Abstract
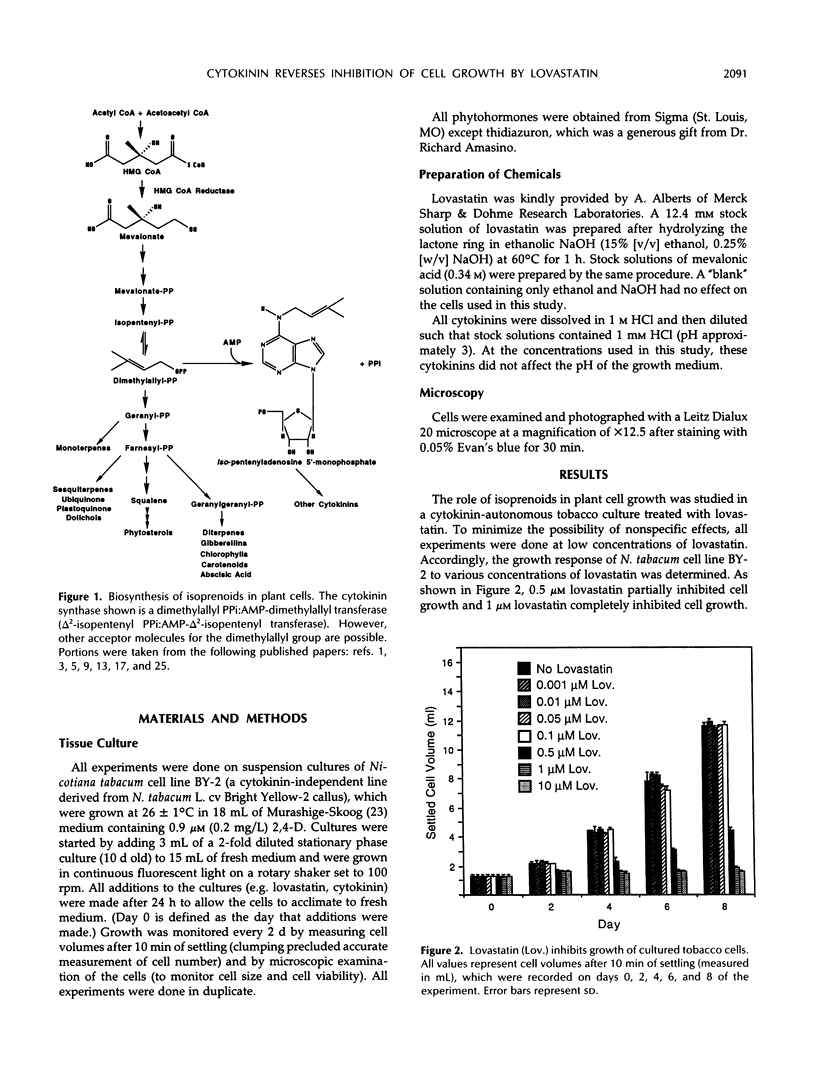
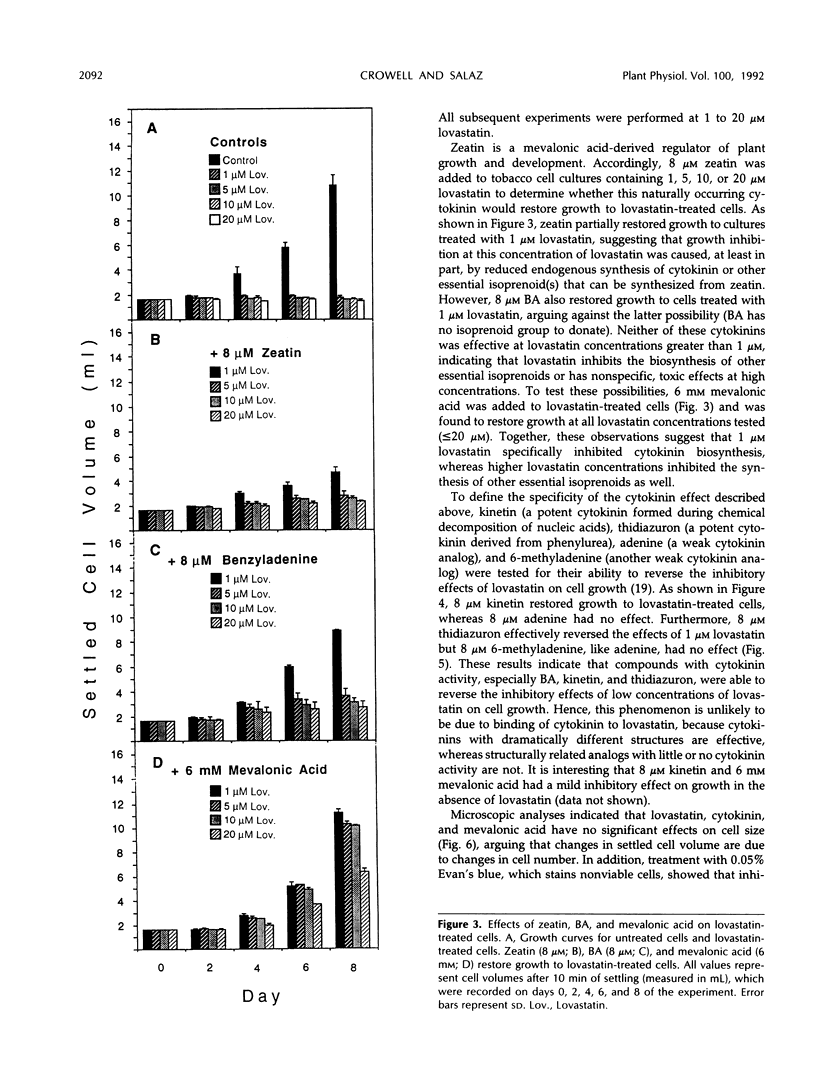
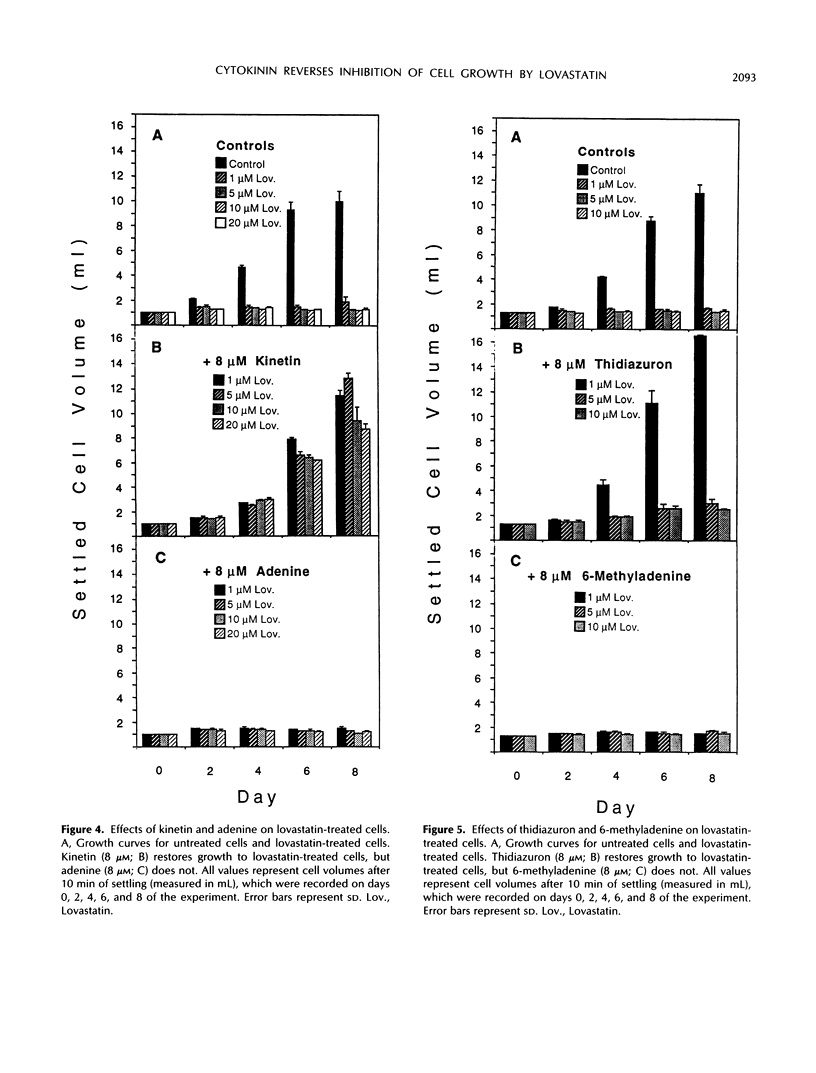
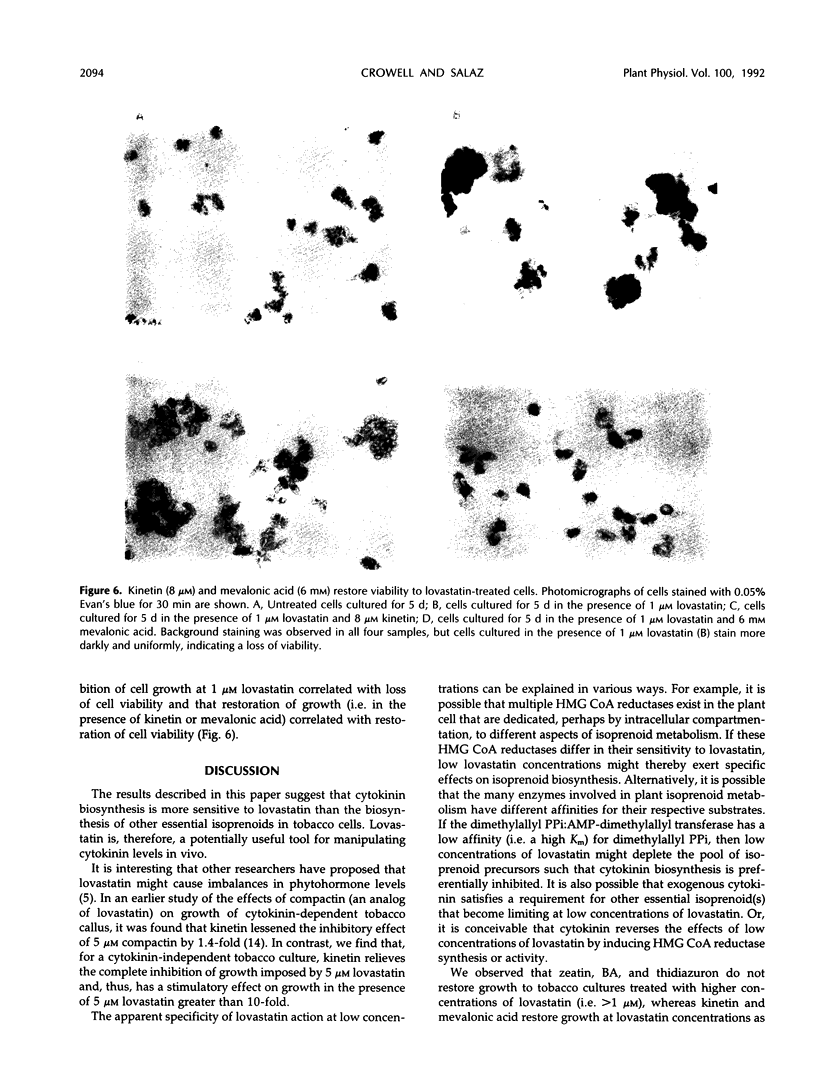
De novo synthesis of mevalonic acid, which is catalyzed by 3-hydroxy-3-methylglutaryl coenzyme A reductase, is the first committed step in the formation of isoprenoid compounds. Various studies have shown that mevalonic acid-derived compounds are required for growth of plant and animal cells, a conclusion supported by the observation that cells treated with lovastatin (a potent inhibitor of 3-hydroxy-3-methylglutaryl coenzyme A reductase) cease growth. We show that Nicotiana tabacum BY-2 cells, which require exogenous auxin for growth in culture but do not require exogenous cytokinin, are growth inhibited by 1 μm lovastatin. However, these cells are capable of growing in the presence of 1 μm lovastatin if 8 μm zeatin is supplied in the medium. Furthermore, benzyladenine, kinetin, and thidiazuron effectively reverse the inhibition of growth of these cells at 1 μm lovastatin, whereas adenine and 6-methyladenine have no effect. These results demonstrate that restoration of growth to lovastatin-treated cells is cytokinin specific and is not caused by metabolism of cytokinin into other isoprenoid compounds. Cytokinin does not effectively reverse the effects of higher concentrations of lovastatin, but mevalonic acid does, consistent with the hypothesis that cytokinin biosynthesis is more sensitive to lovastatin than the biosynthesis of other essential isoprenoid compounds in tobacco cells. This observation suggests that lovastatin can be used to induce cytokinin dependence in cytokinin-autonomous tobacco cell cultures.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akiyoshi D. E., Klee H., Amasino R. M., Nester E. W., Gordon M. P. T-DNA of Agrobacterium tumefaciens encodes an enzyme of cytokinin biosynthesis. Proc Natl Acad Sci U S A. 1984 Oct;81(19):5994–5998. doi: 10.1073/pnas.81.19.5994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberts A. W., Chen J., Kuron G., Hunt V., Huff J., Hoffman C., Rothrock J., Lopez M., Joshua H., Harris E. Mevinolin: a highly potent competitive inhibitor of hydroxymethylglutaryl-coenzyme A reductase and a cholesterol-lowering agent. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3957–3961. doi: 10.1073/pnas.77.7.3957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach T. J. Hydroxymethylglutaryl-CoA reductase, a key enzyme in phytosterol synthesis? Lipids. 1986 Jan;21(1):82–88. doi: 10.1007/BF02534307. [DOI] [PubMed] [Google Scholar]
- Bach T. J., Lichtenthaler H. K. Mechanisms of inhibition by mevinolin (MK 803) of microsome-bound radish and of partially purified yeast HMG-CoA reductase (EC.1.1.1.34). Z Naturforsch C. 1983 Mar-Apr;38(3-4):212–219. doi: 10.1515/znc-1983-3-410. [DOI] [PubMed] [Google Scholar]
- Bach T. J., Lichtenthaler H. K. Mevinolin: a highly specific inhibitor of microsomal 3-hydroxy-3-methylglutaryl-coenzyme A reductase of radish plants. Z Naturforsch C. 1982 Jan-Feb;37(1-2):46–50. doi: 10.1515/znc-1982-1-209. [DOI] [PubMed] [Google Scholar]
- Bauw G., De Loose M., Inzé D., Van Montagu M., Vandekerckhove J. Alterations in the phenotype of plant cells studied by NH(2)-terminal amino acid-sequence analysis of proteins electroblotted from two-dimensional gel-separated total extracts. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4806–4810. doi: 10.1073/pnas.84.14.4806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooker J. D., Russell D. W. Regulation of microsomal 3-hydroxy-3-methylglutaryl coenzyme A reductase from pea seedlings: rapid posttranslational phytochrome-mediated decrease in activity and in vivo regulation by isoprenoid products. Arch Biochem Biophys. 1979 Nov;198(1):323–334. doi: 10.1016/0003-9861(79)90425-9. [DOI] [PubMed] [Google Scholar]
- Brown M. S., Goldstein J. L. Multivalent feedback regulation of HMG CoA reductase, a control mechanism coordinating isoprenoid synthesis and cell growth. J Lipid Res. 1980 Jul;21(5):505–517. [PubMed] [Google Scholar]
- CRELIN E. S. Mitosis in adult cartilage. Science. 1957 Apr 5;125(3249):650–650. doi: 10.1126/science.125.3249.650. [DOI] [PubMed] [Google Scholar]
- Chen C. M., Leisner S. M. Cytokinin-modulated gene expression in excised pumpkin cotyledons. Plant Physiol. 1985 Jan;77(1):99–103. doi: 10.1104/pp.77.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowell D. N., Kadlecek A. T., John M. C., Amasino R. M. Cytokinin-induced mRNAs in cultured soybean cells. Proc Natl Acad Sci U S A. 1990 Nov;87(22):8815–8819. doi: 10.1073/pnas.87.22.8815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein J. L., Brown M. S. Regulation of the mevalonate pathway. Nature. 1990 Feb 1;343(6257):425–430. doi: 10.1038/343425a0. [DOI] [PubMed] [Google Scholar]
- Maltese W. A. Posttranslational modification of proteins by isoprenoids in mammalian cells. FASEB J. 1990 Dec;4(15):3319–3328. doi: 10.1096/fasebj.4.15.2123808. [DOI] [PubMed] [Google Scholar]
- Meins F., Jr Habituation: heritable variation in the requirement of cultured plant cells for hormones. Annu Rev Genet. 1989;23:395–408. doi: 10.1146/annurev.ge.23.120189.002143. [DOI] [PubMed] [Google Scholar]
- Russell D. W., Davidson H. Regulation of cytosolic HMG-CoA reductase activity in pea seedlings: contrasting responses to different hormones, and hormone-product interaction, suggest hormonal modulation of activity. Biochem Biophys Res Commun. 1982 Feb 26;104(4):1537–1543. doi: 10.1016/0006-291x(82)91426-7. [DOI] [PubMed] [Google Scholar]
- Vögeli U., Chappell J. Inhibition of a plant sesquiterpene cyclase by mevinolin. Arch Biochem Biophys. 1991 Jul;288(1):157–162. doi: 10.1016/0003-9861(91)90178-l. [DOI] [PubMed] [Google Scholar]



