Background and Objectives
Patients with sickle cell disease (SCD) are prone to symptomatic neurologic complications. Previous studies reported accrual of neural injury starting at early age, even without having symptomatic neurologic events. The aim of this study was to assess the prevalence and risk factors of abnormal neurologic findings in patients with SCD with no history of major symptomatic neurologic events.
Methods
Our study extracted patients diagnosed with SCD from the Cooperative Study of Sickle Cell Disease. Patients who underwent a neurologic evaluation were included in our analysis. Patients with previous documented major symptomatic neurologic events were excluded. We compared patients with SCD with abnormal neurologic findings with those without in terms of clinical and laboratory parameters using multivariate binary logistic regression.
Results
A total of 3,573 patients with SCD were included (median age = 11 [IQR = 19] years, male = 1719 [48.1%]). 519 (14.5%) patients had at least one abnormal neurologic finding. The most common findings in descending order were abnormal reflexes, gait abnormalities, cerebellar dysfunction, language deficits, nystagmus, abnormal muscle tone and strength, Romberg sign, Horner syndrome, and intellectual impairment. History of eye disease (odds ratio [OR] = 2.76, 95% confidence interval [CI] = 1.63–4.68) and history of osteomyelitis (OR = 2.55, 95% CI 1.34–4.84) were the strongest predictors of abnormal neurologic findings, followed by smoking (OR = 1.59, 95% CI 1.08–2.33), aseptic necrosis (OR = 1.57, 95% CI 1.06–2.33), hand-foot syndrome (OR = 1.48, 95% CI 1.04–2.12), and male sex (OR = 1.42, 95% CI 1.01–2.02).
Discussion
Neurologic deficits are relatively common in patients with SCD, even without documented major neurologic insults. They range from peripheral and ophthalmic deficits to central and cognitive disabilities. Patients with SCD should have early regular neurologic evaluations and risk factor modification, particularly actively promoting smoking cessation.
TAKE-HOME POINTS
→ Neurologic deficits are common in SCD, even without major neurologic insults.
→ Eye disease and osteomyelitis strongly predict abnormal neurologic findings.
→ Smoking cessation promotion is important in preventing neurologic deficits.
Introduction
Sickle cell disease (SCD) is the most common form of hereditary hemolytic anemia and is most prevalent in the Middle East, sub-Saharan Africa, and India.1,2 Patients with SCD are prone to a wide range of early neurologic complications such as ischemic strokes, transient ischemic attack, seizures, posterior reversible encephalopathy syndrome, and cerebral venous sinus thrombosis.3,4 Even in the absence of such events, silent cerebral infarcts (SCI) are a common cause of neurologic decline and found in nearly 22% of patients with SCD.5 Although usually clinically covert, SCI are associated with a high risk of recurrence of future vascular events.6 SCI are also associated with poor school and cognitive functioning, subtle visual-motor speed deficits, and higher neurologic soft sign (NSS; sensory integration and motor coordination impairment) scores.7-9 Moreover, evidence suggests that children with SCD with normal MRIs may still experience neurocognitive impairments.10 Most of the previous studies have assessed the effect of seizures, meningitis, and strokes on the neurologic function in SCD; however, reports are scarce regarding effects of SCD on the nervous system in patients without such major events.11-15
Given its high prevalence, transcranial Doppler ultrasound is used in screening protocols to assess the future risk of stroke to guide decisions to pursue chronic transfusion therapy.16 Unfortunately, this is not always feasible in middle-income and low-income countries, where SCD is the most prevalent.17,18 Even in the lack of accessible chronic transfusion therapy, other interventions can be implemented especially pertaining to stroke prevention, school accommodations, and surveillance for developmental delay and cognitive impairments.19 As such, it would be extremely useful to use basic clinical parameters to predict future neurologic decline.
Other than NSS scores, clinical parameters such as lower hemoglobin concentration, higher systolic blood pressure, and male sex have shown to be associated with SCI in patients with SCD.5,20 Studies exploring the importance of physical examination, a very basic element of patient assessment, in predicting neurologic decline, are very limited. Studies exploring that do not specify whether patients have had a history of neurologic event or whether their neurologic involvement was silent. In addition, they showed that NSS may reliably identify the presence of minor MRI lesions and identify the population at risk of developing strokes.7,21
Therefore, this study aimed to explore the prevalence of abnormal neurologic findings in patients with SCD with no prior documented history of major neurologic events. We further attempt to identify clinical parameters that predict such abnormal findings. This has the potential to assist in identifying those at risk, especially in low-income countries, where more expensive screening methods are not widely available.
Methods
Study Design and Population Sample
This retrospective sample of patients with SCD was derived from the multicenter Cooperative Study of Sickle Cell Disease (CSSCD), which was conducted from 1977 to 1995. The methods for CSSCD have been previously reported.22,23 Access to data was obtained from the Biologic Specimen and Data Repository Information Coordinating Center.24 Patients with SCD were enrolled in 3 phases, and this study was limited to those enrolled in phase 1 (n = 4,085), which spanned from 1978 to 1988 (recruitment ended in 1981, except for infants younger than 6 months, who were still enrolled until 1988). Of those enrolled during phase 1, only those who underwent a neurologic evaluation and had no prior major neurologic events (cerebrovascular accidents, seizures, and meningitis) were included in the analysis (n = 3,573). We compared clinical and laboratory parameters in patients with abnormal neurologic findings with those of patients without such findings. At study entry, medical history, physical examination, and laboratory tests were obtained. Patients were then annually (4 annual visits) assessed for the development of acute events, and laboratory tests were collected (e.g., hemoglobin, red blood cells, white blood cells, platelets, reticulocytes, hemoglobin F, albumin, and lactate dehydrogenase). The mean value of these laboratory variables was calculated from the initial visit to the fourth annual visit when available. The hematology staff members performed the neurologic physical examination. Those who were discovered to have significant abnormalities were assessed by a neurologist for confirmation of findings. All patients underwent a neurologic evaluation on entry or at their first annual visit. The possible predictors of abnormal neurologic findings assessed were age, sex, race, hemoglobin genotype, the presence of non-neurologic complications of SCD (e.g., acute chest syndrome, pneumonia, eye disease, and average number of pain crises), smoking history, family history of seizures or stroke, related laboratory tests, and previous transfusions or operations, such as cholecystectomy, splenectomy, hip replacement, and laser eye therapy. Previous eye disease includes glaucoma, cataract, uveitis, and retinopathy.25
Outcomes
The primary outcome was to determine the prevalence of abnormal neurologic findings in patients with SCD who have no known previous neurologic events. Secondary outcomes included assessing potential predictors of such abnormal neurologic findings and evaluating the implications of the results on clinical practice.
Standard Protocol Approvals, Registrations, and Patient Consents
Approval to access the CSSCD study data was obtained from the BIOLINCC (Biologic Specimen and Data Repository Information Coordinating Center) website (biolincc.nhlbi.nih.gov/home/), which is an open access data repository. Ethical approval was obtained from the Research Ethics Committee, School of Medicine, Jordan University of Science and Technology.
Statistical Analysis
Statistical analyses were performed using SPSS (version 25, IBM corporation, Armonk, NY). To assess the difference in clinical parameters between patients with normal and abnormal neurologic examination, the Mann-Whitney U test was used for continuous variables and the chi-square test for categorical variables. Data for continuous variables were recorded as median (interquartile range [IQR]). Differences in clinical parameters that were statistically significant on univariate analysis were further assessed using a multivariate binary logistic regression model. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated for each variable. Significance was set at p value <0.05.
Data Availability
Datasets used and/or analyzed in this study can be accessed from the BIOLINCC (Biologic Specimen and Data Repository Information Coordinating Center) website: biolincc.nhlbi.nih.gov/studies/csscd/.
Results
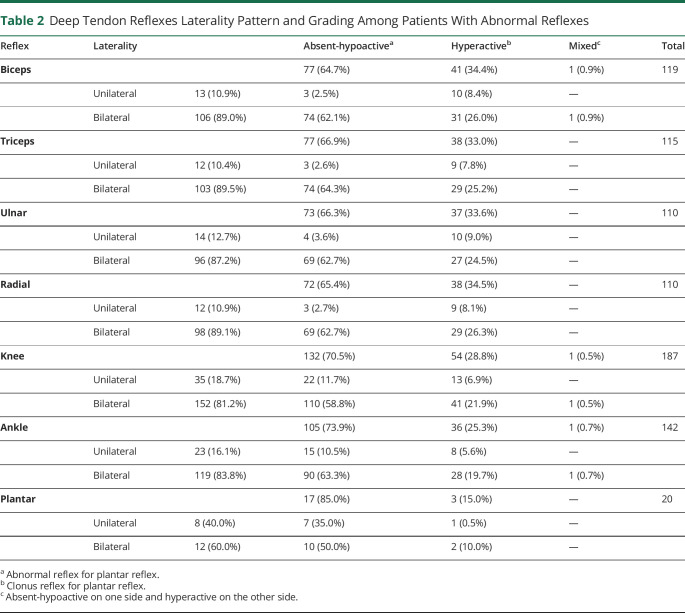
A total of 3,573 patients with SCD met the inclusion criteria and were included in our study (median age = 11 [IQR = 19] years, male = 1719 [48.1%]). Of them, 519 (14.5%) patients had at least 1 abnormal neurologic finding on examination (382 [10.7%] with only 1 abnormal finding and 137 [3.8%] with at least 2). The total number of patients assessed for each element of the full neurologic examination is listed in Table 1. The most commonly observed abnormal neurologic finding was an abnormal deep tendon reflex (n = 238/2,829 [8.4%]; knee (n = 187/2,822 [6.6%]), ankle (n = 142/2,821 [5.0%]), biceps (n = 119/2,822 [4.2%]), triceps (n = 115/2,817 [4.1%]), ulnar (n = 110/2,809 [3.9%]), radial (n = 110/2,816 [3.9%]), and plantar (n = 20/2,818 [0.7%]). Abnormal gait was observed in 91/2,810 (3.2%) patients (3 (0.1%) were unable to walk, 11 (0.4%) had broad-based ataxia, and 77 (2.7%) had a limp) while abnormal cerebellar function was found in 117/2,734 patients (4.3%). Other less frequent abnormal findings included nystagmus 62/2,812 (2.2%), abnormal muscle tone and strength 59/2,831 (2.1%), Romberg sign 27/2,776 (1.0%), Horner syndrome 29/2,813 (1.0%), language deficits 23/938 (2.5%), and intellectual impairment 10/2,037 (0.5%). The number of patients who had at least 1 abnormal deep tendon reflex were 238 of 2,829 (8.4%). Among them, abnormal reflexes were predominantly bilateral (ranging from 81.2% to 89.5%), except for the plantar reflex, which showed a bilateral occurrence in 60% of cases. The study revealed a predominance of absent-hypoactive reflexes. For upper extremity reflexes, including biceps, triceps, ulnar, and radial reflexes, the percentages of absent-hypoactive responses ranged between 64.7% and 66.9%. Similarly, lower extremity reflexes, such as knee, ankle, and plantar reflexes, displayed substantial absent-hypoactive patterns, ranging from 70.5% to 85.0% (Table 2). The ratio of bilateral to unilateral involvement for absent-hypoactive reflexes was notably higher than for hyperactive reflexes for all the included sites (except for plantar reflexes): biceps (24.6 vs 3.1), triceps (24.6 vs 3.2), ulnar (17.2 vs 2.7), radial (23.0 vs 3.2), knee (5.0 vs 3.1), ankle (6.0 vs 3.5), and plantar (1.4 vs 2.0) reflexes. This indicates that hyporeflexia is associated more with bilateral involvement and hyperreflexia is associated with both bilateral and unilateral involvement.
Table 1.
Frequency of Abnormal Neurologic Findings Among Patients With SCD Without Previous Neurologic Insults

| Abnormal neurologic finding | N (%) | Total examined |
| Number of abnormal neurologic findings | 3,573 | |
| None | 3054 (85.5) | |
| One | 382 (10.7) | |
| Two or more | 137 (3.8) | |
| Extraocular movement | 14 (0.5) | 2,764 |
| Facial power, sensation, and reflexes | 19 (0.7) | 2,787 |
| Palatal reflex | 6 (0.2) | 2,775 |
| Tongue movements | 5 (0.2) | 2,774 |
| Muscle tone and strength | 59 (2.1) | 2,831 |
| Muscle tone at the right side | 28 (1.0) | 2,828 |
| Muscle tone at the left side | 29 (1.0) | 2,826 |
| Muscle power at the right side | 27 (1.0) | 2,828 |
| Muscle power at the left side | 33 (1.2) | 2,828 |
| Body reflexes | 238 (8.4) | 2,829 |
| Biceps | 119 (4.2) | 2,822 |
| Triceps | 115 (4.1) | 2,817 |
| Ulnar | 110 (3.9) | 2,809 |
| Radial | 110 (3.9) | 2,816 |
| Knee | 187 (6.6) | 2,822 |
| Ankle | 142 (5.0) | 2,821 |
| Plantar | 20 (0.7) | 2,818 |
| Involuntary movement | 9 (0.3) | 2,828 |
| Nystagmus | 62 (2.2) | 2,812 |
| Romberg sign | 27 (1.0) | 2,776 |
| Cerebellar function | 117 (4.3) | 2,734 |
| Horner syndrome | 29 (1.0) | 2,813 |
| Body sensation | 18 (0.6) | 2,775 |
| Tactile | 10 (0.4) | 2,769 |
| Position | 7 (0.3) | 2,731 |
| Vibration | 10 (0.4) | 2,710 |
| Pain present | 11 (0.4) | 2,778 |
| Language deficits | 23 (2.5) | 938 |
| Intellectual function | 10 (0.5) | 2,037 |
| Gait | 91 (3.2) | 2,810 |
| Unable to walk | 3 (0.1) | |
| Broad-based ataxia | 11 (0.4) | |
| Limp | 77 (2.7) |
Table 2.
Deep Tendon Reflexes Laterality Pattern and Grading Among Patients With Abnormal Reflexes
| Reflex | Laterality | Absent-hypoactivea | Hyperactiveb | Mixedc | Total | |
| Biceps | 77 (64.7%) | 41 (34.4%) | 1 (0.9%) | 119 | ||
| Unilateral | 13 (10.9%) | 3 (2.5%) | 10 (8.4%) | — | ||
| Bilateral | 106 (89.0%) | 74 (62.1%) | 31 (26.0%) | 1 (0.9%) | ||
| Triceps | 77 (66.9%) | 38 (33.0%) | — | 115 | ||
| Unilateral | 12 (10.4%) | 3 (2.6%) | 9 (7.8%) | — | ||
| Bilateral | 103 (89.5%) | 74 (64.3%) | 29 (25.2%) | — | ||
| Ulnar | 73 (66.3%) | 37 (33.6%) | — | 110 | ||
| Unilateral | 14 (12.7%) | 4 (3.6%) | 10 (9.0%) | — | ||
| Bilateral | 96 (87.2%) | 69 (62.7%) | 27 (24.5%) | — | ||
| Radial | 72 (65.4%) | 38 (34.5%) | — | 110 | ||
| Unilateral | 12 (10.9%) | 3 (2.7%) | 9 (8.1%) | — | ||
| Bilateral | 98 (89.1%) | 69 (62.7%) | 29 (26.3%) | — | ||
| Knee | 132 (70.5%) | 54 (28.8%) | 1 (0.5%) | 187 | ||
| Unilateral | 35 (18.7%) | 22 (11.7%) | 13 (6.9%) | — | ||
| Bilateral | 152 (81.2%) | 110 (58.8%) | 41 (21.9%) | 1 (0.5%) | ||
| Ankle | 105 (73.9%) | 36 (25.3%) | 1 (0.7%) | 142 | ||
| Unilateral | 23 (16.1%) | 15 (10.5%) | 8 (5.6%) | — | ||
| Bilateral | 119 (83.8%) | 90 (63.3%) | 28 (19.7%) | 1 (0.7%) | ||
| Plantar | 17 (85.0%) | 3 (15.0%) | — | 20 | ||
| Unilateral | 8 (40.0%) | 7 (35.0%) | 1 (0.5%) | — | ||
| Bilateral | 12 (60.0%) | 10 (50.0%) | 2 (10.0%) | — | ||
Abnormal reflex for plantar reflex.
Clonus reflex for plantar reflex.
Absent-hypoactive on one side and hyperactive on the other side.
In upper extremity reflexes, bilateral involvement predominantly featured absent-hypoactive patterns than hyperactive patterns (biceps: 62.1% vs 26.0%, triceps: 64.3% vs 25.2%, ulnar: 62.7% vs 24.5%, radial: 62.7% vs 26.3%), while unilateral cases leaned toward hyperactive patterns than absent-hypoactive patterns (biceps: 8.4% vs 2.5%, triceps: 7.8% vs 2.6%, ulnar: 9.0% vs 3.6%, radial: 8.1% vs 2.7%). Conversely, in lower extremity reflexes, both bilateral and unilateral instances were predominantly marked by absent-hypoactive responses, with hyperactive responses being less frequent.
MRI results were available only for 92 patients. Thus, the data were not sufficient to be entered into the regression model. Approximately 22 of the 92 patients had abnormal brain MRI (brain ischemia or atrophy), 9 (41%) of them had abnormal neurologic findings, and 13 (59%) of them had normal neurologic examination. On the contrary, 70 patients had normal brain MRI: 21 (30%) of them had abnormal neurologic findings and only 49 (70%) of them had normal neurologic examination.
Univariate analysis (Tables 3 and 4) revealed that in comparison with patients with SCD with normal neurologic examination, those with abnormal findings had a significantly higher number of male patients (51.8% vs 47.0%, p = 0.050), were older at study entry (median = 17.0 (IQR = 21.0) years vs 14.0 (IQR = 16.0) years, p < 0.001), had higher smoking rates (47.7% vs 33.5%, p < 0.001), were more likely to be overweight/obese with body mass index ≥ 25 kg/m2 (12.4% vs 7.7%, p < 0.001), had higher systolic blood pressure (median = 108.3 (IQR = 14.8) mm Hg vs 105.8 (IQR = 14.0) mm Hg, p < 0.001), had higher diastolic blood pressure (median = 65.6 (IQR = 12.0) mm Hg vs 64.7 (IQR = 11.0) mm Hg, p = 0.006), and had more painful crises (57.9% vs 47.4%, p < 0.001). Moreover, they were more likely to have a history of one of the following non-neurologic conditions: aseptic necrosis (25.3% vs 12.0%, p < 0.001), eye disease (12.1% vs 6.7%, p < 0.001), osteomyelitis (10.4% vs 5.1%, p < 0.001), hand-foot syndrome (41.2% vs 33.7%, p = 0.004), renal insufficiency (4.4% vs 2.7%, = 0.050), or lung infarction (5.6% vs 3.6%, = 0.050). They were also more likely to have had blood transfusion (65.4% vs 53.7%, p < 0.001). Laboratory data revealed that patients with abnormal neurologic examination had higher reticulocyte count (p = 0.025) and lower hemoglobin F (Hgb F, p = 0.005) and albumin (p < 0.001) levels.
Table 3.
Differences Among Categorical Variables Between Patients With Normal and Patients With Abnormal Neurologic Examination

| Variable | Normal N (%) |
Abnormal N (%) |
p Value |
| Sex (female) | 1237 (53.0) | 248 (48.2) | 0.050 |
| Sex (male) | 1097 (47.0) | 266 (51.8) | 0.050 |
| Hemoglobin SS and SB variants | 1402 (60.4) | 306 (59.8) | 0.789 |
| Race (African American) | 2275 (97.9) | 502 (98.0) | 0.870 |
| BMI ≥25 kg/m2 | 157 (7.7) | 75 (12.4) | <0.001 |
| G6PD deficiency | 110 (9.5) | 29 (11.8) | 0.266 |
| Pneumonia vaccination | 1149 (49.2) | 240 (46.7) | 0.298 |
| Smoker (includes cigarettes, cigars, and pipes) | 589 (33.5) | 183 (47.7) | <0.001 |
| Previous history of | |||
| Eye disease | 140 (6.7) | 54 (12.1) | <0.001 |
| Hematuria | 264 (12.6) | 66 (14.4) | 0.302 |
| Nephrotic syndrome | 16 (3.5) | 45 (2.2) | 0.080 |
| Renal insufficiency | 56 (2.7) | 20 (4.4) | 0.050 |
| Acute priapism | 121 (12.1) | 37 (14.9) | 0.234 |
| Hearing loss | 125 (6.0) | 25 (5.5) | 0.712 |
| Heart disease | 254 (12.2) | 61 (13.6) | 0.399 |
| Aseptic necrosis | 280 (12.0) | 130 (25.3) | <0.001 |
| Hand-foot syndrome | 660 (33.7) | 170 (41.2) | 0.004 |
| Osteomyelitis | 107 (5.1) | 47 (10.4) | <0.001 |
| Spleen sequestration | 227 (11.2) | 53 (12.0) | 0.607 |
| Pneumonia | 1115 (53.7) | 256 (56.9) | 0.223 |
| Lung infarction | 75 (3.6) | 25 (5.6) | 0.050 |
| Liver sequestration | 162 (7.8) | 45 (10.1) | 0.115 |
| Painful crisis | 992 (47.4) | 358 (57.9) | <0.001 |
| Blood transfusion | 1124 (53.7) | 297 (65.4) | <0.001 |
| Family history of | |||
| Stroke | 12 (0.6) | 6 (1.3) | 0.088 |
| Seizures | 19 (0.9) | 6 (1.3) | 0.423 |
Abbreviations: BMI = body mass index; G6PD = glucose-6-phosphate dehydrogenase.
Table 4.
Difference Among Continuous Variables Between Patients With Normal and Patients With Abnormal Neurologic Examination

| Variable | Normal Median (IQR) |
Abnormal Median (IQR) |
p Value |
| SBP (mm Hg) | 105.8 (14.0) | 108.3 (14.8) | <0.001 |
| DBP (mm Hg) | 64.7 (11.0) | 65.6 (12.0) | 0.006 |
| Age at entry visit (y) | 14.0 (16.0) | 17.0 (21.0) | < 0.001 |
| Hemoglobin (g/dL) | 9.0 (2.7) | 9.0 (2.5) | 0.827 |
| RBC (*1012/L) | 3.0 (1.4) | 3.0 (1.3) | 0.999 |
| WBC (*109/L) | 10.7 (4.6) | 10.9 (4.2) | 0.431 |
| Platelets (*109/L) | 387.9 (184.4) | 378.6 (194.3) | 0.608 |
| Reticulocytes (%) | 9.2 (8.4) | 9.9 (9.2) | 0.025 |
| Hemoglobin F (%) | 4.7 (6.6) | 3.7 (5.6) | 0.005 |
| Albumin (g/dL) | 4.4 (0.4) | 4.2 (0.5) | 0.001 |
| LDH (mg/dL) | 374.8 (302.6) | 360.0 (255.8) | 0.246 |
Abbreviations: DBP = diastolic blood pressure; IQR = interquartile range; LDH = lactate dehydrogenase; RBC = red blood cells; SBP = systolic blood pressure; WBC = white blood cells.
Using logistic regression, we then explored whether the aforementioned significant variables were predictors of abnormal neurologic examination (Table 5). The logistic regression model was statistically significant, χ2 (17) = 84.68, p < 0.001, explained 12.5% (Nagelkerke R2) of the variance, and correctly classified 82.6% of cases. Sensitivity was 9.9%, specificity was 98.6%. History of eye disease (OR = 2.76, p < 0.001, 95% CI 1.63–4.68) and history of osteomyelitis (OR = 2.55, p = 0.004, 95% CI 1.34–4.84) were the strongest predictors of abnormal neurologic findings, followed by smoking (OR = 1.59, p = 0.018, 95% CI 1.08–2.33), aseptic necrosis (OR = 1.57, p = 0.024, 95% CI 1.06–2.33), hand-foot syndrome (OR = 1.48, p = 0.029, 95% CI 1.04–2.12), and male sex (OR = 1.42, p = 0.050, 95% CI 1.01–2.02).
Table 5.
Binary Logistic Regression Predicting the Likelihood of Having an Abnormal Neurologic Examination Among Patients With SCD Who Did Not Experience a Previous Major Neurologic Event

| Variable | Odds ratio | 95% CI | p Value |
| Sex (male) | 1.42 | 1.01–2.02 | 0.050 |
| Age at entry visit (y) | 1.01 | 0.99–1.03 | 0.179 |
| Smoking | 1.59 | 1.08–2.33 | 0.018 |
| BMI ≥25 kg/m2 | 1.36 | 0.80–2.32 | 0.254 |
| SBP | 0.99 | 0.97–1.02 | 0.881 |
| DBP | 0.99 | 0.96–1.02 | 0.647 |
| Previous history of | |||
| Eye disease | 2.76 | 1.63–4.68 | <0.001 |
| Aseptic necrosis | 1.57 | 1.06–2.33 | 0.024 |
| Osteomyelitis | 2.55 | 1.34–4.84 | 0.004 |
| Hand-foot syndrome | 1.48 | 1.04–2.12 | 0.029 |
| Renal insufficiency | 1.54 | 0.66–3.59 | 0.310 |
| Blood transfusion | 1.17 | 0.79–1.74 | 0.427 |
| Lung infarction | 1.49 | 0.70–3.16 | 0.293 |
| Painful crisis | 1.05 | 0.75–1.49 | 0.743 |
| Blood and liver tests | |||
| Reticulocytes (%) | 1.01 | 0.97–1.03 | 0.753 |
| Hemoglobin F (%) | 0.97 | 0.94–1.01 | 0.276 |
| Albumin <3.5 (g/dL) | 1.05 | 0.66–1.68 | 0.818 |
Abbreviations: BMI = body mass index; CI = confidence interval; DBP = diastolic blood pressure; LDH = lactate dehydrogenase; SBP = systolic blood pressure.
Discussion
By virtue of their disease process, patients with SCD commonly experience overt neurologic events such stroke or seizures or covert events such as SCI, which despite being silent, carry tremendous risk for developing future stroke and neurologic decline. Thus, identifying clinical parameters that predict neurocognitive deficits, especially in those with asymptomatic involvement, may be particularly helpful. We first assessed the prevalence of abnormal neurologic physical findings, representative of neurologic involvement, in patients with SCD with no history of neurologic event and found that 14.5% had at least 1 abnormal finding, most commonly an abnormal deep tendon reflex. We then assessed which clinical parameters significantly predicted an abnormal neurologic examination in patients with SCD and found that history of eye disease, osteomyelitis, smoking, aseptic necrosis, hand-foot syndrome, and male sex were all significant predictors. Closely monitoring patients with SCD with such attributes may help prevent neurologic decline, especially in places where early screening may not be feasible.
Given that in high-income countries nowadays virtually all patients with SCD are routinely monitored with brain MRI and TCD, and this level of monitoring may not be readily accessible in low–middle-income countries due to various constraints. The strength of this study is that it summarizes the neurologic examination findings in asymptomatic patients with SCD with neurologic involvement and other clinical findings predictive of neurologic involvement. This is particularly useful in low–middle-income countries, where such patients should be referred to tertiary centers for imaging and further testing, whenever feasible.
The most common abnormal neurologic finding in our SCD cohort was an abnormal deep tendon reflex, mostly absent-hypoactive and most commonly in the lower limbs (namely knee and ankle). Hyperreflexia indicates CNS involvement and most likely results from SCD-related effects on cerebral circulation. On the contrary, hyporeflexia is rather unique because it suggests SCD-related peripheral nerve involvement.26 This is further supported by the finding that most of the hyporeflexia involvement was bilateral in nature. Evidence from animal studies demonstrates diffuse sciatic nerve myelin instability, which could be attributed to increased permeability of the blood-nerve barrier leading to the accumulation of demyelinating factors.27 Although most studies explore central neurologic complications of SCD,28 there is evidence to suggest that SCD creates a neurotoxic environment for peripheral nerves.27 Moreover, the etiology of abnormal gait in SCD may be central due to cerebellar infarcts, leading to manifestations of broad-based ataxia and additional cerebellar signs, or due to peripheral neuropathy. Further studies exploring the peripheral neurologic complications of SCD may improve our understanding of disability patterns and the nature of pain in this population.
Silent cerebral infarcts are an extremely common cause of unexplained neurologic decline in SCD. Studies have shown that, by their 6th birthday, 27% of SCD will have silent cerebral infarcts.5 This number will increase to 37% and 39% by the 14th and 18th birthdays, respectively.4 Apart from the direct effect of sickling and occlusion on brain vessels, the chronic hypoxic environment might distort the cerebral circulation in a unique way. Sickling episodes and the subsequent hypoxia will induce endothelial hyperplasia and nonatherosclerotic vascular stenosis. Ultimately, new small collateral vessels resembling “puff of smoke” will form or what is called Moyamoya syndrome.29,30 All in all, the aforementioned structural changes will result in a gradual chronic neurocognitive decline even without punctuating acute neurologic events.31
The MRI findings in our study showed that 30% (21/70) of patients who had normal brain MRI were found to have abnormal neurologic findings. This was consistent with the findings from other studies, which suggested that children with SCD with normal MRIs may still experience neurocognitive impairments.10 It is worth noting that some of this 30% may be due to peripheral processes, such as peripheral neuropathy. Therefore, to better understand the underlying mechanisms contributing to these neurologic findings, further investigations into the specific etiology of the abnormalities observed in this subgroup of patients are warranted. Thus, it is important to screen patients with SCD for neurologic involvement using regular neurologic examination and transcranial Doppler ultrasound in addition to MRI to facilitate early detection and intervention to prevent subsequent neurologic decline.16 Relying solely on MRI may not be sufficient to detect all potential neurologic impairments because certain peripheral factors can contribute to the observed abnormalities.
Our multivariable model demonstrated that history of eye disease, osteomyelitis, smoking, aseptic necrosis, hand-foot syndrome, and male sex were all significant predictors of abnormal neurologic findings. These predictors reflect a general scheme of vasculopathy, endothelial dysfunction, and microinfarcts and is consistent with previous studies.4,32
Osteomyelitis, hand-foot syndrome, and aseptic necrosis episodes reflect poor disease control and result in more sickling and hemolysis, which releases more inflammatory mediators and vascular growth factors, commonly resulting in diffuse vasculopathy.32 The strong association between these bone pathologies and abnormal neurologic findings may also be suggestive of fat embolus syndrome, wherein bone marrow necrosis causes nontraumatic shedding of multiple fat emboli, which travel throughout the venous system, resulting in various organ-specific manifestations.33 Should the emboli reach the arterial system, a range of cerebral manifestations may occur, from mild confusion or anxiety to overt focal symptoms or even encephalopathy.34-36 Moreover, in those with painful episodes, subtle neurologic symptoms may be overlooked in the context of the overall clinical presentation and thus often go unnoticed for years, allowing them to progress. In addition, certain patients with SCD may experience chronic or neuropathic pain, necessitating the use of potent pain medications, such as opioids, which might obscure the presence of underlying neurologic abnormalities.37 Thus, our findings suggest that patients recovering from osteomyelitis or aseptic necrosis should be evaluated by a neurologist to document any neurologic deficits, in the hope of curbing further decline.
One of the strongest predictors of abnormal neurologic examination in our cohort of patients with SCD was history of eye disease. Previous reports have shown that patients with SCD have a heightened risk of neuro-ophthalmic complications involving the retina, visual tract, and even the cortex.25,38 The development of the visual pathway and other cortical functions, such as movement perception, reading and writing, are closely linked.39 Retinal neurons are subject to the same SCD-related insults that affect the CNS, and given the reciprocal interaction between the retina and the rest of the visual pathway, retinal insults may have negative neurotropic effects on other visual pathway components.39,40As such, the eye can be viewed as a window to the CNS, and examining the fundus and retina may provide an affordable and practical means of evaluating and stratifying patients with SCD according to their risk of subsequent neurologic decline.
Our study reported abnormal neurologic findings in nearly half (47.7%) of smokers, which was significantly higher than those in nonsmokers. Previous studies have linked smoking to increased frequency of acute chest syndrome episodes and community-acquired pneumonia in patients with SCD.41 Children exposed to tobacco smoke were found to have had more sickle cell crises requiring hospitalization than unexposed children.42 This could be related to the fact that exposure to tobacco products increases vaso-occlusive disease through increasing vascular inflammation, platelet aggregation, and expression of endothelial adhesion molecules.43 An association between smoking and neurologic manifestations could thus be explained by the fact that neurologic findings in patients with SCD are mostly the result of vaso-occlusive events, which are more likely to occur in smokers. It is also possible that an acute neurologic insult may have occurred because of hypoxia in the setting of an acute chest syndrome or chest infection. A direct effect of smoking on the CNS through the generation of free radicals and inflammatory mediators in the general population was also previously suggested.44 More studies are yet to be conducted to observe the presence and absence of abnormal neurologic manifestations in patients with SCD exposed to smoking. Considering that it is a modifiable risk factor, efforts should be directed at educating the general population and patients with SCD to avoid both firsthand and secondhand smoking.
We found that there was a significantly increased risk of abnormal neurologic findings in male patients. This was consistent with one study that reported an increased risk of silent cerebral infarct in male patients with sickle cell anemia.20 Many factors may have contributed to these observed effects because male patients seem to have higher overall blood pressure readings than female patients. Although our study found no association between higher blood pressure readings and reported abnormal neurologic manifestations, this remains debatable because other studies have recognized the association.20,45 Smoking status may also be a contributor to the observed effects because previous studies have suggested that men use tobacco products at a higher rate than women.46 On the contrary, one study found no significant difference in the prevalence of CVA between male and female patients with SCD.47
Fetal hemoglobin inhibits polymerization of deoxy sickle hemoglobin (HbS), and this is the rationale behind therapies such as hydroxyurea.48 We found that a lower percentage of fetal hemoglobin (HbF) was not predictive of abnormal neurologic findings. Previous study findings have been inconsistent; some similarly demonstrate that HbF does not associate with silent infarct risk in patients with SCD with no history of CVA,20,49,50 while others suggest that HbF may protect against silent white matter changes (WMC), decreasing the likelihood of WMC being present and their severity. It may therefore be beneficial to increase HbF levels in adult patients with WMC who have no history of CVA.51,52 Some data suggest that the clinical relevance of HbF in predicting SCD severity is questionable, considering that it is the distribution of HbF among F cells that matters; only when the total HbF concentration is near 30% is it possible for the number of protected cells—those with sufficient concentrations to inhibit HbS polymerization—to approach 70%.53 In addition, higher HbF levels are associated with reduced rates of acute painful episodes, leg ulcers, osteonecrosis, acute chest syndrome, and reduced disease severity. However, it had no clear association with silent cerebral infarction, among others.54 Thus, HbF is not capable of uniformly modulating all complications of sickle cell disease, which is likely related to the variable pathophysiologic processes underlying these complications.
Several studies have demonstrated an association between elevated relative systolic blood pressure and SCI in patients with SCD.20 Patients with SCD have lower blood pressure than the general population, which may be explained partly by the compensatory systemic vasodilatation resulting from chronic anemia. Elevated systolic blood pressure thus may offset this process.55 In our SCD cohort, we found that systolic blood pressure was higher among patients with abnormal physical examination; however, the difference was not statistically significant. This can be due to the exclusion of patients with more severe disease, such as those with overt stroke or seizures, which might have limited our ability to detect clinically significant associations.
Given the high risk of strokes in patients with SCD, early screening is implemented to help prevent them.19 Noninvasive transcranial Doppler ultrasound is the primary method by which patients are risk stratified.56 Based on transcranial Doppler velocity (TDC) measures, patients may be placed the patient may be considered for transfusion therapy or other treatment for prevention of stroke.21,57 This has been shown to considerably reduce risk of stroke.58,59 Despite its utility, however, it is not without limitations. TDC measures are operator dependent, can vary from time to time and most importantly, access is not readily available in lower income countries. This is specially relevant given that the highest prevalence of SCD is in countries of Africa.17 Moreover, it is ineffective in predicting silent cerebral infarcts, for which MRI is sought instead. Even if chronic transfusion therapy was not feasible in these countries, special considerations and interventions can be implemented that can help improve the neurologic morbidity. We recommend conducting regular neurologic examinations to patients with SCD, especially in resource-poor countries where screening transcranial Doppler or MRI is not readily available60; in addition, particular consideration with more frequent neurologic evaluations should be given to those who have risk factors of developing abnormal neurologic findings. Thus, future screening guidelines should rely not only on neuroimaging but should take into consideration the neurologic history, examination findings, and potential risk factors.
This study has several limitations. First, 98.3% of our sample are of African American ethnicity. However, this closely resembles the real world because nearly 93% of patients with SCD are of African descent.61 Second, although we excluded 3 major neurologic events that commonly occur in patients with SCD (meningitis, stroke, and seizures), we did not take into account other potential causes of abnormal neurologic findings such as genetic disorders, congenital abnormalities, perinatal hypoxia, trauma, or undocumented stroke/meningitis. Third, neurologic assessment is subjective because it is evaluator dependent. Due to the retrospective nature of data, there is lack of standardization of physical examination findings. Moreover, some of the details of physical examination findings were limited to what was described in the original study, such as the exact description of cerebellar signs. In addition, some of the physical examination findings could be attributed to defects outside of the neurologic system, such as gait problems, which could be due to musculoskeletal abnormalities. Fourth, we did not account for whether patients are on hydroxyurea or on prophylactic transfusions. In addition, our sample did not use a control group (people without SCD), which would have helped make us more confident about the origin of abnormal neurologic findings. We also had insufficient data on medications or brain imaging findings. Being on hydroxyurea, for example, would alter the baseline risk considerably and alter the clinical parameters assessed. In addition, specific medications might interact differently with the disease's pathophysiology, leading to varying neurologic manifestations. Moreover, it would have been helpful to see how abnormal neurologic examination findings correlated with lesions on imaging, which could help establish a stronger correlation between neurologic abnormalities and specific clinical symptoms and to determine the localization and extent of the disease burden and tailor specific interventions for these patients. Finally, a prospective study could provide further insights into the concepts raised in this study and potentially address the limitations more comprehensively. Nevertheless, our large multicenter sample with a relatively good documentation of neurologic findings and follow-up of various potential predictors provides a reliable description of this unique population.
Neurologic deficits are relatively common in patients with SCD, even without having a documented neurologic insult. These manifestations range from peripheral and ophthalmic deficits to the more prominent central and cognitive disabilities. The large variability of manifestations suggests a multidimensional neuropathology, involving chronic hypoxia, demyelination, and fat embolization, and not merely the old paradigm of vasculopathy and cerebrovascular events. Therefore, it seems that the current screening guidelines are inadequate in monitoring neurologic decline in patients with SCD. Patients with SCD should have regular neurologic evaluations, including comprehensive neurologic examination, retinal examination, and risk factor modification. The frequency of follow-ups should probably be tailored based on risk factors suggested by this study and other similar studies. Before implementing these screening protocols, long-term longitudinal studies are needed to evaluate the impact of these potential risk factors more clearly. Moreover, the feasibility and cost-effectiveness of these screening visits and potential protective interventions should be extensively studied.
Acknowledgment
The authors thank the Biologic Specimen and Data Repository Information Coordinating Center (BioLINCC) for providing the data for the Cooperative Study of Sickle Cell Disease (CSSCD). biolincc.nhlbi.nih.gov/studies/csscd/.
Appendix. Authors

| Name | Location | Contribution |
| Mohammed B. Nawaiseh, MD | Department of Ophthalmology, Jordanian Royal Medical Services, Amman, Jordan | Drafting/revision of the article for content, including medical writing for content; study concept or design; and analysis or interpretation of data |
| Ahmed M. Yassin, MD | Neurology, Jordan University of Science and Technology, Irbid, Jordan | Drafting/revision of the article for content, including medical writing for content; study concept or design |
| Mohammed Q. Al-Sabbagh, MD | Neurology, Kansas University Medical Center, Kansas City | Drafting/revision of the article for content, including medical writing for content; analysis or interpretation of data |
| Ahmad AlNawaiseh, MD | Internal Medicine, St. Elizabeth's Medical Center, Boston, MA | Drafting/revision of the article for content, including medical writing for content; analysis or interpretation of data |
| Hadil Zureigat, MD | Internal Medicine, Cleveland Clinic, OH | Drafting/revision of the article for content, including medical writing for content; analysis or interpretation of data |
| Dina Aljbour AlMajali, MD | Internal medicine, Henry Ford Health System, Detroit, MI | Drafting/revision of the article for content, including medical writing for content |
| Rund R. Haddadin, MD | Department of Internal Medicine, JCESOM, Marshall University, WV | Drafting/revision of the article for content, including medical writing for content; analysis or interpretation of data |
| Mohammad El-Ghanem, MD | Department of Clinical Sciences, College of Medicine, University of Houston, HCA Northwest Medical Center, TX | Drafting/revision of the article for content, including medical writing for content |
| Mohammad Abu-Rub, MD | Neurology, George Washington University, Washington, DC | Drafting/revision of the article for content, including medical writing for content |
Study Funding
The authors report no targeted funding.
Disclosure
The authors report no relevant disclosures. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/cp.
References
- 1.Azar S, Wong TE. Sickle cell disease: a brief update. Med Clin. 2017;101(2):375-393. doi: 10.1016/j.mcna.2016.09.009 [DOI] [PubMed] [Google Scholar]
- 2.Ware RE, de Montalembert M, Tshilolo L, Abboud MR. Sickle cell disease. Lancet. 2017;390(10091):311-323. doi: 10.1016/S0140-6736(17)30193-9 [DOI] [PubMed] [Google Scholar]
- 3.Matondo LO, Kija E, Manji KP. Neurocognitive functioning among children with sickle cell anemia attending SCA clinic at MNH, Dar es Salaam, Tanzania. Neurol Res Int. 2020:3636547. doi: 10.1155/2020/3636547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Farooq S, Testai FD. Neurologic complications of sickle cell disease. Curr Neurol Neurosci Rep. 2019;19(4):17. doi: 10.1007/s11910-019-0932-0 [DOI] [PubMed] [Google Scholar]
- 5.Thust SC, Burke C, Siddiqui A. Neuroimaging findings in sickle cell disease. Br J Radiol. 2014;87(1040):20130699. doi: 10.1259/bjr.20130699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Houwing ME, Grohssteiner RL, Dremmen MH, et al. Silent cerebral infarcts in patients with sickle cell disease: a systematic review and meta-analysis. BMC Medicine. 2020;18(1):393. doi: 10.1186/s12916-020-01864-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Melek I, Akgul F, Duman T, Yalcin F, Gali E. Neurological soft signs as the stroke risk in sickle cell disease. Tohoku J Exp Med. 2006;209(2):135-140. doi: 10.1620/tjem.209.135 [DOI] [PubMed] [Google Scholar]
- 8.Schatz J, Brown RT, Pascual JM, Hsu L, DeBaun MR. Poor school and cognitive functioning with silent cerebral infarcts and sickle cell disease. Neurology. 2001;56(8):1109-1111. doi: 10.1212/wnl.56.8.1109 [DOI] [PubMed] [Google Scholar]
- 9.Kral MC, Brown RT, Hynd GW. Neuropsychological aspects of pediatric sickle cell disease. Neuropsychol Rev. 2001;11(4):179-196. doi: 10.1023/a:1012901124088 [DOI] [PubMed] [Google Scholar]
- 10.Hijmans CT, Grootenhuis MA, Oosterlaan J, Heijboer H, Peters M, Fijnvandraat K. Neurocognitive deficits in children with sickle cell disease are associated with the severity of anemia. Pediatr Blood Cancer. 2011;57(2):297-302. doi: 10.1002/pbc.22892 [DOI] [PubMed] [Google Scholar]
- 11.Verduzco LA, Nathan DG. Sickle cell disease and stroke. Blood. 2009;114(25):5117-5125. doi: 10.1182/blood-2009-05-220921 [DOI] [PubMed] [Google Scholar]
- 12.Zainel A, Mitchell H, Sadarangani M. Bacterial meningitis in children: neurological complications, associated risk factors, and prevention. Microorganisms. 2021;9(3):535. doi: 10.3390/microorganisms9030535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nawaiseh M, Shaban A, Abualia M, et al. Seizures risk factors in sickle cell disease. The cooperative study of sickle cell disease. Seizure. 2021;89:107-113. doi: 10.1016/j.seizure.2021.05.009 [DOI] [PubMed] [Google Scholar]
- 14.Nottidge VA. Pneumococcal meningitis in sickle cell disease in childhood. Am J Dis Child. 1983;137(1):29-31. doi: 10.1001/archpedi.1983.02140270025008 [DOI] [PubMed] [Google Scholar]
- 15.Sykes RM. Sickle cell disease, pneumococcal meningitis and paraplegia with recovery. Ann Tropical Paediatrics. 1998;18(1):63-64. doi: 10.1080/02724936.1998.11747929 [DOI] [PubMed] [Google Scholar]
- 16.Venkataraman A, Adams RJ. Neurologic complications of sickle cell disease. Handb Clin Neurol. 2014;120:1015-1025. doi: 10.1016/B978-0-7020-4087-0.00068-1 [DOI] [PubMed] [Google Scholar]
- 17.Diallo D, Tchernia G. Sickle cell disease in Africa. Curr Opin Hematol. 2002;9(2):111-116. doi: 10.1097/00062752-200203000-00005 [DOI] [PubMed] [Google Scholar]
- 18.Grosse SD, Odame I, Atrash HK, Amendah DD, Piel FB, Williams TN. Sickle cell disease in Africa: a neglected cause of early childhood mortality. Am J Prev Med. 2011;41(6 suppl 4):S398-S405. doi: 10.1016/j.amepre.2011.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DeBaun MR, Jordan LC, King AA, et al. American Society of Hematology 2020 guidelines for sickle cell disease: prevention, diagnosis, and treatment of cerebrovascular disease in children and adults. Blood Adv. 2020;4(8):1554-1588. doi: 10.1182/bloodadvances.2019001142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DeBaun MR, Sarnaik SA, Rodeghier MJ, et al. Associated risk factors for silent cerebral infarcts in sickle cell anemia: low baseline hemoglobin, sex, and relative high systolic blood pressure. Blood. 2012;119(16):3684-3690. doi: 10.1182/blood-2011-05-349621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mercuri E, Faundez JC, Roberts I, et al. Neurological ‘soft’signs may identify children with sickle cell disease who are at risk for stroke. Eur J Pediatr. 1995;154(2):150-156. doi: 10.1007/bf01991921 [DOI] [PubMed] [Google Scholar]
- 22.Gaston M, Rosse WF. The cooperative study of sickle cell disease: review of study design and objectives. Am J Pediatr Hematol Oncol. 1982;4(2):197-201. [PubMed] [Google Scholar]
- 23.Gaston M, Smith J, Gallagher D, et al. Recruitment in the cooperative study of sickle cell disease (CSSCD). Control Clin Trials. 1987;8(4 suppl l):131-140. doi: 10.1016/0197-2456(87)90016-x [DOI] [PubMed] [Google Scholar]
- 24.Biologic Specimen and Data Repository Information Coordinating Center. 2020. Accessed May 1, 2023. biolincc.nhlbi.nih.gov/home/
- 25.AlRyalat SA, Nawaiseh M, Aladwan B, Roto A, Alessa Z, Al-Omar A. Ocular manifestations of sickle cell disease: signs, symptoms and complications. Ophthalmic Epidemiol. 2020;27(4):259-264. doi: 10.1080/09286586.2020.1723114 [DOI] [PubMed] [Google Scholar]
- 26.Okuyucu EE, Turhanoglu A, Duman T, Kaya H, Melek IM, Yilmazer S. Peripheral nervous system involvement in patients with sickle cell disease. Eur J Neurol. 2009;16(7):814-818. doi: 10.1111/j.1468-1331.2009.02592.x [DOI] [PubMed] [Google Scholar]
- 27.Sadler KE, Lewis TR, Waltz TB, Besharse JC, Stucky CL. Peripheral nerve pathology in sickle cell disease mice. Pain Rep. 2019;4(4):e765. doi: 10.1097/PR9.0000000000000765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ballas SK, Lieff S, Benjamin LJ, et al. ; Investigators, Comprehensive Sickle Cell Centers. Definitions of the phenotypic manifestations of sickle cell disease. Am J Hematol. 2010;85(1):6-13. doi: 10.1002/ajh.21550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lawrence C, Webb J. Sickle cell disease and stroke: diagnosis and management. Curr Neurol Neurosci Rep. 2016;16(3):27. doi: 10.1007/s11910-016-0622-0 [DOI] [PubMed] [Google Scholar]
- 30.Griessenauer CJ, Lebensburger JD, Chua MH, et al. Encephaloduroarteriosynangiosis and encephalomyoarteriosynangiosis for treatment of moyamoya syndrome in pediatric patients with sickle cell disease. J Neurosurg Pediatr. 2015;16(1):64-73. doi: 10.3171/2014.12.PEDS14522 [DOI] [PubMed] [Google Scholar]
- 31.Piel FB, Steinberg MH, Rees DC. Sickle cell disease. N Engl J Med. 2017;376(16):1561-1573. doi: 10.1056/NEJMra1510865 [DOI] [PubMed] [Google Scholar]
- 32.Morris CR. Vascular risk assessment in patients with sickle cell disease. Haematologica. 2011;96(1):1-5. doi: 10.3324/haematol.2010.035097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gibbs WN, Opatowsky MJ, Burton EC. AIRP best cases in radiologic-pathologic correlation: cerebral fat embolism syndrome in sickle cell β-thalassemia. Radiographics. 2012;32(5):1301-1306. doi: 10.1148/rg.325115055 [DOI] [PubMed] [Google Scholar]
- 34.Mijalski C, Lovett A, Mahajan R, Sundararajan S, Silverman S, Feske S. Cerebral fat embolism: a case of rapid-onset coma. Stroke. 2015;46(12):e251-e253. doi: 10.1161/STROKEAHA.115.011440 [DOI] [PubMed] [Google Scholar]
- 35.Nathan CL, Aamodt WW, Yalamarti T, Dogon C, Kinniry P. Cerebral fat embolism syndrome in sickle cell disease without evidence of shunt. eNeurologicalSci. 2019;14:19-20. doi: 10.1016/j.ensci.2018.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gupta B, Kaur M, D’souza N, et al. Cerebral fat embolism: a diagnostic challenge. Saudi J Anaesth. 2011;5(3):348-352. doi: 10.4103/1658-354X.84122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ramsay Z, Bartlett R, Ali A, Grant J, Gordon-Strachan G, Asnani M. Sickle cell disease and pain: is it all vaso-occlusive crises? Clin J Pain. 2021;37(8):583-590. doi: 10.1097/AJP.0000000000000949 [DOI] [PubMed] [Google Scholar]
- 38.Manara R, Dalla Torre A, Lucchetta M, et al. Visual cortex changes in children with sickle cell disease and normal visual acuity: a multimodal magnetic resonance imaging study. Br J Haematol 2021;192(1):151-157. doi: 10.1111/bjh.17042 [DOI] [PubMed] [Google Scholar]
- 39.Grasso PA, Gallina J, Bertini C. Shaping the visual system: cortical and subcortical plasticity in the intact and the lesioned brain. Neuropsychologia. 2020;142:107464. doi: 10.1016/j.neuropsychologia.2020.107464 [DOI] [PubMed] [Google Scholar]
- 40.Pahl DA, Green NS, Bhatia M, et al. Optical coherence tomography angiography and ultra-widefield fluorescein angiography for early detection of adolescent sickle retinopathy. Am J Ophthalmol. 2017;183:91-98. doi: 10.1016/j.ajo.2017.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Young RC Jr, Rachal RE, Hackney RL Jr, Uy CG, Scott RB. Smoking is a factor in causing acute chest syndrome in sickle cell anemia. J Natl Med Assoc. 1992;84(3):267-271. [PMC free article] [PubMed] [Google Scholar]
- 42.West DC, Romano PS, Azari R, Rudominer A, Holman M, Sandhu S. Impact of environmental tobacco smoke on children with sickle cell disease. Arch Pediatr Adolesc Med. 2003;157(12):1197-1201. doi: 10.1001/archpedi.157.12.1197 [DOI] [PubMed] [Google Scholar]
- 43.Cohen RT, DeBaun MR, Blinder MA, Strunk RC, Field JJ. Smoking is associated with an increased risk of acute chest syndrome and pain among adults with sickle cell disease. Blood. 2010;115(18):3852-3854. doi: 10.1182/blood-2010-01-265819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Naik P, Cucullo L. Pathobiology of tobacco smoking and neurovascular disorders: untied strings and alternative products. Fluids Barriers CNS. 2015;12(1):25. doi: 10.1186/s12987-015-0022-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pegelow CH, Colangelo L, Steinberg M, et al. Natural history of blood pressure in sickle cell disease: risks for stroke and death associated with relative hypertension in sickle cell anemia. Am J Med. 1997;102(2):171-177. doi: 10.1016/s0002-9343(96)00407-x [DOI] [PubMed] [Google Scholar]
- 46.Higgins ST, Kurti AN, Redner R, et al. A literature review on prevalence of gender differences and intersections with other vulnerabilities to tobacco use in the United States, 2004-2014. Prev Med. 2015;80:89-100. doi: 10.1016/j.ypmed.2015.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ohene-Frempong K, Weiner SJ, Sleeper LA, et al. Cerebrovascular accidents in sickle cell disease: rates and risk factors. Blood. 1998;91(1):288-294. [PubMed] [Google Scholar]
- 48.Charache S. Mechanism of action of hydroxyurea in the management of sickle cell anemia in adults. In: Seminars in Hematology; 1997:15-21. [PubMed] [Google Scholar]
- 49.Kinney TR, Sleeper LA, Wang WC, et al. Silent cerebral infarcts in sickle cell anemia: a risk factor analysis. The Cooperative Study of Sickle Cell Disease. Pediatrics. 1999;103(3):640-645. doi: 10.1542/peds.103.3.640 [DOI] [PubMed] [Google Scholar]
- 50.Hindmarsh PC, Brozovic M, Brook CG, Davies SC. Incidence of overt and covert neurological damage in children with sickle cell disease. Postgrad Med J. 1987;63(743):751-753. doi: 10.1136/pgmj.63.743.751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Calvet D, Tuilier T, Mélé N, et al. Low fetal hemoglobin percentage is associated with silent brain lesions in adults with homozygous sickle cell disease. Blood Adv. 2017;1(26):2503-2509. doi: 10.1182/bloodadvances.2017005504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sarnaik SA, Casella JF, Barton BA, et al. Elevated systolic blood pressure and low fetal hemoglobin are risk factors for silent cerebral infarcts in children with sickle cell anemia. Blood. 2009;114(22):262. doi: 10.1182/blood.v114.22.262.262 [DOI] [Google Scholar]
- 53.Steinberg MH, Chui DH, Dover GJ, Sebastiani P, Alsultan A. Fetal hemoglobin in sickle cell anemia: a glass half full? Blood. 2014;123(4):481-485. doi: 10.1182/blood-2013-09-528067 [DOI] [PubMed] [Google Scholar]
- 54.Steinberg MH, Forget BG, Higgs DR, Weatherall DJ. Disorders of Hemoglobin: Genetics, Pathophysiology, and Clinical Management. Cambridge University Press; 2009. [Google Scholar]
- 55.Rodgers GP, Walker EC, Podgor MJ. Is “relative” hypertension a risk factor for vaso-occlusive complications in sickle cell disease? Am J Med Sci. 1993;305(3):150-156. doi: 10.1097/00000441-199303000-00004 [DOI] [PubMed] [Google Scholar]
- 56.Adam R, Nichols F, Carl E, et al. The use of transcutneous ultrasonogrophy to predict stroke in sickle cell disease. N Eng J Med. 1992;326:605-610. [DOI] [PubMed] [Google Scholar]
- 57.Sheehan VA, Hansbury EN, Smeltzer MP, Fortner G, McCarville MB, Aygun B. Transcranial Doppler velocity and brain MRI/MRA changes in children with sickle cell anemia on chronic transfusions to prevent primary stroke. Pediatr Blood Cancer. 2013;60(9):1499-1502. doi: 10.1002/pbc.24569 [DOI] [PubMed] [Google Scholar]
- 58.DeBaun MR, Gordon M, McKinstry RC, et al. Controlled trial of transfusions for silent cerebral infarcts in sickle cell anemia. N Engl J Med. 2014;371(8):699-710. doi: 10.1056/NEJMoa1401731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bernaudin F, Verlhac S, Arnaud C, et al. Impact of early transcranial Doppler screening and intensive therapy on cerebral vasculopathy outcome in a newborn sickle cell anemia cohort. Blood. 2011;117(4):1130-1140; quiz 1436. doi: 10.1182/blood-2010-06-293514 [DOI] [PubMed] [Google Scholar]
- 60.Wang WC, Gallagher DM, Pegelow CH, et al. Multicenter comparison of magnetic resonance imaging and transcranial Doppler ultrasonography in the evaluation of the central nervous system in children with sickle cell disease. J Pediatr Hematol Oncol. 2000;22(4):335-339. doi: 10.1097/00043426-200007000-00010 [DOI] [PubMed] [Google Scholar]
- 61.Pokhrel A, Olayemi A, Ogbonda S, Nair K, Wang JC. Racial and ethnic differences in sickle cell disease within the United States: from demographics to outcomes. Eur J Haematol. 2023;110(5):554-563. doi: 10.1111/ejh.13936 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Datasets used and/or analyzed in this study can be accessed from the BIOLINCC (Biologic Specimen and Data Repository Information Coordinating Center) website: biolincc.nhlbi.nih.gov/studies/csscd/.