Abstract
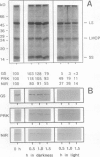
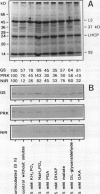
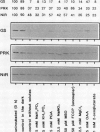
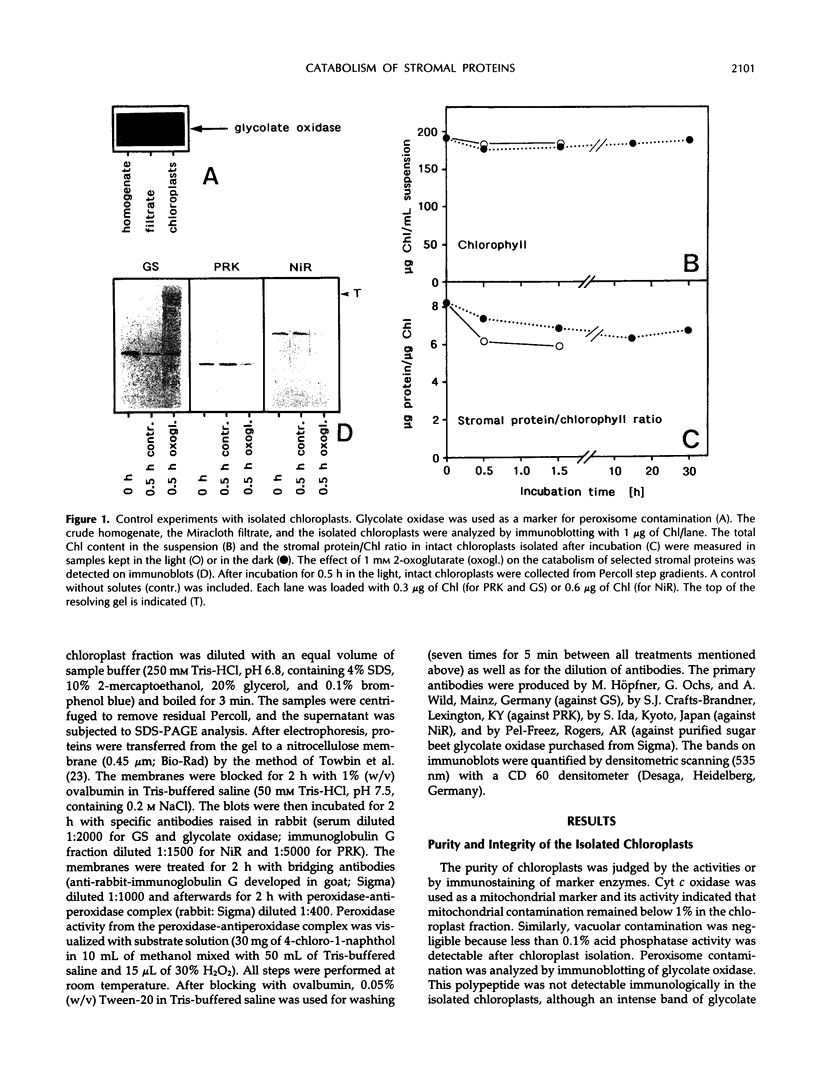
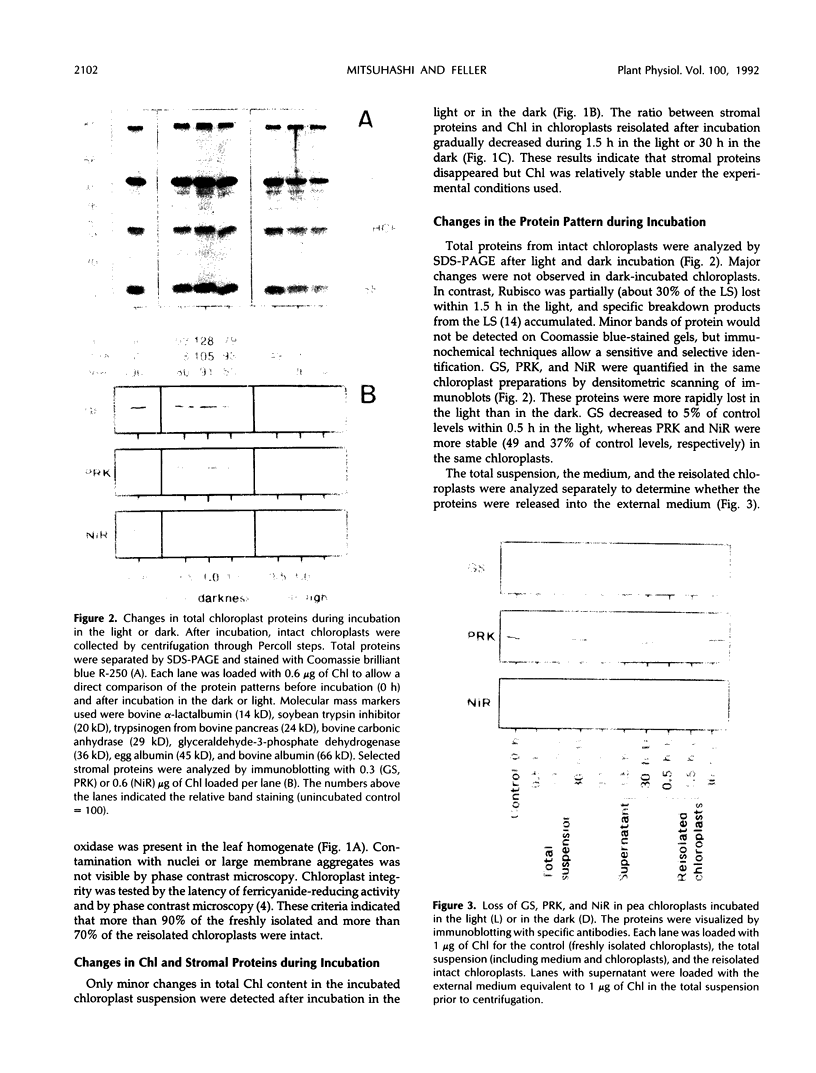
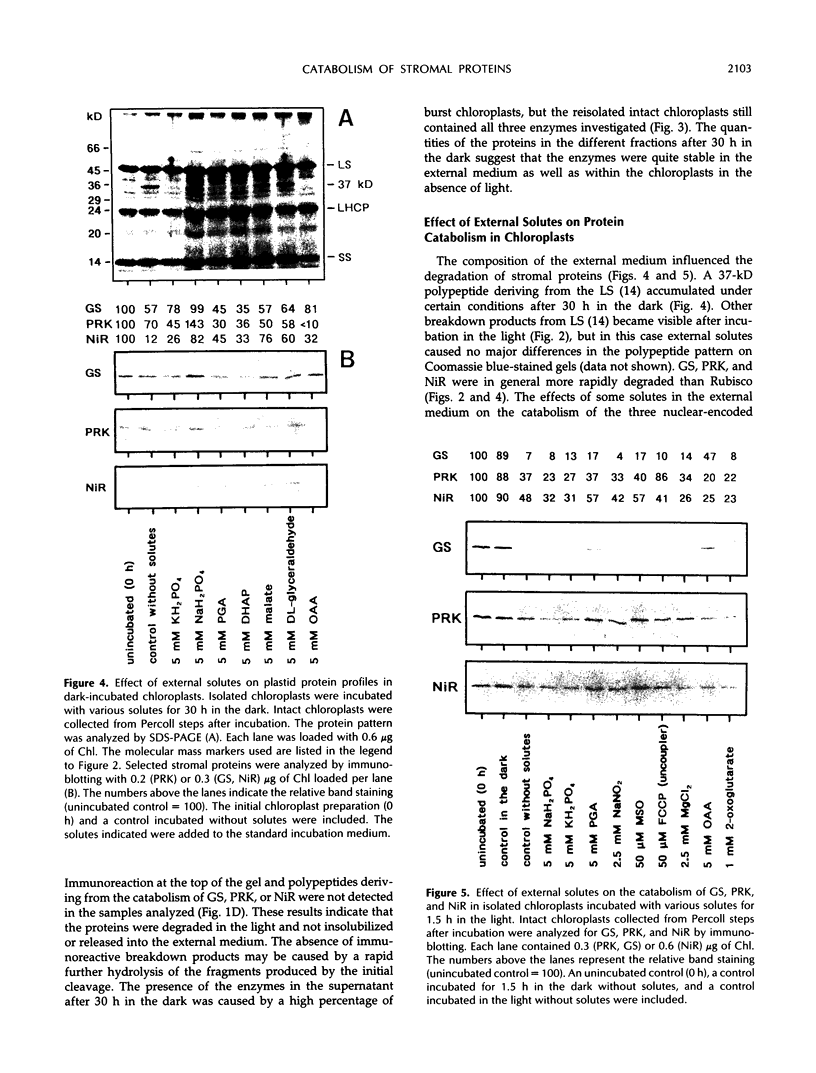
The catabolism of nuclear-encoded stromal proteins was investigated in intact chloroplasts isolated mechanically from pea (Pisum sativum) leaves. Glutamine synthetase, phosphoribulokinase, and nitrite reductase (quantified by immunoblotting) were more rapidly degraded in the light than in the dark. Furthermore, the degradation rates depended on exogenously supplied metabolites. For example, 2-oxoglutarate accelerated the catabolism of all three enzymes in chloroplasts incubated in the light, whereas oxaloacetate stabilized glutamine synthetase and at the same time destabilized the other two enzymes.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Choe H. T., Thimann K. V. The Metabolism of Oat Leaves during Senescence: III. The Senescence of Isolated Chloroplasts. Plant Physiol. 1975 May;55(5):828–834. doi: 10.1104/pp.55.5.828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Liu X. Q., Jagendorf A. T. Neutral peptidases in the stroma of pea chloroplasts. Plant Physiol. 1986 Jun;81(2):603–608. doi: 10.1104/pp.81.2.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayfield S. P., Nelson T., Taylor W. C. The Fate of Chloroplast Proteins during Photooxidation in Carotenoid-Deficient Maize Leaves. Plant Physiol. 1986 Nov;82(3):760–764. doi: 10.1104/pp.82.3.760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musgrove J. E., Elderfield P. D., Robinson C. Endopeptidases in the stroma and thylakoids of pea chloroplasts. Plant Physiol. 1989 Aug;90(4):1616–1621. doi: 10.1104/pp.90.4.1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt G. W., Mishkind M. L. The transport of proteins into chloroplasts. Annu Rev Biochem. 1986;55:879–912. doi: 10.1146/annurev.bi.55.070186.004311. [DOI] [PubMed] [Google Scholar]
- Schnarrenberger C., Oeser A., Tolbert N. E. Development of Microbodies in Sunflower Cotyledons and Castor Bean Endosperm during Germination. Plant Physiol. 1971 Nov;48(5):566–574. doi: 10.1104/pp.48.5.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters S. P., Noble E. R., Dalling M. J. Intracellular Localization of Peptide Hydrolases in Wheat (Triticum aestivum L.) Leaves. Plant Physiol. 1982 Mar;69(3):575–579. doi: 10.1104/pp.69.3.575. [DOI] [PMC free article] [PubMed] [Google Scholar]