Abstract
Natural products have been important parts of traditional medicine since ancient times, with various promising health effects. Leea aequata (L. aequata), a natural product, has been widely used for treating several diseases due to its promising pharmacological activities. Therefore, the present study aimed to explore the phytochemical profiling and molecular docking of the antioxidant-rich part of L. aequata leaves and its antiproliferative activity. L. aequata leaves were extracted with methanol, followed by fractionation with the respective solvents to obtain the petroleum ether, chloroform, ethyl acetate, and aqueous fractions. The antioxidant activity was evaluated by spectrophotometric methods. The cytotoxic and antiproliferative activities were detected using MTT colorimetric and confocal microscopy methods, respectively. Phytochemical compositions were analyzed using gas chromatography‒mass spectrometry analysis. Computer aided (molecular docking SwissADME, AdmetSAR and pass prediction) analyses were undertaken to sort out the best-fit phytochemicals present in the plant responsible for antioxidant and anticancer effects. Among the fractions, the ethyl acetate fraction was the most abundant polyphenol-rich fraction and showed the highest antioxidant, reducing power, and free radical scavenging activities. Compared to untreated MCF-7 cells, ethyl acetate fraction-treated MCF-7 cells showed an increase in apoptotic characteristics, such as membrane blebbing, chromatin condensation, and nuclear fragmentation, causing apoptosis and decreased proliferation of HeLa and MCF-7 cells. Furthermore, gas chromatography mass spectrometry data revealed that the ethyl acetate fraction contained 16 compounds, including methyl esters of long-chain fatty acids, which are the major chemical constituents. Moreover, hexadecanoic acid, methyl ester; 9-octadecenoic acid (Z)-, methyl ester; 9,12-octadecadienoic acid, methyl ester (Z, Z) and phenol, 2,4-bis(1,1-dimethylethyl) are known to have antioxidant and cytotoxic activity, as confirmed by computer-aided models. A strong correlation was observed between the antioxidant and polyphenolic contents and the anticancer activity. In conclusion, we explored the possibility that L. aequata could be a promising source of antioxidants and anticancer agents with a high phytochemical profile.
Keywords: Leea aequata, Antioxidant, Oxidative stress, Free radical, Cytotoxicity, Anticancer
Highlights
-
•
GC-MS analysis was performed to identify the major compounds from L. aequata
-
•
EAF was the highest phenolic and flavonoid-rich fraction
-
•
EAF significantly reduced HeLa and MCF-7 cell proliferation
-
•
Phenol-2,4-bis-1,1-dimethylethyl showed effective antioxidant and anticancer activity
-
•
A positive correlation was observed among polyphenolics, antioxidant and anticancer activities
1. Introduction
Plant secondary metabolites have been used since ancient times to prevent and treat various human diseases. Due to their wide range of clinical applications and excellent therapeutic efficacy, researchers strive to exploit secondary metabolites as a novel source in drug discovery [[1], [2], [3], [4], [5]]. Extensive research has been performed to identify novel agents from natural sources such as plants, fruits, vegetables, extracts, and biomolecules to develop effective and natural therapeutic approaches for preventing and treating various cancers because cancer led to nearly 10 million deaths worldwide in 2020, or nearly one in six deaths, and its incidence is increasing daily [6]. According to cancer statistics for 2023, 1,958,310 new cancer cases and 609,820 cancer deaths are projected to occur in the United States [7]. Moreover, the American Cancer Society estimates that by 2040, the number of new cases of cancer is expected to grow to 27.5 million, and the number of deaths is expected to be 16.3 million globally, an increase of 60 % from 2020 [8]. Unfortunately, even though some cancers are treatable, current therapies may not be able to adequately treat cancer and its related complications. In the new era, immunotherapy, targeted therapy, radiotherapy, and surgical treatments have come to the forefront, some of which are highly selective. Moreover, chemotherapy agents are nonselective, widely used, and highly toxic to both healthy and cancerous cells and tissues, causing serious side effects and health-related complications [9]. Hence, the identification of a novel and natural anticancer agent with minimal adverse effects on normal cellular processes and patients is urgently needed [10].
The production of reactive oxygen species (ROS) is a normal cellular process in living organisms. However, ROS also play pivotal role in the growth and proliferation of cancer cells and can alter the redox homeostasis of cancer. The impaired balance between the generation and scavenging of ROS produces a condition known as oxidative stress (OS), which induces cancer development [11]. However, it has been established that ROS scavengers block cancer cell proliferation [12,13]. Accumulated literature has suggested that polyphenols, one of the main antioxidants (termed “natural antioxidants”), have certain anticancer properties [11]. Therefore, this research is focused on searching for potential natural antioxidants rather than synthetic antioxidants, as natural antioxidants are less toxic, easier to obtain, and more cost-effective.
Antioxidants can delay, prevent, or cure oxidative damage by scavenging free radicals and terminating the propagation of free radical chain reactions [[14], [15], [16]]. Thus, antioxidants can protect normal cells from cancer and reduce the risks of tumorigenesis by reducing OS and other parameters of cell damage. Recently, it has been reported that nutraceuticals rich in antioxidants kill cancer cells by producing pro-oxidative activity [17]. Carotenoids, also called tetraterpenoids, found in fruits, vegetables, and other plant-based products may function as pro-oxidants and cause cancer cells to undergo ROS-mediated apoptosis. Basically, when carotenoids are administered with cytotoxic drugs that cause ROS, they can reduce the toxic effects of these drugs on healthy cells (through an antioxidant mechanism) without affecting the cytotoxicity of drugs to cancer cells (a pro-oxidant mechanism) [18]. Moreover, phenolic and aromatic ring-containing antioxidants are the most effective antioxidants, as they release H• to stabilize the radical intermediate in free radical chain reactions [14,19]. Therefore, antioxidant-enriched herbal supplements can confer protection by decreasing OS and inflammatory processes [4,20,21]. Over 8000 polyphenolic compounds and more than 6000 flavonoids have been identified in various plants. From 1981 to 2014, approximately 136 drugs were recorded as anticancer drugs worldwide, and of these, 83 % were from natural sources [22]. Some evidence suggests that several commonly used anticancer medications come from natural sources. For example, drugs such as curcumin (diferuloylmethane) [23] and paclitaxel [24] were derived from the rhizome of Curcuma longa Linn and the bark of Taxus brevifolia Nutt, while pomiferin and sulforaphane were isolated from Maclura pomifera [25]. Thymoquinone, isolated from the seeds of Nigella sativa, possesses potent anticancer and free radical scavenging abilities in both animal models and cell culture systems [[26], [27], [28]].
Leea aequata (L. aequata) is a traditional medicinal plant belonging to the Leeaceae family. It is widely distributed throughout Bangladesh, Bhutan, Cambodia, China, India, Malaysia, Myanmar, Nepal, the Philippines, Thailand, and Vietnam [29]. In Bangladesh, the plant is distributed in the forests of the Chittagong and Sylhet divisions [30]. It possesses phenolics, flavonoids, saponins, tannins, glycosides, and steroids [9,31]. L. aequata is traditionally used for the treatment of many diseases [30]. The roots, tubers, and stems are used as mucilaginous and astringents [32]. The bark and roots are used as astringents and anthelmintics, as well as to treat indigestion, jaundice, chronic fever, and malaria [33]. The roots, pounded with milk, are often prescribed for consumption; pounded with rice water, they are often prescribed for leukorrhea. The whole plant is used to treat mercury poisoning, along with fever and cough in influenza [32]. The leaves are used as antiseptics and wound-healing agents [32]. A paste of fresh leaves is applied externally to treat skin diseases and wounds [29]. The essential oil obtained from the whole plant is used to treat tuberculosis [32]. The leaves have anticonvulsant, antinociceptive, and anthelmintic properties [30,33]. Additionally, many other species of the genus Leea have been shown to produce anticancer activity against several cancer cells [34] and to show antitumor activity in mouse models [34]. In our previous study, we successfully isolated two glycosides, 7-O-methylmearnsitrin and roseoside A, from L. aequata leaves, demonstrating that both compounds significantly inhibited cell proliferation in the HeLa cell line [31].
L. aequata, as a natural product, has been widely used by local practitioners for the treatment of different diseases, and many other species of the Leea genus possess potential pharmacological properties. Hence, these findings encouraged us to investigate the phytochemical profiling and pharmacological activity of this particular plant. In this study, we investigated cytotoxic activity against HeLa (cervical cancer) and MCF-7 (breast cancer) cell lines and in vitro antioxidant activity against cell-free conditions using different fractions of L. aequata leaf extracts. In addition, it was investigated for its phytochemical profile, the most effective antioxidant, and cytotoxic fractions.
2. Materials and methods
2.1. Materials and reagents used in the different assays
The substances used in this study were methanol, ethyl acetate, chloroform, petroleum ether, Folin-Ciocalteu reagent, sodium carbonate (Na2CO3), gallic acid (GA), 1,1-diphenyl-2-picrylhydrazyl (DPPH), butylated hydroxytoluene (BHT), sulfuric acid, sodium phosphate, catechin (CA), and ammonium molybdate, which were purchased from Duksan Pure Chemicals Co. Limited in South Korea. Dulbecco's modified Eagle's medium (DMEM), fetal bovine serum (FBS), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), phosphate-buffered saline (PBS), 4′,6-diamidino-2-phenylindole (DAPI), annexin V–fluorescein isothiocyanate (FITC), propidium iodide (PI), and dimethyl sulfoxide (DMSO) were purchased from Sigma-Aldrich in India.
2.2. Plant Collection and identification
L. aequata leaves were collected from Sylhet, a metropolitan city located in the northeastern region of Bangladesh, and identified by an expert taxonomist at the Bangladesh National Herbarium, where a voucher specimen (DACB Accession Number 45396) was deposited.
2.3. Preparation of plant leaves
Collected leaves were washed with fresh tap water to remove dirt and debris before being shade dried for several days with occasional sun drying. The leaves were then dried in an oven (YPO-072, Yuanyao, China) for 24 h at a considerably low temperature (30 °C) for better grinding. The dried materials were ground into a coarse powder using a grinding machine (JY2A-4, Single Phase Motor, China) and stored at room temperature (RT) until needed.
2.4. Extraction of plant leaves
Before selecting the appropriate solvent, the dried coarse powdered materials were extracted with several solvents, such as ethanol, ethyl acetate, and chloroform. Based on the percentage yield, methanol was selected for extraction. The dried powder of the L. aequata leaves (approximately 500 g) was deposited into an amber-colored extraction bottle (with a capacity of 2.5 L) and soaked with 100 % methanol (3 × 1.5 L). The sealed bottle was shaken and stirred occasionally. The final extract was filtered separately through cotton and Whatman No. 1 filter paper (Whatman/GE Healthcare Companies, UK) and concentrated under reduced pressure via a rotary evaporator (Bibby Steriling Ltd., UK) at 45 °C to give the crude methanolic extract (CME) (80 g). The CME (35 g) was dissolved in methanol and water and successively fractionated with petroleum ether, chloroform, and ethyl acetate to produce PEF (4.86 g), CHF (3.79 g), EAF (9.45 g), and AQF (15.1 g).
2.5. Determination of polyphenolic content
2.5.1. Determination of total phenolic content
The total phenolic content was determined using the Folin-Ciocalteu reagent (FCR) as previously described [11], with gallic acid (GA) used as a standard. Briefly, different concentrations of 0.4 mL plant extracts or standard were mixed in test tubes with 2 mL FCR (10X dilution in deionized water). Sodium carbonate solution (7.5 %, 3 mL) was added, and the mixture was incubated for 30 min at 25 °C. The absorbance of each solution at 760 nm was measured against a blank using a spectrophotometer (Shimadzu UV-1900 spectrophotometer, Japan). The total content of phenolic compounds in the different extracts was expressed as mg GA equivalent (GAE) per gram of dry extract and was calculated using the calibration curve equation y = 0.102 x + 0.0268 (R2 = 0.9996) by formula C = (x × V)/M, where C is the total content of phenolic compounds as mg GAE in each gram of the dried extract, x is the GAE concentration in mg/mL present in the sample, V is the final volume of the solution in mL, and M is the mass of the sample in the final solution in g.
2.5.2. Determination of total flavonoid content
The total flavonoid content of the different extractives was determined through the colorimetric method using catechin (CA) as a standard [11]. In brief, 0.5 mL of plant extracts or standard at different concentrations was mixed with 150 μL of 5 % sodium nitrate and 2.5 mL of distilled water in different test tubes and incubated for 5 min at RT. AlCl3 (10 %, 0.3 mL) was added to each test tube and incubated for 6 min at RT. NaOH (4 %, 1 mL) was added to the mixture and incubated at RT for 15 min. The absorbance of each solution at 510 nm was then measured against a blank using a spectrophotometer (Shimadzu UV-1900 spectrophotometer, Japan). The total content of flavonoid compounds in the different extracts was calculated as catechin equivalent (CAE) per g of dry extract from the standard curve equation y = 0.0264x - 0.038, R2 = 0.9994 by formula C = (x × V)/M, where C is the total content of flavonoid compounds as mg CAE in each gram of the dried extract, x is the CAE concentration in mg/mL present in the sample, V is the final volume of the solution in mL, and M is the mass of the sample in the final solution in g.
2.6. In vitro antioxidant assay
2.6.1. Determination of total antioxidants
The total antioxidant capacity of the different extractives was determined according to a previous research method [11], with slight modifications. In summary, different concentrations of plant extracts or standard CA (0.5 mL) were mixed with 3 mL of a reaction mixture containing 0.6 M sulfuric acid, 28 mm sodium phosphate, and 1 % ammonium molybdate. The mixtures were then incubated at 95 °C for 10 min. After cooling the mixtures at RT, the absorbance of each solution at 695 nm was measured against a blank using a spectrophotometer (Shimadzu UV-1900 spectrophotometer, Japan). The absorbance of the reaction mixture was directly proportional to the total antioxidant capacity. In this study, extracts or standard at five different concentrations ranging from 12.5 to 150 μg/mL were taken for each antioxidant assay. Concentrations were selected based on trial and error to fit the range of concentrations that can fully represent the rational change in antioxidant activity with increasing sample concentration.
2.6.2. Ferric-reducing power capacity
The total antioxidant capacity of the different extractives was estimated as described in a previous study [11]. First, 0.25 mL of plant extracts or standard ascorbic acid (AA) of different concentrations was mixed with 0.625 mL of potassium buffer (0.2 M, pH 6.6) and 0.625 mL of 1 % potassium ferricyanide [K3Fe(CN)6]. The mixtures were incubated for 20 min at 50 °C. Trichloroacetic acid (TCA) (10 %, 0.625 mL) was added to each test tube and centrifuged at 3000 rotations per minute (rpm) for 10 min. Then, 1.8 mL of the supernatant was withdrawn from each test tube and mixed with 1.8 mL of distilled water, followed by the addition of 0.36 mL of a 0.1 % ferric chloride (FeCl3) solution to each diluted reaction mixture. Finally, the absorbance of each solution and the standard at 700 nm were measured against a blank using a spectrophotometer (Shimadzu UV-1900 spectrophotometer, Japan). The absorbance of the reaction mixture was directly proportional to the total antioxidant capacity.
2.6.3. 1,1-Diphenyl-2-picrylhydrazyl radical scavenging assay
The free radical scavenging abilities of the extracts/standard were tested using the 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging assay, as described in previous research [11]. Briefly, 2.4 mL of a methanol solution containing 0.1 mM DPPH was mixed with 1.6 mL of plant extracts or standard butylated hydroxytoluene (BHT) dissolved in methanol at different concentrations. The mixtures were incubated at RT for 30 min in the dark. The absorbance of the test and standard solutions was measured at 517 nm using a spectrophotometer (Shimadzu UV-1900 spectrophotometer, Japan). A typical blank solution containing the same solution mixture without extracts or standard was incubated under the same conditions as the rest of the sample solutions. The percentage (%) scavenging activity of DPPH radicals was calculated using the equation % I = {(Ac – As)/Ac} × 100, where Ac is the absorbance of the control and As is the absorbance of the extracts or standard. Finally, all scavenging percentages were plotted against concentrations, and the IC50 was calculated.
2.6.4. Hydroxyl radical scavenging assay
The hydroxyl radical scavenging activity of the extracts or standard was determined using a previously described method [11]. Hydroxyl radicals were generated using the Fe3+-ascorbate-EDTA-H2O2 system (Fenton reaction). The assay was based on the quantification of the 2-deoxy-d-ribose degradation product, which forms a pink chromogen upon heating with thiobarbituric acid (TBA) at a low pH. The reaction mixture contained 0.8 mL of phosphate buffer solution (50 mmol/L, pH 7.4), 0.2 mL of extracts or standard CA at different concentrations (6.25–100 μg/mL), 0.2 mL of EDTA (1.04 mmol/L), 0.2 mL of FeCl3 (1 mmol/L), and 0.2 mL of 2-deoxy-d-ribose (28 mmol/L) in the test tubes. The mixtures were kept in a water bath at 37 °C, and the reaction was prompted by adding 0.2 mL of AA (2 mmol/L) and 0.2 mL of H2O2 (10 mmol/L). After incubation at 37 °C for 1 h, 1.5 mL of TBA (10 g/L) was added to the reaction mixture, followed by 1.5 mL of HCl (25 %). The mixture was heated at 100 °C for 15 min and then cooled with water. The absorbance of the solution was measured at 532 nm using a spectrophotometer (Shimadzu UV-1900 spectrophotometer, Japan). The hydroxyl radical scavenging activity was evaluated using the inhibition percentage of 2-deoxy-d-ribose oxidation on hydroxyl radicals. The percentage of hydroxyl radical scavenging activity was calculated according to the following formula: % of hydroxyl radical scavenging activity = {(Ac - As)/Ac} × 100, where Ac is the absorbance of the control and As is the absorbance of the extracts or standard. The experiment was repeated three times at each concentration.
2.7. In vitro biological studies of L. aequata
2.7.1. Lipid peroxidation inhibition assay
The degree of lipid peroxidation was assayed by estimating the TBA-reactive substances in accordance with earlier studies [35]. The long adult Swiss albino mice, each weighing approximately 35 g, were anesthetized with 70 % (v/v) ethanol in 0.9 % sterile saline in the ventral chest region for deep anesthesia. The excised mouse livers were homogenized with a homogenizer in ice-cold phosphate buffer (50 mM, pH 7.4) to produce a 1/10 homogenate. The homogenate was centrifuged at 12,000 rpm for 15 min at 4 °C. The supernatant was used as a liposome for an in vitro lipid peroxidation assay. Then, 0.5 mL of supernatant and 0.3 mL of extracts or standard CA at different concentrations were mixed with 1 mL of 0.15 M KCl and 200 μL of 0.4 mM FeCl3 and put in an incubator at 37 °C for 30 min. Afterward, 2 mL of ice-cold TBA-TCA-HCl-BHT solution was immediately added to each test tube to stop the reaction, and the tubes were then heated at 90 °C for 60 min. After cooling on ice and centrifugation at 3000 rpm for 5 min, the supernatants were removed, and their absorbance at 532 nm was measured using a spectrophotometer (Shimadzu UV-1900 spectrophotometer, Japan). A control experiment was performed in the presence of distilled water without extracts or standard. The percentage (%) of the scavenging activity of lipid peroxide radicals was calculated from the equation % I = {(Ac - As)/Ac} × 100, where Ac is the absorbance of the control and As is the absorbance of the extract/standard. Finally, the percentage of scavenging was plotted against the concentration, and the IC50 was calculated.
2.7.2. Cell culture
Both HeLa and MCF-7 cells were purchased from the American Type Culture Collection, USA. MCF-7 was the first cell line for breast cancer, and HeLa was the first human cell line. Due to their widespread expansion into an essential experimental tool in cancer research, these cell lines were made possible by their discovery. They also provide an endless source of a homogeneous cell population that can self-replicate in conventional cell culture medium for cancer research [36]. The cells were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10 % fetal bovine serum in an atmosphere with 5 % CO2 at 37 °C. Healthy and exponentially growing cells were used in all the described experiments.
2.7.3. Cytotoxic assay
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) was used to assess cell proliferation in HeLa and MCF-7 cells, as described by Rahman et al. [12] and Islam et al. [37]. Briefly, 1 × 104 HeLa cells were incubated in 96-well plates in the presence of various concentrations (125–500 μg/mL) of fractions and the standards vincristine sulphate (VS) and 5-fluorouracil (5-FU) for 48 h; on the other hand, 1 × 104 MCF-7 cells/well were incubated in 96-well plates at concentration ranges of 50–200 μg/mL. Following treatment, 20 μL of MTT (5 mg/mL dissolved in phosphate-buffered saline) was added to each well and incubated for an additional 4 h at 37 °C. The purple‒blue MTT formazan precipitate was dissolved in 200 μL of dimethyl sulfoxide, and the optical density was measured at 570 nm using a microplate reader (VarioSkan Flash 2.4.3, Thermo Fisher Scientific). The percentage of cell viability or inhibition of cell growth was calculated using the following formula: OD of samples/OD of controls × 100.
2.7.4. Observation of morphological changes and nuclear damage in MCF-7 cells
Cell apoptosis (nuclear condensation and fragmentation) was morphologically examined under a fluorescence microscope (Olympus X71, Korea) using a previously described method [35]. Apoptotic morphology was examined using Hoechst 33342 staining. In summary, MCF-7 cells were grown in DMEM for 24 h before being exposed to EAF for the same amount of time. PBS was used to wash the cells three times before staining them with Hoechst 33342 at a concentration of 0.1 mg/mL for 20 min in a darkened room at 37 °C. PBS was then used to wash the cells once more.
2.7.5. Apoptosis assay
4′,6-Diamidino-2-phenylindole (DAPI), annexin V-FITC, and propidium iodide (PI) triple fluorescence staining for cancer cell apoptosis measurement were performed according to the method described by Mostofa et al. [31]. Briefly, HeLa cells were cultured in DMEM containing 100 nM EAF samples. After 48 h of treatment, the cells were washed twice with 0.01 M phosphate-buffered saline and suspended in 200 μL binding buffer. The cells were then incubated with 10 μL DAPI, 10 μL annexin V-FITC, and 5 μL PI for 30 min at 4 °C in the dark. DAPI, annexin V-FITC, and PI fluorescence were immediately observed under an FV1000 confocal laser scanning microscope (Olympus, Tokyo, Japan).
MCF-7 cells were seeded into each well of a 96-well plate and incubated for 24 h in DMEM with 5 % CO2 at 37 °C. The cells were then treated with EAF for 48 h. After that, MCF-7 cells were stained with FITC-labeled annexin V/PI (Bioscience, USA). Finally, the stained plate was examined under a fluorescence microscope (Olympus IX71, Japan).
2.8. Gas Chromatography‒Mass spectrometry analysis
The sample was placed in a test tube and mixed with a boron trifluoride methanol solution for 1 h at a controlled 70 ± 2 °C. The samples containing boron trifluoride methanol solution were cooled to room temperature with hexane, and unsaturated sodium chloride was mixed and vortexed for 1 min to facilitate the settlement of higher molecular weight components such as ketones and water molecules, with an upper layer containing the fatty acid methyl esters. These were then deposited in mini-vials for analysis through GC.
The bioactive compounds from the EAF were analyzed using GC-MS with electron-impact ionization (EI) on a gas chromatograph (GC-17A, Shimadzu Corporation, Kyoto, Japan) coupled to a mass spectrometer (GC-MS TQ 8040, Shimadzu Corporation). A fused silica capillary column (Rxi-5 ms; 0.25 m film, 30 m long, 0.32 mm internal diameter) coated with DB-1 (J&W) was used. The inlet temperature was 260 °C, and the oven temperature was set at 70 °C (0 min); 10 °C and 150 °C (5 min); 12 °C and 200 °C (15 min); and 12 °C and 220 °C (5 min), with a hold time of 10 min. The flow rate of the column was 0.6 mL/min of helium gas at a constant pressure of 90 kPa. The aux (GC-MS interface) temperature was 280 °C. The MS was set to scan mode with a scanning range of 40–350 amu, while the ionization mode was EI type, and the mass range was set within 50–550 m/z. The sample (3 μL) was injected in splitless mode. The total GC-MS run time was set to 29.33 min, and compounds in the peak areas were identified by comparison with those in the database of the GC‒MS library (version NIST 08-S).
2.9. In silico molecular docking
2.9.1. Protein preparation
The 3D crystal structures of caspase-3 (PDB: 5IAE) for HeLa [38,39], the epidermal growth factor receptor (EGFR) kinase domain (PDB: 2ITY) [40], glutathione reductase (PDB: 3GRS), and urate oxidase (PDB: 1R4U) were downloaded in PDB format from the Protein Data Bank [41]. The structures were then prepared and refined through the process described by Uddin et al. and Adnan et al. [42,43].
2.9.2. Ligand preparation
Identified compounds from EAF were collected from PubChem databases in a spatial data file. The 3D configurations for these compounds were built using the LigPrep wizard in Maestro Schrödinger (v11.1) with optimized potentials for liquid simulations-3 [44] force fields. Their ionization states were generated at pH 7.0 ± 2.0 using Epic in Schrödinger's Suite. Up to 32 possible stereoisomers per ligand were retained.
2.9.3. Receptor grid generation
Receptor grids were calculated for prepared proteins so that various ligand poses would bind within the predicted active site during docking. In Glide, the default parameters set the van der Waals radius scaling factor at 1 and the partial charge cutoff at 0.25. A cubic box of specific dimensions centered on the centroid of the active site residues (reference ligand active site) was generated for the receptor. The bounding box was set to 14 Å × 14 Å × 14 Å for docking experiments [45,46].
2.9.4. Glide standard precision ligand docking
Standard precision flexible ligand docking was carried out in the Glide of Schrödinger-Maestro (v11.1), within which penalties were applied to noncis/trans amide bonds. The van der Waals scaling factor and partial charge cutoff were selected to be 0.80 and 0.15, respectively, for ligand atoms. Final scoring was performed on energy-minimized poses and displayed as glide scores. The best-docked pose with the lowest glide score value was recorded for each ligand.
2.9.5. 5. In silico determination of pharmacokinetic parameters by SwissADME
The pharmacokinetic parameters or drug-like properties of the identified compounds were evaluated using the SwissADME (absorption, distribution, metabolism, and excretion) online tool (http://www.swissadme.ch/). ADME properties depict the compound's acceptability in the body, which is determined by Lipinski's rule of five.
2.9.6. In silico toxicological property prediction by AdmetSAR
The toxicological characteristics of the identified compounds were determined using the AdmetSAR online tool (http://lmmd.ecust.edu.cn/admetsar1/predict/), since toxicity presents a major concern during the development of novel drug therapies. In this study, Ames toxicity, carcinogenic properties, acute oral toxicity, and rat acute toxicity were predicted using Veber rules.
2.9.7. In silico online study of the prediction of activity spectra for substances
The identified compounds from EAF were examined to evaluate their anticancer, antiviral, free radical scavenging, lipid peroxidase inhibitory, and antioxidant properties using the prediction of activity spectra for substances (PASS) online (http://www.way2drug.com/PassOnline/) [47].
2.10. Statistical analysis
All statistical tests and graphical evaluations were performed using GraphPad Prism (version 8.4.3). Data are presented as the mean ± standard deviation (SD) from triplicate experiments. The data for significant differences between the test and control groups were described using a one-way analysis of variance, followed by Dunnett's post hoc test. The statistical and graphical analyses were conducted using Microsoft Excel 2007 (Roselle, Illinois, USA) and R version 2.15.1 (http://www.r-project.org/). Experimental results were examined further for the Pearson correlation coefficient of phenolics with antioxidant and cell viability assays. p values < 0.05, < 0.01 and < 0.001 were considered statistically significant.
3. Results
3.1. Determination of polyphenolic content
3.1.1. Determination of total phenolic content
The total phenolic contents in CME and its four fractions (PEF, CHF, EAF, and AQF) of L. aequata leaves were determined using FCR (Fig. 1A). The results showed that EAF possesses the highest phenolic content (267 ± 9 mg GAE/g of the dried sample). AQF (241 ± 17 mg GAE/g of the dried extract) and CME (233 ± 17 mg GAE/g of the dried extract) were also rich sources of phenolic content. However, CHF (39 ± 1 mg GAE/g of the dried extract) and PEF (25 ± 0 mg GAE/g of the dried extract) were comparatively poor sources of phenolic content.
Fig. 1.
Polyphenolic contents of CME and its different fractions. (A) Total phenolic (mg of GAE/gm of the dried sample) and (B) flavonoid contents (mg of CAE/gm of the dried sample) of CME and its four fractions (PEF, CHF, EAF and AQF). Data are expressed as the mean ± SD (n = 3) for all tested dosages. Values with (**p < 0.01) were considered statistically significant. The methanolic extract of L. aequata (CME), petroleum ether (PEF), chloroform (CHF), ethyl acetate (EAF), and aqueous (AQF) fractions were used.
3.1.2. Determination of total flavonoid content
The total flavonoid contents in CME and its four fractions were determined using the well-known aluminumchloride colorimetric method. Fig. 1B represents the total flavonoid content of the extractives expressed as mg CAE/g of the dried extract. The flavonoid contents of CME, PEF, CHF, EAF, and AQF were 131 ± 2, 61 ± 1, 82 ± 3, 153 ± 2, and 68 ± 2 mg CAE/g of the dried extractives, respectively. In a comparison of the total flavonoid contents among the fractions, EAF was observed to contain the highest number of flavonoids, followed by CME, CHF, AQF, and PEF.
3.2. In vitro antioxidant assay
3.2.1. Total antioxidant activity
The total antioxidant activity of the different extractives and standard (CA) was assessed using the phosphomolybdenum method based on the reduction of Mo (V1) to Mo (V). The total antioxidant activity of CME and its four fractions, as well as the standard (CA), is depicted in Fig. 2A. Among the fractions, EAF (1 ± 0.03) possessed the highest antioxidant activity, which was similar to that of the CA standard (1.06 ± 0.09). This was followed by CME (0.89 ± 0.05), AQF (0.79 ± 0.01), PEF (0.31 ± 0.05), and CHF (0.23 ± 0.02) at a concentration of 100 μg/mL. These results demonstrated that all extracts of CME had noticeable antioxidant properties; however, these properties were dependent on concentration.
Fig. 2.
Comparative antioxidant activity of CME and its different fractions. (A) Total antioxidant and (B) Ferric-reducing power capacity of CME and its four fractions (PEF, CHF, EAF and AQF). (C) Comparative DPPH free radical scavenging activity of CME and its different fractions and (D) IC50 (μg/mL) values of CME and its four fractions (PEF, CHF, EAF and AQF) on DPPH. (E) Comparative hydroxyl radical scavenging activity of CME and its different fractions and (F) IC50 (μg/mL) values of CME and its four fractions (PEF, CHF, EAF and AQF) on hydroxyl radical. (G) Comparative lipid peroxidation inhibition of CME and its different fractions and (H) IC50 (μg/mL) values of CME and its four fractions (PEF, CHF, EAF and AQF) on lipid peroxide. Data are expressed as the mean ± SD (n = 3) for all tested dosages. Values with (*p < 0.05 and **p < 0.01) were considered statistically significant. The methanolic extract of L. aequata (CME), petroleum ether (PEF), chloroform (CHF), ethyl acetate (EAF), and aqueous (AQF) fractions were used.
3.2.2. Ferric-reducing power capacity
The reductive capabilities of the extractives and standard (AA) are shown in Fig. 2B. Research has documented that a higher absorbance indicates a higher reducing capacity, while increased absorbance functions in a concentration-dependent manner [4]. The absorbance of the EAF was 2.77 ± 0.01, which was similar to that of standard AA (3.26 ± 0) at a concentration of 50 μg/mL. Similar to the results of total antioxidant activity, EAF showed the highest reducing capacity, followed by AQF (absorbance 1.78 ± 0.19), CHF (absorbance 0.83 ± 0.09), and PEF (absorbance 0.213 ± 0.11), at a concentration of 50 μg/mL. These results demonstrated that CME and its fractions had a significant iron-reducing capacity, a result that was also concentration-dependent.
3.2.3. 1,1-Diphenyl-2-picrylhydrazyl radical scavenging activity
DPPH radical scavenging activity is based on the ability of the extractives to scavenge stable DPPH radicals containing odd electrons. The results of DPPH radical scavenging of CME and its four fractions and standard (BHT) are given in Fig. 2C and D. Among the fractions, EAF showed the highest and most significant free radical scavenging activity. The IC50 values of EAF, AQF, and CME were found to be 5.11 ± 0.04, 6.92 ± 0.19, and 6.09 ± 0.13 μg/mL, respectively, which were higher than that of the standard BHT (IC50: 7.93 ± 0.19 μg/mL). However, CHF and PEF showed moderate scavenging activity, with IC50 values of 24.97 ± 1.2 μg/mL and 9.52 ± 0.17 μg/mL, respectively. This observation demonstrated that all the extracts from the CME of L. aequata leaves scavenged DPPH-free radicals.
3.2.4. Hydroxyl radical scavenging activity
In the hydroxyl radical scavenging assay, the ability of the CME and fraction extracts to remove hydroxyl radicals in solution was evaluated quantitatively through the colorimetric method using CA as the standard, and the percentage of scavenging activity was calculated. The results of the analysis of the hydroxyl radical scavenging activity of different extractives and standards are shown in Fig. 2E and F. The IC50 values of CME, PEF, CHF, EAF, AQF, and standard CA were 121, 475, 325, 38, 165, and 170 μg/mL, respectively. This result demonstrated that EAF, AQF, and CME showed higher activity compared to the standard. Among the extracts, EAF showed significant radical scavenging activity with an IC50 value of 38 μg/mL, more than four times lower than that of the standard. A lower IC50 indicates a higher scavenging capacity. Our results therefore clearly show that the EAF of the CME of L. aequata leaves can scavenge free radicals at a significant level.
3.3. In vitro biological studies of L. aequata
3.3.1. Lipid peroxidation inhibition assay
The results of lipid peroxidation inhibition of the extracts of CME and standard CA are shown in Fig. 2G and H. CME, PEF, CHF, EAF, AQF, and CA showed lipid peroxidation inhibition percentages of 58 ± 0, 52 ± 0, 54 ± 0, 63 ± 1, 59 ± 1, and 69 ± 1, respectively, at a concentration of 100 μg/mL. Among the extracts, EAF showed the highest inhibitory activity with an IC50 value of 40 μg/mL, similar to that of CA (35 μg/mL). However, AQF showed moderate inhibitory activity with an IC50 value of 53 μg/mL. The CHF and PEF fractions showed less inhibitory activity. As with other results, all inhibitory activities were concentration-dependent.
3.3.2. Cytotoxicity activity of L. aequata
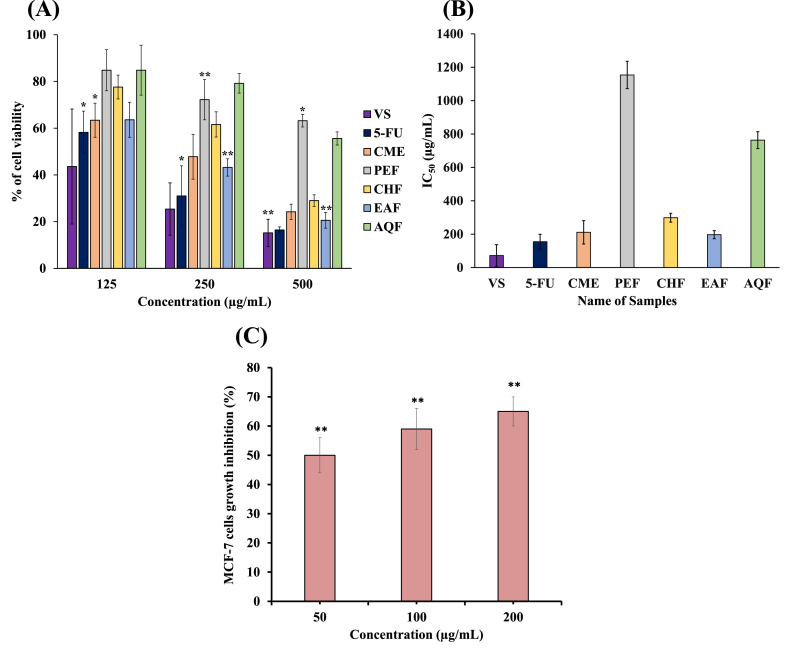
The in vitro anticancer activity of CME and its four fractions and the standards, VS and 5-FU, was determined in the HeLa cell line. EAF showed the highest cytotoxic activity (23 % cell viability at a concentration of 500 μg/mL) with an IC50 value of 197 μg/mL; the standards, VS and 5-FU, showed 15 % and 16 % cell viability at a concentration of 500 μg/mL with IC50 values of 72 μg/mL and 155 μg/mL, respectively. CME and CHF also showed moderate cytotoxicity, with 28 % and 32 % cell viability, respectively (Fig. 3A and B). As with previous results, cytotoxic activity was concentration-dependent. Moreover, EAF treatment in MCF-7 cells caused a 65 % inhibition of the growth of MCF-7 cells at a concentration of 200 μg/mL (Fig. 3C).
Fig. 3.
(A) Percentage (%) of viable cells of CME and its four fractions (PEF, CHF, EAF, and AQF) from L. aequata leaves and standards on HeLa cells. (B) Measurement of the IC50 of samples and standards in HeLa cells. (C) Cell growth inhibition of MCF-7 cells by EAF. Data are expressed as the mean ± SD (n = 3) for all tested dosages. Values with *p < 0.05 and **p < 0.01 were considered statistically significant.
3.3.3. Effect of EAF on MCF-7 cell morphology
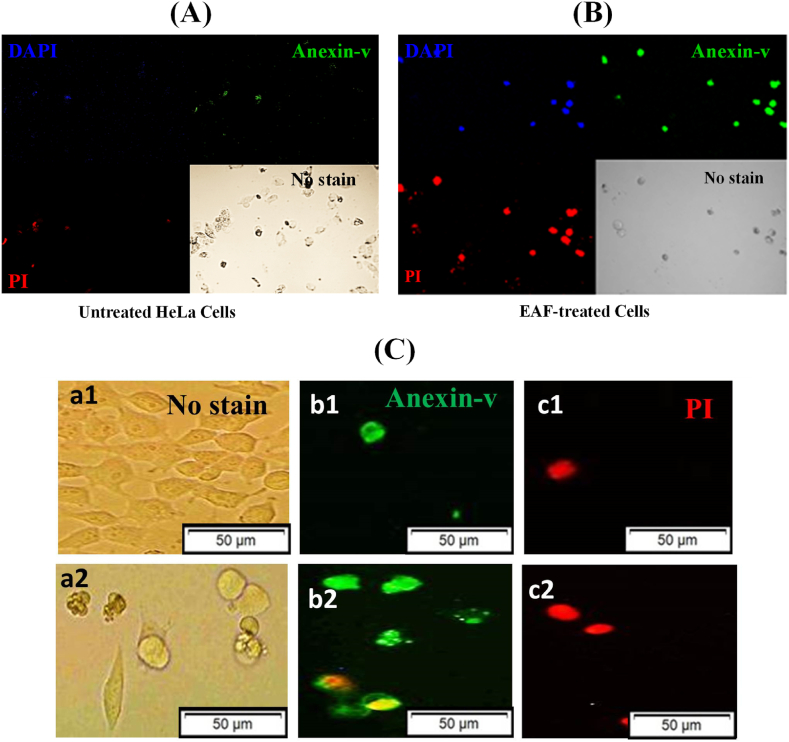
Apoptosis is defined phenotypically by deoxyribonucleic acid (DNA) fragmentation, cell shrinkage, chromatin compaction, blebbing of the plasma membrane, and cell collapse of minute, intact fragments (apoptotic bodies). According to the results of our study, EAF treatment resulted in all of these phenotypes in MCF-7 cells but not in control cells that were left untreated (Fig. 4A (b and d) versus 4A (a and c)), indicating that EAF causes apoptosis in MCF-7 cells.
Fig. 4.
Cell morphological changes detected by Hoechst 33342 staining. (a) and (c) untreated MCF-7 cells. (b) and (d) EAF-treated MCF-7 cells.
3.3.4. Determination of HeLa and MCF-7 cell apoptosis
The effect of EAF on HeLa cell apoptosis was evaluated using DAPI, annexin-V FITC, and PI triple fluorescence staining. Annexin V-FITC and PI signals were barely detectable in the untreated control cells, while treated cells exhibited strong fluorescence densities, indicating that EAF has the capacity to induce HeLa cell apoptosis (Fig. 5A and B). EAF treatment in MCF-7 cells also led to the induction of apoptosis compared with untreated MCF-7 cells (Fig. 5C (a2, b2, and c2) versus 5C (a1, b1, and c1)).
Fig. 5.
(A) Measurement of apoptosis in untreated HeLa cells. (B) Measurement of apoptosis in EAF-treated HeLa cells. (C) Detection of apoptosis in MCF-7 cells by FITC-annexin V/PI staining. (a1), (b1), and (c1) Optical and fluorescence microscopy images in the presence of annexin V and PI, respectively, of untreated MCF-7 cells. (a2), (b2), and (c2) Optical and fluorescence microscopic images of EAF-treated MCF-7 cells in the presence of annexin V and PI. Green and red colors indicate early and late apoptosis, respectively. Pictures were captured at 20x magnification.
3.4. Correlation and regression of phenolic and flavonoid contents with antioxidant and cell viability potential
An excellent positive association was observed during the correlation and regression study between the phenolic and flavonoid content of CME and its four extractives and their radical scavenging activity and cell viability. Table 1, Table 2 present the correlation and regression values (*P < 0.05, **P < 0.01 and ***P < 0.001 indicate a moderate, strong and extremely strong positive correlation, respectively) of phenolic and flavonoid content with the antioxidant and cell viability potential. Therefore, since it was the most antioxidant-rich and effective cytotoxic fraction, EAF was selected for GC-MS profiling to determine which compounds might be most responsible for the apoptosis of HeLa and MCF-7 cells.
Table 1.
Correlation coefficients between the total phenolic contents and antioxidants as well as HeLa and MCF-7 cell proliferation.
| Total Phenolic Contents (Correlation R2) |
|||||||
|---|---|---|---|---|---|---|---|
| Assays | DPPH | Hydroxyl | Lipid Peroxide | TAC | FRPC | MTT assay in HeLa cells | MTT assay in MCF-7 cells |
| CME | 0.55856 | 0.93479** | 0.89227* | 0.9888*** | 0.90741* | 0.99414* | – |
| PEF | 1.9483 | 0.97567** | 0.9105* | 0.92046** | 0.97598** | 0.92154 | – |
| CHF | 0.14652** | 0.95742** | 0.88646* | 0.97608** | 0.99748*** | 0.99996** | – |
| EAF | 0.0808 | 0.66511 | 0.8493* | 0.99566*** | 0.88279* | 0.97599 | 0.90977* |
| AQF | 0.8171 | 0.94858** | 0.85996* | 0.99387*** | 0.99265*** | 0.97742 | – |
Here, *P < 0.05, **P < 0.01 and ***P < 0.001 indicate a moderate, strong and extremely strong positive correlation, respectively. Where DPPH assay = 2,2-diphenyl-1-picrylhydrazyl assay, TAC = total antioxidant capacity, FRPC = ferric-reducing power capacity and MTT assay = 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide assay.
Table 2.
Correlation coefficients between the total flavonoid content and antioxidants as well as HeLa and MCF-7 cell proliferation.
| Total Flavonoid Contents (Correlation R2) |
|||||||
|---|---|---|---|---|---|---|---|
| Assays | DPPH | Hydroxyl | Lipid Peroxide | TAC | FRPC | MTT assay in HeLa cells | MTT assay in MCF-7 cells |
| CME | 0.55856 | 0.93479** | 0.89227* | 0.9888*** | 0.90741* | 0.99414* | – |
| PEF | 0.66765 | 0.97567** | 0.9105* | 0.92046** | 0.97598** | 0.92154 | – |
| CHF | 0.97345** | 0.95742** | 0.88646* | 0.97608** | 0.99748*** | 0.99996** | – |
| EAF | 0.4918 | 0.66511 | 0.8493* | 0.99566*** | 0.88279** | 0.97599 | 0.90977* |
| AQF | 0.55222 | 0.94858** | 0.85996* | 0.99387*** | 0.99265*** | 0.97742 | – |
Here, *P < 0.05, **P < 0.01 and ***P < 0.001 indicate a moderate, strong and extremely strong positive correlation, respectively. Where DPPH assay = 2,2-diphenyl-1-picrylhydrazyl assay, TAC = total antioxidant capacity, FRPC = ferric-reducing power capacity and MTT assay = 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide assay.
3.5. Gas Chromatography‒Mass spectrometry analysis
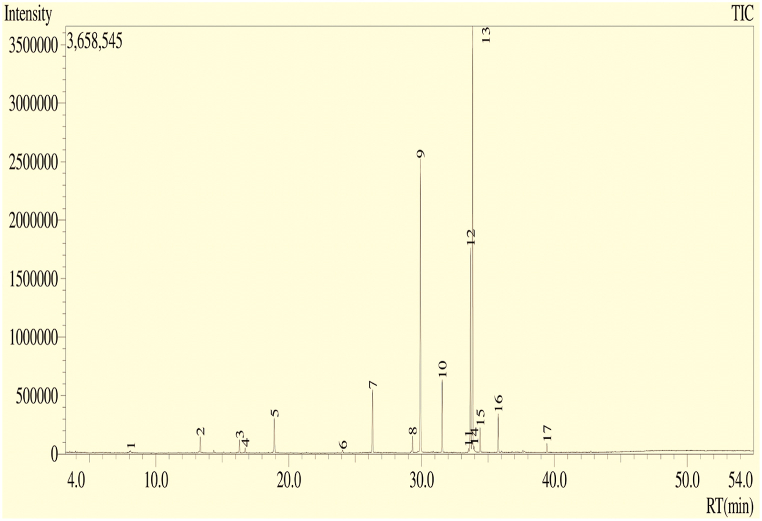
The GC-MS profile of EAF (shown in Table 3) was found to contain 16 different compounds (Fig. 6). The retention times revealed the nature and structures of the compounds. The compounds were of different chemical classes. Hexadecanoic acid; methyl ester (42.5 %); 9-octadecenoic acid (Z)-; methyl ester (22.08 %); 9,12-octadecadienoic acid; methyl ester (11.74 %); E-15-heptadecenal (4.7 %); 1-heneicosanol (4.28 %); E-14-hexadecenal (3.22 %); methyl stearate (2.56 %); and phenol, 2,4-bis(1,1-dimethylethyl)- (2.21 %), were the major constituents. Additionally, 9-hexadecenoic acid, methyl ester; (Z)-; dodecanoic acid, methyl ester; nonacos-1-ene; tridecanoic acid, 12-methyl-, methyl ester; 11-octadecenoic acid, methyl ester; cyclopropane, nonyl-; and n-heptadecanol-1 were also present in the plant.
Table 3.
GC‒MS profiling of EAF of L. aequata leaves.
| Name | R. Time | m/z | Area | Concentration (%) |
|---|---|---|---|---|
| Cyclopropane, nonyl- | 8.103 | 55 | 9221 | 0.22 % |
| Phenol, 2,4-bis(1,1-dimethylethyl)- | 16.291 | 191 | 91164 | 2.21 % |
| Dodecanoic acid, methyl ester | 16.724 | 74 | 32031 | 0.78 % |
| E−14-Hexadecenal | 18.908 | 55 | 132956 | 3.22 % |
| Tridecanoic acid, 12-methyl-, methyl ester | 24.056 | 74 | 18405 | 0.45 % |
| E−15-Heptadecenal | 26.307 | 55 | 193904 | 4.70 % |
| 9-Hexadecenoic acid, methyl ester, (Z)- | 29.318 | 55 | 38562 | 0.93 % |
| Hexadecanoic acid, methyl ester | 29.912 | 74 | 1754372 | 42.50 % |
| 1-Heneicosanol | 31.551 | 55 | 176606 | 4.28 % |
| n-Heptadecanol-1 | 33.535 | 55 | 9258 | 0.22 % |
| 9,12-Octadecadienoic acid, methyl ester | 33.681 | 67 | 484592 | 11.74 % |
| 9-Octadecenoic acid (Z)-, methyl ester | 33.843 | 55 | 911366 | 22.08 % |
| 11-Octadecenoic acid, methyl ester | 33.95 | 55 | 11021 | 0.27 % |
| Methyl stearate | 34.407 | 74 | 105541 | 2.56 % |
| 1-Heneicosanol | 35.768 | 57 | 81769 | 1.98 % |
| Nonacos-1-ene | 39.436 | 57 | 19057 | 0.46 % |
Fig. 6.
The GC‒MS profile of EAF was obtained from GC‒MS with the electron impact ionization (EI) method.
3.6. In silico molecular docking for antioxidant activity
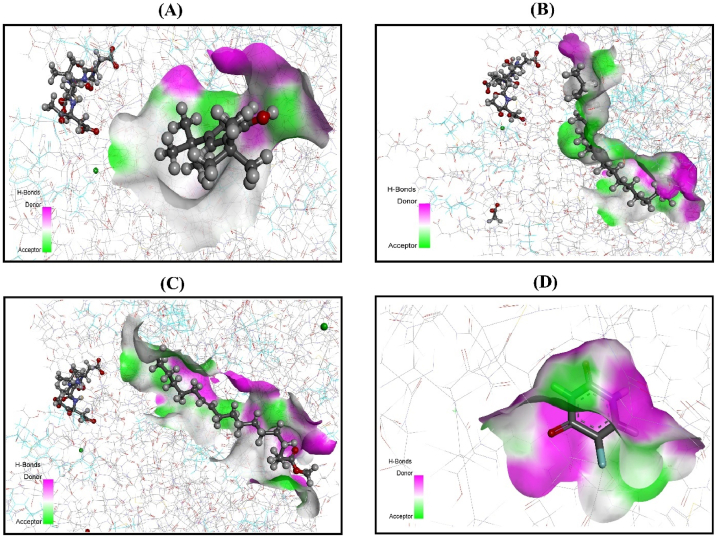
The outcomes of the molecular docking simulation for sixteen selected compounds are represented in Supplementary Table S1. To determine their antioxidant properties, the compounds were subjected to urate oxidase (PDB: 1R4U) and glutathione reductase (PDB: 3GRS). For antioxidant properties, the compounds were docked against urate oxidase (PDB: 1R4U) and glutathione reductase (PDB: 3GRS), where phenol, 2,4-bis(1,1-dimethylethyl), exhibited the highest score (−4.196 kcal/mol) and formed hydrogen bonds with LYS-171 against the urate oxidase receptor. The phenol 2,4-bis(1,1-dimethylethyl) (−5.262 kcal/mol), formed a hydrogen bond with THR-156. Additionally, E−15-heptadecenal (−0.479 kcal/mol) formed two hydrogen bonds, one with THR-57 and another with HOH-490. Furthermore, 9-octadecenoic acid (−0.856 kcal/mol) formed a hydrogen bond with LYS-66. As a result, these compounds exhibited the highest docking score against the glutathione reductase receptor. (Supplementary Fig. S1). The figures expressing the 3D conformation of ligand-receptor interactions for urate oxidase are given in Fig. 7, and the interactions for glutathione reductase are given in Fig. 8. Furthermore, hydrogen and hydrophobic bonds between each compound and protein, as associated with amino acid residues, are expressed in Supplementary Table S2 and Supplementary Table S3.
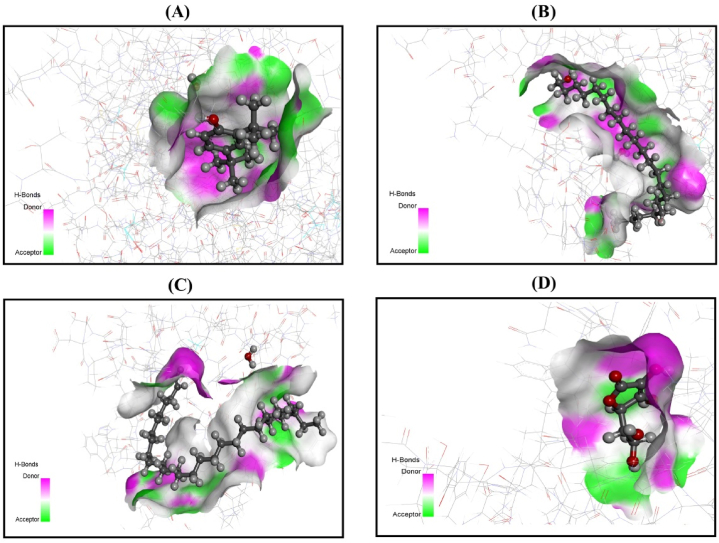
Fig. 7.
3D representation of molecular docking simulation between urate oxidase (PDB:1R4U) and (A) phenol, 2,4-bis(1,1-dimethylethyl)-; (B) 1-heneicosanol; (C) nonacos-1-ene; (D) AA.
Fig. 8.
3D representation of molecular docking simulation between glutathione reductase (PDB: 3GRS) and (A) Phenol, 2,4-bis(1,1-dimethylethyl)-; (B) 1-Heneicosanol; (C) Nonacos-1-ene; (D) AA.
3.7. In silico molecular docking for anticancer activity
The outcomes of the molecular docking simulation for sixteen selected compounds are represented in Supplementary Table S1. Two protein structures for HeLa cells and MCF-7 cells, the EGFR kinase domain (PDB: 2ITY) and caspase-3 (PDB: 5IAE), were used to assess the anticancer activity of the selected compounds. Phenol, 2,4-bis(1,1-dimethylethyl), demonstrated the highest docking scores (−5.512 kcal/mol) among the compounds and established a hydrogen bond with ASP-855 in the EGFR kinase domain. Moreover, when considering caspase-3, phenol and 2,4-bis(1,1-dimethylethyl) formed a hydrogen bond with ARG-207. Additionally, 9,12-octadecadienoic acid formed three hydrogen bonds with ASN-208, TRP-214, and PHE-250, while the methyl ester established two hydrogen bonds with PHE-250 and TRP-214. These compounds showed the highest docking scores of −5.631 kcal/mol, −0.672 kcal/mol, and −0.586 kcal/mol, respectively. (Supplementary Fig. S2). The 3D representations for the ligand-receptor complex associated with the EGFR kinase domain are given in Fig. 9, and the representations regarding caspase-3 are given in Fig. 10. Furthermore, the bonds corresponding to the interactions are given in Supplementary Table S4 and Supplementary Table S5.
Fig. 9.
3D representation of molecular docking simulation between the EGFR kinase domain (PDB: 2ITY) and (A) phenol, 2,4-bis(1,1-dimethylethyl)-; (B) 1-heneicosanol; (C) 9,12-octadecadienoic acid, methyl ester; and (D) 5-fluorouracil.
Fig. 10.
3D representation of molecular docking simulation between caspase 3 (PDB: 5IAE) and (A) Phenol, 2,4-bis(1,1-dimethylethyl)-; (B) 1-Heneicosanol; (C) 11-Octadecenoic acid, methyl ester; (D) 5-Fluorouracil.
3.8. Determination of pharmacokinetic parameters by SwissADME and toxicological property prediction by AdmetSAR
The ADME properties of the selected compounds were evaluated based on Lipinski's rule of five. The data regarding each parameter were retrieved from the SwissADME online server and are given in Table 4. Three of the compounds were determined to violate more than one parameter of the standard. This evaluation suggested that all the compounds possessed medicinal properties and favorable oral bioavailability, with the exception of hexadecanoic acid, methyl ester, 11-octadecenoic acid, methyl ester, and nonacos-1-ene. Moreover, the toxicological characteristics of each selected compound were evaluated using the AdmetSAR online server and are given in Table 5. The results indicated that all the compounds were not Ames toxic and had little or no acute toxicity, which indicates that all the compounds are safe for administration in the human body.
Table 4.
Physicochemical properties of the identified compounds from EAF for good oral bioavailability.
| Compound |
Lipinski Rules |
Lipinski's Violations |
||||
|---|---|---|---|---|---|---|
| MW |
HBA |
HBD |
Log P |
MR |
||
| ≤500 | ≤10 | ≤5 | ≤5 | 40–130 | ≤1 | |
| Cyclopropane, nonyl- | 168.32 | 0 | 0 | 4.73 | 57.68 | 0 |
| Phenol,2,4-bis(1,1-dimethylethyl)- | 206.32 | 1 | 1 | 3.09 | 67.01 | 0 |
| Dodecanoic acid, methyl ester | 214.34 | 2 | 0 | 4.10 | 65.89 | 0 |
| E−14-Hexadecenal | 238.41 | 1 | 0 | 5.13 | 78.75 | 1 |
| Tridecanoic acid, 12-methyl-, methyl ester | 242.40 | 2 | 0 | 4.75 | 75.50 | 0 |
| E−15-Heptadecenal | 252.44 | 1 | 0 | 5.50 | 83.56 | 1 |
| 9-Hexadecenoic acid, methyl ester, (Z)- | 268.43 | 2 | 0 | 5.26 | 84.64 | 1 |
| Hexadecanoic acid, methyl ester | 270.45 | 2 | 9 | 5.54 | 85.12 | 2 |
| 1-Heneicosanol | 312.57 | 1 | 1 | 7.25 | 104.22 | 1 |
| n-Heptadecanol-1 | 256.5 | 1 | 1 | 7.8 | 82.34 | 1 |
| 9,12-Octadecadienoic acid, methyl ester | 294.47 | 2 | 0 | 5.69 | 93.98 | 1 |
| 9-Octadecenoic acid (Z)-, methyl ester | 296.49 | 2 | 0 | 5.95 | 94.26 | 1 |
| 11-Octadecenoic acid, methyl ester | 296.49 | 2 | 9 | 5.95 | 94.26 | 2 |
| Methyl stearate | 298.50 | 2 | 0 | 6.24 | 94.73 | 1 |
| Nonacos-1-ene | 406.77 | 0 | 0 | 11.21 | 141.04 | 2 |
MW, molecular weight (g/mol); HBA, hydrogen bond acceptor; HBD, hydrogen bond donor; Log P, lipophilicity; MR, molar refractivity.
Table 5.
Toxicological properties of identified compounds from EAF.
| Parameters |
||||
|---|---|---|---|---|
| Compound | Ames toxicity | Carcinogens | Acute oral | Rat Acute Toxicity |
| Cyclopropane, nonyl- | NAT | NC | III | 1.5283 |
| Phenol, 2,4-bis(1,1-dimethylethyl)- | NAT | NC | III | 2.2064 |
| Dodecanoic acid, methyl ester | NAT | Carcinogens | III | 1.4915 |
| E−14-Hexadecenal | NAT | NC | III | 1.6100 |
| Tridecanoic acid, 12-methyl-, methyl ester | NAT | NC | III | 1.5702 |
| E−15-Heptadecenal | NAT | NC | III | 1.6100 |
| 9-Hexadecenoic acid, methyl ester, (Z)- | NAT | Carcinogens | III | 1.7357 |
| Hexadecanoic acid, methyl ester | NAT | Carcinogens | III | 1.4915 |
| 1-Heneicosanol | NAT | NC | III | 1.5561 |
| n-Heptadecanol-1 | NAT | NC | III | 1.5561 |
| 9,12-Octadecadienoic acid, methyl ester | NAT | Carcinogens | III | 1.7357 |
| 9-Octadecenoic acid (Z)-, methyl ester | NAT | Carcinogens | III | 1.7357 |
| 11-Octadecenoic acid, methyl ester | NAT | Carcinogens | III | 1.7357 |
| Methyl stearate | NAT | Carcinogens | III | 1.4915 |
| Nonacos-1-ene | NAT | Carcinogens | III | 1.3452 |
NAT, Non-Ames toxic; NC, Noncarcinogenic; Category-I (LD50 ≤ 50 mg/kg); Category-II (50 mg/kg < LD50 < 500 mg/kg); Category-III (500 mg/kg < LD50 < 5000 mg/kg); Category- IV (5000 mg/kg < LD50).
3.9. Online study of the prediction of activity spectra for substances
The PASS prediction study was conducted through the PASS online server. All compounds exhibited a greater Pa value than Pi value (Supplementary Table S6).
4. Discussion
Plants are the most abundant sources of natural antioxidants, including polyphenols, which can be used as anticancer, antimicrobial, antiulcer, antiarthritic, and antiangiogenic agents [9,47]. Phenolic and flavonoid compounds are potential sources of antioxidants, which may play a key role in preventing cellular damage caused by free radicals [48,49]. A balance between free radicals and antioxidants is necessary for proper physiological function [37]. An imbalance of free radicals during cellular processes leads to OS, which in turn can cause serious damage to many important cellular macromolecules, such as proteins and DNA. However, the overproduction of free radicals can be balanced by the action of endogenous antioxidants as well as natural and synthetic antioxidants [37]. Antioxidants exert their effects through several mechanisms: (i) prevention of chain initiation, which is necessary for the formation of free radicals; (ii) chelation of transition metal ion catalysts; (iii) decomposition of peroxidases; (iv) prevention of continued hydrogen abstraction; and (v) radical scavenging [50].
Several phytoconstituents found in the leaves of L. aequata have been suggested to have potential as antioxidants, free radical scavengers, and sources of cytotoxic action. One of the most striking findings of this study was that the phenolic and flavonoid components of CME and its fractions were explored in large amounts. The total antioxidant activity of different extractives was determined by measuring the reduction power of Mo (VI) to Mo (V) and the subsequent formation of a green phosphate/Mo (V) complex. In this way, we detected a discrepancy in the total antioxidant activities of leaves of L. aequata reported in the literature, which could be attributed to differences in fractions.
High consumption of foods rich in polyphenols may have a significant impact on the prevention of noncommunicable diseases (NCDs), including cancer [51]. Evidence in this area has motivated researchers to examine antioxidants for the prevention and treatment of diseases and the maintenance of human health [52,53]. According to a study, certain flavonoids and their related polyphenols greatly enhanced phosphomolybdate scavenging activity [37]. One of the most important findings of this study was that all extracts demonstrated positive antioxidant activity in a dose-dependent manner. In particular, the EAF of L. aequata leaves showed the highest antioxidant activity due to the presence of a large number of polyphenols. The literature has demonstrated that the antioxidant capacity of citrus is due to the presence of phenolics, flavonoids, and AA. Supporting this, Alara et al. reported that the phenolic compounds β-glucogallin and apocynin isolated from Vernonia cinerea leaves showed antioxidant activity, similar to our study [54].
A compound's ferric-reducing power capacity is considered another prominent indicator of anti-radical activity [55]. The mechanism of this capacity is associated with the presence of a reductant that donates a hydrogen atom following ROS breakdown. In this study, EAF showed the highest ferric-reducing power capacity through its reduction of the Fe3+/ferricyanide complex into the ferrous form, identified during monitoring through the formation of the blue-green complex at 700 nm. This result fits the findings of existing research on this topic [31]. The reducing power of the EAF was significant (p < 0.05), most likely due to the presence of abundant phenolic constituents, which could stabilize and block free radical chain reactions by donating electrons.
The most typical technique used to assess antioxidant activity is DPPH radical scavenging activity. Antioxidant activity depends on the donation of hydrogen or an electron to stabilize the purple-colored DPPH free radical into a reduced, pale yellow form of DPPH [56]. A stable diamagnetic molecule is formed when the DPPH radical accepts an electron or hydrogen radical, leading to changes in the color of the solution from blue to yellow. This color-changing technique is a widely accepted in vitro method because of its simplicity, stability, and reproducibility [57]. In this study, CME and all of its fractions showed significant DPPH radical scavenging activity (p < 0.01). Their radical scavenging capacities, with IC50 values of different extractives and standard BHT, were in the following order: EAF > CME > AQF > BHT > CHF > PEF. EAF had a significant IC50 value compared to the standard BHT (p < 0.01). In the literature, phenolic and flavonoid-rich natural antioxidants reduce DPPH radicals by donating hydrogen ions [58].
Hydroxyl radicals are highly associated with ROS, causing detrimental effects on proteins, lipids, and nucleic acids. ROS can damage cellular components by reacting with polyunsaturated fatty acids and adding a double bond to DNA bases, leading to carcinogenesis [19,58]. In the present study, CME and its extracts showed promising hydroxyl radical scavenging activity and were capable of protecting deoxyribose in a dose-dependent manner. Several studies have shown that the hydroxyl radical activity of an extract is directly proportional to its antioxidant activity [4]. In this study, we found that EAF, AQF, and CME possessed higher hydroxyl radical scavenging activity than CA (standard). Surprisingly, the scavenging activity of EAF was four times higher than that of the CA standard. EAF can therefore serve as a potent hydroxyl radical scavenger and shield cells and cellular components. Previous studies have demonstrated that extracts capable of scavenging hydroxyl radicals can potentially inhibit lipid peroxidation and break free radical chain reactions [59].
Lipid peroxides are highly reactive and more stable, causing harm to both the sites of generation and diffusion. High concentrations of lipid peroxide in the body are responsible for many life-threatening diseases, including cancer. Therefore, limiting the generation of lipid peroxide is beneficial for controlling these serious illnesses [9]. Antioxidant-rich fractions are directly linked to the prevention of lipid peroxidation and the scavenging of hydroxyl radicals. In this study, EAF was shown to significantly inhibit lipid peroxidation due to the presence of lipid-soluble antioxidant compounds, which could donate protons to stabilize lipid radicals or scavenge lipid peroxyl radicals and thus terminate subsequent chain propagation reactions. Current evidence suggests that L. aequata leaves are enriched with polyphenolics and can combat the damage associated with ROS due to their reduced power capacity and radical scavenging activity [31].
Cancer develops through cellular damage, for which ROS are mainly responsible. Since cancer cells are less prone to death [12], many naturally occurring compounds, such as alkaloids, phenolics, flavonoids, and saponins, exhibit anticancer activity by inhibiting cell proliferation and angiogenesis, causing cell cycle arrest, and inducing cell apoptosis [12,60,61]. Furthermore, ROS are responsible for modifying DNA-protein crosslinks, base and sugar lesions, strand breaks, and base-free sites [62]. Antioxidants can scavenge ROS, leading to cancer prevention. Our findings revealed that EAF exhibited more profound cytotoxic activity that was quite similar to that of the standards, VS and 5-FU. The presence of higher amounts of flavonoids, saponins, and other compounds in the EAF could influence cytotoxic activity. The fraction's dose-dependent activity showed that the presence of a high concentration of compounds resulted in a lower percentage of HeLa cell viability. Similar results were observed when EAF was used to examine its potential cytotoxicity toward MCF-7 cells. EAF significantly reduced the proliferation of MCF-7 cells. Additionally, EAF treatment caused MCF-7 cells to undergo morphological changes that indicate apoptosis, such as DNA breakage, cell shrinkage, and DNA condensation [31]. Compared to untreated MCF-7 cells, these morphological changes were visible, and they are consistent with prior observations [63]. This study also used fluorescent labeling to assess how EAF affects the apoptosis of HeLa and MCF-7 cells. We observed negligible signals in the untreated control cells. However, cells treated with EAF showed strong fluorescence densities, indicating that EAF induces apoptosis in both HeLa and MCF-7 cells. Thus, by decreasing free radicals and oxidative stress, antioxidants play a role in ameliorating DNA damage, reducing the rate of abnormal cell division, and decreasing mutagenesis. Therefore, many antioxidant-rich plants possess anticancer activity.
Since the EAF of Leea aqueata showed the highest antioxidative and cytotoxic properties among the CME fractions, EAF was subjected to GC-MS analysis to identify the compounds present and their proportions in the plant. Hexadecanoic acid methyl ester (42.5 %) and 9-octadecenoic acid (Z)-methyl ester (22.08) are the major constituents of EAF with antioxidant and anticancer properties. A recent study reported that Leea indica contained different types of phytoconstituents detected with the HPLC-ESI-microTOF-Q-MS/MS analysis [64]. Zhao et al. reported that the compound 9-octadecenoic acid (Z)-methyl ester could prevent cancer via some mechanisms. It has also been reported that unsaturated fatty acid esters possess significant antioxidant properties [65] and are deposited on the lipid layer of the cell membrane and mitochondria. Thus, this compound showed cytotoxicity by increasing the permeability of cells due to the lack of integrity in the cellular structure. Moreover, another constituent in L. aequata, phenol, 2,4-bis(1,1-dimethylethyl)-, exhibits antioxidant and antitumor activity [9]. Mostofa et al. reported that two glycosides isolated from L. aequata leaves showed promising anticancer activity [9], suggesting that phytoconstituents in L. aequata might be a significant source of antioxidants and cytotoxic agents. This finding was confirmed by the in silico molecular docking performed during the study.
Molecular docking is an integral segment in the structural molecular biology discipline and is designed to evaluate probable ligand-protein synergy through a computational approach [66]. This process further reveals the investigated compounds’ mechanisms of action and provides an efficient way to discover novel active medicinal agents as remedies for various diseases. A molecular docking simulation was used in this study to associate and reciprocate the findings with the current in vitro study. Sixteen compounds from the EAF of L. aequata leaves were selected to conduct molecular docking simulations for antioxidant and anticancer activity. Two proteins, urate oxidase (PDB: 1R4U) and glutathione reductase (PDB: 3GRS), were selected as ligand targets for antioxidant activity. When all the compounds from the GC-MS data were docked against urate oxidase and phenol, 2,4-bis(1,1-dimethylethyl) produced the highest score (−4.196). Moreover, when the compounds were in complex with glutathione reductase, the same compound was found to have the best outcome (−5.262). The interaction of phenol, 2,4-bis(1,1-dimethylethyl)- with urate oxidase formed two π-alkyl bonds with Arg-176 and His-256, as well as a conventional hydrogen bond with Lys-171. On the other hand, when interacting with glutathione reductase, this compound formed only a conventional hydrogen bond with Thr-156. The docking score for this compound produced a result very close to that of the standard drug, AA. This finding suggested that the compound could serve as a potential antioxidant agent.
Furthermore, the EGFR kinase domain (PDB: 2ITY) and caspase-3 (PDB: 5IAE) were used as receptors for the experimental compounds to determine their anticancer activity. Following the docking simulation, phenol (2,4-bis(1,1-dimethylethyl)) had the best docking score (−5.512) when interacting with the EGFR kinase domain. In the case of caspase-3, the same compound was found to have the best result (−5.631). Compared to the standard drug 5-FU, the examined compound possessed a better score than the standard for both proteins. Phenol, 2,4-bis(1,1-dimethylethyl), was determined to have formed a conventional hydrogen bond with Asp-855 and an alkyl bond with Leu-718. Furthermore, two π-alkyl bonds with Leu-844 and Val-726 were observed when the compound interacted with the active site of the EGFR kinase domain. When interacting with caspase-3 receptors, this compound formed one conventional hydrogen bond with Arg-207, a π-π stacked bond with Trp-206, and a π-alkyl bond with Tyr-204. Other compounds also showed a positive affinity for each of these four distinctive proteins for anticancer and antioxidant activity.
Each of these compounds was additionally evaluated for ADME and toxicological analysis. According to Lipinski's rule of five, orally administered agents should satisfy the following criteria: (i) molecular weight ≤500; (ii) hydrogen bond donor ≤5; (iii) hydrogen bond acceptor ≤10; (iv) logP ≤5; and (v) molar refractivity of 40–130 [58]. This rule prescribes that effective medicinal agents must fall within these parameters to have good oral bioavailability [11,67]. In this study, ADME analysis based on Lipinski's rule of five revealed that all the examined compounds satisfied the given criteria, with the exceptions of hexadecenoic acid, methyl ester, 11-octadecenoic acid, methyl ester, and nonacos-1-ene. Additionally, toxicity evaluation of the selected compounds revealed that all of them were nontoxic according to the Ames test and had negligible hazardous qualities. On the other hand, some of the compounds were discovered to be carcinogenic in the toxicological assessment. However, they are non-Ames toxic and relatively safe in terms of their LD50 value. In regard to carcinogenicity, the probabilities for the compounds extrapolated from the AdmetSAR database are as follows: dodecanoic acid, methyl ester (0.5347); 9-hexadecenoic acid, methyl ester, (Z) (0.5217); hexadecanoic acid, methyl ester (0.5347); 9,12-octadecadienoic acid, methyl ester (0.5217); 9-octadecenoic acid (Z)-, methyl ester (0.5217); 11-octadecenoic acid, methyl ester (0.5217); methyl stearate (0.5347); and nonacos-1-ene (0.6000). As the data depict, the probability of a carcinogenic property is almost half of 1, which means that the event is less likely to happen. Therefore, we can conclude that there is less of a chance that these compounds will cause cancer [68]. In addition, the total phenolic and flavonoid contents of the EAF of L. aequata leaves showed a significant and strong positive correlation (p < 0.001 and 0.05) with antioxidant (DPPH) and cell viability assays. Our results were consistent with those of Islam et al., who reported a strong relationship between total phenolic content and DPPH radical scavenging [37]. Thus, it is evident from the molecular docking analysis that the identified compounds from L. aequata have significant antioxidant and anticancer properties and represent a potential source of medicinal agents for treating cancer and other ROS-related complications.
5. Conclusion
In conclusion, the in vitro cell-free investigation in this study revealed that the EAF of L. aequata is a potential source of polyphenols, which have shown significant antioxidant and anti-ROS activities. In addition to antioxidant and anti-ROS activities in a dose-dependent manner, EAF markedly reduced both HeLa and MCF-7 cell proliferation and altered their cellular morphology, which was due to the induction of apoptosis in HeLa and MCF-7 cells during the study analysis. According to the GC-MS results, EAF contained 16 critical phytoconstituents. Of these, phenol-2,4-bis-1,1-dimethylethyl was found to be the most effective antioxidant and cytotoxic compound, which was confirmed by molecular docking in computer-aided models.
Therefore, we provide novel insights into the future development of natural ROS-inducing anticancer agents for cancer by discovering the composition of L. aequata and its pharmacological characteristics and activities. Although it has promising effects on cancer with its antioxidant and cytotoxic effects, it may not be accurate to claim that the precise mechanisms and pathways driving these effects are those that underlie EAF's anticancer properties. Further investigation, including randomized controlled trials, is therefore needed to pinpoint the phytochemicals of L. aequata that accurately reveal their mechanisms of action and efficacy in cancer. In addition, the availability of these natural products in certain regions may be limited in terms of generalizing the results of this study to all over the world and converting them to clinical applications. Nevertheless, L. aequata is thought to deserve further investigation because it contains a significant amount of antioxidant and cytotoxic compounds.
Fundings
The authors would like to extend their sincere appreciation to the Researchers Supporting Project Number (RSP2023R301), King Saud University, Riyadh, Saudi Arabia.
Institutional review Board statement
The Animal Ethical Committee, Rajshahi University (27/08/RUBCMB) and the Committee of Cell Research of Rajshahi Medical College, Bangladesh (ref. RMC/ER/2010–2013/01) approved the current research.
Data availability statement
All data related to this research are available within the manuscript.
CRediT authorship contribution statement
Md Golam Mostofa: Writing – original draft, Methodology, Investigation, Formal analysis, Data curation. A.S.M. Ali Reza: Writing – review & editing, Writing – original draft, Validation, Methodology, Investigation, Formal analysis, Data curation. Zidan Khan: Writing – review & editing, Validation. Mst Shirajum Munira: Writing – review & editing, Validation. Mst Mahfuza Khatoon: Software, Investigation, Data curation. Syed Rashel Kabir: Methodology, Investigation, Data curation. Md Golam Sadik: Writing – review & editing. Duygu Ağagündüz: Writing – review & editing. Raffaele Capasso: Funding acquisition. Mohsin Kazi: Writing – review & editing, Funding acquisition. AHM Khurshid Alam: Writing – original draft, Visualization, Supervision, Software, Resources, Project administration, Methodology, Investigation, Data curation, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors wish to thank the Department of Pharmacy, University of Rajshahi, for their kind support in the progress of the research. A preprint has previously been published [1]. The authors would like to extend their sincere appreciation to the Researchers Supporting Project Number (RSP2023R301), King Saud University, Riyadh, Saudi Arabia.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e23400.
Contributor Information
Md Golam Mostofa, Email: gm.sajol@gmail.com.
A.S.M. Ali Reza, Email: alirezaru@gmail.com.
Zidan Khan, Email: zidankhan9090@gmail.com.
Mst Shirajum Munira, Email: monira683382@gmail.com.
Mst Mahfuza Khatoon, Email: mahfuza.pharmacy@gmail.com.
Syed Rashel Kabir, Email: rashelkabir@ru.ac.bd.
Md Golam Sadik, Email: gsadik2@yahoo.com.
Duygu Ağagündüz, Email: duyguturkozu@gazi.edu.tr.
Raffaele Capasso, Email: rafcapas@unina.it.
Mohsin Kazi, Email: mkazi@ksu.edu.sa.
AHM Khurshid Alam, Email: khurshid.jaist@gmail.com.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
References
- 1.Mostofa MG., Reza ASA., Khan Z., Tsukahara T., Alam AK., Sadik MG. 2021. The Apoptosis-Inducing Antiproliferative Activity and Quantitative Phytochemical Profiling of Polyphenol-Rich Part of Leea Aequata L. Leaves. [DOI] [Google Scholar]
- 2.Moni J.N.R., Adnan M., Tareq A.M., Kabir M.I., Reza A.S.M.A., Nasrin M.S., Chowdhury K.H., Sayem S.A.J., Rahman M.A., Alam A.H.M.K., Alam S.B., Sakib M.A., Oh K.K., Cho D.H., Capasso R. Therapeutic potentials of syzygium fruticosum fruit (seed) reflected into an array of pharmacological assays and prospective receptors-mediated pathways. Life 2021. 2021;11:155. doi: 10.3390/LIFE11020155. 155. 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Islam M.S., Rashid M.M., Ahmed A.M.A., Ali Reza A.S.M., Rahman M.A., Choudhury T.R. The food ingredients of different extracts of Lasia spinosa (L.) Thwaites can turn it into a potential medicinal food. NFS Journal. 2021;25:56–69. doi: 10.1016/J.NFS.2021.11.002. [DOI] [Google Scholar]
- 4.Ali Reza A.S.M., Hossain M.S., Akhter S., Rahman M.R., Nasrin M.S., Uddin M.J., Sadik G., Khurshid Alam A.H.M. In vitro antioxidant and cholinesterase inhibitory activities of Elatostema papillosum leaves and correlation with their phytochemical profiles: a study relevant to the treatment of Alzheimer's disease. BMC Compl. Alternative Med. 2018;18 doi: 10.1186/S12906-018-2182-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nasrin S., Islam M.N., Tayab M.A., Nasrin M.S., Siddique M.A.B., Bin Emran T., Reza A.S.M.A. Chemical profiles and pharmacological insights of Anisomeles indica Kuntze: an experimental chemico-biological interaction. Biomed. Pharmacother. 2022;149 doi: 10.1016/J.BIOPHA.2022.112842. [DOI] [PubMed] [Google Scholar]
- 6.Hirko K.A. Addressing global cancer care inequities using implementation science and community-engaged research approaches. EMJ Innov Innovations. 2022;2022 doi: 10.33590/EMJINNOV/10018969. [DOI] [Google Scholar]
- 7.Siegel R.L., Miller K.D., Wagle N.S., Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73:17–48. doi: 10.3322/CAAC.21763. [DOI] [PubMed] [Google Scholar]
- 8.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. doi: 10.3322/CAAC.21442/FULL. [DOI] [PubMed] [Google Scholar]
- 9.Rahman MdM., Hossain A.S.M.S., Mostofa MdG., Khan M.A., Ali R., Mosaddik A., Sadik MdG., Alam A.H.M.K. Evaluation of anti-ROS and anticancer properties of Tabebuia pallida L. Leaves. Clinical Phytoscience. 2019;5 doi: 10.1186/s40816-019-0111-5. [DOI] [Google Scholar]
- 10.Lichota A., Gwozdzinski K. Anticancer activity of natural compounds from plant and marine environment. International Journal of Molecular Sciences 2018. 2018;19:3533. doi: 10.3390/IJMS19113533. 3533. 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reza A.S.M.A., Haque MdA., Sarker J., S Nasrin Mst, Rahman MdM., Tareq A.M., Khan Z., Rashid M., Sadik MdG., Tsukahara T., Alam A.K. Antiproliferative and antioxidant potentials of bioactive edible vegetable fraction of Achyranthes ferruginea Roxb. in cancer cell line. Food Sci. Nutr. 2021;9:3777–3805. doi: 10.1002/FSN3.2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rahman M.M., Reza A.S.M.A., Khan M.A., Sujon K.M., Sharmin R., Rashid M., Sadik M.G., Reza M.A., Tsukahara T., Capasso R., Mosaddik A., Gobe G.C., Alam A.K. Unfolding the apoptotic mechanism of antioxidant enriched-leaves of Tabebuia pallida (lindl.) miers in EAC cells and mouse model. J. Ethnopharmacol. 2021;278 doi: 10.1016/j.jep.2021.114297. [DOI] [PubMed] [Google Scholar]
- 13.Hossen M.A., Reza A.S.M.A., Ahmed A.M.A., Islam M.K., Jahan I., Hossain R., Khan M.F., Maruf M.R.A., Haque M.A., Rahman M.A. Pretreatment of Blumea lacera leaves ameliorate acute ulcer and oxidative stress in ethanol-induced Long-Evan rat: a combined experimental and chemico-biological interaction. Biomed. Pharmacother. 2021;135 doi: 10.1016/J.BIOPHA.2020.111211. [DOI] [PubMed] [Google Scholar]
- 14.Tan B.L., Norhaizan M.E., Liew W.P.P., Rahman H.S. Antioxidant and oxidative stress: a mutual interplay in age-related diseases. Front. Pharmacol. 2018;9 doi: 10.3389/FPHAR.2018.01162/BIBTEX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ahmed A.M.A., Rahman M.A., Hossen M.A., Reza A.S.M.A., Islam M.S., Rashid M.M., Rafi M.K.J., Siddiqui M.T.A., Al-Noman A., Uddin M.N. Epiphytic Acampe ochracea orchid relieves paracetamol-induced hepatotoxicity by inhibiting oxidative stress and upregulating antioxidant genes in in vivo and virtual screening. Biomed. Pharmacother. 2021;143 doi: 10.1016/J.BIOPHA.2021.112215. [DOI] [PubMed] [Google Scholar]
- 16.Rashid M.M., Rahman M.A., Islam M.S., Hossen M.A., Reza A.S.M.A., Ahmed A.M.A., Alnajeebi A.M., Babteen N.A., Khan M., Aboelenin S.M., Soliman M.M., Habib A.H., Alharbi H.F. Incredible affinity of Kattosh with PPAR-γ receptors attenuates STZ-induced pancreas and kidney lesions evidenced in chemicobiological interactions. J. Cell Mol. Med. 2022;26:3343–3363. doi: 10.1111/JCMM.17339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prasad S., Gupta S.C., Tyagi A.K. Reactive oxygen species (ROS) and cancer: role of antioxidative nutraceuticals. Cancer Lett. 2017;387:95–105. doi: 10.1016/J.CANLET.2016.03.042. [DOI] [PubMed] [Google Scholar]
- 18.Shin J., Song M.H., Oh J.W., Keum Y.S., Saini R.K. Pro-oxidant actions of carotenoids in triggering apoptosis of cancer cells: a review of emerging evidence. Antioxidants. 2020;9:1–17. doi: 10.3390/ANTIOX9060532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Uddin A.B.M.N., Hossain F., Reza A.S.M.A., Nasrin M.S., Alam A.H.M.K. Traditional uses, pharmacological activities, and phytochemical constituents of the genus Syzygium: a review. Food Sci. Nutr. 2022;10:1789–1819. doi: 10.1002/fsn3.2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chow A.Y. Cell cycle control by oncogenes and tumor suppressors: driving the transformation of normal cells into cancerous cells. Nature Education. 2010;3:7. [Google Scholar]
- 21.Jahan I., Sakib S.A., Alam N., Majumder M., Sharmin S., Reza A.S.M.A. Pharmacological insights into Chukrasia velutina bark: experimental and computer-aided approaches. Animal Model Exp Med. 2022;5:377–388. doi: 10.1002/AME2.12268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Amaral R.G., dos Santos S.A., Andrade L.N., Severino P., Carvalho A.A. Natural products as treatment against cancer: a historical and current vision. Clinics in Oncology. 2019;4:1–5. [Google Scholar]
- 23.Jackson S.J.T., Murphy L.L., Venema R.C., Singletary K.W., Young A.J. Curcumin binds tubulin, induces mitotic catastrophe, and impedes normal endothelial cell proliferation. Food Chem. Toxicol. 2013;60 doi: 10.1016/j.fct.2013.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Malik S., Cusidó R.M., Mirjalili M.H., Moyano E., Palazón J., Bonfill M. Production of the anticancer drug taxol in Taxus baccata suspension cultures: a review. Process Biochemistry. 2011;46 doi: 10.1016/j.procbio.2010.09.004. [DOI] [Google Scholar]
- 25.Son I.H., Chung I.M., Lee S.I., Yang H.D., Moon H.I. Pomiferin, histone deacetylase inhibitor isolated from the fruits of Maclura pomifera. Bioorg Med Chem Lett. 2007;17 doi: 10.1016/j.bmcl.2007.06.060. [DOI] [PubMed] [Google Scholar]
- 26.Gali-Muhtasib H., Roessner A., Schneider-Stock R. Thymoquinone: a promising anti-cancer drug from natural sources. Int. J. Biochem. Cell Biol. 2006;38:1249–1253. doi: 10.1016/J.BIOCEL.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 27.Babar Z.M., Jaswir I., Tareq A.M., Ali Reza A.S.M., Azizi W.M., Hafidz M., Ahfter F., Hasan M., Farhad S., Uddin M.M.R., Ichwan S.J.A., Ahmed Q.U., Taher M., Mawa I. In vivo anxiolytic and in vitro anti-inflammatory activities of water-soluble extract (WSE) of Nigella sativa (L.) seeds. Nat. Prod. Res. 2021;35 doi: 10.1080/14786419.2019.1667348. [DOI] [PubMed] [Google Scholar]
- 28.Babar Z.U.M., Jaswir I., Mahamad Maifiah M.H., Ismail S., Ahmad Raus R., Tareq A.M., Ahfter F., Faraque A., Reza A.S.M.A., Sayeed M.A., Hossain MdM., Uddin M., Hossain M.S., Farhad S., Azizi W.M., Arief S.J., Ahmed Q.U., Mawa I. The thrombolytic and cytotoxic effects of Nigella sativa (L.) seeds: the prophetic medicine. International Journal of Halal Research. 2020;2 doi: 10.18517/ijhr.2.2.70-77.2020. [DOI] [Google Scholar]
- 29.Tun N.L., Hu D.B., Xia M.Y., Zhang D.D., Yang J., Oo T.N., Wang Y.H., Yang X.F. Chemical constituents from ethanoic extracts of the aerial parts of Leea aequata L., a traditional folk medicine of Myanmar. Nat Prod Bioprospect. 2019;9:243–249. doi: 10.1007/s13659-019-0209-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Halder S., Saqueeb N., Qais N. Antinociceptive and anthelmintic activities of leaves of Leea aequata. Dhaka Univ. J. Pharm. Sci. 2018;17:251–255. doi: 10.3329/dujps.v17i2.39183. [DOI] [Google Scholar]
- 31.Rahim A., Mostofa M.G., Sadik M.G., Rahman M.A.A., Khalil M.I., Tsukahara T., Nakagawa-Goto K.K., Alam A.K. The anticancer activity of two glycosides from the leaves of Leea aequata L. Nat. Prod. Res. 2020;0:1–5. doi: 10.1080/14786419.2020.1798661. [DOI] [PubMed] [Google Scholar]
- 32.Khare C.P. 2004. Indian Herbal Remedies: Rational Western Therapy, Ayurvedic, and Other Traditional Usage. Botany, Khare, C.P. [Google Scholar]
- 33.Suwarso E., Ginting N., Sudarmi, Manurung V.D. In vitro anticonvulsant effect of ethyl acetate fraction of titanus leaf (Leea aequata L.) on isolated colon. Asian J. Pharmaceut. Clin. Res. 2018;11:248–251. doi: 10.22159/ajpcr.2018.v11s1.26614. [DOI] [Google Scholar]
- 34.Wong Y.H., Abdul Kadir H., Ling S.K. 2012. Bioassay-guided Isolation of Cytotoxic Cycloartane Triterpenoid Glycosides from the Traditionally Used Medicinal Plant Leea Indica, Evidence-Based Complementary and Alternative Medicine; p. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alam A.H.M.K., Hossain A.S.M.S., Khan M.A., Kabir S.R., Reza M.A., Rahman M.M., Islam M.S., Rahman M.A.A., Rashid M., Sadik M.G. The antioxidative fraction of white mulberry induces apoptosis through regulation of p53 and NFκB in EAC cells. PLoS One. 2016;11:1–18. doi: 10.1371/journal.pone.0167536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Holliday D.L., Speirs V. Choosing the right cell line for breast cancer research. Breast Cancer Res. 2011;13 doi: 10.1186/bcr2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Islam S., Nasrin S., Khan M.A., Hossain A.S.M.S., Islam F., Khandokhar P., Mollah M.N.H., Rashid M., Sadik G., Rahman M.A.A., Alam A.H.M.K. Evaluation of antioxidant and anticancer properties of the seed extracts of Syzygium fruticosum Roxb. growing in Rajshahi, Bangladesh. BMC Compl. Alternative Med. 2013;13:1–10. doi: 10.1186/1472-6882-13-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maciag J.J., Mackenzie S.H., Tucker M.B., Schipper J.L., Swartz P., Clark A.C. Tunable allosteric library of caspase-3 identifies coupling between conserved water molecules and conformational selection. Proc Natl Acad Sci U S A. 2016;113 doi: 10.1073/pnas.1603549113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Luo Z., Valeru A., Penjarla S., Liu B., Khan I. Synthesis, anticancer activity and molecular docking studies of novel pyrido[1,2-a]pyrimidin-4-one derivatives. Synth. Commun. 2019;49 doi: 10.1080/00397911.2019.1619773. [DOI] [Google Scholar]
- 40.Zhao G.F., Huang Z.A., Du X.K., Yang M.L., Huang D.D., Zhang S. Molecular docking studies of Traditional Chinese Medicinal compounds against known protein targets to treat non-small cell lung carcinomas. Mol. Med. Rep. 2016;14 doi: 10.3892/mmr.2016.5350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Berman H.M., Battistuz T., Bhat T.N., Bluhm W.F., Bourne P.E., Burkhardt K., Feng Z., Gilliland G.L., Iype L., Jain S., Fagan P., Marvin J., Padilla D., Ravichandran V., Schneider B., Thanki N., Weissig H., Westbrook J.D., Zardecki C. The protein Data Bank. Acta Crystallogr D Biol Crystallogr. 2002;58:899–907. doi: 10.1107/S0907444902003451. [DOI] [PubMed] [Google Scholar]
- 42.Uddin M.J., Reza A.S.M.A., Abdullah-Al-Mamun M., Kabir M.S.H., Nasrin M.S., Akhter S., Arman M.S.I., Rahman M.A. Antinociceptive and anxiolytic and sedative effects of methanol extract of anisomeles indica: an experimental assessment in mice and computer aided models. Front. Pharmacol. 2018;9 doi: 10.3389/fphar.2018.00246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Adnan M., Chy M.N.U., Kamal A.T.M.M., Chowdhury K.A.A., Rahman M.A., Ali Reza A.S.M., Moniruzzaman M., Rony S.R., Nasrin M.S., Azad M.O.K., Park C.H., Lim Y.S., Cho D.H. Intervention in neuropsychiatric disorders by suppressing inflammatory and oxidative stress signal and exploration of in silico studies for potential lead compounds from holigarna caustica (Dennst.) oken leaves. Biomolecules. 2020;10 doi: 10.3390/biom10040561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Harder E., Damm W., Maple J., Wu C., Reboul M., Xiang J.Y., Wang L., Lupyan D., Dahlgren M.K., Knight J.L., Kaus J.W., Cerutti D.S., Krilov G., Jorgensen W.L., Abel R., Friesner R.A. OPLS3: a force field providing broad coverage of drug-like small molecules and proteins. J Chem Theory Comput. 2016;12 doi: 10.1021/acs.jctc.5b00864. [DOI] [PubMed] [Google Scholar]
- 45.Castro A.L.G., Cruz J.N., Sodré D.F., Correa-Barbosa J., Azonsivo R., de Oliveira M.S., de Sousa Siqueira J.E., da Rocha Galucio N.C., de Oliveira Bahia M., Burbano R.M.R., do Rosário Marinho A.M., Percário S., Dolabela M.F., Vale V.V. Evaluation of the genotoxicity and mutagenicity of isoeleutherin and eleutherin isolated from Eleutherine plicata herb. using bioassays and in silico approaches. Arab. J. Chem. 2021;14 doi: 10.1016/j.arabjc.2021.103084. [DOI] [Google Scholar]
- 46.Santana de Oliveira M., Pereira da Silva V.M., Cantão Freitas L., Gomes Silva S., Nevez Cruz J., de Aguiar Andrade E.H. Extraction yield, chemical composition, preliminary toxicity of bignonia nocturna (bignoniaceae) essential oil and in silico evaluation of the interaction. Chem. Biodivers. 2021;18 doi: 10.1002/cbdv.202000982. [DOI] [PubMed] [Google Scholar]
- 47.Filimonov D.A., Lagunin A.A., Gloriozova T.A., Rudik A.V., Druzhilovskii D.S., Pogodin P.V., Poroikov V.V. Prediction of the biological activity spectra of organic compounds using the pass online web resource. Chem Heterocycl Compd (N Y) 2014;50 doi: 10.1007/s10593-014-1496-1. [DOI] [Google Scholar]
- 48.Rahman M.M., Uddin M.J., Ali Reza A.S.M., Tareq A.M., Bin Emran T., Simal‐gandara J. Ethnomedicinal value of antidiabetic plants in Bangladesh: a comprehensive review. Plants. 2021;10 doi: 10.3390/plants10040729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tareq A.M., Farhad S., Neshar Uddin A.B.M., Hoque M., Nasrin M.S., Uddin M.M.R., Hasan M., Sultana A., Munira M.S., Lyzu C., Moazzem Hossen S.M., Ali Reza A.S.M., Bin Emran T. Chemical profiles, pharmacological properties, and in silico studies provide new insights on Cycas pectinata. Heliyon. 2020;6 doi: 10.1016/j.heliyon.2020.e04061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Khan M.A., Rahman M.M., Sardar M.N., Arman M.S.I., Islam M.B., Khandakar M.J.A., Rashid M., Sadik G., Alam A.H.M.K. Comparative investigation of the free radical scavenging potential and anticancer property of Diospyros blancoi (Ebenaceae) Asian Pac. J. Trop. Biomed. 2016;6 doi: 10.1016/j.apjtb.2016.03.004. [DOI] [Google Scholar]
- 51.Zhang Y.J., Gan R.Y., Li S., Zhou Y., Li A.N., Xu D.P., Bin Li H., Kitts D.D. Antioxidant phytochemicals for the prevention and treatment of chronic diseases. Molecules. 2015;20 doi: 10.3390/molecules201219753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Reza A.S.M.A., Sakib M.A., Nasrin M.S., Khan J., Khan M.F., Hossen M.A., Ali M.H., Haque M.A. Lasia spinosa (L.) thw. attenuates chemically induced behavioral disorders in experimental and computational models. Heliyon. 2023;9 doi: 10.1016/j.heliyon.2023.e16754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tareq A.M., Hossain M.M., Uddin M., Islam F., Khan Z., Karim M.M., Lyzu C., Ağagündüz D., Reza A.S.M.A., Bin Emran T., Capasso R. Chemical profiles and pharmacological attributes of Apis cerana indica beehives using combined experimental and computer-aided studies. Heliyon. 2023;9 doi: 10.1016/j.heliyon.2023.e15016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Alara O.R., Abdurahman N.H., Ukaegbu C.I., Azhari N.H. Vernonia cinerea leaves as the source of phenolic compounds, antioxidants, and anti-diabetic activity using microwave-assisted extraction technique. Ind. Crops Prod. 2018;122:533–544. doi: 10.1016/j.indcrop.2018.06.034. [DOI] [Google Scholar]
- 55.Oliveira I., Sousa A., Ferreira I.C.F.R., Bento A., Estevinho L., Pereira J.A. Total phenols, antioxidant potential and antimicrobial activity of walnut (Juglans regia L.) green husks. Food Chem. Toxicol. 2008;46 doi: 10.1016/j.fct.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 56.Hossain M.S., Ali Reza A.S.M., Rahaman M.M., Nasrin M.S., Rahat M.R.U., Islam M.R., Uddin M.J., Rahman M.A. Evaluation of morning glory (Jacquemontia tamnifolia (L.) Griseb) leaves for antioxidant, antinociceptive, anticoagulant and cytotoxic activities. J. Basic Clin. Physiol. Pharmacol. 2018;29 doi: 10.1515/jbcpp-2017-0042. [DOI] [PubMed] [Google Scholar]
- 57.Christodoulou M.C., Orellana Palacios J.C., Hesami G., Jafarzadeh S., Lorenzo J.M., Domínguez R., Moreno A., Hadidi M. Spectrophotometric methods for measurement of antioxidant activity in food and pharmaceuticals. Antioxidants. 2022;11 doi: 10.3390/antiox11112213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Das R.R., Rahman M.A., Al-Araby S.Q., Islam M.S., Rashid M.M., Babteen N.A., Alnajeebi A.M., Alharbi H.F.H., Jeandet P., Rafi M.K.J., Siddique T.A., Uddin M.N., Zakaria Z.A. The antioxidative role of natural compounds from a green coconut mesocarp undeniably contributes to control diabetic complications as evidenced by the associated genes and biochemical indexes. Oxid. Med. Cell. Longev. 2021;2021 doi: 10.1155/2021/9711176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Khan M.A., Rahman A.A., Islam S., Khandokhar P., Parvin S., Islam M.B., Hossain M., Rashid M., Sadik G., Nasrin S., Mollah M.N.H., Alam A.H.M.K. A comparative study on the antioxidant activity of methanolic extracts from different parts of Morus alba L. (Moraceae) BMC Res. Notes. 2013;6:24. doi: 10.1186/1756-0500-6-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Haque M.A., Reza A.S.M.A., Nasrin M.S., Rahman M.A. Pleurotus highking mushrooms potentiate antiproliferative and antimigratory activity against triple-negative breast cancer cells by suppressing Akt signaling. Integr. Cancer Ther. 2020;19 doi: 10.1177/1534735420969809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ali Reza A.S.M., Nasrin M.S., Hossen M.A., Rahman M.A., Jantan I., Haque M.A., Sobarzo-Sánchez E. Mechanistic insight into immunomodulatory effects of food-functioned plant secondary metabolites. Crit. Rev. Food Sci. Nutr. 2023;63 doi: 10.1080/10408398.2021.2021138. [DOI] [PubMed] [Google Scholar]
- 62.Pizzino G., Irrera N., Cucinotta M., Pallio G., Mannino F., Arcoraci V., Squadrito F., Altavilla D., Bitto A. Oxidative stress: harms and benefits for human health. Oxid. Med. Cell. Longev. 2017 doi: 10.1155/2017/8416763. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kabir S.R., Nabi M.M., Haque A., Zaman R.U., Mahmud Z.H., Reza M.A. Pea lectin inhibits growth of Ehrlich ascites carcinoma cells by inducing apoptosis and G2/M cell cycle arrest in vivo in mice. Phytomedicine. 2013;20 doi: 10.1016/j.phymed.2013.06.010. [DOI] [PubMed] [Google Scholar]
- 64.Singh D., Siew Y.Y., Chong T.I., Yew H.C., Ho S.S.W., Lim C.S.E.S., Tan W.X., Neo S.Y., Koh H.L. Identification of Phytoconstituents in Leea indica (Burm. F.) Merr. Leaves by high performance liquid chromatography micro time-of-flight mass spectrometry. Molecules. 2019;24 doi: 10.3390/molecules24040714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Erwin E., Pusparohmana W.R., Sari I.P., Hairani R., Usman U. vol. 7. 2018. p. F1000Res. (Phytochemical and Antioxidant Activity Evaluation of the Bark of Tampoi (Baccaurea Macrocarpa) [version 1; Peer Review: 3 Approved with Reservations, 1 Not Approved]). 1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Khan S., Nazir M., Raiz N., Saleem M., Zengin G., Fazal G., Saleem H., Mukhtar M., Tousif M.I., Tareen R.B., Abdallah H.H., Mahomoodally F.M. Phytochemical profiling, in vitro biological properties and in silico studies on Caragana ambigua stocks (Fabaceae): a comprehensive approach. Ind. Crops Prod. 2019;131 doi: 10.1016/j.indcrop.2019.01.044. [DOI] [Google Scholar]
- 67.Hoque M.A., Ahmad S., Chakrabarty N., Khan M.F., Kabir M.S.H., Brishti A., Raihan M.O., Alam A.H.M.K., Haque M.A., Nasrin M.S., Haque M.A., Reza A.S.M.A. Antioxidative role of palm grass rhizome ameliorates anxiety and depression in experimental rodents and computer-aided model. Heliyon. 2021;7 doi: 10.1016/j.heliyon.2021.e08199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bhat V., Chatterjee J. vol. 49. 2021. (The Use of in Silico Tools for the Toxicity Prediction of Potential Inhibitors of SARS-CoV-2, ATLA Alternatives to Laboratory Animals). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data related to this research are available within the manuscript.