Abstract
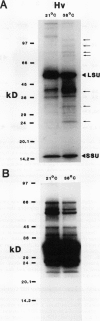
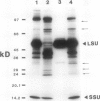
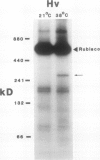
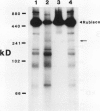
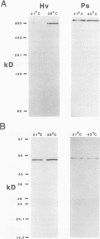
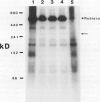
In vivo radiolabeling of chloroplast proteins in barley (Hordeum vulgare L. cv Corvette) leaves and their separation by one-dimensional electrophoresis revealed at least seven heat-shock proteins between 24 and 94 kD, of which most have not been previously identified in this C3 species. Fractionation into stromal and thylakoid membrane components showed that all chloroplast heat-shock proteins were synthesized on cytoplasmic ribosomes, translocated into the chloroplast, and located in the stroma. Examination of stromal preparations by native (nondissociating) polyacrylamide gel electrophoresis revealed the presence of a high-molecular mass heat-shock protein complex in barley. This complex was estimated to be 250 to 265 kD in size. Dissociation by denaturing polyacrylamide gel electrophoresis revealed a single protein component, a 32-kD heat-shock protein. The synthesis of this protein and the formation of the heat-shock protein complex were dependent on functional cytoplasmic ribosomes. Immunological studies showed that the heat-shock protein complex did not contain any proteins homologous to the α-subunit of ribulose bisphosphate carboxylase oxygenase subunit-binding protein. Other features about the complex included the absence of nucleic acid (RNA or DNA) and its nondissociation in the presence of Mg2+/ATP. These results suggest that the heat-shock protein complex in barley chloroplasts is a homogeneous octamer of 32-kD subunits.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernstein H. D., Poritz M. A., Strub K., Hoben P. J., Brenner S., Walter P. Model for signal sequence recognition from amino-acid sequence of 54K subunit of signal recognition particle. Nature. 1989 Aug 10;340(6233):482–486. doi: 10.1038/340482a0. [DOI] [PubMed] [Google Scholar]
- Chen Q., Lauzon L. M., DeRocher A. E., Vierling E. Accumulation, stability, and localization of a major chloroplast heat-shock protein. J Cell Biol. 1990 Jun;110(6):1873–1883. doi: 10.1083/jcb.110.6.1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q., Vierling E. Analysis of conserved domains identifies a unique structural feature of a chloroplast heat shock protein. Mol Gen Genet. 1991 May;226(3):425–431. doi: 10.1007/BF00260655. [DOI] [PubMed] [Google Scholar]
- Clarke A. K., Critchley C. Synthesis of early heat shock proteins in young leaves of barley and sorghum. Plant Physiol. 1990 Oct;94(2):567–576. doi: 10.1104/pp.94.2.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firgaira F. A., Cotton R. G., Danks D. M. Isolation and characterization of dihydropteridine reductase from human liver. Biochem J. 1981 Jul 1;197(1):31–43. doi: 10.1042/bj1970031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gegenheimer P. Preparation of extracts from plants. Methods Enzymol. 1990;182:174–193. doi: 10.1016/0076-6879(90)82016-u. [DOI] [PubMed] [Google Scholar]
- Glaczinski H., Kloppstech K. Temperature-dependent binding to the thylakoid membranes of nuclear-coded chloroplast heat-shock proteins. Eur J Biochem. 1988 May 2;173(3):579–583. doi: 10.1111/j.1432-1033.1988.tb14038.x. [DOI] [PubMed] [Google Scholar]
- Grimm B., Ish-Shalom D., Even D., Glaczinski H., Ottersbach P., Ohad I., Kloppstech K. The nuclear-coded chloroplast 22-kDa heat-shock protein of Chlamydomonas. Evidence for translocation into the organelle without a processing step. Eur J Biochem. 1989 Jul 1;182(3):539–546. doi: 10.1111/j.1432-1033.1989.tb14861.x. [DOI] [PubMed] [Google Scholar]
- Hemmingsen S. M., Ellis R. J. Purification and properties of ribulosebisphosphate carboxylase large subunit binding protein. Plant Physiol. 1986 Jan;80(1):269–276. doi: 10.1104/pp.80.1.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingolia T. D., Craig E. A. Four small Drosophila heat shock proteins are related to each other and to mammalian alpha-crystallin. Proc Natl Acad Sci U S A. 1982 Apr;79(7):2360–2364. doi: 10.1073/pnas.79.7.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ish-Shalom D., Kloppstech K., Ohad I. Light regulation of the 22 kd heat shock gene transcription and its translation product accumulation in Chlamydomonas reinhardtii. EMBO J. 1990 Sep;9(9):2657–2661. doi: 10.1002/j.1460-2075.1990.tb07450.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloppstech K., Meyer G., Schuster G., Ohad I. Synthesis, transport and localization of a nuclear coded 22-kd heat-shock protein in the chloroplast membranes of peas and Chlamydomonas reinhardi. EMBO J. 1985 Aug;4(8):1901–1909. doi: 10.1002/j.1460-2075.1985.tb03869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloppstech K., Ohad I. Heat-shock protein synthesis in Chlamydomonas reinhardi. Translational control at the level of initiation of a poly(A)-rich-RNA coded 22-KDa protein in a cell-free system. Eur J Biochem. 1986 Jan 2;154(1):63–68. doi: 10.1111/j.1432-1033.1986.tb09359.x. [DOI] [PubMed] [Google Scholar]
- Krishnasamy S., Mannan R. M., Krishnan M., Gnanam A. Heat shock response of the chloroplast genome in Vigna sinensis. J Biol Chem. 1988 Apr 15;263(11):5104–5109. [PubMed] [Google Scholar]
- Lindquist S., Craig E. A. The heat-shock proteins. Annu Rev Genet. 1988;22:631–677. doi: 10.1146/annurev.ge.22.120188.003215. [DOI] [PubMed] [Google Scholar]
- Lubben T. H., Gatenby A. A., Donaldson G. K., Lorimer G. H., Viitanen P. V. Identification of a groES-like chaperonin in mitochondria that facilitates protein folding. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7683–7687. doi: 10.1073/pnas.87.19.7683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMullin T. W., Hallberg R. L. A highly evolutionarily conserved mitochondrial protein is structurally related to the protein encoded by the Escherichia coli groEL gene. Mol Cell Biol. 1988 Jan;8(1):371–380. doi: 10.1128/mcb.8.1.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musgrove J. E., Johnson R. A., Ellis R. J. Dissociation of the ribulosebisphosphate-carboxylase large-subunit binding protein into dissimilar subunits. Eur J Biochem. 1987 Mar 16;163(3):529–534. doi: 10.1111/j.1432-1033.1987.tb10900.x. [DOI] [PubMed] [Google Scholar]
- Neidhardt F. C., VanBogelen R. A., Vaughn V. The genetics and regulation of heat-shock proteins. Annu Rev Genet. 1984;18:295–329. doi: 10.1146/annurev.ge.18.120184.001455. [DOI] [PubMed] [Google Scholar]
- Neumann D., Nover L., Parthier B., Rieger R., Scharf K. D., Wollgiehn R., zur Nieden U. Heat shock and other stress response systems of plants. Results Probl Cell Differ. 1989;16:1–155. [PubMed] [Google Scholar]
- Neumann D., Scharf K. D., Nover L. Heat shock induced changes of plant cell ultrastructure and autoradiographic localization of heat shock proteins. Eur J Cell Biol. 1984 Jul;34(2):254–264. [PubMed] [Google Scholar]
- Nieto-Sotelo J., Vierling E., Ho T. H. Cloning, sequence analysis, and expression of a cDNA encoding a plastid-localized heat shock protein in maize. Plant Physiol. 1990 Aug;93(4):1321–1328. doi: 10.1104/pp.93.4.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nover L., Munsche D., Neumann D., Ohme K., Scharf K. D. Control of ribosome biosynthesis in plant cell cultures under heat-shock conditions. Ribosomal RNA. Eur J Biochem. 1986 Oct 15;160(2):297–304. doi: 10.1111/j.1432-1033.1986.tb09971.x. [DOI] [PubMed] [Google Scholar]
- Nover L., Scharf K. D., Neumann D. Cytoplasmic heat shock granules are formed from precursor particles and are associated with a specific set of mRNAs. Mol Cell Biol. 1989 Mar;9(3):1298–1308. doi: 10.1128/mcb.9.3.1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nover L., Scharf K. D., Neumann D. Formation of cytoplasmic heat shock granules in tomato cell cultures and leaves. Mol Cell Biol. 1983 Sep;3(9):1648–1655. doi: 10.1128/mcb.3.9.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nover L., Scharf K. D. Synthesis, modification and structural binding of heat-shock proteins in tomato cell cultures. Eur J Biochem. 1984 Mar 1;139(2):303–313. doi: 10.1111/j.1432-1033.1984.tb08008.x. [DOI] [PubMed] [Google Scholar]
- Raschke E., Baumann G., Schöffl F. Nucleotide sequence analysis of soybean small heat shock protein genes belonging to two different multigene families. J Mol Biol. 1988 Feb 20;199(4):549–557. doi: 10.1016/0022-2836(88)90300-2. [DOI] [PubMed] [Google Scholar]
- Rothman J. E. Polypeptide chain binding proteins: catalysts of protein folding and related processes in cells. Cell. 1989 Nov 17;59(4):591–601. doi: 10.1016/0092-8674(89)90005-6. [DOI] [PubMed] [Google Scholar]
- Salinovich O., Montelaro R. C. Reversible staining and peptide mapping of proteins transferred to nitrocellulose after separation by sodium dodecylsulfate-polyacrylamide gel electrophoresis. Anal Biochem. 1986 Aug 1;156(2):341–347. doi: 10.1016/0003-2697(86)90263-0. [DOI] [PubMed] [Google Scholar]
- Schuster G., Even D., Kloppstech K., Ohad I. Evidence for protection by heat-shock proteins against photoinhibition during heat-shock. EMBO J. 1988 Jan;7(1):1–6. doi: 10.1002/j.1460-2075.1988.tb02776.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Süss K. H., Yordanov I. T. Biosynthetic cause of in vivo acquired thermotolerance of photosynthetic light reactions and metabolic responses of chloroplasts to heat stress. Plant Physiol. 1986 May;81(1):192–199. doi: 10.1104/pp.81.1.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vierling E., Harris L. M., Chen Q. The major low-molecular-weight heat shock protein in chloroplasts shows antigenic conservation among diverse higher plant species. Mol Cell Biol. 1989 Feb;9(2):461–468. doi: 10.1128/mcb.9.2.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vierling E., Key J. L. Ribulose 1,5-Bisphosphate Carboxylase Synthesis during Heat Shock. Plant Physiol. 1985 May;78(1):155–162. doi: 10.1104/pp.78.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vierling E., Mishkind M. L., Schmidt G. W., Key J. L. Specific heat shock proteins are transported into chloroplasts. Proc Natl Acad Sci U S A. 1986 Jan;83(2):361–365. doi: 10.1073/pnas.83.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vierling E., Nagao R. T., DeRocher A. E., Harris L. M. A heat shock protein localized to chloroplasts is a member of a eukaryotic superfamily of heat shock proteins. EMBO J. 1988 Mar;7(3):575–581. doi: 10.1002/j.1460-2075.1988.tb02849.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viitanen P. V., Lubben T. H., Reed J., Goloubinoff P., O'Keefe D. P., Lorimer G. H. Chaperonin-facilitated refolding of ribulosebisphosphate carboxylase and ATP hydrolysis by chaperonin 60 (groEL) are K+ dependent. Biochemistry. 1990 Jun 19;29(24):5665–5671. doi: 10.1021/bi00476a003. [DOI] [PubMed] [Google Scholar]
- Weng J., Wang Z. F., Nguyen H. T. Nucleotide sequence of a Triticum aestivum cDNA clone which is homologous to the 26 kDa chloroplast-localized heat shock protein gene of maize. Plant Mol Biol. 1991 Aug;17(2):255–258. doi: 10.1007/BF00039500. [DOI] [PubMed] [Google Scholar]