Abstract
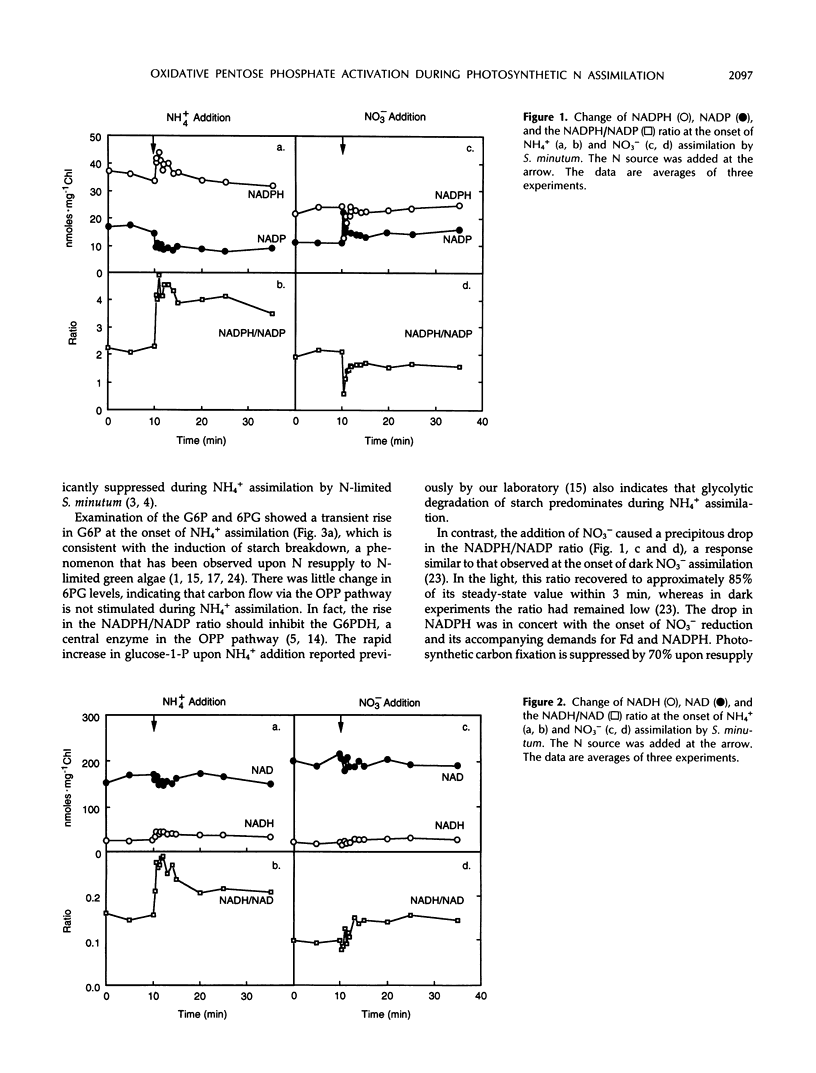
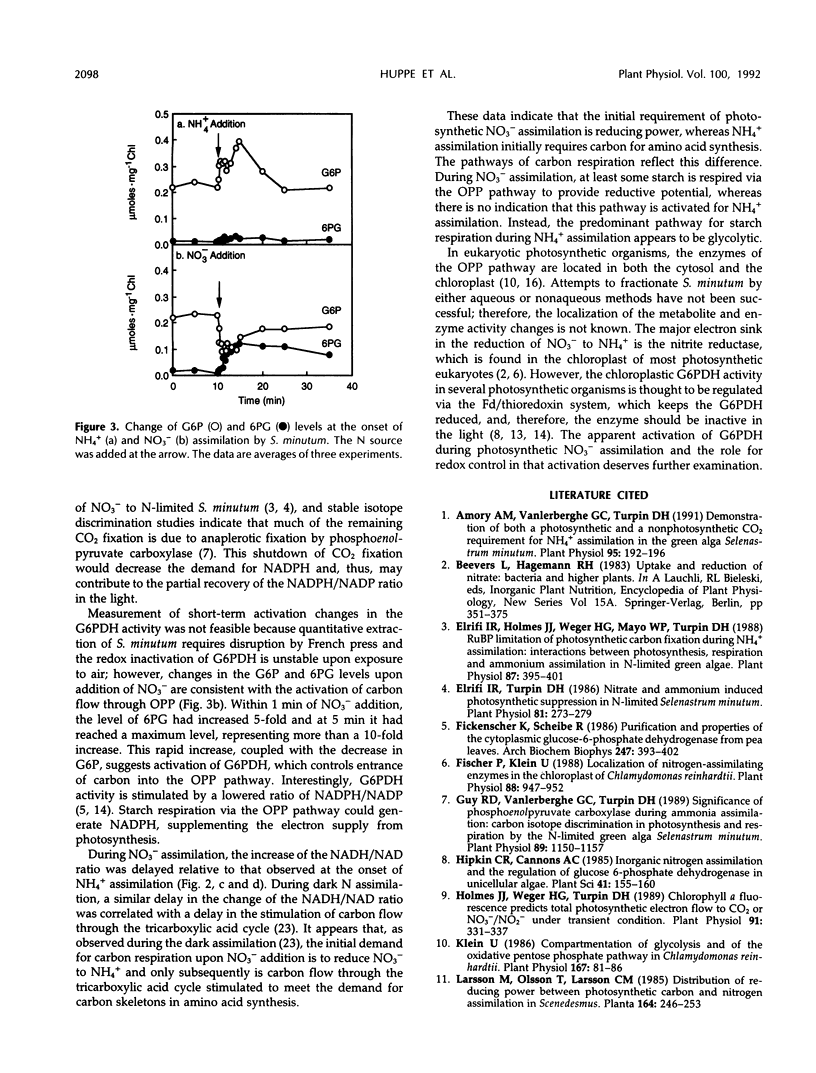
Addition of NO3− to N-limited Selenastrum minutum during photosynthesis resulted in an immediate drop in the NADPH/NADP ratio and a slower increase of the NADH/NAD ratio. These changes were accompanied by a rapid decrease in glucose-6-phosphate and increase in 6-phosphogluconate, indicating activation of glucose-6-phosphate dehydrogenase and a role for the oxidation pentose phosphate pathway during photosynthetic NO3− assimilation. In contrast, the short-term changes in pyridine nucleotides and metabolites during photosynthetic assimilation of NH4+ were not consistent with a stimulation of the oxidative pentose phosphate pathway.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amory A. M., Vanlerberghe G. C., Turpin D. H. Demonstration of Both a Photosynthetic and a Nonphotosynthetic CO(2) Requirement for NH(4) Assimilation in the Green Alga Selenastrum minutum. Plant Physiol. 1991 Jan;95(1):192–196. doi: 10.1104/pp.95.1.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elrifi I. R., Holmes J. J., Weger H. G., Mayo W. P., Turpin D. H. RuBP Limitation of Photosynthetic Carbon Fixation during NH(3) Assimilation : Interactions between Photosynthesis, Respiration, and Ammonium Assimilation in N-Limited Green Algae. Plant Physiol. 1988 Jun;87(2):395–401. doi: 10.1104/pp.87.2.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elrifi I. R., Turpin D. H. Nitrate and Ammonium Induced Photosynthetic Suppression in N-Limited Selenastrum minutum. Plant Physiol. 1986 May;81(1):273–279. doi: 10.1104/pp.81.1.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fickenscher K., Scheibe R. Purification and properties of the cytoplasmic glucose-6-phosphate dehydrogenase from pea leaves. Arch Biochem Biophys. 1986 Jun;247(2):393–402. doi: 10.1016/0003-9861(86)90598-9. [DOI] [PubMed] [Google Scholar]
- Fischer P., Klein U. Localization of Nitrogen-Assimilating Enzymes in the Chloroplast of Chlamydomonas reinhardtii. Plant Physiol. 1988 Nov;88(3):947–952. doi: 10.1104/pp.88.3.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy R. D., Vanlerberghe G. C., Turpin D. H. Significance of Phosphoenolpyruvate Carboxylase during Ammonium Assimilation: Carbon Isotope Discrimination in Photosynthesis and Respiration by the N-Limited Green Alga Selenastrum minutum. Plant Physiol. 1989 Apr;89(4):1150–1157. doi: 10.1104/pp.89.4.1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes J. J., Weger H. G., Turpin D. H. Chlorophyll a Fluorescence Predicts Total Photosynthetic Electron Flow to CO(2) or NO(3)/NO(2) under Transient Conditions. Plant Physiol. 1989 Sep;91(1):331–337. doi: 10.1104/pp.91.1.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheibe R., Anderson L. E. Dark modulation of NADP-dependent malate dehydrogenase and glucose-6-phosphate dehydrogenase in the chloroplast. Biochim Biophys Acta. 1981 Jun 12;636(1):58–64. doi: 10.1016/0005-2728(81)90075-x. [DOI] [PubMed] [Google Scholar]
- Scheibe R., Geissler A., Fickenscher K. Chloroplast glucose-6-phosphate dehydrogenase: Km shift upon light modulation and reduction. Arch Biochem Biophys. 1989 Oct;274(1):290–297. doi: 10.1016/0003-9861(89)90441-4. [DOI] [PubMed] [Google Scholar]
- Smith R. G., Vanlerberghe G. C., Stitt M., Turpin D. H. Short-Term Metabolite Changes during Transient Ammonium Assimilation by the N-Limited Green Alga Selenastrum minutum. Plant Physiol. 1989 Oct;91(2):749–755. doi: 10.1104/pp.91.2.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanlerberghe G. C., Feil R., Turpin D. H. Anaerobic Metabolism in the N-Limited Green Alga Selenastrum minutum: I. Regulation of Carbon Metabolism and Succinate as a Fermentation Product. Plant Physiol. 1990 Nov;94(3):1116–1123. doi: 10.1104/pp.94.3.1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanlerberghe G. C., Horsey A. K., Weger H. G., Turpin D. H. Anaerobic Carbon Metabolism by the Tricarboxylic Acid Cycle : Evidence for Partial Oxidative and Reductive Pathways during Dark Ammonium Assimilation. Plant Physiol. 1989 Dec;91(4):1551–1557. doi: 10.1104/pp.91.4.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanlerberghe G. C., Huppe H. C., Vlossak K. D., Turpin D. H. Activation of Respiration to Support Dark NO(3) and NH(4) Assimilation in the Green Alga Selenastrum minutum. Plant Physiol. 1992 Jun;99(2):495–500. doi: 10.1104/pp.99.2.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weger H. G., Turpin D. H. Mitochondrial Respiration Can Support NO(3) and NO(2) Reduction during Photosynthesis : Interactions between Photosynthesis, Respiration, and N Assimilation in the N-Limited Green Alga Selenastrum minutum. Plant Physiol. 1989 Feb;89(2):409–415. doi: 10.1104/pp.89.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]


