Abstract
Several authors have reported finding retained primitive reflexes (RPRs) in individuals with autism spectrum disorders (ASD). This case report describes the reduction of RPRs and changes in cognitive function after transcutaneous electrical nerve stimulation (TENS) of muscle. Three individuals were examined in a study at the Institute for Neurology and Neurosurgery in Havana, Cuba. Two child neurologists, not involved in the study, conducted clinical examinations on each participant and diagnosed each with ASD based on DSM-V criteria and the Autism Diagnostic Interview-Revised (an autism evaluation tool). Each child with ASD possessed a triad of impairments in three domains: social interaction, communication, and repetitive behaviour. Individuals were evaluated by quantitative electroencephalographic measures and tested by standardised cognitive function tests before and after 12 weeks of intervention. These interventions were associated with reduced ASD symptoms in the three domains, significant changes in qEEG network connectivity and significantly improved performance on standardised cognitive tests.
Keywords: Clinical neurophysiology, Child and adolescent psychiatry (paediatrics), Developmental paediatrocs, Child and adolescent psychiatry
Background
Sensory/motor reflexes present at birth are known as primitive or infantile reflexes. Primitive reflexes, which help with survival after birth and in the early years of a child’s development, are unconscious motor responses that originate in the brainstem.1 2 It is assumed that the main function of primitive reflexes post partum is to allow infants to move and react to their environment without a fully developed motor cortex at birth. By 4–6 months of age, these central nervous system (CNS) motor responses should be eventually inhibited as the brain develops and replaces them with voluntary motor responses.2 3 However, they may reappear in the presence of neurological disease, or as we propose, they can be retained, acting as a sign of maturational issues related to autism spectrum disorder (ASD). Regardless of participant age, the ability of an infant to engage with objects and individuals through actions and communicative gestures is highly correlated with the maintenance of primordial reflexes.
A significant feature of those with ASD is the ‘unevenness’ of cognitive function.4 It has been proposed elsewhere that the diverse aberrant behaviours noted in ASD and in other neurobehavioral disorders can be understood better by viewing ASD in the context of functional brain dysconnectivity of the kind that has been noted in minimally conscious states5 6 and even in sleep,7 or as reported in individuals with dyslexia.8 9
It has also been demonstrated that this functional disconnection can relate to a cortical maturational imbalance between networks.10 It has been hypothesised that a delay in cortical maturation in certain networks may result in an increased cortical maturation and development in other networks, leading to developmental asynchrony and unevenness of functional skills.10 11 Some authors12 13 consider RPRs as predictive of diffuse cerebral dysfunction since these signs are significantly correlated with cognitive deficits in a wide age range of individuals as determined by the Halstead-Reitan neuropsychological test battery.12 In a large-scale post hoc analysis of children with RPRs, their presence was found to be highly correlated with cognitive impairment.14 Considering that cognitive functions tend to be lateralised, cognitive deficits could assist in localising specific areas of treatment interventions for those individuals with ASD that could lead to the diminishing of RPRs with concomitant cognitive improvement.10 14 15
In adult and geriatric cases, returned primitive reflexes are associated with frontotemporal dementia with cognitive effects but not so with lesions in other cortical areas.16–18 This then makes a connection between frontal lobe function, cognition, and the existence of retained or returned primitive reflexes also known as frontal release signs. We observed that the interventions described below can reduce ASD symptoms in the three domains along with significant changes in qEEG network connectivity and significantly improved performance on standardised cognitive tests. In this article, we report that the outcome of transcutaneous electrical nerve stimulation (TENS) of muscles linked with RPRs can reduce RPRs and be associated with objective changes in cognitive function.
Three children were examined in a study at the Institute for Neurology and Neurosurgery in Havana, Cuba. Two child neurologists, not involved in the study, conducted clinical examinations on each child and confirmed in each a diagnosis of ASD based on DSM-V criteria and the Autism Diagnostic Interview-Revised.19 The Institute of Neurology and Neurosurgery Ethics Committee and the Institutional Review Board for the University of Haifa approved the proposed research project (INN2020-41). The children’s parents provided informed consent for each child and separately for themselves.
Case presentation
Case 1
Case 1 is a right-sided child in middle childhood with measured intelligence within the normal range, tall for his chronological age and overweight. Apgar score at birth was 9/9. In utero and after birth and breast feeding, he was exposed to a home environment in which cigarette smoking and alcohol intake was reported throughout the pregnancy. A history of low weight was reported during the first trimester and an ultrasound at 16 weeks gestational age (GA) revealed fetal hydrocephalus, which had resolved at 24 weeks GA. During the third trimester, a diagnosis of anaemia and urinary tract infection was made, and the mother received treatment with oral iron supplementation and antibiotics. A caesarean section with blood transfusion was performed at 39 weeks GA. Birth weight was 3700 g, and height 53 cm.
When he was a toddler, his parents noticed that he had little interest in playing with his siblings and kindergarten classmates. He was significantly delayed in language, and when he began to speak during early childhood, he always did so in the third person singular. He never used ‘I‘ or ‘me‘. He crawled at the end of early childhood and walked as a toddler. He also presented difficulty in pointing (joint attention); when he wanted something. Instead of pointing, he led the person by the hand to the place or to the item he wanted. He also walked on tiptoe. This stopped during late early childhood. For many years, he exhibited motor stereotypies (ie, repetitive, rhythmic, but purposeless movements), ASD behaviour and flapping of his hands. He frequently cried spontaneously, especially when the television, cell phone or laptop was taken away from him. From the time he was born until the end of early childhood, he slept little and woke up constantly in the early morning, every 1 or 2 hours. He was frequently irritable, demonstrating low frustration tolerance. His choice of food was unvarying. This behaviour continues to the present, which has led him to be overweight for his age.
His teachers report that he does not speak like other children in his classroom, because he has a marked accent, reflective of the cartoons he watches on television (ie, scripting). He currently presents with difficulties in playing games and in social interaction, showing little interest in group activities or imaginary games. His social behaviour is significantly below age level as are his non-verbal communication abilities. His parents report his having an ‘addiction‘ to video games. His behaviour is monotonous and at times, inflexible and hyperactive. His thinking is rigid.
Physical examination
Case 1 was alert and oriented, demonstrating no apparent distress. Parents were present in during the examination and testing. His pulse was 107/min with normal sinus rhythm, blood pressure 110/80 mm Hg, respiratory rate 22/min, temperature 36.6°C. O2 saturation was 95% in room air. Examination of his head, eyes, ears, nose and throat (HEENT) was normal, and his pupils were equally round and reactive to light. Extraocular muscles were intact, and conjunctivae were pink. Oral mucous membranes were moist and without lesion. Tympanic membranes were intact bilaterally. There was no jugular venous distention. There was no lymphadenopathy. On examination of his lungs, breath sounds were normal—without rhonchi, rales or wheezes. On examination of his heart, there were no murmurs or gallops and no rub. Distal pulses were 2+ and no carotid bruits were noted. The abdomen was soft on palpation and non-tender. There was no distention. Bowel sounds were present. There was no organomegaly and no abdominal mass. There were no peritoneal signs. On examination of the limbs, there was no peripheral oedema, focal long bone tenderness or deformity. The patient’s skin was warm and dry, without rash or lesion.
Neurological examination
Case 1 was evaluated by two child neurologists, and he was free of other treatments before and during the 12-week treatment phase. He was alert, attentive and oriented. He had clear speech, with a unique accent. Comprehension was somewhat diminished, but he recalled 3/3 objects after 5 min. Visual fields were full to confrontation. Fundoscopic examination was normal with sharp discs. The pupils were 4 mm and briskly reactive to light. Visual acuity was 20/20 bilaterally. The extraocular muscles were intact with no nystagmus or ptosis. Facial sensation was intact to pinprick in all three divisions bilaterally. His face was symmetric with normal eye closure and smile. Hearing was normal to rubbing fingers. The palate elevated symmetrically, and phonation was normal. Head turning and shoulder shrug were intact. The tongue was midline with normal movements and no atrophy. There was no pronator drift of out-stretched arms, and muscle bulk and tone were found to be normal. Strength was full (score of 5 out of 5) bilaterally. Light touch, pinprick, position and vibration senses were intact in the fingers and toes. Rapid alternating movements and fine finger movements were intact. There was no dysmetria on finger-to-nose and heel-knee-shin examination. There were no abnormal or extraneous movements, and the Romberg sign was absent. Posture was normal and gait steady with normal steps, base, arm swing and turning. Heel and toe walking were normal. Tandem gait was normal with one eye closed. A diagnosis of ASD was confirmed and with a classical triad of impairments in the three domains that included: social interaction, communication and repetitive behaviour, based on DSM-V criteria and the Autism Diagnostic Interview-Revised.
Case 2
Case 2 is a male in middle childhood with measured intelligence within the normal range, and weight and height appropriate for his age and gender. His previous medical history includes bronchial asthma. Apgar score at birth was 9/9. As a fetus, gestation was complicated by gestational diabetes, requiring insulin treatment. A caesarean section was performed at 38 weeks GA. His birth weight was 3300 g and his was height 56 cm.
The child had delayed psychomotor development, both in walking and in speaking. He was observed to have difficulty in socialising as a toddler. He crawled at the end of infancy and started walking as a toddler. His first words were spoken in early childhood. He exhibited stereotypical as well as repetitive behaviours, unmotivated laughter and crying and racing thoughts (thoughts that tangentially jump from one to the next). He was always interested in the same objects, which he always aligned, grouped and ordered in the same way. He walked on tiptoe until the end of early childhood. From the time he started school until the present, he has demonstrated poor socialisation with his classmates, as well as difficulty in understanding and following teachers’ directions. He does not understand jokes or comic stories. At school, he is always isolated, alone, does not participate in free time activities with his classmates and remains in the classroom while others leave. Teachers have noticed that most of the time he plays with a pencil or any object, spinning it senselessly or arranging his supplies on the desk without paying attention to voices or calls, disconnected from his environment. His teachers report that he speaks very differently from the rest of the children, since he speaks with an accent from other countries or from the cartoons he watches on television. He has a great affinity for television, tablets, computers and cell phones. He spends hours in his own world and most times he does not get up to eat. He enjoys singing, however.
Physical examination
Case 2 was alert and cooperative and parents were present in during the examination and testing. His pulse was 84/min, blood pressure 135/85 mm Hg, respiration rate 18/min, temperature 36.1°C and room air O2 saturation 96%—all within normal limits. HEENT was normal and non-icteric sclera were noted. His pupils were equally round and reactive to light. The conjunctivae appeared well perfused. Convergent strabismus was detected. Examination of the oropharynx was normal. The mucous membranes were moist. No jugular venous distention was observed. Some soft and mobile cervical lymphadenopathy was found. The chest wall was non-tender. On auscultation, regular heart rate and rhythm was evidenced without murmurs. The lungs were clear to auscultation bilaterally. The patient’s abdomen was soft, non-tender and non-distended. Bowel sounds were normal. No organomegaly or masses were appreciated. There were no peritoneal signs. The skin was warm and dry. No rash, oedema, petechiae or purpura were present. There was no focal long bone tenderness. Case 2 had with genus recurvatum or hyperextended knees.
Neurological examination
Case 2 was evaluated by two child neurologists and was free of other treatments before and during the 12-week treatment phase. Case 2 was alert, cooperative and oriented with clear speech with a particular accent. Comprehension was somewhat diminished, but he could recall 3/3 of objects after 5 min. Visual fields were full to confrontation. Fundoscopic examination was normal, with sharp discs. Both pupils were 4 mm and briskly reactive to light. No nystagmus and no ptosis were found. Convergent strabismus was diagnosed. Facial sensation was intact to pinprick in all three divisions bilaterally. His face was symmetric with normal eye closure and smile. His hearing was normal to rubbing fingers. The palate was elevated symmetrically. Phonation was normal. Head turning and shoulder shrug were intact, and the tongue was midline with normal movements and no atrophy. There was no pronator drift of out-stretched arms. Muscle bulk and tone were normal. Strength was full bilaterally (5 out of 5). In the fingers and toes, light touch, pinprick, location sensation and vibration sense were all intact. Rapid alternating movements and fine finger movements were also intact. There was no dysmetria on finger-to-nose and heel-knee-shin examination. There were no abnormal or extraneous movements. Romberg sign was absent. Case 2’s posture was normal, and his gait was steady with normal steps, base, arm swing and turning. Heel and toe walking were normal. Tandem gait was normal when the patient closed one of his eyes. Case 2 had a classical triad of impairments in the three domains that included: social interaction, communication and repetitive behaviour, based on DSM-V criteria and the Autism Diagnostic Interview-Revised.
Case 3
Case 3 is a male in late adolescence with intelligence within the normal range, and with a height of 195 cm and weight of 70 kg. His mother had a normal pregnancy. His birth weight was 4700 g and height 54 cm. His mother was in her early 30s at the time of her pregnancy. She did not remember the date of her last menstruation; therefore, by ultrasound, it was calculated as a pregnancy of 40 weeks. However, the child showed post-term signs at birth and was a macro-fetus, so it was assumed by the attending obstetrician at the time that the pregnancy period had lasted more than 42 weeks.
At 4 months post partum, his mother noticed that Case 3’s gaze was different compared with other infants of his age, demonstrating ‘lost gaze’. The sun reportedly bothered him. When he began ablactation, it was difficult for him to eat. He was irritable and cried often. He did not know how to chew. As a result, his diet comprised porridge and pureed vegetables for years.
He began to walk as a toddler. He reportedly suffered from a significant language delay, pronouncing his first word, at the end of early childhood. Later, he demonstrated verbal rituals and compulsions, as well as hand and finger mannerisms. He did not socialise with others, never demonstrated a social smile, and hid frequently under tables. He had difficultly sustaining eye contact. His racing thoughts were marked, and food whims persisted, refusing hard food and requiring food to be pureed. He would vomit if his mother changed his diet, resulting in a non-varying and limited array of foods. He had difficulty socialising with others. From a young age, he was bothered by noises such as food processors and drills. His behaviour patterns became increasingly restricted, repetitive and stereotyped. He learnt to read and write relatively late in middle childhood. From the time of his childhood, he manifested frequent tantrums. He often jumped for no apparent reason, a behaviour that persists.
Currently, Case 3 manifests abnormal social relationships, difficulty with conversation, both for comprehension and expression, and demonstrates little interest in his surroundings. He shows poor eye contact, reduced non-verbal communication and infrequent social expression. His behaviours are largely inflexible and routine, and he demonstrates limited interests except for television and certain video games.
Physical examination
Case 3 was alert and oriented, in no apparent distress. Parents were present in during the examination and testing. His pulse was 78/min and in sinus rhythm, blood pressure 120/65 mm Hg, respiratory rate 16/min and temperature 36.8°C. His O2 saturation was 99% on room air. HEENT was normal with pupils equally round and reactive to light. The extraocular muscles were intact. The conjunctivae were pink. Oral mucous membranes were moist without lesions. Tympanic membranes were within normal limits bilaterally. There was no jugular venous distention. There was no lymphadenopathy. The lungs were clear bilaterally without rhonchi, rales or wheeze. On examination of the heart, the heart rate was regular with no murmurs, gallops or rub. Distal pulses were 2+. No carotid bruits were appreciated. The abdomen completely soft, non-tender and non-distended. Bowel sounds were normal and there was no organomegaly. There was no palpable abdominal mass. There were no peritoneal signs. On examination of the limbs, there was no peripheral oedema and no focal long bone tenderness or deformity. The skin was warm and dry, without a rash or lesions.
Neurological examination
Case 3 was evaluated by two child neurologists and was free of alternate treatments before and during the 12-week treatment phase. Case 3 was alert, attentive and oriented with clear speech, but with a peculiar accent. Comprehension was markedly diminished, but he recalled 3/3 objects after 5 min. Visual fields were full to confrontation. Fundoscopic examination was normal with sharp discs. Both pupils were 4 mm and briskly reactive to light. Visual acuity was 20/20 bilaterally. The extraocular muscles were intact. There was no nystagmus or ptosis. Facial sensation was intact to pinprick in all three divisions bilaterally. The face was symmetric with normal eye closure and smile. His hearing was normal to rubbing fingers. The palate was elevated symmetrically. Head turning and shoulder shrug were intact. The tongue was midline with normal movements and no atrophy. There was no pronator drift of out-stretched arms and muscle bulk, and tone were normal. Strength was unimpaired bilaterally (5 out of 5 bilaterally). In the fingers and toes, light touch, pinprick, location sensation and vibration sense were all intact. Rapid alternating movements and fine finger movements were also intact. There was no dysmetria on finger-to-nose and heel-knee-shin examination. There were no abnormal or extraneous movements. Romberg sign was absent. His posture was normal and gait steady with normal steps, base, arm swing and turning. Heel and toe walking were normal. Tandem gait was normal when the patient closed one of his eyes. A diagnosis of ASD was confirmed. He had a classical triad of impairments in the three domains that included: social interaction, communication and repetitive behaviour, based on DSM-V criteria and the Autism Diagnostic Interview-Revised.
Investigations
The investigations performed are described in detail below. At all times, all three children were accompanied by their parents. The children and parents were briefed in full about the texts and gave their consent to proceed. The testing took approximately 1 hour for each child.
Reflex testing and stimulation
All three children had the following reflexes examined clinically by two neurologists in a private clinical examination room with their parents present and included both symmetric and asymmetric reflexes: asymmetric tonic neck reflex (ATNF), symmetric tonic neck reflex (STNR), Spinal Galant, Babinski, Palmer Grasp, Rooting and tonic labyrinthine neck reflex (TNR). Abnormality was defined as an individual exhibiting two or greater RPRs. Reflexes were graded on a scale of 0–4 based on clinical judgement (0=0 fully integrated; 1=25%; retained; 2=50% retained; 3=75%; 4=100% completely retained when tested by three examiners. The testing procedures are described more fully in table 1 and in online supplemental appendix 1.2 3 10–12
Table 1.
Reflex testing and intervention procedures (greater detail about the methods may be found in online supplemental appendix 1)
| Reflex repetitions | |||
| Reflex | Method of evoking reflex |
Reflex stimulation procedures | Frequency of repetition |
| Tonic Labyrinthine (TLR) | Lay on the back with legs flexed up and arms wrapped around legs; head on ground to start. The head flexed, and body rocked forward as far as possible. | Standing feet together eyes closed, bend head back wait 5 s, look for sway forward, back or fall, then repeat with head forward, or Extend head and roll backwards and then roll forward. |
x 10×3 /day |
| Asymmetric Tonic Neck Reflex (ATNR) | Lying on stomach, head turned to one side, arm and leg are extended straight on side of head turn, arm and leg on opposite side flexed | Hands and knees turn head all the way to right beyond end range, look for left elbow bend, then repeat to the left. | x 10×3 /day |
| Symmetric Tonic Reflex (STNR) | On hands and knees, eyes open, bend head back and look upwards then bend head down to look through the knees; | Patient on knees place hands on patient chin and forehead then head moved up and down through full range, look for elbow bend with flexion and legs bend with extension of head. | x 10×3 /day |
| Babinski | Roll Tennis Ball on bottom outside of foot. | Stroke upwards with hard end of paintbrush laterally and at bottom of foot. Stop below ball of foot, look for toe extension and flare. | 20 Left foot, 10 Right: x 3 /day |
| Rooting | Suck on straw or suck and blow lips. | Brushstroke from cheek to corner of mouth then across both lips x five then from chin toward corner of mouth and across lips x 5 x’s; repeat on contralateral face. | 30 s to 1 minute x 3 /day |
| Snout | Press on filtrum (space between nose and upper lip). Look for pursing of lips. | x 103 /day | |
| Spinal Galant | Lying on the back, arms at side and legs together. Open arms and legs together slowly as far as possible bringing hands together over head and legs spread apart simultaneously. lowly return to original position 10 repetitions | Stroke side of spine downwards with hard side of a brush little more lateral 10 times both sides, look for lateral flexion toward side of stroking. | x 10×3 /day |
| Moro | Sitting on chair, start from fetal position, hands curled in fist and head bent forward. Right wrist over left wrist and right ankle over left (crisscrossed). Open all the way bending head back with arms and legs stretched then return to fetal position crossing left wrist over right and left ankle over right repeat and to original position for 10 repetitions | Lying face up, hold head and bend it forward quickly moving head downwards ‘Approx. 1–2 inches 10 reps. Additionally clap hands loudly behind the individuals head to evoke startle. Look for movement of arms and hands up and out or arms and legs. |
x 10×3 /day |
| Palmer Grasp | Squeeze tennis ball with hand | Use brush or vibration to draw X on hand with the hard side of the brush. Look for flexion of hand. |
x 20 left, x 10 right x 3 /day |
| Sensory Stimulation | |||
| Vestibular Ocular Reflex (VOR) | VOR exercises to the left (head turn): | While fixating on object (eg, pencil, finger, or looking in mirror turn head left-ward as far as possible while keeping eyes fixed on object. | x 10×2 /day |
| Post-rotational nystagmus testing (PRNG) (Spinning) |
Spinning | Spin fast clockwise for 10 rotations of 2 s/rotation and slow spin counterclockwise @ 6 s/rotation repeated until nystagmus evoked | 10 rotations each direction x 3 /day |
| Optokinetic reflex | Optodrum | Use animals or black and white stripes to right and down only generate don mobile phone | 30 3 /day. |
| Light stimulation | Penlight | Shine light in corner of left eye | x 10 for 3 s -x 3 /day |
| Combined sensory stimulation | MetroTimer | Flashing light @ 54 beats/min in corner of left eye only for 1 min After 1 month sound and light together 30 s after light and sound separately for 30 s. |
x three times/day |
| Tactile stimulation | Brushing | Brush on left arm and legs for right hemisphere dominant or vice versa for left dominant | x10 times on each limb x 3 /day |
| Transcutaneous electrical nerve stimulation (TENS) | Pulsed electrodermal stimulation and Pulsed light stimulation |
Wireless pads placed on left upper back between shoulder blade and spine. TENS unit set to mode 6. Starting at 10 min x 2 /day. Increase intensity until individual felt light tingling ensuring absence of muscle contraction. Initially administered separately from pulsed light stimulation. At fifth wk. (when both administered for 30 min) then combined for 30 min. simultaneously, increasing by 10 min. every other week up to 60 min. for both simultaneously. |
x 2 /day (Every other week added 10 min until 60 min) x 2 /day |
bcr-2023-255285supp001.pdf (162.5KB, pdf)
Cognitive and behavioural testing
The Spanish editions of the following standardised tests were performed: The Wechsler Individualised Achievement Testing-III (WIAT-III),20 Wechsler Intelligence Scale for Children-Fourth Edition (WISC-IV),21 ADI-R (Autism Diagnostic Interview-Revised-Spanish Edition)19 22 with the data represented in table 2.
Table 2.
Results of primitive reflex and cognitive testing prior to and after 12 week intervention
| CASE | Prior to treatment | Post-treatment (12 WeekS) | ||||||||||||||
| PRP026 | PRP096 | PRP162 | PRP167 | PRP026 | PRP096 | PRP162 | PRP167 | |||||||||
| REFLEX | ||||||||||||||||
| ATNR | 3 | 3 | 3 | 0 | 0 | 0 | 0 | 0 | ||||||||
| Spinal Galant | 3 | 4 | 2 | 0 | 0 | 0 | 0 | 0 | ||||||||
| STNR | 3 | 4 | 3 | 0 | 0 | 0 | 0 | 0 | ||||||||
| Moro | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||||||||
| Babinski | 2R | 2L | 4R | 4L | 2R | 2L | 0R | 0L | 0R | 0L | 0R | 0L | 0R | 0L | 0R | 0L |
| Rooting | 2R | 2L | 3R | 3L | 3R | 3L | 0R | 0L | 0R | 0L | 0R | 0L | 0R | 0L | 0R | 0L |
| Palmer | 2R | 2L | 4R | 4L | 2R | 2L | 0R | 0L | 0R | 0L | 0R | 0L | 0R | 0L | 0R | 0L |
| WISC/WAIS IQ FS | 96 | 85 | 85 | 122 | 118 | 102 | 106 | 123 | ||||||||
| Pict. Comp. | 8 | 11 | 10 | 13 | 14 | 9 | 13 | 13 | ||||||||
| Block Des | 10 | 8 | 10 | 11 | 12 | 10 | 15 | 11 | ||||||||
| Matrix | 8 | 7 | 7 | 12 | 13 | 11 | 8 | 12 | ||||||||
| Info | 8 | 4 | 9 | 19 | 11 | 10 | 11 | 19 | ||||||||
| Similarities | 13 | 12 | 10 | 19 | 9 | 14 | 12 | 19 | ||||||||
| Digit Span | 0 | 0 | 10 | 16 | 0 | 0 | 9 | 16 | ||||||||
Hearing and vestibular function
All children in Cuba with ASD are routinely tested by brainstem auditory evoked potentials to rule out auditory impairment. Cranial nerve function was also included as part of the evaluation protocol as was the case in the patients evaluated in this report.
Quantitative electroencephalography (qEEG)
The three children were examined in the fashion reported in previous studies.23 24 qEEG is concerned with the numerical analysis of EEG data and was employed to examine shared activity between rhythms incorporating and magnitude synchrony for all individuals prior to and after treatment (12 weeks later). The data in figures 1–3 (B) represent the absolute power in μV and the colouration represents the distribution of the alpha activity prior to and after treatment. Testing was performed with the children’s parents present throughout all testing.
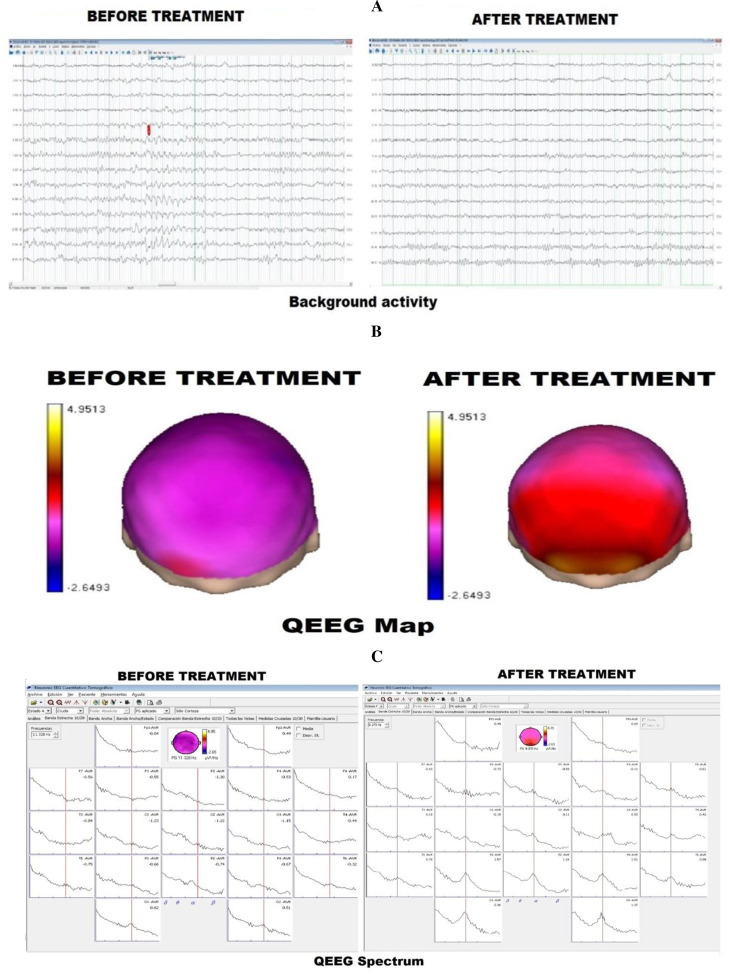
Figure 1.
Case 1: (A) Presents the EEG background activity both before and after treatment. After treatment, the background activity is better organised with the appearance of the alpha rhythm, with its typical wax and waning pattern. (B) The qEEG spectrum before and after treatment. Before treatment, the qEEG spectrum is poorly delineated; after treatment, the spectrum is clearly outlined with a dominant alpha pattern. (C) qEEG map. Before treatment, alpha absolute power is not significantly present in the left hemisphere, but after the treatment, the qEEG map demonstrates a dominant alpha pattern.
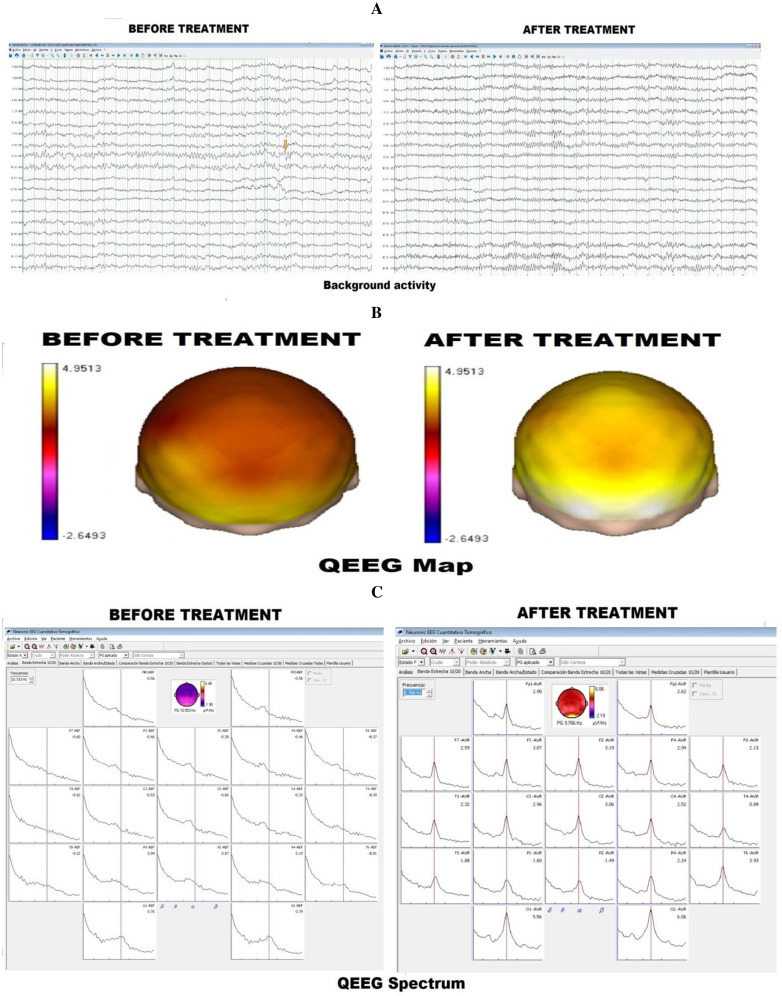
Figure 2.
Case 2: (A) Background activity: Before treatment, the background activity is poorly organised, with focal paroxysmal activity. After treatment, the background activity is very well organised, demonstrating the absence of paroxysmal activity. (B) qEEG spectrum. The qEEG spectrum prior to treatment is middle-to-well delineated, but after treatment, a dominant alpha pattern. (C) qEEG map. qEEG map before treatment demonstrates low alpha absolute power values, and after treatment, a dominant alpha pattern is evidenced.
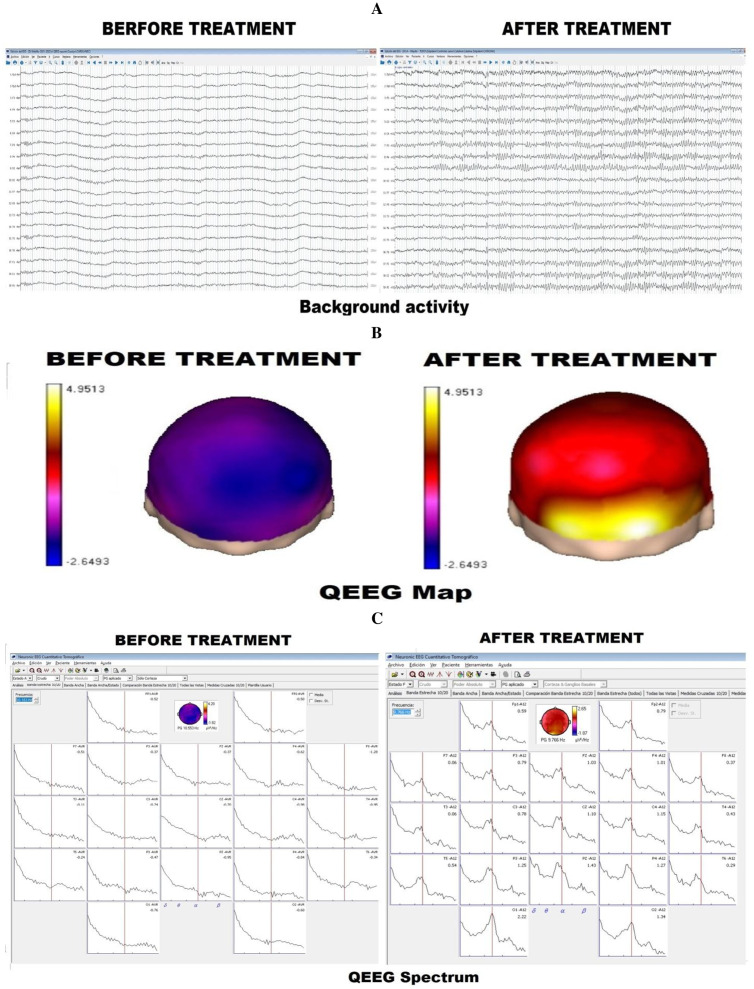
Figure 3.
Case 3: (A) Background activity. Before treatment, the background activity is poorly organised but well outlined after treatment. (B) qEEG spectrum. Before treatment, the qEEG spectrum is poorly delineated but demonstrates a dominant alpha pattern after treatment. (C) qEEG map. Before treatment, the qEEG map demonstrates significantly low values of alpha absolute power, a dominant alpha pattern is evidenced after treatment.
qEEG records were obtained employing the MEDICID 05 system (Neuronic S.A.), with the following technical parameters: gain of 20 000, filters bandpass between 0.3 and 70 Hz, a 60 Hz ‘notch’ filter, level of noise 2 μv RMS and an environmental temperature of approximately 23°C. A sampling period of 5 ms was used.
Nineteen monopolar derivations of the International 10/20 System (FP1, FP2, F3, F4, C3, C4, P3, P4, O1, O2, F7, F8, T3, T4, T5, T6, Fz, Cz, Pz) were recorded using scalp electrodes of copper coated with silver chloride, with linked ear lobes as a reference. Electrode-skin impedance was less than 10 KΩ. The total time of collection was 20 min, with eyes closed. The children were awake, recumbent and relaxed. Artefact free segments of 2.56 s duration were selected through visual editing by an expert electroencephalographer, who also eliminated obvious changes such as drowsiness. Each testing session lasted approximately 1 hour.
The complex cross-spectra covariance matrix was calculated with Fast Fourier Transform (FFT). In this way, time domain EEG data were transformed to the frequency domain for each of the 49 discrete frequencies from 0.39 Hz to 70 Hz in steps of 0.39 Hz. Subsequently, broad-band spectral parameters were calculated for the classic frequency bands, defined as: delta: 0.5–3.5 Hz, theta: 4–7.5 Hz, alpha:8–12.5 Hz, beta: 13–30 Hz and gamma: 30 to 70 Hz; total power: 0.5–70 Hz.
Absolute power (AP) from these classic frequency bands, from eyes-closed records, were obtained for all individuals. The AP represents the energy content in each frequency band in the whole spectrum and is represented graphically as the area of those frequency bands under the power spectrum. Z transform was applied to these data, and the probabilistic statistical significance maps were obtained. Coherence values for the discrete frequencies corresponding to every EEG band were averaged.23 24
RPR-reduction stimulation procedures
Table 1 represents the methods of evoking reflexes and intervention procedures employed for all three participants.
Treatment
The treatment involved the integration of primitive reflexes by evoking them repetitively and by TENS. stimulation of muscle linked with RPRs that was associated with reduction of the presence of RPRs. The examination and treatment procedures are outlined in table 1. All three children’s parents were present throughout.
Outcome and follow-up
Table 2 reports the results of the neuropsychological testing before and after the 12- week treatment programme. The children remain under follow-up at Institute for Neurology and Neurosurgery in Havana and the improvements in all three cases have been sustained.
Figures 1–3 report the observed changes in the qEEG both prior to and after the 12 week intervention programme for all participants.
Discussion
If RPRs are retained beyond the normal developmental period, they have the potential to disrupt maturation processes and reduce the brain’s ability to process sensory information effectively. In other words, the persistence of primary reflexes beyond the normal timespan (12 months post partum) can reflect interference with subsequent development and may be indicative of neurological dysfunction.
The persistence of primitive reflexes is significantly associated with an infant’s performance in both interaction with objects (ie, actions) and with people (ie, communicative gestures) implying that a high persistence of RPRs inversely correlate with low scores in motor repertoire (General Movement Assessment) independent of the infants’ age.11 25 26 Motor proficiency testing comprises 18 tasks divided into four areas that include: locomotion, stability, object control and fine movement skills. Performance of these tasks is scored. The higher the movement skill level for each of the tasks evaluated, the higher children score on such assessment protocols. This finding is consistent with previous studies, revealing that the persistence of the ATNR, another primitive reflex, is associated with reduced fine motor (such as fingering, shaking, rotating and transferring objects across the midline) and gross motor (such as rolling, creeping, crawling, riding a bicycle and catching or kicking a ball) abilities.25 26
A reduction in motor activity, spatial exploration, experience-dependent plasticity, RPRs and delayed postural reflexes may be associated with a global immaturity of cortical network function in childhood.27 A more specific imbalance in maturity would be expected if there were an asymmetric development of RPRs. If there existed unilateral RPRs and unilateral delay of postural reflexes, we would expect an asymmetric development and maturity of the brain and nervous system since this would alter muscle tone or produce asymmetry of tone, which could alter sensory and muscle feedback, which is thought to be a significant driving factor in brain development.28
Several reports suggest that connectivity differs between children and adults with autism. For example, autistic children may have unusually strong connections in several brain networks; autistic adults tend to show weaker connections in some of the same networks.23 24 29
One of the most interesting features of those with ASD is the ‘unevenness‘ of cognitive abilities. It is a view held by many authors that an effective way to explain the diverse behavioural effects noted in ASD and many other neurobehavioural disorders is by understanding that a significant contribution to ASD is a functional disconnection between areas of the brain30–36 not unlike what is seen in sleep or in minimally conscious states.3 23 Functional asymmetry within widespread cortical networks could result in decreased temporal coherence in certain networks and enhanced temporal coherence in other functional networks.
The persistence of primitive reflexes through childhood and adulthood has been associated with brain injury and various developmental disorders.13 16–18 RPRs have also been documented in several functional neurological disorders of childhood that are not associated with any specific neurological insult or pathology.13 15 We think that the presence of RPRs is representative of a maturational delay, which is also reflected in structural and functional changes and various motor and cognitive delays.
Normally, after the first few months of life, the feedback created by primitive reflex-generated movement leads ultimately to the inhibition of primitive reflexes and the activation of more complex subsequent postural reflexes, resulting in a more complex interaction with the environment, which in turn leads to greater sensory feedback, thereby activating genes that allow for the creation of integration and coordination between various cortical networks. As cortical networks become increasingly connected and integrated, their interaction speeds up and their synchronisation improves, allowing more areas to be stimulated at the same time. If cortical maturity and motor coordination are delayed, which may happen due to the abnormal persistence of primitive reflexes, the brain will not continue to grow and develop at a normal rate, thereby delaying the emergence of its more mature functions.
Because the brain’s hemispheres develop at distinct rates and durations, combined with the aberrant, asymmetric persistence of basic reflexes, a maturational imbalance can occur in which one hemisphere matures normally while the other is delayed. This can create large imbalances in synchronisation and temporal coherence, decreasing the ability for large cortical networks to have effective coherent function between the two hemispheres (ie, effective shared activity).37 This can result in a functional disconnection syndrome, which can present with varied symptoms depending on the time, hemisphere, and degree of the maturational delay.
RPRs may be one of the first signs of abnormal or delayed cortical maturation, and consequently of ASD and other neurobehavioral issues.38 39 Rooting and sucking reflexes, as well as a number of other primitive reflex responses, are present from birth if not before.2 A newborn’s failure to bond to its mother and suckle, which is not uncommon in newborns with developmental delays and delays or asymmetry of turning over between approximately 3–5 months of age, may be one of the earliest markers of ASD.40–42 Numerous therapists recommend interventions that activate or reproduce primal reflexes to address a variety of neurobehavioral illnesses.43 44
We think that participating in the activities described in the frequency section of table 1 and in the online supplemental appendix with treatment provided 10 times three times per day for 12 weeks, will boost sensory input and feedback to the nervous system, promoting synaptogenesis and neuroplasticity in more rostral and complex areas of the brain.45 46 We suggest that this is associated with inhibition through descending propriospinal connections, which would ordinarily result in more intricate individualised volitional regulation of movement, encouraging growth and cortical maturation.47 Finally, this can be associated with the release of ‘bottom-up interference’ which can delay cortical maturation and hamper effective top-down regulation, eventually blocking primitive reflexes.2 3 10 23 These types of intervention have garnered support.15 48–53
We think that the presence of RPRs, as well as the developmental milestones that may be delayed or absent as a result, are early indicators of developmentally delayed children in general, and those with ASD in particular. As a result, we offer clinical support for the notion that hemispheric-specific interventions can significantly reduce the presence of RPRs and consequently will have measurable and significant positive effects on both motor and cognitive function.
One of the limitations of the study is that the WISC-IV Spanish edition was employed with an interval of 12 weeks between testing and retesting. In clinical environments, it is recommended that at least a year if not longer should exist between initial testing and retesting.54 For screening purposes, older versions of the WISC have maintained effective intertest reliability over a mean test-retest interval of 28 and 35 days.55 In a more recent study, for test–retest reliability with a mean test–retest interval of 32 days, the overall index in the subindices in a sample of 243 children aged 6–16 years was high to moderate.56
In the case of these three patients with ASD, follow-up was recommended by the treating physicians to examine if there is a long-term effect and that is now being studied over longer periods under controlled conditions and an analysis of a large data base of interventions has been reported.57
Learning points.
It is common to find retained primitive reflexes in autism spectrum disorder.
Retained primitive reflexes apparently can be found at all ages.
Treatment interventions can lead to the disappearance of retained primitive reflexes, with concomitant cognitive improvement.
A rearrangement of functional connectivity might explain the improvement of individuals with autism spectrum disorder and the disappearance of retained primitive reflexes. Examining primitive reflexes after the first year of life may be an important practice.
Footnotes
Contributors: The following authors were responsible for drafting of the text, sourcing and editing of clinical images, investigation results, drawing original diagrams and algorithms, and critical revision for important intellectual content: GL, RJM, EC. The following authors gave final approval of the manuscript: RJM, GL, CM, EC.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Case reports provide a valuable learning resource for the scientific community and can indicate areas of interest for future research. They should not be used in isolation to guide treatment choices or public health policy.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Consent obtained from parent(s)/guardian(s)
References
- 1.Bartlett D, Piper M, Okun N, et al. Primitive reflexes and the determination of fetal presentation at birth. Early Hum Dev 1997;48:261–73. 10.1016/s0378-3782(97)01865-3 [DOI] [PubMed] [Google Scholar]
- 2.Melillo R, Leisman G. Neurobehavioral disorders of childhood: an evolutionary perspective. New York, NY: Springer, 2010. 10.1007/978-1-4419-1231-2 [DOI] [Google Scholar]
- 3.Melillo R, Leisman G. Autistic spectrum disorders as functional disconnection syndrome. Rev Neurosci 2009;20:111–31. 10.1515/revneuro.2009.20.2.111 [DOI] [PubMed] [Google Scholar]
- 4.Russo N, Flanagan T, Iarocci G, et al. Deconstructing executive deficits among persons with autism: implications for cognitive neuroscience. Brain Cogn 2007;65:77–86. 10.1016/j.bandc.2006.04.007 [DOI] [PubMed] [Google Scholar]
- 5.Demertzi A, Soddu A, Laureys S. Consciousness supporting networks. Curr Opin Neurobiol 2013;23:239–44. 10.1016/j.conb.2012.12.003 [DOI] [PubMed] [Google Scholar]
- 6.Fernández-Espejo D, Soddu A, Cruse D, et al. A role for the default mode network in the bases of disorders of consciousness. Ann Neurol 2012;72:335–43. 10.1002/ana.23635 [DOI] [PubMed] [Google Scholar]
- 7.Cirelli C, Tononi G. Is sleep essential PLoS Biol 2008;6:e216. 10.1371/journal.pbio.0060216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leisman G, Ashkenazi M. Aetiological factors in Dyslexia: IV. cerebral hemispheres are functionally equivalent. Int J Neurosci 1980;11:157–64. 10.3109/00207458009147581 [DOI] [PubMed] [Google Scholar]
- 9.Leisman G, Zenhausern R. Integratory systems deficits in developmental dyslexia. Neuropsychology and Cognition 1982;2:507. 10.1007/978-94-009-7654-2 [DOI] [Google Scholar]
- 10.Melillo R, Leisman G, Machado C, et al. Retained primitive reflexes and potential for intervention in autistic spectrum disorders. Front Neurol 2022;13:922322. 10.3389/fneur.2022.922322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leisman G, Melillo R, Melillo T, et al. Taking sides: asymmetries in the evolution of human brain development in better understanding autism spectrum disorder. Symmetry 2022;14:2689. 10.3390/sym14122689 [DOI] [Google Scholar]
- 12.Koziol LF, Budding DE, Chidekel D. From movement to thought: executive function, embodied cognition, and the cerebellum. Cerebellum 2012;11:505–25. 10.1007/s12311-011-0321-y [DOI] [PubMed] [Google Scholar]
- 13.Damasceno A, Delicio AM, Mazo DFC, et al. Primitive reflexes and cognitive function. Arq Neuro-Psiquiatr 2005;63:577–82. 10.1590/S0004-282X2005000400004 [DOI] [PubMed] [Google Scholar]
- 14.Konicarova J, Bob P. Principle of dissolution and primitive reflexes in ADHD. Act Nerv Super 2013;55:74–8. 10.1007/BF03379598 [DOI] [Google Scholar]
- 15.Pecuch A, Gieysztor E, Wolańska E, et al. Primitive reflex activity in relation to motor skills in healthy preschool children. Brain Sci 2021;11:967. 10.3390/brainsci11080967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seraji-Bzorgzad N, Paulson H, Heidebrink J. Neurologic examination in the elderly. Handb Clin Neurol 2019;167:73–88. 10.1016/B978-0-12-804766-8.00005-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Franco JG, Trzepacz PT, Velásquez-Tirado JD, et al. Discriminant performance of dysexecutive and frontal release signs for delirium in patients with high dementia prevalence: implications for neural network impairment. J Acad Consult Liaison Psychiatry 2021;62:56–69. 10.1016/j.psym.2020.04.002 [DOI] [PubMed] [Google Scholar]
- 18.Karimianpour A, Nagpal SJS, Parker K. Palmomental reflex. Am J Med Sci 2015;350:e2. 10.1097/MAJ.0000000000000440 [DOI] [PubMed] [Google Scholar]
- 19.Couteur AL, Lord C, Rutter M. Entrevista para el diagnóstico del autismo-revisada: ADI-R. Madrid: TEA, 2006. [Google Scholar]
- 20.Vaughan-Jensen J, Adame C, McLean L, et al. Test review: Wechsler individual achievement test. J Psychoeduc Assess 2011;29:286–91. 10.1177/0734282910385645 [DOI] [Google Scholar]
- 21.Clinton A. Test review: Wechsler, D.(2005). Wechsler intelligence scale for Children− four edition Spanish. J Psychoeduc Assess 2007;25:285–92. [Google Scholar]
- 22.Lebersfeld JB, Swanson M, Clesi CD, et al. Systematic review and meta-analysis of the clinical utility of the ADOS-2 and the ADI-R in diagnosing autism spectrum disorders in children. J Autism Dev Disord 2021;51:4101–14. 10.1007/s10803-020-04839-z [DOI] [PubMed] [Google Scholar]
- 23.Machado C, Estévez M, Leisman G, et al. QEEG spectral and coherence assessment of autistic children in three different experimental conditions. J Autism Dev Disord 2015;45:406–24. 10.1007/s10803-013-1909-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Machado C, Rodríguez R, Estévez M, et al. Anatomic and functional connectivity relationship in autistic children during three different experimental conditions. Brain Connect 2015;5:487–96. 10.1089/brain.2014.0335 [DOI] [PubMed] [Google Scholar]
- 25.Gieysztor EZ, Sadowska L, Choińska AM, et al. Trunk rotation due to persistence of primitive reflexes in early school-age children. Adv Clin Exp Med 2018;27:363–6. 10.17219/acem/67458 [DOI] [PubMed] [Google Scholar]
- 26.Gieysztor EZ, Choińska AM, Paprocka-Borowicz M. Persistence of primitive reflexes and associated motor problems in healthy preschool children. Arch Med Sci 2018;14:167–73. 10.5114/aoms.2016.60503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vogel AC, Power JD, Petersen SE, et al. Development of the brain’s functional network architecture. Neuropsychol Rev 2010;20:362–75. 10.1007/s11065-010-9145-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Longman D, Stock JT, Wells JCK. A trade-off between cognitive and physical performance, with relative preservation of brain function. Sci Rep 2017;7:13709. 10.1038/s41598-017-14186-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rane P, Cochran D, Hodge SM, et al. Connectivity in autism: a review of MRI connectivity studies. Harv Rev Psychiatry 2015;23:223–44. 10.1097/HRP.0000000000000072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lanillos P, Oliva D, Philippsen A, et al. A review on neural network models of schizophrenia and autism spectrum disorder. Neural Netw 2020;122:338–63. 10.1016/j.neunet.2019.10.014 [DOI] [PubMed] [Google Scholar]
- 31.Uddin LQ. Brain mechanisms supporting flexible cognition and behavior in adolescents with autism spectrum disorder. Biol Psychiatry 2021;89:172–83. 10.1016/j.biopsych.2020.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Song Y, Epalle TM, Lu H. Characterizing and predicting autism spectrum disorder by performing resting-state functional network community pattern analysis. Front Hum Neurosci 2019;13:203. 10.3389/fnhum.2019.00203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lombardo MV, Eyler L, Moore A, et al. Default mode-visual network hypoconnectivity in an autism subtype with pronounced social visual engagement difficulties. Elife 2019;8:e47427. 10.7554/eLife.47427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fan L-Y, Booth JR, Liu M, et al. Developmental differences in neural connectivity for semantic processing in youths with autism. J Child Psychol Psychiatry 2021;62:1090–9. 10.1111/jcpp.13373 [DOI] [PubMed] [Google Scholar]
- 35.Geschwind DH, Levitt P. Autism spectrum disorders: developmental disconnection syndromes. Curr Opin Neurobiol 2007;17:103–11. 10.1016/j.conb.2007.01.009 [DOI] [PubMed] [Google Scholar]
- 36.Courchesne E, Pierce K. Why the frontal cortex in autism might be talking only to itself: local over-connectivity but long-distance disconnection. Curr Opin Neurobiol 2005;15:225–30. 10.1016/j.conb.2005.03.001 [DOI] [PubMed] [Google Scholar]
- 37.Melillo R, Leisman G, Machado C, et al. The relationship between retained primitive reflexes and Hemispheric Connectivity in autism spectrum disorders. Brain Sci 2023;13:1147. 10.3390/brainsci13081147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hallahan B, Daly EM, McAlonan G, et al. Brain morphometry volume in autistic spectrum disorder: a magnetic resonance imaging study of adults. Psychol Med 2009;39:337–46. 10.1017/S0033291708003383 [DOI] [PubMed] [Google Scholar]
- 39.Floris DL, Wolfers T, Zabihi M, et al. Atypical brain asymmetry in autism-A candidate for clinically meaningful stratification. Biol Psychiatry Cogn Neurosci Neuroimaging 2021;6:802–12. 10.1016/j.bpsc.2020.08.008 [DOI] [PubMed] [Google Scholar]
- 40.Baranek GT, Watson LR, Boyd BA, et al. Hyporesponsiveness to social and nonsocial sensory stimuli in children with autism, children with developmental delays, and typically developing children. Dev Psychopathol 2013;25:307–20. 10.1017/S0954579412001071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Duchan E. Child developmental delays and disorders: motor delay. FP Essent 2021;510:11–6. [PubMed] [Google Scholar]
- 42.Kobayashi S, Maki T, Kunimoto M. Clinical symptoms of bilateral anterior cerebral artery territory infarction. J Clin Neurosci 2011;18:218–22. 10.1016/j.jocn.2010.05.013 [DOI] [PubMed] [Google Scholar]
- 43.Chandradasa M, Rathnayake L. Retained primitive reflexes in children, clinical implications and targeted home-based interventions. Nurs Child Young People 2020;32:37–42. 10.7748/ncyp.2019.e1132 [DOI] [PubMed] [Google Scholar]
- 44.Grigg I, Ivashko-Pachima Y, Hait TA, et al. Tauopathy in the young autistic brain: novel biomarker and therapeutic target. Transl Psychiatry 2020;10:228. 10.1038/s41398-020-00904-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.So C, Pierluissi E. Attitudes and expectations regarding exercise in the hospital of hospitalized older adults: a qualitative study. J Am Geriatr Soc 2012;60:713–8. 10.1111/j.1532-5415.2012.03900.x [DOI] [PubMed] [Google Scholar]
- 46.Thompson A, Murphy D, Dell’Acqua F, et al. Impaired communication between the motor and somatosensory homunculus is associated with poor manual dexterity in autism spectrum disorder. Biol Psychiatry 2017;81:211–9. 10.1016/j.biopsych.2016.06.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Watkins MW, Smith LG. Long-term stability of the Wechsler intelligence scale for children—fourth edition. Psychol Assess 2013;25:477–83. 10.1037/a0031653 [DOI] [PubMed] [Google Scholar]
- 48.Teitelbaum P, Teitelbaum O, Nye J, et al. Movement analysis in infancy may be useful for early diagnosis of autism. Proc Natl Acad Sci USA 1998;95:13982–7. 10.1073/pnas.95.23.13982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Torres EB, Brincker M, Isenhower RW, et al. Autism: the micro-movement perspective. Front Integr Neurosci 2013;7:32. 10.3389/fnint.2013.00032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Robledo J, Donnellan AM, Strandt-Conroy K. An exploration of sensory and movement differences from the perspective of individuals with autism. Front Integr Neurosci 2012;6:107. 10.3389/fnint.2012.00107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gandotra A, Kotyuk E, Szekely A, et al. Fundamental movement skills in children with autism spectrum disorder: a systematic review. Research in Autism Spectrum Disorders 2020;78:101632. 10.1016/j.rasd.2020.101632 [DOI] [Google Scholar]
- 52.Foster NC, Bennett SJ, Causer J, et al. Getting off to a shaky start: specificity in planning and feedforward control during sensorimotor learning in autism spectrum disorder. Autism Res 2020;13:423–35. 10.1002/aur.2214 [DOI] [PubMed] [Google Scholar]
- 53.Wójtowicz D, Ptak A, Świtkowska S, et al. Effectiveness of sensory stimulation among children with impaired psychomotor development: a pilot study. Pq 2021;29:67–72. 10.5114/pq.2020.100285 [DOI] [Google Scholar]
- 54.Irwin DO. Reliability of the Wechsler intelligence scale for children. J Educational Measurement 1966;3:287–92. 10.1111/j.1745-3984.1966.tb00891.x [DOI] [Google Scholar]
- 55.Andrikopoulos V. Exploring the validity and reliability of the WISC-IV: a review of the literature. Jsss 2021;8:101. 10.5296/jsss.v8i2.18166 [DOI] [Google Scholar]
- 56.Melillo R, Leisman G, Mualem R, et al. Persistent childhood primitive reflex reduction effects on cognitive, sensorimotor, and academic performance in ADHD. Front Public Health 2020;8:431835. 10.3389/fpubh.2020.431835 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 57.Melillo R, Leisman G, Machado C, et al. Retained primitive reflexes and potential for intervention in autistic spectrum disorders. Front Neurol 2022;13. 10.3389/fneur.2022.922322 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bcr-2023-255285supp001.pdf (162.5KB, pdf)