Abstract
For Sinorhizobium meliloti (also known as Rhizobium meliloti) AK631 to establish effective symbiosis with alfalfa, it must be able to synthesize a symbiotically active form of its K antigen, a capsular polysaccharide containing a Kdo (3-deoxy-d-manno-octulosonic acid) derivative. Previously isolated mutants defective in the synthesis of K antigen are resistant to bacteriophage φ16-3. By screening ca. 100,000 Tn5-mutagenized R. meliloti bacteria for resistance to bacteriophage φ16-3, we isolated 119 mutants, 31 of which could not be complemented by genes previously identified as being required for K-antigen synthesis. Of these 31 new mutants, 13 were symbiotically defective and lacked the K antigen. Through genetic and phenotypic analyses, we have grouped these mutants into four distinct classes. Although all of these mutants lack the K antigen, many also have altered lipopolysaccharides (LPS), suggesting that the biochemical pathways for the synthesis of K antigen and LPS have common enzymatic steps. In addition, we have found that these and other classes of K-antigen-defective mutants of S. meliloti AK631 exhibit unique patterns of sensitivities to phage strains to which the parental strain was resistant. Our studies have identified new classes of genes required for both the synthesis of K antigen and the symbiotic proficiency of S. meliloti AK631. Some of these classes of genes also play a role in LPS synthesis.
In order to invade the nodules it elicits on alfalfa (Medicago sativa), Sinorhizobium meliloti (also known as Rhizobium meliloti) must synthesize at least one of three polysaccharides: succinoglycan, exopolysaccharide (EPS) II, or a symbiotically active form of the strain-specific K antigen (4, 15–17). Strains which produce symbiotically appropriate forms of any one of these three polysaccharides are capable of forming healthy, nitrogen-fixing nodules. In contrast, S. meliloti mutants that fail to synthesize at least one of these polysaccharides in symbiotically appropriate forms will produce ineffective or Fix− nodules, which contain few bacteria and which are incapable of fixing nitrogen on alfalfa host plants.
The fact that K antigen can replace succinoglycan or EPS II was discovered through the study of a derivative of S. meliloti Rm41 known as AK631. This strain cannot synthesize succinoglycan or EPS II due to a mutation in the exoB (galE) gene, which is required for the synthesis of both of these polysaccharides (2, 3). However, strain AK631 is still capable of forming fully effective nodules on alfalfa because AK631 produces a form of K antigen which can functionally replace succinoglycan or EPS II during nodule invasion (12, 13, 15–17). So far, two gene regions that are required for the production of K antigen have been identified. The genes rkpABCDEFGHIJ are clustered in the rkp-1 region, and rkpZ is located by itself in a distinct genetic region. However, the fact that none of these genes were predicted to be glycosyltransferases, as have been found in the succinoglycan (5, 6, 14) and EPS II (1) biosynthetic regions, suggested that there must be additional genetic loci that encode functions required for K-antigen biosynthesis.
The lytic phage φ16-3 will infect S. meliloti AK631, which produces K antigen, but not AK631 mutants which lack K antigen (13). The role that K antigen plays in sensitivity to this phage is unknown, but in a simple model the K antigen would act as a ligand for a receptor on the phage particle. We therefore reasoned that resistance to phage φ16-3 could be used as a powerful selection factor in isolating additional mutations in genes required for the synthesis of K antigen. Using this method, we carried out a large-scale genetic study that resulted in the identification of four new, phenotypically and genetically distinct classes of K-antigen mutations which are not complemented by plasmids carrying the rkp-1 region or the rkpZ+ gene.
Screening for mutations in genes involved in K-antigen synthesis by selecting for mutants with resistance to phage φ16-3.
To select phage-resistant mutants of S. meliloti AK631, bacteria were mutagenized with Tn5 and plated on LB plates spread with 109 PFU of phage φ16-3. Colonies which grew on these plates were picked and restreaked on plates spread with phage φ16-3 to confirm phage resistance. In this manner, 20 independent pools of bacteria representing approximately 100,000 genomes were screened, which resulted in 119 phage φ16-3-resistant mutants.
To determine whether the mutants isolated in our screen carried defects in previously characterized loci, we carried out complementation tests with plasmids containing the rkp-1 region or the rkpZ gene. Thirty-one of these 119 mutants were not made sensitive to φ16-3 by complementation with either plasmid, and so we focused on these mutants for further study.
Since S. meliloti AK631 requires K antigen during the invasion of symbiotic nodules on alfalfa, we inoculated alfalfa seedlings with these mutants to test their ability to form nitrogen-fixing nodules. These mutants segregated into two groups. One group consisted of 13 mutants that formed white round nodules and resulted in yellow alfalfa plants with stunted growth. Since these plants could not fix nitrogen, the AK631 derivatives in this group are described as having a Fix− phenotype. The other group consisted of 18 mutants that formed wild-type, elongated pink nodules and resulted in healthy green plants. This suggests that these mutations either do not affect K antigen or else affect K antigen in a fashion that does not interfere with its symbiotic role. Since it seemed likely that the mutants in the first group would have mutations in hitherto-unidentified K-antigen synthesis genes, we focused our efforts on analyzing the Fix−, φ16-3-resistant mutants.
Analysis of K antigen and LPS from Fix−, phage φ16-3-resistant mutants reveals three distinct classes of K antigen mutations.
We analyzed the K antigen from our novel Fix−, phage φ16-3-resistant mutants to determine whether their inability to form nitrogen-fixing nodules correlated with a defect in K-antigen production. The cell-associated polysaccharides were extracted with hot phenol-water, and the water-soluble extracts were analyzed by polyacrylamide gel electrophoresis (PAGE) and alcian blue-silver staining (Fig. 1). All 13 of the Fix− mutants we had isolated failed to produce K antigen, indicating that all of these mutants had defects in previously unidentified genes involved in K-antigen synthesis.
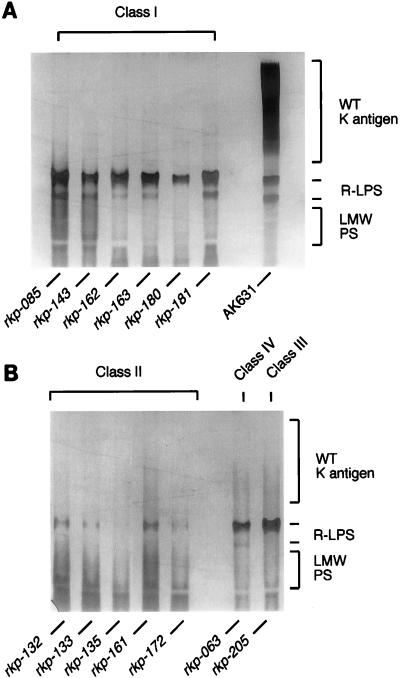
FIG. 1.
PAGE analysis of phenol-water-extracted material from AK631 and class I mutants (A) and class II, III, and IV mutants (B). K antigen and LPS were visualized via alcian blue-silver staining. The four classes of mutants can be readily distinguished from the parental strain, AK631, by their lack of K antigen. Classes can be distinguished through the properties of their R-LPSs. In class I and class III mutants, the R-LPS is indistinguishable from that of the wild type (note that the lower R-LPS band is absent in the rkp-180 lane due to underloading of the lane). In the class II mutants, the R-LPS separates into the phenol phase during the phenol-water extraction. In the case of the class IV mutants, the R-LPS runs similarly to that of the exoB+ parental strain of AK631. WT, wild type; LMW PS, lower-molecular-weight polysaccharide.
Unexpectedly, we found that some of these mutants are also defective in lipopolysaccharide (LPS) production. On the basis of these LPS defects, we were able to subdivide the 13 Fix− mutants into three phenotypic classes. Mutants in class I lack the K antigen but produce LPS that appears similar to that produced by the parental strain AK631; i.e., the rough-LPS (R-LPS) migrates as two diffuse bands (Fig. 1A). It is important to note that the R-LPS from the parental strain, AK631, migrates slightly further due to the presence of the K antigens; also, the rkp-180 mutant appears to have only one band due to less total carbohydrate being loaded. This is similar to the previously isolated K-antigen mutations in the rkp-1 region and in the rkpZ gene. Seven of the 13 mutants, rkp-085, rkp-143, rkp-162, rkp-163, rkp-180, rkp-181 (Fig. 1A), and rkp-063 (Fig. 1B), fall into this class. However, the rkp-063 mutant has a phenotype which make it distinct from other members of this class, as will be discussed below. In contrast, both class II and III mutants produce LPS which is different from that produced by the parental strain (Fig. 1B). Class II includes the five mutants rkp-132, rkp-133, rkp-135, rkp-161, and rkp-172. During the process of extracting the polysaccharides from these mutants with hot phenol-water, we noticed that the LPS from these mutants had the unusual characteristic of separating predominantly into the phenol phase rather than into the aqueous phase. We confirmed this observation by dialyzing the phenol phase and running the resulting products on PAGE gels (data not shown). Class III consisted of one K-antigen-deficient mutant, rkp-205, which was unusual in that its LPS resembled that found in the exoB+ parent of AK631 rather than that of AK631 itself. Like the exoB+ strain, the rkp-205 mutant has only one LPS band which, unlike the LPS of class II mutants, does not segregate into the phenol phase. The fact that class II and class III mutants displayed alterations in their LPS as well as being deficient in K antigen may indicate that the biochemical pathways for K antigen and LPS have common steps and that these mutations are defects in genes involved in those common steps. The set of 13 Fix−, K-antigen-deficient mutants also shared one other interesting feature. In every case, they produced a very-high-mobility polysaccharide that migrated in a ladder pattern at the bottom of the lane. The nature of this material is currently under investigation.
Absence of K antigen alters the susceptibility of S. meliloti AK631 to several phages.
Since AK631 mutants lacking K antigen are resistant to φ16-3, we decided to examine whether they had altered sensitivity to other S. meliloti phages (Table 1). With the exception of φ16-3 and φM12H1, the phages we tested had been isolated based on their ability to form plaques on another S. meliloti strain, SU47. We found that none of these phages would form plaques on AK631, the parental strain in our genetic study. φM12H1 is a derivative of φM12 capable of infecting both Rm41, the parental strain of AK631, and strain SU47.
TABLE 1.
Different classes of Fix−, K-antigen-deficient mutants have distinctive phage sensitivity profilesa
| Parental or mutant strain or class | Genotype | Sensitivityb to:
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| φM1 | φM5 | φM6 | φM7 | φM9 | φM10 | φM11 | φM12 | φM12H1 | φM14 | φM19 | φ16-3 | ||
| Parental strain AK631 | Wild type | R | R | R | R | R | R | R | R | +++ | R | R | +++ |
| Mutants | |||||||||||||
| rkp-1 | rkpA | R | R | + | +++ | ++ | +++ | +++ | +++ | +++ | +++ | +++ | R |
| rkpI | R | R | + | +++ | ++ | +++ | +++ | +++ | +++ | +++ | +++ | R | |
| rkpJ | R | R | + | +++ | +++ | +++ | +++ | +++ | +++ | ++ | +++ | R | |
| Class I | rkp-085 | R | R | R | +++ | R | + | +++ | ++ | +++ | + | ++ | R |
| rkp-143 | R | R | R | +++ | R | + | +++ | ++ | ++ | + | +++ | R | |
| rkp-162 | R | R | R | +++ | R | + | +++ | ++ | +++ | + | +++ | R | |
| rkp-163 | R | R | R | +++ | + | + | +++ | ++ | +++ | + | ++ | R | |
| rkp-180 | R | R | R | +++ | R | + | +++ | +++ | +++ | + | +++ | R | |
| rkp-181 | R | R | R | +++ | R | + | +++ | ++ | +++ | + | ++ | R | |
| Class II | rkp-132 | R | R | R | +++ | + | R | ++ | ++ | +++ | R | ++ | R |
| rkp-133 | R | R | R | +++ | + | R | ++ | ++ | +++ | R | ++ | R | |
| rkp-135 | R | R | R | +++ | + | R | +++ | +++ | +++ | R | ++ | R | |
| rkp-161 | R | R | R | +++ | + | R | +++ | ++ | +++ | R | ++ | R | |
| rkp-172 | R | R | R | +++ | + | R | ++ | ++ | +++ | R | ++ | R | |
| Class III | rkp-205 | R | R | R | ++ | + | + | ++ | ++ | ++ | + | ++ | R |
| Class IV | rkp-063 | R | R | R | + | R | R | +++ | ++ | +++ | R | ++ | R |
AK631 is resistant to all phage except φ16-3 and φM12H1. Mutations in genes located in the rkp-1 region, rkpA674, rkpI634, and rkpJ671, rendered AK631 sensitive to almost all phages tested with the exception of φM1 and φM5. Mutants in groups I, II, III, and IV also showed increased sensitivities to most phages tested. The mutants from the rkp-1 region and from groups I, II, III, and IV all show distinctive profiles of phage sensitivity and resistance.
Symbols: R, strain resistant to phage; +++, phage forms clear spot on bacteria; ++, phage will form slightly turbid spot on bacteria; +, phage will form a very turbid spot on bacteria and forms plaques with a reduced efficiency.
To determine whether AK631 is insensitive to the Rm1021-specific phages shown in Table 1 because of its ability to synthesize K antigen, we tested the sensitivities to various phages of the AK631 mutant strains containing the previously characterized K-antigen mutations rkpA674, rkpI634, and rkpJ671, all of which are in the rkp-1 region (9, 10, 13). Both the rkpA674 and rkpI634 mutants are unable to synthesize K antigen (9, 10, 13). rkpJ671 is unable to export K antigen to the cell surface (10, 13). Interestingly, we found that these mutations render AK631 sensitive to many SU47-specific phages, including φM7, φM9, φM10, φM11, φM12, φM14, and φM19. Strains with these mutations also show a slight sensitivity to φM6, but efficient plaque formation did not occur. These data indicate that the production of K antigen by strain AK631 protects it against infection by many S. meliloti phages to which it would otherwise be susceptible. K antigen does confer sensitivity to φ16-3, however, indicating that while K antigen offers AK631 protection against some phages, this polysaccharide renders the strain susceptible to others.
New K-antigen mutants have distinct phage sensitivity profiles which support the mutant classifications determined by PAGE and suggest an additional mutant class.
Like the mutants containing defects in the rkp-1 region analyzed above, the 13 novel Fix− K-antigen mutants we isolated are more sensitive to many of the phages tested (Table 1). Like mutants rkpA674, rkpI634, and rkpJ671, most of our mutants are very sensitive to φM7, φM11, φM12, and φM19. These phages will form plaques on all of our mutants. However, the phage sensitivity profiles of our mutants are different from those of rkp-1 mutants in certain respects. For example, in every case, our mutants were more resistant to φM7, φM10, and φM14 than the rkp-1 mutants we tested. The patterns of phage sensitivity shown by our mutants also differ between classes (Table 1).
The phage sensitivity profile of mutant rkp-063 is particularly interesting. Although when run on PAGE gels the K antigen and LPS from this mutant resemble those of the class I mutants, the phage sensitivity profile of mutant rkp-063 distinguishes it from other members of this class. This strain is unique in having a greatly reduced resistance to φM7 and resistance to both φM9 and φM10. Also, this mutant resembles the class III mutants in being resistant to φM14. Based on these results and on the results of the following sections, we have placed mutant rkp-063 into a distinct class termed class IV.
Different classes of K-antigen-deficient mutants show distinct phenotypes when streaked on Congo red plates.
Congo red dye has been found to bind many types of polysaccharides (19). It has been reported that when streaked on M9-mannitol plates containing 0.05% Congo red, AK631 will form salmon-colored colonies (18). In contrast, an AK631 rkpZ mutant forms dark-red colonies under these conditions (18). We therefore investigated whether this assay could be used to distinguish AK631 strains which had deficiencies in producing K antigen from those that did not. As expected, when we streaked an rkpZ mutant on Congo red plates, it formed dark-red colonies as did the rkpA674, rkpI634, and rkpJ671 mutants. Surprisingly, when we streaked our newly isolated mutants on Congo red plates, they had two distinct phenotypes (Table 2). Mutants from classes III and IV formed dark-red colonies similar to the ones formed by the rkpZ and rkpJ671 mutants. Mutants from classes I and II, however, formed salmon-colored colonies which were almost identical in color to those of AK631, the parental strain. The fact that mutant rkp-063 stains red whereas the class I mutants do not support our decision to place this mutant in a separate class.
TABLE 2.
Mutant classificationsa
| Category | No. of isolates | K antigen | LPS | Congo red result | Phage sensitivity profile | Complementation group |
|---|---|---|---|---|---|---|
| AK631 | NAb | Present | AK631 | Salmon | Distinct | NA |
| rkp-1 | 3 tested | Absent | AK631 | Dark red | Distinct | rkp-1 |
| Class I | 5 | Absent | AK631 | Salmon | Distinct | rkp-3 |
| Class II | 6 | Absent | Altered | Salmon | Distinct | rkp-2 |
| Class III | 1 | Absent | Rm41 | Dark red | Distinct | Unique |
| Class IV | 1 | Absent | AK631 | Dark red | Distinct | Unique |
Genetic analysis of K-antigen mutations.
While performing the experiments described above, we became aware that Kereszt et al. (7, 8) had also isolated a series of φ16-3-resistant mutants, which were Fix− on alfalfa and failed to produce K antigen, that fell into two complementation groups, which they called rkp-2 and rkp-3 (7, 8). Mutations in the rkp-2 region, were complemented by the cosmid pAT330; mutations in the rkp-3 region, was complemented by the overlapping cosmids pAT399 and pAT401 (7, 8). Kereszt et al. generously provided us with these three cosmids prior to publication (7, 8). We were thereby able to perform complementation tests with the mutants that we had isolated by determining whether these cosmids would restore φ16-3 sensitivity to these mutants.
We found that the mutants we had classified as belonging to class I were made sensitive to φ16-3 by complementation with pAT399 and pAT401 and were thus in the rkp-3 region, while mutants we determined as belonging to class II were complemented by pAT330 or in the rkp-2 region. These data indicate that the classes that we had assigned on the basis of phenotypes represent distinct genetic classes of mutants as well.
Our class III and IV mutants were not complemented by the cosmids pAT330, pAT399, and pAT401. To analyze these mutants further, we first screened an Rm41 library for cosmids that complemented the sensitivity of mutants rkp-063 and rkp-205 to φM12. However, we were unable to isolate any complementing cosmids to the mutations in these strains by using this strategy. We also attempted to complement the φ16-3 resistance phenotype directly, by replica plating colonies containing cosmids from the library onto plates spread with φ16-3 and looking for clones which were sensitive to this phage. We screened several thousand clones by this strategy but were unable to isolate any cosmids which complemented mutant rkp-063 or rkp-205. In addition, we provided Putnoky and his colleagues with our class III and class IV mutants, and they also failed to isolate complementing cosmids (11).
Because our Fix−, φ16-3-resistant mutants had been isolated from a population of R. meliloti cells mutagenized with the transposon Tn5, we expected that the majority of the mutants that we had isolated would carry recessive loss-of-function alleles caused by the insertion of Tn5 and thus provide us with a selectable cloning marker. However, when we transduced the Tn5 insertions in mutants rkp-063 and rkp-205 into AK631, in each case we found that the mutation causing the φ16-3-resistant phenotype was unlinked to the Tn5 insertion.
Four classes of novel K-antigen mutants.
After screening approximately 100,000 Tn5-mutagenized derivatives of S. meliloti AK631 for mutants which are resistant to φ16-3, we successfully isolated 13 novel K-antigen-deficient mutants. Genetic and phenotypic analyses placed these mutants into four distinct classes (Table 2). Two of these classes, class III and class IV, each contain only one member and cannot be complemented by a cosmid carrying any known genes required for K-antigen synthesis, including those described in the accompanying report (7). On PAGE gels, rkp-205, the class III mutant, has its own distinct phenotype. Like many of the other K-antigen mutants, rkp-205 produces no K antigen. However, the LPS from this mutant migrates differently from other K-antigen mutants that exhibit LPS defects and, in fact, resembles that observed for strain Rm41, the exoB+ parent of AK631. In contrast, our class IV mutant rkp-063 looks similar to the class I mutants in that it produces no K antigen and has no observable differences in LPS from that of the parental strain AK631. In addition, the class III and class IV mutants have phage resistance profiles that are distinct from the rest of our mutant classes, as well as from each other.
We also identified 11 mutations which we grouped into two additional classes, class I and class II. Class I mutants lack K antigen and produce LPS which looks indistinguishable from that of the parental strain, AK631, on PAGE gels. In this respect, these mutants resemble rkpZ and rkp-1 region mutants. Class I mutants also have similar phage resistance profiles that are distinct from those of the other classes of mutants. Specifically, class I mutants are slightly sensitive to φM10 and φM14 and are resistant to φM9. We have determined that these mutations lie in the region defined by Kereszt et al. as rkp-3 (7, 8), based on the finding that these mutants are complemented by the overlapping cosmids pAT399 and pAT401.
The effects of class II mutations are less specific than those of the class I mutations. Not only do they abolish K-antigen synthesis, but they also have striking effects on LPS synthesis. In addition to producing no K antigen, the majority of the LPS from these mutants segregates into the phenol phase during the extraction process, indicating that it is altered from the LPS of the parental strain. In this respect, these mutations are different from those in any of the previously characterized K-antigen synthesis genes. This class of mutants also has its own profile of phage resistances and sensitivities. Through complementation tests, we have determined that these mutations are located in the rkp-2 region. As described in the accompanying paper, Kereszet et al. (7, 8) sequenced the rkp-2 region and found it to contain two open reading frames organized in monocistronic transcription units. Although they found both of these genes to be required for normal LPS production, only the second one, designated rkpK, is involved in K-antigen synthesis. They have found that RkpK possesses UDP-glucose dehydrogenase activity. Presumably this enzyme is needed for the synthesis of both K antigen and LPS.
It is not yet clear why we were unable to obtain complementing cosmids to the class III and IV mutations. One explanation could be that the phenotypes of these mutants are not the result of simple recessive null mutations but are instead caused by dominant mutations, complex rearrangements, or multiple mutations. Alternatively, these genes may not be present in our cosmid library because they are toxic when present in multiple copies. The fact that these mutations were not caused by Tn5 insertions may reflect difficulty in obtaining null mutations in these genes, perhaps because they are essential. This possibility is supported by the facts that these mutations fell into the smallest groups in our original screen and that class III and class IV mutants were not obtained by Kereszt et al. (7, 8).
We thank Peter Putnoky and his colleagues for their willingness to share data and plasmids prior to publication and Juan González, Hai-Ping Cheng, and Greg York for their useful discussions.
This work was supported by Public Health Service grant GM31030 from the National Institutes of Health.
REFERENCES
- 1.Becker A, Rüberg S, Küster H, Roxlau A A, Keller M, Ivashina T, Cheng H-P, Walker G C, Pühler A. The 32-kilobase exp gene cluster of Rhizobium meliloti directing the biosynthesis of galactoglucan: genetic organization and properties of the encoded gene products. J Bacteriol. 1997;179:1375–1384. doi: 10.1128/jb.179.4.1375-1384.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buendia A M, Enenkel B, Köplin R, Niehaus K, Arnold W, Pühler A. The Rhizobium meliloti exoZ/exoB fragment of megaplasmid 2: ExoB functions as a UDP-glucose-4-epimerase and ExoZ shows homology to NodX of Rhizobium leguminosarum biovar viciae strain TOM. Mol Microbiol. 1991;5:1519–1530. doi: 10.1111/j.1365-2958.1991.tb00799.x. [DOI] [PubMed] [Google Scholar]
- 3.Canter-Cremers H C J, Batley M, Redmond J W, Eydems L, Breedveld M W, Zevenhuizen L P T M, Pees E, Wijffelman C J, Lugtenberg B J J. Rhizobium leguminosarum exoB mutants are deficient in the synthesis of UDP-glucose 4-epimerase. J Biol Chem. 1990;265:21122–21127. [PubMed] [Google Scholar]
- 4.Forsberg L S, Reuhs B L. Structural characterization of the K antigens from Rhizobium fredii USDA257: evidence for a common structural motif, with strain-specific variation, in the capsular polysaccharides of Rhizobium spp. J Bacteriol. 1997;179:5366–5371. doi: 10.1128/jb.179.17.5366-5371.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Glucksmann M A, Reuber T L, Walker G C. Family of glycosyl transferases needed for the synthesis of succinoglycan by Rhizobium meliloti. J Bacteriol. 1993;175:7033–7044. doi: 10.1128/jb.175.21.7033-7044.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Glucksmann M A, Reuber T L, Walker G C. Genes needed for the modification, polymerization, export, and processing of succinoglycan by Rhizobium meliloti: a model for succinoglycan biosynthesis. J Bacteriol. 1993;175:7045–7055. doi: 10.1128/jb.175.21.7045-7055.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kereszt A, Kiss E, Reuhs B L, Carlson R W, Kondorosi A, Putnoky P. Novel rkp gene clusters of Sinorhizobium meliloti involved in capsular polysaccharide production and the invasion of the symbiotic nodule: rkpK gene encodes a UDP-glucose dehydrogenase. J Bacteriol. 1998;180:5426–5431. doi: 10.1128/jb.180.20.5426-5431.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kereszt A, Kiss E, Reuhs B, Carlson R, Kondorosi A, Putnoky P. Abstracts of the 11th International Congress on Nitrogen Fixation 1997, Paris, France. Paris, France: Institut Pasteur; 1997. Isolation and characterization of novel Rhizobium meliloti regions involved in KPS production, abstr. 10.44; p. 111. [Google Scholar]
- 9.Kiss E, Reuhs B L, Kim J S, Kereszt A, Petrovics G, Putnoky P, Dusha I, Carlson R W, Kondorosi A. The rpkGHI and -J genes are involved in capsular polysaccharide production by Rhizobium meliloti. J Bacteriol. 1997;179:2132–2140. doi: 10.1128/jb.179.7.2132-2140.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Petrovics G, Putnoky P, Reuhs B, Kim J, Thorp T A, Noel K D, Carlson R W, Kondorosi A. The presence of a novel type of surface polysaccharide in Rhizobium meliloti requires a new fatty acid synthase-like gene cluster involved in symbiotic nodule development. Mol Microbiol. 1993;8:1083–1094. doi: 10.1111/j.1365-2958.1993.tb01653.x. [DOI] [PubMed] [Google Scholar]
- 11.Putnoky, P. Personal communication.
- 12.Putnoky P, Grosskopf E, Ha D T C, Kiss G B, Kondorosi A. Rhizobium fix genes mediate at least two communication steps in symbiotic nodule development. J Cell Biol. 1988;106:597–607. doi: 10.1083/jcb.106.3.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Putnoky P, Petrovics G, Kereszt A, Grosskopf E, Ha D T C, Banfalvi Z, Kondorosi A. Rhizobium meliloti lipopolysaccharide and exopolysaccharide can have the same function in the plant-bacterium interaction. J Bacteriol. 1990;172:5450–5458. doi: 10.1128/jb.172.9.5450-5458.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reuber T L, Walker G C. Biosynthesis of succinoglycan, a symbiotically important exopolysaccharide of Rhizobium meliloti. Cell. 1993;74:269–280. doi: 10.1016/0092-8674(93)90418-p. [DOI] [PubMed] [Google Scholar]
- 15.Reuhs B L. Acidic capsular polysaccharides (K antigens) of Rhizobium. In: Stacey G, Mullin B, Gresshoff P M, editors. Biology of plant-microbe interactions. St. Paul, Minn: International Society for Molecular Plant-Microbe Interactions; 1996. pp. 331–336. [Google Scholar]
- 16.Reuhs B L, Carlson R W, Kim J S. Rhizobium fredii and Rhizobium meliloti produce 3-deoxy-d-manno-2-octulosonic acid-containing polysaccharides that are structurally analogous to group II K antigens (capsular polysaccharides) found in Escherichia coli. J Bacteriol. 1993;175:3570–3580. doi: 10.1128/jb.175.11.3570-3580.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reuhs B L, Williams M N V, Kim J S, Carlson R W, Côté F. Suppression of the Fix− phenotype of Rhizobium meliloti exoB mutants by lpsZ is correlated to a modified expression of the K polysaccharide. J Bacteriol. 1995;177:4289–4296. doi: 10.1128/jb.177.15.4289-4296.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Williams M N V, Hollingsworth R I, Brzoska P M, Signer E R. Rhizobium meliloti chromosomal loci required for suppression of exopolysaccharide mutants by lipopolysaccharide. J Bacteriol. 1990;172:6596–6598. doi: 10.1128/jb.172.11.6596-6598.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wood P J, Erfle J D, Teather R M. Use of complex formation between Congo red and polysaccharides in detection and assay of polysaccharide hydrolases. Methods Enzymol. 1988;160:59–74. [Google Scholar]