Abstract
Background
Tailored coronavirus disease 2019 (COVID-19) prevention strategies are needed for urban refugee youth in resource-constrained contexts. We developed an 8-wk interactive informational mobile health intervention focused on COVID-19 prevention practices informed by the Risk, Attitude, Norms, Ability, Self-regulation—or RANAS—approach.
Methods
We conducted a pre-post trial with a community-recruited sample of refugee youth aged 16–24 y in Kampala, Uganda. Data were collected before (T1) and immediately following (T2) the intervention, and at the 16-wk follow up (T3), to examine changes in primary (COVID-19 prevention self-efficacy) and secondary outcomes (COVID-19 risk awareness, attitudes, norms and self-regulation practices; depression; sexual and reproductive health [SRH] access; food/water security; COVID-19 vaccine acceptability).
Results
Participants (n=346; mean age: 21.2 [SD 2.6] y; cisgender women: 50.3%; cisgender men: 48.0%; transgender persons: 1.7%) were largely retained (T2: n=316, 91.3%; T3: n=302, 87.3%). In adjusted analyses, COVID-19 prevention self-efficacy, risk awareness, attitudes and vaccine acceptance increased significantly from T1 to T2, but were not sustained at T3. Between T1 and T3, COVID-19 norms and self-regulation significantly increased, while community violence, water insecurity and community SRH access decreased.
Conclusions
Digital approaches for behaviour change hold promise with urban refugee youth but may need booster messaging and complementary programming for sustained effects.
Keywords: COVID-19, global health, humanitarian health, intervention, mHealth, sanitation, water insecurity
Introduction
Despite the largest number of forcibly displaced persons in history, surpassing 89 million at the end of 2021,1 there remain research gaps regarding tailored approaches to coronavirus disease 2019 (COVID-19) prevention programming with this population. These gaps are particularly notable in resource-constrained settings, where the majority of refugees live and may experience contexts of poverty, overcrowding and limited access to water, sanitation and hygiene (WASH), which elevate risks for COVID-19 and other infectious diseases.2 Urban refugee youth are underserved by COVID-19 prevention strategies in contexts such as Uganda,3 which hosts >1.5 million refugees, of whom >100 000 live in Kampala.4 Experiences, priorities and challenges of refugee adolescents and youth accessing WASH and WASH-related interventions are underrepresented in research, and hand and respiratory hygiene interventions at large.5–7 This raises concerns for understanding how we can tailor COVID-19 prevention in humanitarian settings for young people, and also how we can adapt prevention strategies for youth for other infectious diseases.
Reflecting on the future of WASH in the context of COVID-19, Stauber et al.8 (p.1017) describe: ‘We find ourselves at an inflection point in global WASH with an opportunity to build new approaches with potentially more equitable, cost-effective, and scalable solutions. Mobile health (mHealth) technology is an important and innovative tool for WASH advances.’ Such mHealth opportunities include interactive SMS messaging, geotagging and mapping, WhatsApp groups and user/provider feedback.8,9 In South Africa, for instance, WhatsApp was used to provide COVID-19 information and reply to concerns among the general public, and cellphone networks were used for contact tracing.9 Mobile applications were also used for contact tracing by community health workers in Ugandan rural regions10 and in a humanitarian setting in Bangladesh.11
mHealth is cost-effective and congruent with the ways in which youth learn and socialise, and helpful in times of physical distancing. As most urban refugee youth in Kampala have access to mobile phones,12 there is the opportunity to extend the potential benefits of mHealth for WASH and infection control to this group. To address this knowledge gap, we conducted an interactive informational mHealth pre-test/post-test intervention that aimed to increase COVID-19 prevention with urban refugee youth in Kampala, Uganda.
Materials and Methods
Study design and setting
During April–October 2021, we conducted a single-group pre-test/post-test intervention study (Kukaa Salama), nested within a cluster randomised HIV self-testing trial (i.e. Tushirikiane trial)14 among refugee youth living in informal settlements in Kampala, Uganda. A control group design during this lockdown period in Kampala was intentionally not used due to ethical concerns over the potential withholding of any health benefits from this highly vulnerable group during a pandemic.15 Data were collected at three time points: baseline enrolment before the intervention [T1], after the completion of the 8-wk intervention [T2] and at a 16-wk follow-up [T3]. Full details of the Kukaa Salama trial protocol have been published,16 and the trial is registered at ClinicalTrials.gov (NCT04631367).
Participants and recruitment
All participants enrolled in the Tushirikiane trial14 were eligible for enrolment into the Kukaa Salama substudy. Tushirikiane participants were recruited using purposive sampling methods with the support of peer navigators (PNs), who are community-respected self-identified refugees with experience of working as health or peer educators.14 Inclusion criteria for participants into Kukaa Salama were being a Tushirikiane participant, age 16–24 y at the time of Tushirikiane enrolment, living in one of five informal settlements in Kampala (Kabalagala, Kansanga, Katwe, Nsambya, Rubaga), identifying as a refugee or displaced person, speaking one of the study languages (English, French, Swahili, Luganda, Kinyarwanda, Kirundi) and owning or having access to a mobile phone for the duration of the study. Participants were free to withdraw from the Kukaa Salama substudy while remaining in the Tushirikiane trial; however, participants withdrawing from Tushirikiane were automatically withdrawn from Kukaa Salama.
Kukaa Salama intervention description
In this single group pre-test/post-test intervention, all participants received the 8-wk mHealth intervention of COVID-19 prevention messaging. The intervention is detailed elsewhere.16 In brief, the intervention included three complementary mHealth components using a web-based SMS platform hosted by WelTel17,18 as well as moderated group interactions and photo sharing using WhatsApp. The first mHealth component was a weekly check-in message with follow-ups by PNs. The second component was a weekly COVID-19 informational SMS and an accompanying engagement question. The weekly messages were developed based on a formative qualitative research phase involving in-depth interviews with youth and were aligned with the Risk, Attitude, Norms, Ability, Self-regulation (RANAS) framework19 for behaviour change techniques. The weekly messages (Supplementary Table 1) covered the following topics: mental health, vaccine hesitancy, handwashing, mask wearing, economic stressors, symptoms and testing, stigma and recovery, recap and community support.16 All weekly messages were translated, and sent by SMS in the participant's preferred language, as indicated in the T1 pre-intervention survey. To incentivise engagement, participant responses were collected, reviewed and synthesized by the PNs and the most common responses were shared with participants at the end of each week. The third component was a small-group WhatsApp chat facilitated by PNs. Within each group, participants were prompted to share multimedia images (e.g. photos, memes, GIFs) related to the weekly topic. At each survey time point, participants were offered a small honorarium (∼$5 CAD) and a COVID-19 prevention parcel including a face mask, soap, hand sanitiser and a small food parcel (the total parcel was worth approximately $10 CAD). These honorarium amounts were decided as contextually appropriate through community consultation with the community-based implementing partner.
Data collection and outcome measures
Data were collected using standardised questionnaires administered by trained research assistants in person or by telephone at each time point. Interviews were conducted in all study languages and data were recorded using a tablet-based survey application (SurveyCTO, Doblity, Cambridge, MA, USA). Sociodemographic data were collected at T1, and linked to baseline data collected from the Tushirikiane trial. Data on primary outcomes (ability to practise COVID-19 prevention) and secondary outcomes (COVID-19 risk awareness; attitudes towards COVID-19; perceived COVID-19 norms; COVID-19 self-regulation practices; depression; sexual and reproductive health practices; and food and water insecurity) were collected at each time point. At T3, we also collected information on participants’ experiences and satisfaction with mHealth intervention components.
Primary outcome: ability to practise COVID-19 prevention
Ability to practise COVID-19 prevention was measured as self-reported self-efficacy to practise hand hygiene, respiratory hygiene and physical distancing. Specifically, we used five self-efficacy questions covering ability, confidence and adherence from the RANAS framework13,19–21 applied to COVID-19 (present study Cronbach's α=0.83). At each time point, participants’ scores were calculated by taking the mean of the five question items; scores ranged from 1 to 4, with higher scores indicating higher self-efficacy.
Secondary outcomes
Questions from the RANAS framework were also adapted for the following secondary outcomes: COVID-19 risk awareness, attitudes towards COVID-19, perceived COVID-19 norms and COVID-19 self-regulation practices.13,19–21 We collected two risk awareness outcomes: (i) personal perceived risk, which was assessed using the question ‘How likely do you think you are to catch COVID-19?’, with responses coded using a four-point Likert scale (not at all likely to very likely); and (ii) risk awareness, which assessed participants’ knowledge of COVID-19 symptoms, severity and routes of transmission. We measured attitudes towards COVID-19 at the community level through six questions covering perceived community attitudes towards transmission, infection and prevention practices (present study Cronbach's α=0.64), and at the individual level through three questions covering personal feelings towards COVID-19 prevention practices (present study Cronbach's α=0.65). COVID-19 norms were measured through nine questions assessing participants’ perceived behaviours approved by others (i.e. social pressures) towards COVID-19 prevention practices, transmission and stigma (modified scale, eight of nine original questions; present study Cronbach's α=0.61). COVID-19 self-regulation was measured through three questions assessing participants’ action plans for implementing COVID-19 prevention practices (present study Cronbach's α=0.83). At each time point participants’ scores were calculated by taking the mean of the question items for each outcome; scores ranged from 1 to 4, with higher scores indicating a better outcome.
The patient health questionnaire nine-item (PHQ-9) scale was used to measure depression symptoms22 (present study Cronbach's α=0.89). We collected three outcomes related to sexual and reproductive health (SRH) experiences and practices: personal experience with intimate partner violence (IPV), perceived changes in community violence and perceived changes in community access to SRH services. Experiences of physical/sexual IPV were assessed using an adapted short form of the Conflict Tactics Scale.23 Participants were categorised as yes if they reported experiencing physical and/or sexual IPV in the last 3 months, otherwise they were categorised as no. We assessed increases in community violence on a scale of 0 to 3, with participants reporting yes or no to if they perceived an increase in community violence against each of women, men and children since lockdown. Access to SRH services was measured on a scale of 0 to 2, with participants reporting yes/no to if they perceived reduced community access to SRH services since lockdown. Food insecurity was assessed using a single item question about how often participants went to sleep hungry because they did not have enough food to eat (categorised: ever/never).24 Water insecurity was assessed using a single item question asking if participants did not have enough water when needed for handwashing/bathing in the previous 2 wk (categorised: yes/no).
Post-hoc analysis: vaccine acceptance
Given the rapid development and distribution of COVID-19 vaccines from study development to rollout, we collected data on COVID-19 vaccine acceptance and conducted a post-hoc analysis. This was assessed using a single item four-point Likert scale question (‘not at all likely’ to ‘very likely’) on COVID-19 vaccine acceptance with demonstrated effectiveness and availability.25
mHealth satisfaction: user experience and lifestyle consequence
At T3, we measured participants’ experiences and satisfaction with mHealth intervention components (i.e. SMS informational messages, WhatsApp multimedia group chat) using two subscales of the mHealth satisfaction questionnaire: (i) the six-item usability experience subscale (present study Cronbach's α=0.77); and (ii) the four-item lifestyle consequence subscale (present study Cronbach's α=0.78).26 Scores were calculated by taking the mean of items for each subscale; scores ranged from 1 to 3, with higher scores indicating higher usability/better lifestyle consequences.
Power and sample size
A sample size of 52 participants (104 datapoints) was required to detect a medium effect size of 0.4 between pairs with a power of 80% and type 1 error rate of 5%, and assuming a correlation between pre-test/post-test responses of 0.5. Based on participant retention rates, we anticipated that at least 85% (n=340) of the Tushirikiane cohort (n=404) would participate in Kukaa Salama. This gave us sufficient power for conducting this analysis, as well as for covariate adjustment.
Statistical analyses
We used descriptive statistics to characterise the study population at baseline, stratified by gender. Number and proportion are reported for each categorical factor and mean and SD are reported for each continuous factor. Differences in sociodemographic factors between participants retained and those lost to follow-up were examined using χ2 or Fisher's exact tests for categorical variables and t tests for continuous variables. Among those still in the study at T3, we describe participants’ self-reported use of, and satisfaction with, the SMS and WhatsApp components of the intervention.
To measure changes in outcomes over time, we used generalised estimating equation (GEE) models with robust standard errors accounting for within-subject correlation using an unstructured correlation matrix.27 Logistic GEE models were used for categorical outcomes and linear GEE models were used for continuous outcomes with the time as the primary exposure. Each model was first conducted without adjustment, followed by adjustment for a priori determined variables of gender, age and settlement. To assess the moderating effect of engagement with the mHealth intervention, we examined interactions between time and intervention engagement on pre-/post-score changes of the primary outcome (COVID-19 prevention practices ability). Participants’ mHealth engagement was divided into terciles (low, medium, high) based on the number of weeks they responded to the weekly SMS and participated in WhatsApp groups.
Intervention effects are expressed as crude and adjusted ORs (aORs) or 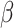 coefficients (a
coefficients (a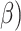 , along with 95% CIs. All regression analyses were performed using intention to treat. For scale outcomes with missing item data, we used participant mean imputation by assigning the mean of the answered items to the missing items. Participant mean imputation has been shown to be valid and produce unbiased results when implemented for missing scale items.24 All analyses were two-sided with a significance level of p≤0.05, and were conducted in Stata 16.1 (StataCorp, College Station, TX, USA).
, along with 95% CIs. All regression analyses were performed using intention to treat. For scale outcomes with missing item data, we used participant mean imputation by assigning the mean of the answered items to the missing items. Participant mean imputation has been shown to be valid and produce unbiased results when implemented for missing scale items.24 All analyses were two-sided with a significance level of p≤0.05, and were conducted in Stata 16.1 (StataCorp, College Station, TX, USA).
Results
Participant characteristics
A total of 346 refugee youths (mean age: 21.2 [SD: 2.6] y) were enrolled into Kukaa Salama (Figure 1). About one-half identified as cisgender women (n=174, 50.3%) and just under one-half identified as cisgender men (n=166, 48.0%), with a small portion identifying as transgender (n=6, 1.7%) (Table 1). Baseline characteristics were similar between men and women, but differed among those identifying as transgender. Participant retention dropped from T1 to T2 (n=316, 91.3%) and T3 (n=302, 87.3%), with participants reporting leaving the study sites and/or becoming unavailable. Overall sociodemographic characteristics of participants retained compared with those lost to follow-up were similar, but those living in Uganda for >10 y were more likely to be lost at T2 and those living in Rubaga settlement were more likely to be lost at T3 (Supplementary Table 2; Supplementary Table 3).
Figure 1.
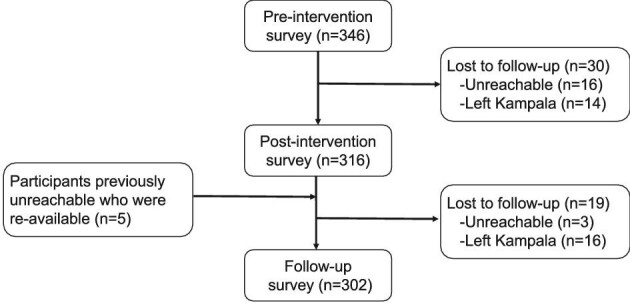
Flowchart of participation of refugee youth enrolled in Kukaa Salama, a mobile health intervention for increasing COVID-19 prevention practices in Kampala, Uganda, 2021.
Table 1.
Baseline sociodemographic characteristics of participants enrolled in Kukaa Salama, Kampala, Uganda, 2021 (n=346)
| Total | Men | Women | Transgender | |
|---|---|---|---|---|
| N=346 | N=166 | N=174 | N=6 | |
| Age, y, mean (SD) | 21.2 (2.6) | 21.7 (2.6) | 20.6 (2.5) | 24.8 (0.4) |
| Place of birth, n (%) | ||||
| Democratic Republic of Congo | 229 (66.2) | 100 (60.2) | 129 (74.1) | 0 (0.0) |
| Burundi | 59 (17.0) | 30 (18.1) | 24 (13.8) | 5 (83.3) |
| Sudan/South Sudan | 12 (3.5) | 10 (6.0) | 2 (1.2) | 0 (0.0) |
| Others1 | 46 (13.3) | 26 (15.7) | 19 (10.9) | 1 (16.7) |
| Length of time in Uganda, n (%) | ||||
| 1–5 y | 132 (38.2) | 64 (38.6) | 68 (39.1) | 0 (0.0) |
| 6–10 y | 125 (36.1) | 61 (36.8) | 62 (35.6) | 2 (33.3) |
| >10 y | 89 (25.7) | 41 (24.7) | 44 (25.3) | 4 (66.7) |
| Employment status, n (%) | ||||
| No employment | 179 (51.7) | 83 (50.0) | 91 (52.3) | 5 (83.3) |
| Student | 78 (22.5) | 33 (19.9) | 45 (25.9) | 0 (0.0) |
| Employed (paid/unpaid) | 89 (25.7) | 50 (30.1) | 38 (21.8) | 1 (16.7) |
| Highest level of education, n (%) | ||||
| Less than secondary | 106 (30.6) | 35 (21.1) | 71 (40.8) | 0 (0.0) |
| Some secondary | 211 (61.0) | 115 (69.3) | 91 (52.3) | 5 (83.3) |
| Secondary + | 29 (8.4) | 16 (9.6) | 12 (6.9) | 1 (16.7) |
| Main water source, n (%) | ||||
| Piped | 186 (53.8) | 90 (54.2) | 92 (52.9) | 4 (66.7) |
| Public tap | 130 (37.6) | 66 (39.8) | 64 (36.8) | 0 (0.0) |
| Well/spring | 24 (6.9) | 8 (4.8) | 16 (9.2) | 0 (0.0) |
| Tanker truck | 6 (1.7) | 2 (1.2) | 2 (1.2) | 2 (33.3) |
| Settlement, n (%) | ||||
| Kabalagala/Kansanga | 98 (28.3) | 50 (30.1) | 48 (27.6) | 0 (0.0) |
| Katwe/Nsambye | 103 (29.8) | 45 (27.1) | 58 (33.3) | 0 (0.0) |
| Rubaga | 145 (41.9) | 71 (42.8) | 68 (39.1) | 6 (100.0) |
1Others includes Uganda, Kenya, Rwanda and Somalia.
Primary outcome: COVID-19 prevention self-efficacy
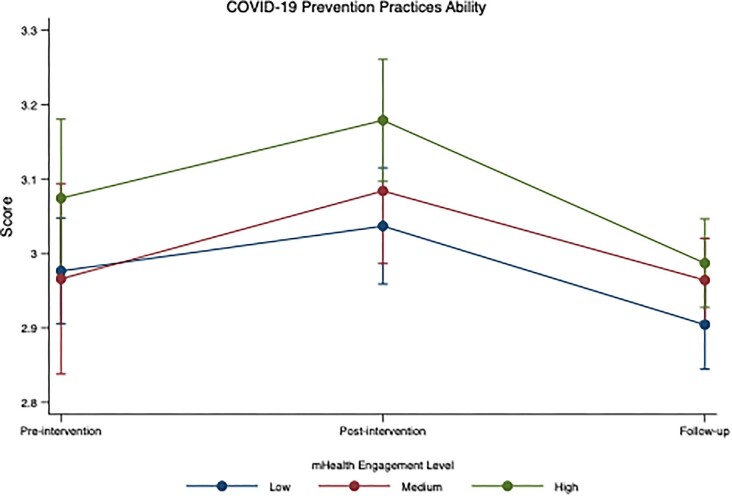
At baseline, participants reported a moderately high level of COVID-19 prevention self-efficacy, referring to the ability to practise COVID-19 prevention (mean: 3.0, SD: 0.5). In adjusted analyses, this increased significantly from T1 to T2 (aβ=0.09; 95% CI 0.03 to 0.15; p=0.003), but reduced at T3 (aβ=-0.06; 95% CI -0.12 to 0.00; p=0.037) (Table 2). Participants with higher mHealth engagement levels reported higher scores for ability to practise COVID-19 prevention at all three time points (Figure 2). Although participants with high and medium engagement levels reported greater increases between T1 and T2, there was no significant difference in scores over time by different engagement levels (e.g. medium, high) (p-value for interaction=0.623) (Figure 2, Supplementary Table 4).
Table 2.
Distribution of COVID-19 prevention outcomes across time points and effectiveness of mHealth intervention among Kukaa Salama participants, Kampala, Uganda, 2021
| Unadjusted model1 | Adjusted model2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Outcome | Time period | N | Mean (SD) | β | 95% CI | p | aβ | 95% CI | p |
| COVID-19 prevention self-efficacy | |||||||||
| Pre-intervention | 346 | 3.0 (0.5) | ref | ref | |||||
| Post-intervention | 316 | 3.1 (0.4) | 0.09 | 0.03, 0.15 | 0.003 | 0.09 | 0.03, 0.15 | 0.003 | |
| Follow-up | 302 | 2.9 (0.3) | −0.06 | −0.12, −0.01 | 0.030 | −0.06 | −0.12, 0.00 | 0.037 | |
| COVID-19 personal perceived risk | |||||||||
| Pre-intervention | 344 | 1.8 (0.9) | ref | ref | |||||
| Post-intervention | 314 | 1.8 (0.9) | 0.01 | −0.10, 0.12 | 0.836 | 0.01 | −0.10, 0.12 | 0.852 | |
| Follow-up | 302 | 1.8 (0.8) | 0.01 | −0.10, 0.12 | 0.913 | 0.00 | −0.11, 0.11 | 0.985 | |
| COVID-19 risk awareness | |||||||||
| Pre-intervention | 346 | 3.4 (0.9) | ref | ref | |||||
| Post-intervention | 316 | 3.5 (0.7) | 0.10 | 0.02, 0.17 | 0.010 | 0.10 | 0.03, 0.18 | 0.008 | |
| Follow-up | 302 | 3.5 (0.7) | 0.06 | −0.02, 0.14 | 0.166 | 0.07 | −0.02, 0.15 | 0.125 | |
| COVID-19 community attitudes | |||||||||
| Pre-intervention | 346 | 2.5 (0.5) | ref | ref | |||||
| Post-intervention | 315 | 2.5 (0.5) | −0.01 | −0.06, 0.05 | 0.773 | −0.01 | −0.06, 0.05 | 0.809 | |
| Follow-up | 302 | 2.6 (0.4) | 0.03 | −0.02, 0.08 | 0.248 | 0.03 | −0.02, 0.09 | 0.221 | |
| COVID-19 personal attitude | |||||||||
| Pre-intervention | 346 | 3.1 (0.5) | ref | ref | |||||
| Post-intervention | 315 | 3.2 (0.3) | 0.12 | 0.06, 0.18 | <0.001 | 0.12 | 0.06, 0.18 | <0.001 | |
| Follow-up | 302 | 3.1 (0.3) | 0.01 | −0.05, 0.06 | 0.864 | 0.00 | −0.05, 0.06 | 0.883 | |
| COVID-19 norms | |||||||||
| Pre-intervention | 346 | 2.4 (0.4) | ref | ref | |||||
| Post-intervention | 316 | 2.6 (0.2) | 0.19 | 0.14, 0.23 | <0.001 | 0.19 | 0.14, 0.23 | <0.001 | |
| Follow-up | 302 | 2.5 (0.2) | 0.14 | 0.10, 0.19 | <0.001 | 0.14 | 0.09, 0.19 | <0.001 | |
| COVID-19 self-regulation | |||||||||
| Pre-intervention | 346 | 3.2 (0.5) | ref | ref | |||||
| Post-intervention | 316 | 3.4 (0.5) | 0.21 | 0.15, 0.27 | <0.001 | 0.21 | 0.15, 0.27 | <0.001 | |
| Follow-up | 302 | 3.2 (0.5) | 0.07 | 0.01, 0.13 | 0.018 | 0.07 | 0.01, 0.13 | 0.021 | |
| Depression | |||||||||
| Pre-intervention | 346 | 5.9 (5.8) | ref | ref | |||||
| Post-intervention | 316 | 6.4 (5.2) | 0.53 | −0.03, 1.09 | 0.064 | 0.53 | −0.02, 1.09 | 0.061 | |
| Follow-up | 302 | 6.2 (5.0) | 0.15 | −0.45, 0.76 | 0.617 | 0.20 | −0.40, 0.81 | 0.510 | |
| Increased community violence | |||||||||
| Pre-intervention | 346 | 1.1 (1.1) | ref | ref | |||||
| Post-intervention | 315 | 1.0 (1.1) | −0.12 | −0.26, 0.02 | 0.085 | −0.12 | −0.26, 0.01 | 0.076 | |
| Follow-up | 302 | 0.9 (1.1) | −0.25 | −0.39, −0.10 | 0.001 | −0.26 | −0.40, −0.11 | 0.001 | |
| Reduced community SRH access | |||||||||
| Pre-intervention | 346 | 0.6 (0.9) | ref | ref | |||||
| Post-intervention | 315 | 1.0 (0.9) | 0.37 | 0.25, 0.49 | <0.001 | 0.37 | 0.25, 0.49 | <0.001 | |
| Follow-up | 302 | 0.8 (0.9) | 0.17 | 0.03, 0.30 | 0.015 | 0.15 | 0.02, 0.29 | 0.022 | |
| N | n (%) | OR | 95% CI | p | aOR | 95% CI | p | ||
| Experiencing IPV | |||||||||
| Pre-intervention | 346 | 21 (6.1) | ref | ref | |||||
| Post-intervention | 315 | 29 (9.2) | 1.57 | 0.97, 2.53 | 0.068 | 1.58 | 0.96, 2.60 | 0.071 | |
| Follow-up | 302 | 16 (5.3) | 0.85 | 0.49, 1.49 | 0.579 | 0.84 | 0.47, 1.50 | 0.557 | |
| Food insecurity | |||||||||
| Pre-intervention | 346 | 226 (65.3) | ref | ref | |||||
| Post-intervention | 316 | 213 (67.4) | 1.09 | 0.87, 1.37 | 0.461 | 1.09 | 0.85, 1.40 | 0.509 | |
| Follow-up | 302 | 214 (70.9) | 1.24 | 0.97, 1.58 | 0.090 | 1.25 | 0.96, 1.63 | 0.103 | |
| Water insecurity | |||||||||
| Pre-intervention | 345 | 168 (48.7) | ref | ref | |||||
| Post-intervention | 316 | 130 (41.1) | 0.74 | 0.58, 0.94 | 0.015 | 0.71 | 0.54, 0.92 | 0.011 | |
| Follow-up | 302 | 129 (42.7) | 0.75 | 0.60, 0.95 | 0.018 | 0.72 | 0.56, 0.93 | 0.012 | |
Abbreviations: aOR, adjusted OR; IPV, intimate partner violence; SRH, sexual and reproductive health.
1Unadjusted intervention effect calculated using generalised estimating equation logistic or linear regression model with an unstructured correlation matrix.
2Adjusted intervention effect calculated using generalised estimating equation logistic or linear regression model with an unstructured correlation matrix, controlling for prespecified covariates (gender, age, settlement).
Bolded p-values reflect statistically significant findings of p<0.05.
Figure 2.
Adjusted effectiveness of mHealth intervention on COVID-19 prevention self-efficacy by mHealth engagement among Kukaa Salama participants, Kampala, Uganda, 2021.
Secondary outcomes
There were modest changes in secondary outcomes across time (Table 2). Participants reported low personal perceived risk of COVID-19 at baseline (mean: 1.8, SD: 0.9), with no significant changes over time. COVID-19 risk awareness was high at baseline (mean: 3.4, SD: 0.9) and significantly increased directly after the intervention (aβ=0.10; 95% CI 0.03 to 0.18; p=0.008), but was not retained during follow-up (p=0.125). There were no significant changes in attitudes towards COVID-19 prevention at the community level; however, there was a significant increase in positive attitudes towards COVID-19 prevention at the personal level at T2 (aβ=0.12; 95% CI 0.06 to 0.18; p<0.001), but this was not sustained at T3 (p=0.883). Norms towards COVID-19 significantly increased between T1 and T2 (aβ=0.19; 95% CI 0.14 to 0.23; p<0.001) and were sustained at T3 (aβ=0.14; 95% CI 0.09 to 0.19; p<0.001), indicating a more positive perception of behaviours approved by others (i.e. social pressures) towards COVID-19 prevention practices. At baseline, participants reported high COVID-19 self-regulation (mean: 3.2, SD: 0.5), and this significantly increased at T2 (aβ=0.21; 95% CI 0.15 to 0.27; p<0.001). Self-regulation attenuated at T3, but was still significantly higher than at baseline (aβ=0.07; 95% CI 0.01 to 0.13; p=0.021), indicating more positive action plans for implementing COVID-19 prevention practices.
There were no significant changes in depression over time (Table 2). Participants reported lower levels of community violence over time, and this was significantly lower at T3 (aβ=-0.26; 95% CI -0.40 to -0.11; p=0.001); however, there were no significant differences in participants’ self-reported experiences with IPV. Participants reported greater reductions in community SRH access at both T2 (aβ=0.37; 95% CI 0.25 to 0.49; p<0.001) and at T3 (aβ=0.15; 95% CI 0.02 to 0.29; p=0.022). At baseline, food insecurity was high (65.3%), and while there were no significant changes, this remained high over time. Water insecurity was also high at baseline (48.7%); however, there were significant decreases at both T2 (aOR=0.71; 95% CI 0.54 to 0.92; p=0.011) and at T3 (aOR=0.72; 95% CI 0.56 to 0.93; p=0.012), indicating that participants reported becoming more water secure.
COVID-19 vaccine acceptance
At baseline, participants reported low levels of acceptance towards a COVID-19 vaccine (mean: 2.1, SD: 1.1). In adjusted analyses, COVID-19 vaccine acceptance increased significantly after the intervention at T2 (aβ=0.15; 95% CI 0.02 to 0.29; p=0.022), but attenuated at T3 (aβ=0.13; 95% CI -0.01 to 0.27; p=0.065) (Supplementary Table 5).
mHealth satisfaction
At the 16-wk follow-up, most participants reported using the WelTel SMS informational messages (n=256, 84.8%), with those using the service reporting a positive user experience (mean: 2.7, SD, 0.4) and positive lifestyle consequences (mean: 2.8, SD: 0.4) (Table 3). A lower proportion of participants reported using the small-group WhatsApp multimedia chats (n=203, 67.4%); however, those using the service also reported positive lifestyle consequences (mean: 2.8, SD: 0.3).
Table 3.
Self-reported use and satisfaction with mHealth intervention among Kukaa Salama participants in follow-up survey, Kampala, Uganda, 2021
| Total | Use of service N (%) |
Scale score Mean (SD) |
|
|---|---|---|---|
| WelTel SMS Informational Messages | 302 | 256 (84.8) | |
| Usability Experience Scale | 2.7 (0.4) | ||
| Lifestyle Consequence Scale | 2.8 (0.4) | ||
| WhatsApp Multimedia Groups | 301 | 203 (67.4) | |
| Lifestyle Consequence Scale | 2.8 (0.3) |
Discussion
Our findings reveal significant increases in several dimensions central to COVID-19 prevention practice uptake (self-efficacy; risk awareness; personal attitudes; vaccine acceptability) after the 8-wk Kukaa Salama intervention; however, these changes were not maintained at the 16-wk follow up. There were sustained effects reported for increased positive COVID-19 norms and COVID-19 self-regulation. Taken together with the findings that most participants engaged with the mHealth modalities, mHealth satisfaction was high, and those with higher mHealth engagement levels reported higher COVID-19 prevention scores across time, it appears that mHealth approaches to COVID-19 behaviour change are feasible and hold promise for health promotion with urban refugee youth in Kampala. These approaches appear to have improved COVID-19 prevention practices initially yet the effects were not sustained, signalling the need for future research to explore the role of information boosters, follow-up reminders and/or complementary programming to bolster initial positive behaviour changes.
These findings corroborate research in other contexts,8,9 including findings from a systematic review of digital interventions for household and community infection prevention.28 While we did not identify mHealth interventions conducted with urban refugee youth, a study with university students in Germany informed by the Health Action Process framework focused on behaviour change techniques for handwashing and found sustained increases at the 86-d follow-up.29 Taken together with the dearth of hand and respiratory hygiene studies in general with adolescents/youth in humanitarian settings,5–7 findings highlight the need for longer-term randomised controlled trials (RCTs) that explore various ways of supplementing, supporting and scaffolding digital strategies with this population.
Our findings that participants reported reduced SRH access maps onto global scoping review findings that document reduced access to, and uptake of, SRH services, particularly among marginalised populations.30 Participants reported reduced perceived community-level violence that could be linked to Kampala's long lockdowns, which restricted movement. While water insecurity reduced over time, a significant proportion of participants (>40%) consistently had insufficient water to engage in COVID-19 prevention practices such as handwashing. However, barriers to accessing water during lockdowns may have been addressed over time as communities adapted to find alternative sources.31 Persistent food insecurity remained high across time points, requiring a structural intervention. For instance, cash transfers implemented with refugee settlement-based adults in Uganda found positive effects on mental health and food security—but not on COVID-19 preventive practices32—suggesting the potential for integrating behaviour change digital strategies with structural interventions. We also did not observe changes in depression: food24 and water33 insecurity were previously linked to refugee youths’ depression and may need to be addressed to improve mental health in the context of resource scarcity. This also indicates that a COVID-19 prevention mHealth study is not sufficiently tailored to reduce depression among this population.
Strengths and limitations
This study has some limitations. First, with no control group we are not able to account for socioenvironmental changes regarding COVID-19 over time. For instance, the Delta variant wave in Uganda occurred over this timeframe and could have impacted practices beyond the intervention; however, we note that participants’ perceived risk remained low at each time point. Second, the non-random sample limits generalisability. It is plausible that participants already enrolled in an HIV study may have fewer barriers to research participation and higher health literacy than refugee youth not engaged in research. Third, there was differential loss to follow-up by length of time in Uganda and settlement. This requires further exploration to better understand mobility and migration patterns during the pandemic among refugee youth, and implications for health promotion. Fourth, vaccines were not readily available during the study, hence we conducted post-hoc analysis for vaccine acceptance as that was not an originally planned analysis; as only 31% of Uganda's general population were fully vaccinated for COVID-19 as of July 2022,34 our findings can inform future vaccine rollout.
Despite these limitations, our study is unique in developing and evaluating a RANAS13 theoretically informed mHealth intervention in the pandemic with urban refugee youth, a group understudied in hand and respiratory hygiene literature at large.5–7 Results document that many COVID-19 prevention practice outcomes changed over the short term and thus our study can inform future mHealth approaches. Future studies, for instance, could employ RCT designs and combination intervention packages with structural approaches to tackle entrenched food and water insecurity.
Conclusions
It is important to promote hand and respiratory hygiene in urban slums and humanitarian settings beyond COVID-19 to reduce global infectious disease burdens.35 For instance, lessons learned from our study about using mHealth approaches—such as providing information boosters and reminders—can be used for other infectious disease emergencies with urban refugee youth, such as cholera and Ebola outbreaks. Thus community-based youth mHealth strategies such as Kukaa Salama, with high uptake and satisfaction, offer insight into the potential role for mHealth in advancing infection prevention and control. There remains an urgent need to better understand social determinants and multilevel factors—spanning individual, community and structural domains—shaping urban refugee youth health practices in a pandemic.
Supplementary Material
Acknowledgements
We would like to acknowledge the support and contributions of: Young African Refugees for Integral Development (YARID), Uganda Ministry of Health, Uganda National AIDS Control Program, Dr. Gabby Serafini (WelTel), Mildmay Uganda, Organization for Gender Empowerment and Rights Advocacy (OGERA Uganda), Most At Risk Population Initiative, Uganda Office of the Prime Minister Department of Refugees, Tushirikiane Research Team and Peer Navigators (Gabriella Nzulungi; Sabrina Gamwany; Hillary Nuwamanya; Bibishe Hakiza; Justin Paluku; Bella Nshimirimana; Claudine Ndoole; Priscilla Asiimwe; Angelique Kipenda; Faith Musubaho; Phiona Nattabi; Joyeux Mugisho).
Contributor Information
Carmen H Logie, Factor-Inwentash Faculty of Social Work, University of Toronto, Toronto, Ontario M5S 1V4, Canada; Women's College Research Institute, Women's College Hospital, Toronto, Ontario M5S 1B2, Canada; Centre for Gender & Sexual Health Equity, Vancouver, BC V6Z 2K5, Canada.
Moses Okumu, School of Social Work, University of Illinois Urbana-Champaign, Urbana, Illinois 61820, United States; School of Social Sciences, Uganda Christian University, Mukono, Uganda.
Isha Berry, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario M5T 3M7, Canada.
Jean-Luc Kortenaar, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario M5T 3M7, Canada.
Robert Hakiza, Young African Refugees for Integral Development (YARID), Kampala, Uganda.
Daniel Kibuuka Musoke, International Research Consortium (IRC), Kampala, Uganda.
Brenda Katisi, Young African Refugees for Integral Development (YARID), Kampala, Uganda.
Aidah Nakitende, International Research Consortium (IRC), Kampala, Uganda.
Peter Kyambadde, National AIDS and STI Control Programme, Ministry of Health, Kampala, Uganda; Most at Risk Population Initiative, Mulago Hospital, Kampala, Uganda.
Richard Lester, Department of Medicine, University of British Columbia, Vancouver, British Columbia V6T 1Z3, Canada.
Amaya G Perez-Brumer, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario M5T 3M7, Canada.
Zerihun Admassu, Factor-Inwentash Faculty of Social Work, University of Toronto, Toronto, Ontario M5S 1V4, Canada.
Lawrence Mbuagbaw, Department of Health Research Methods, Evidence and Impact, McMaster University, Hamilton, Ontario L8S 4L8, Canada; Department of Anesthesia, McMaster University, Hamilton, ON L8N 3Z5, Canada; Department of Pediatrics, McMaster University, Hamilton, ON L8S 4K1, Canada; Biostatistics Unit, Father Sean O'Sullivan Research Centre, St Joseph's Healthcare, Hamilton, ON L8G 5E4, Canada; Centre for Development of Best Practices in Health (CDBPH), Yaoundé Central Hospital, Yaoundé, Cameroon; Division of Epidemiology and Biostatistics, Department of Global Health, Stellenbosch University, Cape Town, South Africa.
Authors’ contributions
Conceived study: CHL, MO, RH. Designed study protocol: CHL, MO, RH, LM. Collected data: IB, LK, DKM, BK, AN. Analyzed data: IB, LK, ZA. Interpretation of findings: CHL, MO, IB, JK, RH, DKM, BK, AN, PK, RL, APB, ZA, LM. Drafted manuscript: CHL, IB. All authors read and approved the final manuscript. CHL, MO, and IB are guarantors of the paper.
Funding
This study is funded by the International Development Research Center (# 109549-001). CHL is also funded by the Canada Research Chairs programme (#Tier 2), Canada Foundation for Innovation (#JELF) and the Ontario Ministry of Research and Innovation (ERA).
Competing interests
RL is an academic physician-researcher and also has interests in a non-profit and private company social enterprise, WelTel Inc, that develops and provides digital health software. He is not being paid or otherwise compensated by WelTel for this project. No other authors declare a conflict of interest.
Ethical approval
The trial protocol was approved by the Research Ethics Board of the University of Toronto (Protocol Number: 37496), Mildmay Uganda Research Ethics Committee (Ref: 0806–2019) and the Uganda National Council for Science & Technology (Ref: HS2716). All participants provided written informed consent.
Data availability
Data is available from the contact author upon reasonable request and receiving required ethical approval.
References
- 1. UNHCR . Figures at a Glance. 2022. Copenhagen, Denmark: United Nations High Commissioner for Refugees. 2019. Available at: https://www.unhcr.org/figures-at-a-glance.html [accessed June 8, 2023]. [Google Scholar]
- 2. Singh L, Singh NS, Nezafat Maldonado B, et al. What does ‘leave no one behind’ mean for humanitarian crises-affected populations in the COVID-19 pandemic? BMJ Glob Health. 2020;5:e002540. [Google Scholar]
- 3. Bukuluki P, Mwenyango H, Katongole SP, et al. The socio-economic and psychosocial impact of Covid-19 pandemic on urban refugees in Uganda. Soc Sci Humanit Open. 2020;2:100045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. UNHCR . Uganda - Refugee Statistic June 2022 - Kampala. 2022. Copenhagen, Denmark: United Nations High Commissioner for Refugees. [Google Scholar]
- 5. Nasreen S, Azziz-Baumgartner E, Gurley ES, et al. Prevalent high-risk respiratory hygiene practices in urban and rural Bangladesh: High-risk respiratory hygiene practices. Trop Med Int Heal. 2010;15:762–71. [DOI] [PubMed] [Google Scholar]
- 6. Sultana F, Nizame FA, Southern DL, et al. Pilot of an elementary school cough etiquette intervention: Acceptability, feasibility, and potential for sustainability. Am J Trop Med Hyg. 2017;97:1876–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Stebbins S, Cummings DAT, Stark JH, et al. Reduction in the incidence of influenza A but not influenza B associated with use of hand sanitizer and cough hygiene in schools: A randomized controlled trial. Pediatr Infect Dis J. 2011;30:921–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Stauber CE, Brown J, Bourgeois AG, et al. Mobile health technologies are essential for reimagining the future of water, sanitation, and hygiene. Am J Trop Med Hyg. 2022;106:1017–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Verhagen LM, de Groot R, Lawrence CA, et al. COVID-19 response in low- and middle-income countries: Don't overlook the role of mobile phone communication. Int J Infect Dis. 2020;99:334–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kok MO, Terra T, Van Der Hoeven M, et al. Using telehealth to support 3500 community health workers in rural Uganda: A mixed-methods study. BMC Health Serv Res. 2023;23(1):284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ahmed AI, Kaiser A, Jayal G, et al. COVID-19 and the fear of other unknowns: Challenges and lessons learned from a digital contact tracing activity in the Rohingya camps in Cox's Bazar, Bangladesh. J Glob Health Rep. 2022;6:1–9. [Google Scholar]
- 12. Okumu M, Logie C, Ansong D, et al. Exploring the protective value of using sexting for condom negotation on condom use determinants and practices among forcibly displaced adolescents in the slums of Kampala, Uganda. AIDS Behavior. 2022;26:3538–50. [DOI] [PubMed] [Google Scholar]
- 13. Contzen N, Mosler HJ.. The RANAS Approach to Systematic Behavior Change: Methodological Fact Sheet 1. Dübendorf, Switzerland: Eawag, Swiss Federal Institute of Aquatic Science and Technology; 2015. [Google Scholar]
- 14. Logie C, Okumu M, Hakiza R, et al. Mobile health-supported HIV self-testing strategy among Urban refugee and displaced youth in Kampala, Uganda: Protocol for a cluster randomized trial (Tushirikiane, Supporting Each Other). JMIR Res Protoc. 2021;10(2):e26192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Digitale JC, Stojanovski K, McCullo. ch CE, et al. Study designs to assess real-world interventions to prevent COVID-19. Front Public Health. 2021;9:657976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Logie CH, Okumu M, Berry I, et al. Kukaa Salama (Staying Safe): Study protocol for a pre/post-trial of an interactive mHealth intervention for increasing COVID-19 prevention practices with urban refugee youth in Kampala, Uganda. BMJ Open. 2021;11:e055530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lester RT, Ritvo P, Mills EJ, et al. Effects of a mobile phone short message service on antiretroviral treatment adherence in Kenya (WelTel Kenya1): A randomised trial. Lancet. 2010;376:1838–45. [DOI] [PubMed] [Google Scholar]
- 18. van der Kop ML, Muhula S, Nagide PI, et al. Articles effect of an interactive text-messaging service on patient retention during the first year of HIV care in Kenya (WelTel Retain): An open-label, randomised parallel-group study. Lancet Public Health. 2018;3(3):143–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mosler H-J. A systematic approach to behavior change interventions for the water and sanitation sector in developing countries: A conceptual model, a review, and a guideline. Int J Environ Health Res. 2012;22:431–49. [DOI] [PubMed] [Google Scholar]
- 20. Contzen N, Meili IH, Mosler H-J.. Changing handwashing behaviour in southern Ethiopia: A longitudinal study on infrastructural and commitment interventions. Soc Sci Med 2015;124:103–14. [DOI] [PubMed] [Google Scholar]
- 21. Gamma AE, Slekiene J, von Medeazza G, et al. Contextual and psychosocial factors predicting Ebola prevention behaviours using the RANAS approach to behaviour change in Guinea-Bissau. BMC Public Health. 2017;17:446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kroenke K, Spitzer RL, Williams JBW.. The patient Health Questionnaire-2: Validity of a two-item depression screener. Med Care. 2003;41:1284–92. [DOI] [PubMed] [Google Scholar]
- 23. Straus MA, Douglas EM.. A short form of the revised conflict tactics scales, and typologies for severity and mutuality. Violence Vict. 2007;19:507–20. [DOI] [PubMed] [Google Scholar]
- 24. Logie C, Berry I, Okumu M, et al. The prevalence and correlates of depression before and after the COVID-19 pandemic declaration among urban refugee adolescents and youth in informal settlements in Kampala, Uganda : A longitudinal cohort study. Ann Epidemiol. 2022;66:37–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lazarus J V, Ratzan SC, Palayew A, et al. A global survey of potential acceptance of a COVID-19 vaccine. Nat Med. 2021;27:225–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Melin J, Bonn SE, Pendrill L, et al. A questionnaire for assessing user satisfaction with mobile health apps: Development using rasch measurement theory. JMIR mHealth uHealth. 2020;8:e15909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Vittinghoff E., Glidden DV, Shiboski SC, McCulloch CE. Regression methods in biostatistics. New York: Springer, 2011. [Google Scholar]
- 28. Gold N, Hu XY, Denford S, et al. Effectiveness of digital interventions to improve household and community infection prevention and control behaviours and to reduce incidence of respiratory and/or gastro-intestinal infections: A rapid systematic review. BMC Public Health. 2021;21:1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Keller J, Kwasnicka D, Wilhelm LO, et al. Hand washing and related cognitions following a brief behavior change intervention during the COVID-19 pandemic: A pre-post analysis. Int J Behav Med. 2022;29(5):575–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. VanBenschoten H, Kuganantham H, Larsson EC, et al. Impact of the COVID-19 pandemic on access to and utilisation of services for sexual and reproductive health: A scoping review. BMJ Glob Health. 2022;7:e009594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. UNHCR . Urban Refugees Struggling To Survive as Economic Impact of COVID19 Worsens in East, Horn and Great Lakes of Africa. 2020;4–9. Copenhagen: Denmark: United Nations High Commissioner for Refugees. [Google Scholar]
- 32. Stein D, Bergemann R, Lanthorn H, et al. Cash, COVID-19 and aid cuts: A mixed-method impact evaluation among South Sudanese refugees registered in Kiryandongo settlement, Uganda. BMJ Glob Health. 2022;7:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Logie CH, Okumu M, Loutet M, et al. Associations between water insecurity and depression among refugee adolescents and youth in a humanitarian context in Uganda: Cross-sectional survey findings. Int Health. 2023;15(4):474–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Institute for Health Metrics and Evaluation . COVID-19 Health Data Uganda. 2022. New York, NY.Available at: https://www.healthdata.org/sites/default/files/covid_briefs/190_briefing_Uganda.pdf [Accessed June 1, 2023]. [Google Scholar]
- 35. United Nations Children's Fund and World Health Organization . State of the World's Hand Hygiene: A Global Call to Action To Make Hand Hygiene a Priority in Policy and Practice. New York, NY: UNICEF; 2021. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is available from the contact author upon reasonable request and receiving required ethical approval.