Abstract
Introduction
Preterm birth is a leading cause of perinatal morbidity and mortality. During the COVID-19 pandemic, reduction in rates of preterm birth in women exposed to viral mitigation measures was reported by multiple studies. In addition, others and we observed a more pronounced reduction of preterm birth in women who had previously experienced a preterm birth. The aim of this pilot study is to establish the feasibility of a lifestyle intervention based on viral mitigation measures in high-risk pregnancies, with the ultimate aim to reduce the incidence of preterm birth.
Methods and analysis
One hundred pregnant women, enrolled in antenatal clinics at two tertiary maternity centres in Melbourne, Australia, who have had a previous preterm birth between 22 and 34 weeks gestation will be recruited. This is a two-arm, parallel group, open-label randomised controlled feasibility trial: 50 women will be randomised to the intervention group, where they will be requested to comply with a set of lifestyle changes (similar to the viral mitigation measures observed during the pandemic). Another 50 women will be randomised to the control group, where they will undergo standard pregnancy care. The primary outcome of this trial is feasibility, which will be assessed by measuring patient eligibility rate, recruitment rate, compliance rate and data completion rate. Secondary outcomes include incidence of preterm birth, maternal satisfaction, maternal quality of life and other pregnancy outcomes. Standard methods in statistical analysis for randomised controlled trials on an intention to treat basis will be followed.
Ethics and dissemination
This trial has been approved by the Monash Human Research Ethics Committee; approval reference number RES-22-0000-122A. Study findings will be reported and submitted to peer-reviewed journals for publication, and presentation at conferences.
Trial registration number
ACTRN12622000753752; Pre-results.
Keywords: COVID-19, neonatology, obstetrics
STRENGTHS AND LIMITATIONS OF THE STUDY.
This study is a randomised controlled trial investigating the feasibility of a pregnancy intervention that mimics viral mitigation measures on preterm birth rates.
Outcomes are measured using both subjective (surveys) and objective (actigraphy device data) measures to provide a comprehensive range of data regarding acceptability of the intervention.
Compliance to the intervention is self-reported.
Due to the nature of the intervention, it is not possible to blind patients or clinicians involved in the recruitment process.
Introduction
Preterm birth, defined as delivery prior to 37 weeks gestation, is the leading cause of perinatal morbidity and mortality worldwide. Globally, approximately 15 million preterm births occur yearly and more than 1 million babies die shortly after birth as a direct result of their prematurity.1 In Australia, 8.6% of deliveries are preterm with the average gestational age at birth being 33 weeks.2 Preterm delivery occurs after the following obstetric precursors: spontaneous preterm labour (40%–45%), preterm premature rupture of membranes (PPROM, 25%–30%) or where delivery is indicated due to maternal or fetal compromise (30%–35%).3 An increasing degree of prematurity is known to correlate with a greater risk of complications including neurodevelopmental delay, cerebral palsy and cardiorespiratory disease.4 Prematurity also has a significant economic impact; 72% of preterm infants will need admission to the neonatal intensive care unit (NICU) where the average length of admission is 28 days and the cost per day is almost US$2000.2 5 Mothers of preterm infants take longer to return to work, have a lower medium income and increased out of pocket healthcare costs.6
The exact causality of preterm birth remains unknown; however, risk factors include previous preterm birth, maternal age, smoking, multiple gestation, gestational diabetes, maternal literacy level and social disadvantage.3 Although we have methods to manage high risk women including progesterone treatments, aspirin and cervical cerclage, overall preterm birth rates have continued to rise in most industrialised nations.7 8
The outbreak of COVID-19 brought the world to a standstill, having drastic social and economic impacts. The first Australian case was detected in Victoria in January 2020 and by March, measures including social distancing, wearing face masks and performing hand hygiene were introduced to mitigate virus spread.9 Unexpectedly, it has been observed around the world that pregnant women exposed to mitigation measures for the COVID-19 virus have had a reduction in preterm birth rates by 20%–30%, with this effect being more pronounced in early preterm birth (<34 weeks).10–12 At Monash Health in Melbourne, an observational study demonstrated a 30% reduction in preterm birth rate prior to 34 weeks (risk ratio (RR) 0.74 (95% CI 0.57 to 0.96; p=0.021). This effect was stronger in women who had experienced a previous preterm birth (RR 0.42, 95% CI 0.21 to 0.82; p=0.008) when compared with parous women who had not experienced a preterm birth (RR 0.93, 95% CI 0.63 to 1.28; p=0.714).13
We hypothesise that in women with a previous preterm birth (<34 weeks), a pregnancy intervention mimicking COVID-19 mitigation measures will reduce the incidence of a subsequent preterm birth. We propose that the mechanism of action behind this effect may be due to a reduction in physical activity, stress, noise or air pollution, medical interventions and/or reduced rates of infection.
While observational studies, including those conducted by our team, have demonstrated that COVID-19 mitigation measures have an effect on preterm birth rates, these findings are inconsistent; it is unclear which aspect of these measures contribute to the phenomenon, and there have been no randomised controlled trials that have further investigated this effect to establish causality. We believe that we have a unique opportunity to study this effect further as we are based in Melbourne, where the population has been subject to some of the harshest lockdowns. However, we must first assess feasibility of such an intervention in pregnancy prior to conducting any larger randomised trials.
Aim
The aim of this study is to investigate the feasibility of a lifestyle intervention in pregnancy that mimics viral mitigation measures in pregnant women who have previously had a preterm birth between 22 and 34 weeks.
Methods and analysis
Study design
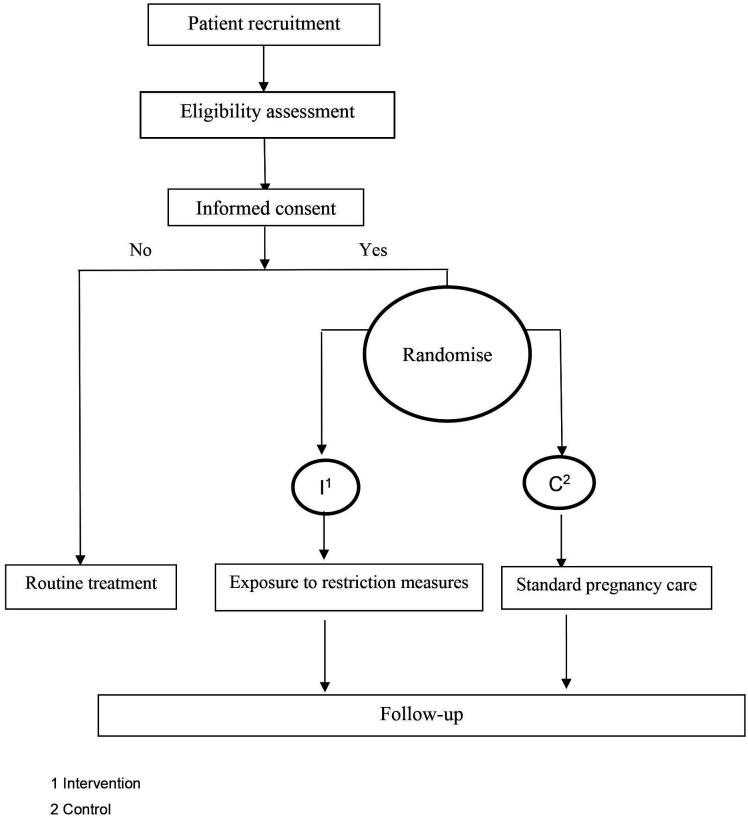
This is a multisite, two-arm open-label randomised controlled clinical trial that will be conducted across tertiary maternity centres in Melbourne, Australia. The Standard Protocol Items: Recommendations for Interventional Trials checklist was used to prepare this report.14 The flow chart of the study design is shown in figure 1. The flow chart of the study design is shown in figure 1. This trial was registered with the Australia New Zealand Clinical Trials Registry on 26 May 2022 (table 1).
Figure 1.
Flow chart.
Table 1.
Trial registration data
| Data category | Information |
| Primary registry and trial identifying number | Australian New Zealand Clinical Trials Registry ACTRN12622000753752 |
| Date registered | 26 May 2022 |
| Source(s) of monetary or material support | Monash Health Monash University |
| Primary sponsor | Monash Health |
| Secondary sponsor | Monash University |
| Contact for public and scientific queries | Associate Professor Daniel Rolnik Email: daniel.rolnik@monash.edu |
| Public title | iPREM Pilot (Isolate to Prevent pretERM birth) |
| Scientific title | Feasibility of a pregnancy intervention mimicking viral transmission mitigation measures on the incidence of preterm birth in high-risk pregnant women enrolled in antenatal clinics in Melbourne, Australia: protocol for a pilot, feasibility randomised trial |
| Countries of recruitment | Australia |
| Health condition(s) studied | Premature birth |
| Intervention(s) | Active comparator: Pregnancy intervention mimicking COVID-19 viral mitigation measures |
| Control comparator: Standard pregnancy care | |
| Key inclusion and exclusion criteria | Inclusion: Pregnant women>18 years, previous preterm birth between 22 and 34 weeks gestation |
| Exclusion criteria: Fetus with major congenital abnormality | |
| Study type | Interventional |
| Allocation: randomised interventional model | |
| Primary purpose: feasibility | |
| Date of first enrolment | July 2022 |
| Target sample size | 100 |
| Recruitment status | Recruiting |
| Primary outcome (s) | Feasibility |
| Secondary outcome(s) | Preterm birth<34 weeks, maternal quality of life and satisfaction and other pregnancy outcomes |
Sample size
Given the primary objective of this trial is to establish feasibility, we aim to recruit up to 100 pregnant women, 50 of whom will be randomised to the intervention group and 50 of whom will be randomised to the control group. We chose this sample size as we estimated that at our initial recruitment site, there may approximately 150–200 eligible women and so a sample size of 100 (ie, half of the eligible population) would be representative of the overall group.
Patient population
Adult pregnant women receiving care at antenatal clinics who are at ‘high risk’ for having a preterm birth, where ‘high risk’ will be defined as having had a previous preterm birth between 22–34 weeks gestation.
Inclusion criteria
Pregnant women, singleton or multiple gestation, will be eligible for this trial if they are aged 18 years or over and have previously delivered a preterm baby between 22+0 and 34+0 gestation, either spontaneously or iatrogenically. Women must primarily speak English and have the ability to read and write.
Exclusion criteria
Pregnant women will be excluded if they are carrying a fetus with one or more major congenital abnormalities.
Recruitment
Pregnant women who are enrolled in each recruitment site’s antenatal clinics will be screened by a clinical team who are familiar with the eligibility criteria. We will make an entry onto the relevant medical records system flagging eligible women. We will also brief clinicians in the clinic on the study details so that they can refer any eligible patients we may have missed in our initial screening process. Additionally, we will display flyers advertising the study in clinic rooms so women who feel they are eligible can contact the research team themselves. When an eligible woman presents to the clinic, we will ask her treating clinician to briefly explain the study and provide her with a patient invitation form. A member of our research team will then approach eligible patients and explain trial details prior to recruitment. Prospective participants will be given 48 hours to consider whether they would like to take part in the trial. If the patient agrees to participate in the trial, they will be asked to sign a written consent form. We will ensure to obtain and store an individual record of all non-recruited patients, including their reasons for exclusion.
No members of the research team will be involved in the care of potential participants at the site of recruitment, ensuring that there are no unequal or dependent relationships. This study will not be blinded to participants as well as nurses, midwives, doctors or investigators who are involved in the recruitment process. The investigator collecting study outcome data once a participant has completed the trial and the investigator who analyses the data will be blinded to the group to which participants were allocated.
Recruitment commenced in June 2022 and is expected to take around 18–24 months for completion.
Randomisation
Pregnant women who are successfully recruited are randomised and allocated on the first day of the trial using Research Electronic Data Capture (REDCap, V.12.4.10, Vanderbilt University) by a member of the research team. REDCap is a secure, web-based data collection and management software that meets Health Insurance Portability and Accountability Act compliance standards.15 16 The randomisation table was generated by using the statistical software Stata, with variable block sizes of 2 and 4.17
Interventions
Intervention Group
This pregnancy intervention is designed to mimic the stage 3 and 4 COVID-19 virus mitigation measures implemented in metropolitan Melbourne, Australia in 2020–2021. Briefly, this involved social distancing, restrictions to movements outside the home unless necessary, imposition of a curfew as well as hygiene recommendations including hand hygiene and mask wearing.9 Originally, alongside their standard pregnancy care, study participants were asked to comply with the following measures for the duration of the intervention.
Refrain from leaving their homes unless required to do so, such as shopping for essentials, to work or study, to seek or give care, for safety purposes (eg, escaping domestic violence) or for outside exercise.
Avoid having visitors to their home unless it is their intimate partner and try to maintain social distancing where possible, that is, a 1.5 m distance between themselves and another party unless in their own home or with an intimate partner.
Wear a face mask or covering when outside their home and perform hand hygiene prior to removing their mask or touching any aspect of their nose or mouth.
Aim to remain at home between 21:00 and 05:00 am unless they are required to leave for work or study or to seek or give care and avoid travelling beyond 5 km of their place of residence except for essential reasons.
Initial recruitment rates were approximately 30%, that is, 30% of eligible participants consented to take part in the trial and the majority of eligible participants who declined to take part did so as they felt that the intervention was too strict. In order to increase recruitment, the research team made the decision to relax the requirements of the intervention. As of now, participants who are assigned to the intervention group will be asked to comply with the following:
Try to minimise the number of visitors to their home and refrain from attending large social gatherings where possible.
Remain in their homes unless required to do so, such as for study/work, shopping for essentials, to seek/give care, for outdoor exercise or if their home environment becomes unsafe in any way (eg, domestic violence).
Wear a face mask/covering when outside their home and perform hand hygiene prior to removing their mask/touching any aspect of their nose or mouth.
Standard pregnancy care will be defined as routine antenatal care appointments, ultrasound scans, pathology and any other investigations or treatments required as determined by the participant’s antenatal care team.
Control group
Participants randomised to the control group will undergo standard pregnancy care without any restrictions.
Participants will begin the trial intervention 2 weeks prior to the gestational age at which the study participant’s previous preterm birth occurred (ie, if they delivered in their previous pregnancy at 32+3 weeks, their first day in the trial will be at 30+3 weeks). If the participant has had multiple preterm births, they will begin the trial 2 weeks prior to the gestational age of their earliest preterm delivery. The maximum gestation for recruitment will be 31 weeks to ensure that the participant is in the trial for at least 3 weeks. Therefore, if they previously gave birth at 33+6 weeks gestation, they will be required to begin the study at 31 weeks gestation. The duration of the intervention will be 6 weeks (ie, 2 weeks prior and 4 weeks post the gestational age of the previous preterm birth) or until 34 weeks gestation or until birth, whichever comes first.
All participants will be required to complete short, online surveys (which will be developed and distributed to their email via REDCap) to assess their hygiene, social contacts, activities, mood and quality of life at baseline and then on a fortnightly basis for the duration of their time in the trial (see online supplemental material).
bmjopen-2023-075703supp001.pdf (133.3KB, pdf)
All participants will also be encouraged to wear an actigraphy device (provided by the study team), similar to a watch, on their non-dominant wrist, 24 hours a day, for the duration of their time in the trial. For the purposes of this study, we have chosen the GENEActiv Original (Activinsights, Kimbolton, UK). It is a 43×40×13 mm water resistant device, which has an inbuilt triaxial piezoelectric accelerometer, light intensity and temperature sensors. The device will be set to record data at a sampling rate frequency of 20 Hz. As sampling rate has a direct impact on the actigraph’s battery life, this will enable the participant to wear the device for 4 weeks without requiring a re-charge. We will configure the device to automatically start recording on the participant’s first day in the trial so they will not be required to push any buttons. If the participant remains in the trial for greater than 4 weeks, a research assistant will collect the old device from them and provide them with a new, charged device. Once the participant has finished their time in the trial, raw data will be downloaded from the devices using the GENEActiv PC Software (V.3.3, Activinsights) as a ‘.bin’ file and analysed.
Patient and public involvement
The study team consulted patients and clinicians at the antenatal clinic where recruitment is taking place for their advice and input into the design of the study.
Outcomes
Primary outcome
Feasibility
We will measure feasibility using the following criteria:
Patient eligibility rate, which will be measured as the proportion of eligible women screened at the antenatal clinic who are expected to consent to taking part in this trial. We have set a predefined target of 50%.
Patient recruitment rate will be measured as the proportion of eligible women who consent to taking part in the study who are randomised (noting that there may be a significant time period between consent and randomisation). We have set a predefined target of 50%.
Compliance rate will be measured as the proportion of participants in the intervention group who are considered to have good compliance with the intervention. This will be measured via fortnightly, participant filled surveys and we will set a predefined target of 75%. On each fortnightly survey, participants will be asked questions pertaining to their compliance with each restriction measure. For example, participants will be asked how often they wore a mask when outside their home and if they were to answer either ‘most of the time’ or ‘all of the time’, we would classify this as having >75% compliance with this restriction measure. Participants must report>75% compliance for all restriction measures in order to be defined has having ‘good compliance’ with the intervention. This will be measured at the end of the trial.
Data completion rate will be measured as the proportion of trial participants who complete the final survey (ie, the survey that the participant is required to complete once they reach an endpoint). This will be measured at the end of the trial. We have set a predefined target of >75%.
Secondary outcomes
Secondary outcomes will include incidence of preterm birth prior to 34 weeks gestation, maternal satisfaction and quality of life as well as other pregnancy outcomes such as pregnancy duration, incidence of stillbirth and incidence of iatrogenic or spontaneous delivery. We will measure maternal satisfaction and quality of life via fortnightly surveys we have developed based on previously validated questionnaires including but not limited to the Beck Depression Inventory, QOL-GRAV (pregnant women’s quality of life questionnaire) and Multidimensional Scale of Perceived Social Support. We will also collect non-dominant wrist raw acceleration data through the actigraphy device. The raw acceleration data will be used to derive physical activity patterns, estimate sleep–wake cycle and verify the compliance with wearing the device.
Other data to be collected
Demographic data collected will include maternal demographic data such as birth country, age, marital status, medical history, drug use, risk factors for preterm birth, gravida/parity, obstetric history, details of current pregnancy, incidence of PPROM, incidence of presumed chorioamnionitis, use of antenatal steroids, mode of delivery and maternal death. We will also collect infant demographic data such as birth weight in grams.
Other neonatal outcomes collected will include incidence of admission to NICU or special care nursery, incidence of NICU stay>48 hours, neonatal morbidity (5-min Apgar score<5, respiratory distress syndrome requiring intubation, grade 3 or 4 intraventricular haemorrhage, neonatal seizures, culture-positive neonatal sepsis, retinopathy of prematurity requiring treatment, necrotising enterocolitis) and neonatal death.
A member of the research team will download data from the actigraphy device from the device after the participant has completed their time in the trial. Once the participant has given birth, secondary and other data will be collected by a member of the research team who is blinded to participant’s allocation.
Data analysis
We will use the open-source package GENEActiv and GENEA In R (GGIR, V.2.5) to translate raw actigraphy data to readable information.18 Data will be downloaded from each device as a .bin file, which will be read and translated to a .csv file by the GGIR package according to predefined parameters. Initially, GGIR will perform sensor calibration in the data collected to check and correct calibration errors in the accelerometer.19 Due to the raw data size, we will set GGIR to summarise the collected 20 Hz sample-rate data to 5 s epochs.
Feasibility targets will be assessed first. Baseline continuous covariates will be expressed as mean and SD or median and IQR depending on the distribution of the data as assessed by inspection of histograms and quantile-quantile plots. Normally distributed continuous variables will be compared between the groups using independent-samples t-test, and non-normally distributed variables will be compared between the trial arms with the Wilcoxon rank-sum test.
Categorical variables will be expressed as counts and percentages and compared between the study groups using the χ2 test or Fisher’s exact test, as appropriate. Descriptive statistics will be reported for assessment of feasibility as previously defined in the study outcomes section.
The effect of the intervention on the odds of preterm birth and other binary pregnancy outcomes will be modelled using univariable logistic regression models and expressed as the OR with 95% CIs. Multivariable models will be used to adjust for covariates with significant imbalances between the groups at baseline, if needed. Analyses will be performed according to an intention-to-treat principle, and secondary per-protocol analysis will be performed including only participants from the intervention group with compliance≥75%.
All statistical analyses will be conducted in the statistical environment R, and p values below 0.05 will be considered statistically significant. We will report findings in accordance with Consolidated Standards of Reporting Trials guidelines.
Adverse events
Serious adverse events (SAEs)
SAEs will be defined as any event required admission to hospital (excluding admission for delivery), maternal or fetal death, fetal malformations, any event that leads to maternal disability and any event where a participant contacts the study team with concerns regarding a serious deterioration in their mental health during the trial.
Adverse events
Any other event reported by a participant or their partner not needing hospital admission or not falling in a category of SAEs.
Data Safety Monitoring Board (DSMB)
An independent DSMB comprising a senior research fellow in obstetrics and gynaecology, a consultant neonatologist and a perinatal epidemiologist has been formed to review the study, the data generated and ensure safety of all participants. Members of the DSMB do not have a vested financial, scientific, or other conflict of interest with this trial. All SAEs will be reported to the DSMB within 24–48 hours of the team becoming aware of the event.
An interim analysis evaluating the safety of the trial will be conducted after 50 eligible women have been screened. The DSMB will be required to evaluate any adverse events and assess the ongoing safety of the trial. They will have the ability to suspend or terminate the study if required on the basis of a lack of feasibility or any SAEs observed. Given the feasibility nature of the trial and that the interim analysis will only assess safety, no sequential trial adjustments to the alpha level will be made.
Reports to Human Research Ethics Committee (HREC)
Any SAEs that the DSMB deem will necessitate a temporary halt of the trial pending review and any changes to the protocol or patient information consent form (PICF) will be reported to the HREC. We will also ensure to provide an interim report following the recruitment of 50 participants to the trial as well as an annual research progress report.
Ethics and dissemination
Ethics
This study has been approved by the Monash Health HREC and will be conducted in accordance with the approved protocol/amendment(s) and National Health and Medical Research Council’s National Statement on Ethical Conduct in Human Research 2007 (updated 2018). The study will also comply with the Declaration of Helsinki and with the Good Clinical Practice standards. An expert team of senior obstetricians, neonatologists, researchers and psychiatrists were consulted regarding the intervention and protecting the psychological safety of the women in the trial. We have ensured that participants have ample opportunity to contact the study team if they are concerned and have developed the surveys in consultation with a psychiatrist to appropriately assess the quality of life of trial participants.
Data for this trial will be collected from electronical medical records system, surveys completed by participants and from actigraphs used by participants following informed consent. Each participant will be assigned a unique participant identifier which will be the only number that appears on their reports to maintain confidentiality. Only authorised members of the research team will be able to log into the secure web-based portal, REDCap, to input trial data for each participant using their unique participant identifier. Deidentified actigraph device data will be stored in a password-protected computer file, also only accessible to authorised members of the research team. All data will be stored for 15 years after which it will be disposed of via permanent deletion.
Consent
Informed, written consent will be obtained using a specifically designed PICF (see online supplemental material) from potential participants after a member of the research team has ensured that the participant understands the study procedures involved, the potential benefits and/or risks as well as the expected duration of the intervention. Participants will be made aware that their participation is entirely voluntary and that they are free to withdraw at any stage for any reason. The research team member will also inform participants that withdrawal of consent will not affect their relationship with their physician or their right to appropriate medical treatment.
Dissemination
The study results will be disseminated by publication in peer-reviewed journals and presented at conferences as appropriate.
Supplementary Material
Acknowledgments
We would like to thank all the clinic staff at the antenatal clinics in Monash Medical Centre for providing their input into the design of the study as well as for assisting with recruitment.
Footnotes
DLR and AM contributed equally.
Correction notice: This article has been corrected since it was published. Daniel Lorber Rolnik name has been corrected in the list of authors.
Contributors: The initial concept was developed by AM, DLR and BWM. All authors contributed to the design of the study. SSr drafted the ethics application, protocol, case report forms, surveys and manuscript. AM, DLR, BWM, RH, KRP, SSu, RdCP, RTS, FB-J, DM and JS made critical revisions and assisted with ethics application submission, editing of protocol and editing of manuscript. All authors have read and approved the final manuscript and are accountable for its accuracy.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.Blencowe H, Cousens S, Chou D, et al. Born too soon: the global epidemiology of 15 million Preterm births. Reprod Health 2013;10:S2. 10.1186/1742-4755-10-S1-S2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Australian Institute of Health and Welfare . Australian Institute of health and welfare. Impact 2018;2018:80–1. 10.21820/23987073.2018.2.80 [DOI] [Google Scholar]
- 3.Goldenberg RL, Culhane JF, Iams JD, et al. Epidemiology and causes of Preterm birth. The Lancet 2008;371:75–84. 10.1016/S0140-6736(08)60074-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization . Born Too Soon. The Global Action Report on Preterm Birth. Geneva: World Health Organization, 2012. [Google Scholar]
- 5.Zainal H, Dahlui M, Soelar SA, et al. Cost of Preterm birth during initial hospitalization: A care provider’s perspective. PLoS One 2019;14:e0211997. 10.1371/journal.pone.0211997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fox H, Callander E. Cost of Preterm birth to Australian mothers: assessing the financial impact of a birth outcome with an increasing prevalence. J Paediatr Child Health 2021;57:618–25. 10.1111/jpc.15278 [DOI] [PubMed] [Google Scholar]
- 7.Chawanpaiboon S, Vogel JP, Moller A-B, et al. Global, regional, and national estimates of levels of Preterm birth in 2014: a systematic review and Modelling analysis. Lancet Glob Health 2019;7:e37–46. 10.1016/S2214-109X(18)30451-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Newnham JP, Dickinson JE, Hart RJ, et al. Strategies to prevent Preterm birth. Front Immunol 2014;5:584. 10.3389/fimmu.2014.00584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Storen RC. COVID-19: a chronology of state and territory government announcements (up until 30 June 2020). Canberra: Parliamentary Library, 2020. [Google Scholar]
- 10.Philip RK, Purtill H, Reidy E, et al. Unprecedented reduction in births of very low birthweight (VLBW) and extremely low birthweight (ELBW) infants during the COVID-19 Lockdown in Ireland: a ‘natural experiment’ allowing analysis of data from the prior two decades. BMJ Glob Health 2020;5:e003075. 10.1136/bmjgh-2020-003075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Been JV, Burgos Ochoa L, Bertens LCM, et al. Impact of COVID-19 mitigation measures on the incidence of Preterm birth: a national quasi-experimental study. Lancet Public Health 2020;5:e604–11. 10.1016/S2468-2667(20)30223-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matheson A, McGannon CJ, Malhotra A, et al. Prematurity rates during the Coronavirus disease 2019 (COVID-19) pandemic Lockdown in Melbourne, Australia. Obstet Gynecol 2021;137:405–7. 10.1097/AOG.0000000000004236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rolnik DL, Matheson A, Liu Y, et al. Impact of COVID-19 pandemic restrictions on pregnancy duration and outcome in Melbourne, Australia. Ultrasound Obstet Gynecol 2021;58:677–87. 10.1002/uog.23743 Available: https://obgyn.onlinelibrary.wiley.com/toc/14690705/58/5 [DOI] [PubMed] [Google Scholar]
- 14.Chan A-W, Tetzlaff JM, Altman DG, et al. SPIRIT 2013 statement: defining standard protocol items for clinical trials. Ann Intern Med 2013;158:200–7. 10.7326/0003-4819-158-3-201302050-00583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (Redcap)--A Metadata-driven methodology and Workflow process for providing Translational research Informatics support. J Biomed Inform 2009;42:377–81. 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harris PA, Taylor R, Minor BL, et al. The Redcap consortium: building an international community of software platform partners. J Biomed Inform 2019;95:S1532-0464(19)30126-1. 10.1016/j.jbi.2019.103208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.StataCorp . Stata Statistical Software: Release 17. College Station, TX: StataCorp LLC, 2021. [Google Scholar]
- 18.Migueles JH, Rowlands AV, Huber F, et al. GGIR: A research community–driven open source R package for generating physical activity and sleep outcomes from multi-day raw accelerometer data. J Meas Phys Behav 2019;2:188–96. 10.1123/jmpb.2018-0063 [DOI] [Google Scholar]
- 19.van Hees VT, Fang Z, Langford J, et al. Autocalibration of accelerometer data for free-living physical activity assessment using local gravity and temperature: an evaluation on four continents. J Appl Physiol (1985) 2014;117:738–44. 10.1152/japplphysiol.00421.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2023-075703supp001.pdf (133.3KB, pdf)