Abstract
The site-specific recombinase IntI1 found in class 1 integrons catalyzes the excision and integration of mobile gene cassettes, especially antibiotic resistance gene cassettes, with a site-specific recombination system. The integron integrase belongs to the tyrosine recombinase (phage integrase) family. The members of this family, exemplified by the lambda integrase, do not share extensive amino acid identities, but three invariant residues are found within two regions, designated box I and box II. Two conserved residues are arginines, one located in box I and one in box II, while the other conserved residue is a tyrosine located at the C terminus of box II. We have analyzed the properties of IntI1 variants carrying point mutations at the three conserved residues of the family in in vivo recombination and in vitro substrate binding. We have made four proteins with mutations of the conserved box I arginine (R146) and three mutants with changes of the box II arginine (R280); of these, MBP-IntI1(R146K) and MBP-IntI1(R280K) bind to the attI1 site in vitro, but only MBP-IntI1(R280K) is able to excise cassettes in vivo. However, the efficiency of recombination and DNA binding for MBP-IntI1(R280K) is lower than that obtained with the wild-type MBP-IntI1. We have also made two proteins with mutations of the tyrosine residue (Y312), and both mutant proteins are similar to the wild-type fusion protein in their DNA-binding capacity but are unable to catalyze in vivo recombination.
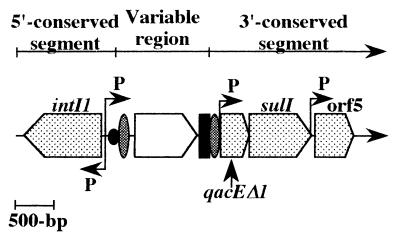
Integrons are DNA elements that capture genes, especially antibiotic resistance genes, by a site-specific recombination system (32). The recombination system consists of a DNA integrase (Int) and two types of recombination sites, attI and attC (59-base element). The integrase gene (int) is located in the 5′ conserved segment of the integron structure (Fig. 1) and is a member of the tyrosine recombinase family (1, 4, 13, 23, 24). Three types of integrases, sharing around 50% identity among themselves, have been identified; they define integron classes 1, 2, and 3 (30). The 5′ conserved segment found in class 1 integrons also contains a promoter region responsible for the expression of inserted cassettes (11, 21) and the recombination site attI1 (31). The 3′ conserved segment of the class 1 integrons includes an ethidium bromide resistance determinant (qacEΔ1), a sulfonamide resistance gene (sulI), an open reading frame (ORF5) of unknown function, and further sequences that differ from one integron to another (5, 6, 28). The 3′ conserved segment of class 2 integrons includes transposition genes (20) while that of class 3 integrons has not yet been studied (2). The variable region, located between the two conserved segments, usually contains antibiotic resistance genes; In0 contains no inserted genes while In21 possesses eight cassettes with ten genes (or ORFs) in this region (5, 16). These genes are part of mobile cassettes which include a recombination site, attC, that differs from one gene to another (18, 33). Incoming genes must be associated with an attC to be recognized by the integron integrase and are preferentially inserted at the recombination site attI1 (11). Cassettes are excised as circular intermediates and integrated at core sites by the action of the integrase (8–10). The core site, defined as GTTRRRY, makes up the 3′ end of attI1 and attC, with the crossover taking place between the G and the first T (19). Antibiotic selection pressure can reveal cassette rearrangements in which a given resistance is nearest the promoter and thus most strongly expressed (10).
FIG. 1.
General structure of class 1 integrons. Cassettes are inserted in the integron variable region by a site-specific recombination mechanism. The attI1 site is shown by a black circle, core sites are represented by ovals, the attC site is indicated by a black rectangle, and promoters are denoted by P. intIl, integrase gene; qacEΔ1, antiseptic resistance gene; sulI, sulfonamide resistance gene; orf5, gene of unknown function.
Site-specific recombination, unlike homologous recombination, is characterized by relatively short, specific DNA sequences and requires only limited homology of the recombining partners (12). Site-specific recombination is an entirely conservative process since all DNA strands that are broken (two per exchange site) are rejoined in a process that involves neither ATP nor DNA synthesis. Homology alignments of site-specific recombinases assign them to two families: the resolvase family, named after the TnpR proteins encoded by the transposons γδ and Tn3, and the integrase family. The integrase family includes over 140 members to date, but they are highly diversified proteins (13, 23). Members of this family, which include the well-studied λ integrase, recombine DNA duplexes by executing two consecutive strand breakage and rejoining steps and a topoisomerization of the reactants. The first pair of exchanges form a four-way Holliday junction and the second pair resolve the junction to complete the recombination. The integrase nucleophile is a conserved tyrosine that becomes associated with a phosphate group on DNA. The cleavage sites on each DNA duplex are separated by 6 to 8 bp with a 5′ stagger, and the tyrosine joins to the 3′ phosphate (17).
The initial definition of the integrase family was based on comparisons of seven sequences, and three invariant residues were identified: an HXXR cluster and a Y residue (4). Alignment of 28 sequences identified a fourth invariant position, occupied by an arginine residue (1). These four conserved residues are found in two boxes located in the second half of the protein. A recent analysis has shown that the conserved histidine is present in 136 of the 147 members (93%); this residue is then not conserved in all members of the family (13). Another recent analysis has identified three patches of residues located around box I, which seem to be important in the secondary structure of these proteins (23). In this study, we analyzed the properties of several mutants of the conserved residues R146, R280, and Y312 of the integron integrase IntI1 in in vivo recombination and in vitro substrate binding.
Construction of plasmids overexpressing mutant MBP-IntI1 fusion proteins.
The plasmids encoding various mutants of MBP-IntI1 were constructed by PCR using pLQ369 (50 ng) as a template (15). Two primer pairs, designed with the OLIGO software package (version 4.1; National Biosciences, Plymouth, Minn.), were used to construct each set of mutants. The R146 mutants were constructed with an XcmI-BamHI primer pair [IntI1(R146)-XcmI, 5′-TTCACCAGCTTCTGTATGGAACGGGCATG(A/G)(A/T)AATCAG-3′; IntI1(R146)-BamHI, 5′-CCGGATCCCTACCTCTCACT-3′], the R280 mutants were constructed with an NruI-XmnI primer pair [IntI1(R280)-NruI, 5′-AGCCGTCGCGAACGAGTGC(C/T)(C/T)GAGGG-3′; IntI1(R280)-XmnI, 5′-ACCCCTAATGAAGTGGTTCGTATCC-3′], and the Y312 mutants were constructed with a AatII-ScaI primer pair [IntI1(Y312)-AatII, 5′-ATTCCGACGTCTCTACTACGATGATTT(C/T)CACGC-3′; pLQ369-ScaI, 5′-ATGCTTTTCTGTGACTGGTG-3′] (restriction sites within primer sequences are underlined). PCR conditions were 10 min at 94°C, three cycles consisting of 45 s at 94°C, 45 s at 47°C, and 90 s at 72°C, 30 cycles consisting of 45 s at 94°C, 45 s at 60°C (50°C for Y312 mutants), and 90 s at 72°C, and a final elongation step of 10 min at 72°C. The XcmI, NruI, and AatII primers were degenerate in one or two positions, so that a single primer could give all mutants. Mutant PCR fragments were digested and cloned directly into pLQ369 digested with the same enzymes, except for the R146 mutant fragments that were subcloned into pLQ364 at first. New mutant PCR fragments were then amplified on these subclones, using IntI1(R146)-BamHI and IntI1(R280)-XmnI primers. These mutant PCR fragments were cleaved with BamHI and XmnI, and the resulting fragments were cloned into pLQ369. This avoids the necessity of partial digestion of pLQ369 with XcmI. Mutant clones were digested with restriction endonucleases and sequenced to determine the mutation.
In vivo recombination.
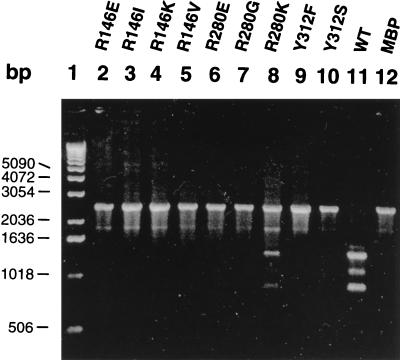
Mutant MBP-IntI1 clones were introduced into Escherichia coli TB1 {F′ araΔ(lac-proAB) rpsL (Strr) [φ80dlacΔ(lacZ)M15] hsdR(rK−mK−)} containing pLQ428 by transformation (Fig. 2 and Table 1). E. coli TB1 cells containing pLQ428 and one of the MBP-IntI1 mutants were grown at 37°C for 3 h in Luria-Bertani medium. Excision of the aacA1-ORFG and/or ORFH cassettes was induced by the overexpression of the malE-intI1 gene by using 0.3 mM isopropyl-β-d-thiogalactopyranoside (IPTG; Sigma Chemical Co.) and by incubation at 37°C for another 3 h. Cell culture was done in the presence of 50 μg of ampicillin per ml, 15 μg of amikacin per ml, and 50 μg of chloramphenicol per ml. Plasmid DNA was then prepared from 5-ml cultures with the Perfect Prep DNA extraction kit (Mandel Corporation). In order to determine the capacity of mutant MBP-IntI1 proteins to excise aacA1-ORFG and/or ORFH cassettes of In21, we used PCR primers pACYC184-5′ (5′-TGTAGCACCTGAAGTCAGCC-3′) and pACYC184-3′ (5′-ATACCCACGCCGAAACAAG-3′) (Fig. 2, primers 1 and 2) to detect the reduction of pLQ428 length. PCR conditions were 10 min at 94°C, 30 cycles consisting of 1 min at 94°C, 1 min at 60°C, and 5 min at 72°C, and a final elongation step of 10 min at 72°C. A major PCR fragment can be seen in each lane containing a DNA preparation from a mutant clone (Fig. 3, lanes 2 to 9). This band is 2,499 bp long and, as determined by restriction enzyme digestions, represents the pLQ428 clone without any cassette excision (data not shown). This band is also observed in the negative control, which is the pMAL-c2 vector without any gene fused to malE (Fig. 3, lane 12).
FIG. 2.
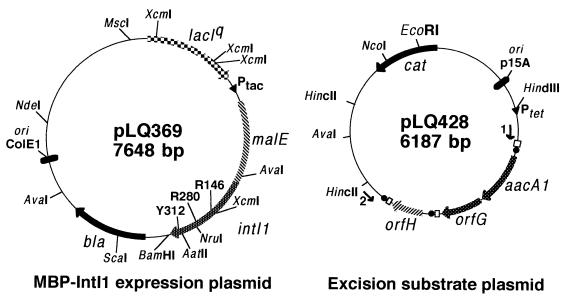
Representation of plasmids used in this study. The positions of the three invariant residues of the integrase family are indicated, along with restriction sites used to construct mutant proteins. Core sites are represented by black circles, and attCs are shown by white boxes. The numbered arrows represent the PCR primers used to detect excision events, pACYC184-5′ (1) and pACYC184-3′ (2). bla, gene encoding β-lactamase; cat, gene encoding chloramphenicol acetyltransferase; intIl, gene encoding the integron integrase (IntI1); malE, gene encoding the maltose binding protein (MBP); ori, origin of replication; Ptac, tac promoter; Ptet, tetracycline promoter. Only relevant restriction sites are indicated.
TABLE 1.
Plasmids used in this study
| Plasmid | Characteristic(s)a | Reference or source |
|---|---|---|
| pLQ363 | 2,190-bp EcoRI-HincII fragment of pLQ161 cloned in pLQ402 (Apr) | 16 |
| pLQ364 | 1,027-bp NcoI-BamHI PCR fragment amplified on pLQ860 and cloned in pET-3d (Apr) | This study |
| pLQ369 | 1,019-bp NdeI-BamHI PCR fragment modified to create a blunt-end 5′-ATG and cloned in pMAL-c2 cut with XmnI-BamHI (Apr) | 15 |
| pLQ376 | pLQ369 MBP-IntI1(R146K) (Apr) | This study |
| pLQ377 | pLQ369 MBP-IntI1(R146E) (Apr) | This study |
| pLQ378 | pLQ369 MBP-IntI1(R146I) (Apr) | This study |
| pLQ379 | pLQ369 MBP-IntI1(R146V) (Apr) | This study |
| pLQ388 | pLQ369 MBP-IntI1(R280G) (Apr) | This study |
| pLQ390 | pLQ369 MBP-IntI1(R280E) (Apr) | This study |
| pLQ391 | pLQ369 MBP-IntI1(R280K) (Apr) | This study |
| pLQ393 | pLQ369 MBP-IntI1(Y312S) (Apr) | This study |
| pLQ394 | pLQ369 MBP-IntI1(Y312F) (Apr) | This study |
| pLQ428 | 2,133-bp EcoRI (filled in)-BglII fragment of pLQ363 cloned in pACYC184 cut with EcoRV-BamHI (Akr Cmr) | This study |
| pLQ860 | 2,900-bp BamHI fragment of pVS1 cloned in pTZ19R (Apr Sulr) | 5 |
Akr, Apr, and Cmr, resistance to amikacin, ampicillin, and chloramphenicol.
FIG. 3.
Electrophoresis of PCR products obtained with the pACYC184 primer pair and 100 ng of DNA preparations from overexpressed cultures on a 1% agarose gel. Lane 1, 1-kb DNA ladder (Gibco BRL); lane 2, DNA preparation of pLQ428-pLQ377 (R146E); lane 3, pLQ428-pLQ378 (R146I); lane 4, pLQ428-pLQ376 (R146K); lane 5, pLQ428-pLQ379 (R146V); lane 6, pLQ428-pLQ390 (R280E); lane 7, pLQ428-pLQ388 (R280G); lane 8, pLQ428-pLQ391 (R280K); lane 9, pLQ428-pLQ394 (Y312F); lane 10, pLQ428-pLQ393 (Y312S); lane 11, pLQ428-pLQ369 (wild type); lane 12, pLQ428-pMAL-c2 (MBP).
The 2,499-bp PCR product was not obtained in the reaction containing the wild-type MBP-IntI1-expressing clone pLQ369 (Fig. 3, lane 11), indicating that there were no remaining full-length pLQ428 molecules. This shows that the wild-type fusion protein is very efficient in site-specific recombination and that all pLQ428 clones have undergone an excision of one or both cassettes. In this PCR, we observed two major bands of 1,341 and 889 bp. The 1,341-bp PCR product was digested with restriction enzymes to show that it represents a pLQ428 clone which has lost the aacA1-ORFG cassette (data not shown). The 889-bp band was also digested with restriction enzymes to show that it represents a pLQ428 clone which has lost both aacA1-ORFG and ORFH cassettes (data not shown). These two PCR products are also observed in the reaction containing the mutant clone pLQ391, which expresses the MBP-IntI1(R280K) fusion protein. This mutant protein is, however, less efficient than the wild-type protein, as seen by the intensity of the PCR products (Fig. 3, lane 8). We were not able to detect a PCR product of 2,047 bp, corresponding to the excision of the ORFH cassette alone; this is not surprising since this event has been shown in another study to be rare (16). It is possible to observe another band in pLQ428-pLQ391 (R280K) and pLQ428-pLQ369 (wild type) PCRs (Fig. 3, lanes 8 and 11); this PCR product is 1,100 bp long and probably represents a recombination event at a secondary site. Restriction enzyme digestions were done on this product, but we were unable to identify its origin. This product results from an event mediated by the integron integrase since it is seen only in reactions containing active proteins. An 1,800-bp PCR band is also present in the negative control and in all PCRs containing a mutant clone. This product appears to be nonspecific, and the fact that it is not seen in the PCR containing the pLQ428-pLQ369 (wild-type) clones probably results from the PCR being more favorable to smaller PCR products.
In vitro substrate binding.
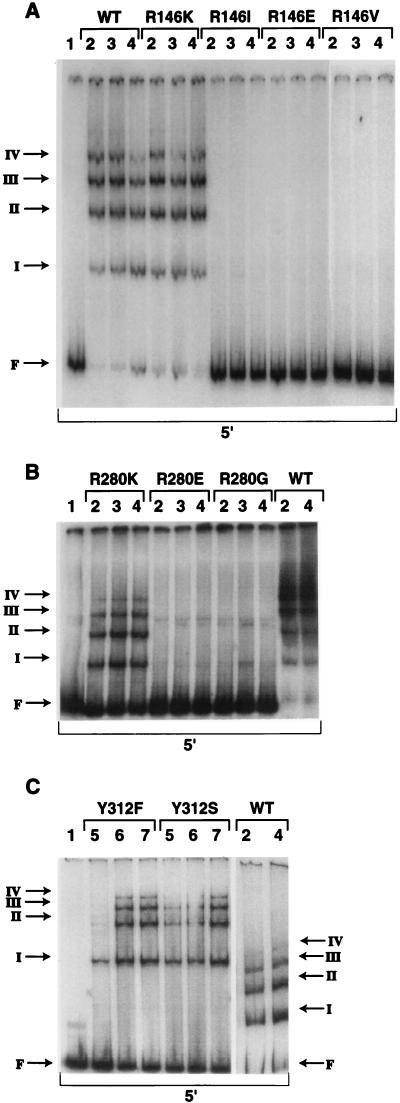
The experiments described above demonstrate that only one of our mutants of IntI1 protein is able to catalyze in vivo recombination. Can all mutant proteins recognize and bind to the IntI1 recombination site in a manner similar to the wild-type protein? To investigate this question, we used purified fusion proteins and a gel retardation assay with the complete attI1 site (5′ site) of the integron. MBP-IntI1 fusion proteins were purified as suggested by New England Biolabs. The concentration of the purified fusion protein was determined by using the Bradford protein assay (Bio-Rad). The protein solution was then made 20% in glycerol and stored at −80°C. The purity of MBP-IntI1 was evaluated as >90% by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (data not shown). Binding reactions were done with labeled 5′-site DNA fragments (20,000 cpm, 0.25 pmol), incubated with different concentrations of MBP-IntI1 in a 10-μl volume containing 10 mM HEPES (K+, pH 8.0), 60 mM KCl, 4 mM MgCl2, 100 μM EDTA (pH 8.0), 100 μg of bovine serum albumin per ml, 250 μM dithiothreitol, 100 ng of poly(dI-dC), and 10% glycerol. Reaction mixtures were incubated at room temperature for 15 min prior to electrophoresis through 4 or 5% prerun, nondenaturing polyacrylamide gels buffered with 0.5× Tris-borate-EDTA. Dried gels were subjected to autoradiography. The wild-type fusion protein and native IntI1 were shown to lead to the same four distinct complexes (I, II, III, and IV) with this DNA substrate (Fig. 4) (15). These complexes represent the binding of four IntI1 molecules to four different sites in the attI1 site (15). Figure 4 shows results obtained with nine mutants of the MBP-IntI1 fusion protein. We observed that MBP-IntI1(R146E), MBP-IntI1(R146I), and MBP-IntI1(R146V) lost their ability to bind to the attI1 site, as no complexes are seen in the gel retardation experiment (Fig. 4A). However, MBP-IntI1(R146K) formed four IntI1-DNA complexes with the 5′ site DNA fragment. The band pattern and the intensity observed with this mutant protein are similar to those observed with the wild-type protein, suggesting that MBP-IntI1(R146K) and MBP-IntI1 bind DNA with similar affinities.
FIG. 4.
Binding of mutant MBP-IntI1 fusion proteins purified from E. coli TB1 to the 5′-site DNA fragment containing the complete attI1 site of the In2 integron (from nucleotide −96 to nucleotide +71, relative to the G residue of the core site as position 0). (A) MBP-IntI1(R146) mutants; (B) MBP-IntI1(R280) mutants; (C) MBP-IntI1(Y312) mutants. A purified labeled fragment was incubated with different concentrations of mutant fusion proteins. Free DNA (F) and protein-DNA complexes (I, II, III, and IV) were separated on 4 or 5% polyacrylamide gels and are indicated by arrows. Lanes 1, free DNA; lanes 2 through 7, purified fusion protein at 250, 375, 500, 12.5, 37.5, and 62.5 nM, respectively. The wild-type (WT) lanes in panel C were from a separate gel.
Competition with a specific fragment with a 30-fold excess of unlabeled DNA competed away all four complexes, while a 100-fold excess of a nonspecific unlabeled DNA fragment did not compete away any complexes, indicating their specificity (data not shown) (15). We observed that MBP-IntI1(R280G) and MBP-IntI1(R280E) lost their ability to bind the 5′-site DNA fragment, while the MBP-IntI1(R280K) could still bind the attI1 site (Fig. 4B). However, the band pattern obtained with this mutant protein is weaker than that obtained with the wild-type integrase. For example, at a protein concentration of 250 nM MBP-IntI1(R280K) (lane 2), we observed the formation of complexes I, II, and III, with a stronger intensity for the fastest-migrating complexes, while the intensity of the fourth complex was very weak. At the same concentration of the wild-type protein, we observed the formation of all four complexes, with a stronger intensity for the slowest-migrating complexes and no unbound DNA. These results show that MBP-IntI1(R280K) binds the attI1 site with a lower affinity than the wild-type fusion protein. As shown in Fig. 4C, both MBP-IntI1(Y312F) and MBP-IntI1(Y312S) lead to the formation of four complexes that migrate similarity to those obtained with wild-type MBP-IntI1, as judged by the gel migration of these complexes. The band pattern observed shows that the binding affinity of these mutant proteins is the same as or even better than that of the wild-type protein.
Relationships with other members of the family.
We found that MBP-IntI1 recombinase in which Arg-146 has been changed to lysine [MBP-IntI1(R146K)] by PCR mutagenesis cannot excise cassettes but can bind to the attI1 site with the same efficiency as the wild-type fusion protein. However, MBP-IntI1(R146I), MBP-IntI1(R146E), and MBP-IntI1(R146V) mutant proteins have completely lost both phenotypes. These findings are different from those for other members of the family. The only mutant protein of the lambda integrase at this residue [λ(R212Q)] binds the core site partially and is not able to catalyze in vivo or in vitro recombination (22). Mutants of the Cre recombinase with a change at this residue [Cre(R173K)] bind DNA as well as the wild-type protein but cannot catalyze in vivo or in vitro recombination (1). Mutants of Flp [Flp(R191K) and Flp(R191E)] bind FRT recombination sites as well as the wild-type protein but cannot carry out in vivo or in vitro recombination, except for the Flp(R191K) protein, which has shown slight activity in in vivo recombination (Table 2) (7, 14, 25). Therefore, the Cre(R173K) and Flp(R146K) mutants have the same phenotype as the MBP-IntI1(R146K) protein. However, the Flp(R191E) mutant protein shows efficient DNA binding while MBP-IntI1(R146E) does not bind to the attI1 site. We interpret these results according to the charge of the Arg-146 residue. The positively charged side chain of this residue makes contact with the DNA, which is negatively charged. This contact is probably important for the good conformation of the protein molecule in positioning the tyrosine residue to perform recombination. When this residue is exchanged for a lysine, DNA contacts are still able to take place because of the charge of the residue, but the side chain is smaller and the lysine is probably not able to position the tyrosine to catalyze recombination. We think that the charge of this residue is very important in the formation of DNA-protein complexes in the integron system, since all other MBP-IntI1 mutants tested are unable to bind DNA. This observation differs from those for Flp, because even when the wild-type residue was replaced by a negatively charged one, it could still bind DNA as well as the wild-type protein (Table 2).
TABLE 2.
Mutational analysis of IntI1 and corresponding residues of other recombinases from the Int family
| Recombinase | Mutation | DNA binding | Recombination | Reference(s) |
|---|---|---|---|---|
| λInt | R212Q | Yesa | No | 22 |
| λInt | Y342F | Yes | No | 22, 26 |
| Flp | R191E | Yes | No | 7 |
| Flp | R191K | Yes | Yes | 7, 14 |
| Flp | R308G | Yes | No | 27 |
| Flp | R308K | Yes | Yesa | 27 |
| Flp | Y343F | Yes | No | 29 |
| Flp | Y343S | Yes | No | 29 |
| Cre | R173K | Yes | No | 1 |
| P2 | R272K | NDb | No | 23 |
| XerC | Y275F | Yes | No | 3 |
| XerD | Y279F | Yes | No | 3 |
| IntI1 | R146E | No | No | This study |
| IntI1 | R146I | No | No | This study |
| IntI1 | R146K | Yes | No | This study |
| IntI1 | R146V | No | No | This study |
| IntI1 | R280E | No | No | This study |
| IntI1 | R280G | No | No | This study |
| IntI1 | R280K | Yes | Yesa | This study |
| IntI1 | Y312F | Yes | No | This study |
| IntI1 | Y312S | Yes | No | This study |
Less efficient than the wild-type protein.
ND, not determined.
We have also made proteins with mutations at position 280; these were MBP-IntI1(R280E), MBP-IntI1(R280G), and MBP-IntI1(R280K). We found that the MBP-IntI1(R280K) mutant protein binds the attI1 site and excises integron cassettes with a lower efficiency than the wild-type MBP-IntI1, while MBP-IntI1(R280E) and MBP-IntI1(R280G) have completely lost both phenotypes. The Flp(R308K) mutant protein has been shown to bind DNA as well as the wild-type protein, but it recombines DNA with a lower efficiency than wild-type Flp (27). Another mutant protein of Flp [Flp(R308G)] has also been shown to bind DNA as well as the wild-type protein, but it was unable to catalyze in vivo or in vitro recombination (27). These results show that Flp(R308K) and MBP-IntI1(R280K) act similarly but that the other Flp mutant [Flp(R308G)] can bind DNA while the MBP-IntI1 mutant [MBP-IntI1(R280G)] cannot (Table 2). We also think that the positive charge of this residue is important for the binding of the recombinase to DNA, but Arg-280 does not seem to be implicated in the positioning of the tyrosine residue, since the MBP-IntI1(R280K) mutant protein can perform recombination.
We found that MBP-IntI1(Y312S) and MBP-IntI1(Y312F) mutant proteins bind the attI1 site with the same efficiency as the wild-type protein but are not able to catalyze in vivo recombination. As expected, these results are the same as those obtained with the lambda integrase [λ(Y342F)], the XerC and XerD recombinases [XerC(Y275F) and XerD(Y279F)], and the Flp recombinases [Flp(Y343S) and Flp(Y343F)] (Table 2) (3, 22, 26, 29). The loss of the catalytic activity of the MBP-IntI1(Y312F) mutant protein is not surprising, since the hydroxyl group of the tyrosine, which is responsible for the nucleophilic attack of the DNA at the recombination site, is not present on the phenylalanine residue. The phenotype of MBP-IntI1(Y312S) indicates that the conformation of the tyrosine residue is important for the good activity of the recombinase, because even if the serine residue has a hydroxyl group, it is not able to catalyze recombination. These results indicate that the integron integrase IntI1 uses the hydroxyl group of the conserved tyrosine (Y312) to catalyze site-specific recombination, like other members of the family. However, in vitro recombination using this mutant protein needs to be done to confirm this.
These results of point mutations show that mutations of the conserved arginines by nonpositively charged residues abolish substrate recognition, unlike the corresponding mutants of other members of the family. However, further mutational analysis, such as of residues around and in patch III, would be interesting, since only integron integrases contain more residues in this region than other members of the family (23). In vitro recombination assays with purified mutant proteins also need to be done in order to study thoroughly the mechanism of site-specific recombination in integrons.
Acknowledgments
We thank France Gagnon for the construction of pLQ428 and technical assistance. We thank Jean Renaud for automatic sequencing and Bénédicte Fournier for helpful discussions. We thank Dominic Esposito for the maintenance of the Tyrosine Recombinases Web Site (http://orac.niggk.nih.gov/www/trhome.html), which has helped us in the statistical analysis of the Int family.
This work was supported by grant MT-13564 from the Medical Research Council (MRC) of Canada to P. H. Roy. A. Gravel and N. Messier held fellowships from MRC Canada.
ADDENDUM IN PROOF
A recent article by C. M. Collis, M.-J. Kim, H. W. Stokes, and R. M. Hall (Mol. Microbiol. 29:477–490, 1998) shows that FLAG-IntI1 mutant proteins R280G and Y312F cannot catalyze cointegrate formation. These results correspond to our results found in in vivo recombination experiments for the same mutant MBP-IntI1 proteins.
REFERENCES
- 1.Abremski K E, Hoess R H. Evidence for a second conserved arginine residue in the integrase family of recombination proteins. Protein Eng. 1992;5:87–91. doi: 10.1093/protein/5.1.87. [DOI] [PubMed] [Google Scholar]
- 2.Arakawa Y, Murakami M, Suzuki K, Ito H, Wacharotayankun R, Ohsuka S, Kato N, Ohta M. A novel integron-like element carrying the metallo-β-lactamase gene blaIMP. Antimicrob Agents Chemother. 1995;39:1612–1615. doi: 10.1128/aac.39.7.1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arciszewska L K, Sherratt D J. Xer site-specific recombination in vitro. EMBO J. 1995;14:2112–2120. doi: 10.1002/j.1460-2075.1995.tb07203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Argos P, Landy A, Abremski K, Egan J B, Håggard-Ljungquist E, Hoess R H, Kahn M L, Kalinoes B, Narayana S V L, Pierson III L S, Sternberg N, Leong J M. The integrase family of site-specific recombinases: regional similarities and global diversity. EMBO J. 1986;5:433–440. doi: 10.1002/j.1460-2075.1986.tb04229.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bissonnette L, Roy P H. Characterization of In0 of Pseudomonas aeruginosa plasmid pVS1, an ancestor of integrons of multiresistance plasmids and transposons of gram-negative bacteria. J Bacteriol. 1992;174:1248–1257. doi: 10.1128/jb.174.4.1248-1257.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown H J, Stokes H W, Hall R M. The integrons In0, In2, and In5 are defective transposon derivatives. J Bacteriol. 1996;178:4429–4437. doi: 10.1128/jb.178.15.4429-4437.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen J-W, Evans B R, Yang S-H, Araki H, Oshima Y, Jayaram M. Functional analysis of box I mutations in yeast site-specific recombinases Flp and R: pairwise complementation with recombinase variants lacking the active-site tyrosine. Mol Cell Biol. 1992;12:3757–3765. doi: 10.1128/mcb.12.9.3757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Collis C M, Grammaticopoulos G, Briton J, Stokes H W, Hall R M. Site-specific insertion of gene cassettes into integrons. Mol Microbiol. 1993;9:41–52. doi: 10.1111/j.1365-2958.1993.tb01667.x. [DOI] [PubMed] [Google Scholar]
- 9.Collis C M, Hall R M. Gene cassettes from the insert region of integrons are excised as covalently closed circles. Mol Microbiol. 1992;6:2875–2885. doi: 10.1111/j.1365-2958.1992.tb01467.x. [DOI] [PubMed] [Google Scholar]
- 10.Collis C M, Hall R M. Site-specific deletion and rearrangement of integron insert genes catalyzed by the integron DNA integrase. J Bacteriol. 1992;174:1574–1585. doi: 10.1128/jb.174.5.1574-1585.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Collis C M, Hall R M. Expression of antibiotic resistance genes in the integrated cassettes of integrons. Antimicrob Agents Chemother. 1995;39:155–162. doi: 10.1128/aac.39.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Craig N L. The mechanism of conservative site-specific recombination. Annu Rev Genet. 1988;22:77–105. doi: 10.1146/annurev.ge.22.120188.000453. [DOI] [PubMed] [Google Scholar]
- 13.Esposito D, Scocca J J. The integrase family of tyrosine recombinases: evolution of a conserved active site domain. Nucleic Acids Res. 1997;25:3605–3614. doi: 10.1093/nar/25.18.3605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Friesen H, Sadowski P D. Mutagenesis of a conserved region of the gene encoding the FLP recombinase of Saccharomyces cerevisiae. A role for arginine 191 in binding and ligation. J Mol Biol. 1992;225:313–326. doi: 10.1016/0022-2836(92)90924-9. [DOI] [PubMed] [Google Scholar]
- 15.Gravel, A., B. Fournier, and P. H. Roy. DNA-complexes obtained with the integron integrase IntI1 at the attI1 site. Nucleic Acids Res., in press. [DOI] [PMC free article] [PubMed]
- 16.Gravel, A., R. Parent, and P. H. Roy. Sequence analysis and cassette mobility of the In21 integron from transposon Tn2424. Submitted for publication.
- 17.Grindley N D F. Site-specific recombination: synapsis and strand exchange revealed. Curr Biol. 1997;7:R608–R612. doi: 10.1016/s0960-9822(06)00314-9. [DOI] [PubMed] [Google Scholar]
- 18.Hall R M, Brookes D E, Stokes H W. Site-specific insertion of genes into integrons: role of the 59-base element and determination of the recombination cross-over point. Mol Microbiol. 1991;5:1941–1959. doi: 10.1111/j.1365-2958.1991.tb00817.x. [DOI] [PubMed] [Google Scholar]
- 19.Hansson K, Sköld O, Sundström L. Non-palindromic attI sites of integrons are capable of site-specific recombination with one another and with secondary targets. Mol Microbiol. 1997;26:441–453. doi: 10.1046/j.1365-2958.1997.5401964.x. [DOI] [PubMed] [Google Scholar]
- 20.Hansson, K., L. Sundström, A. Pelletier, and P. H. Roy. Activation by mutagenesis of a novel integron integrase, IntI2, in Tn7. Submitted for publication.
- 21.Lévesque C, Brassard S, Lapointe J, Roy P H. Diversity and relative strength of tandem promoters for the antibiotic-resistance genes of several integrons. Gene. 1994;142:49–54. doi: 10.1016/0378-1119(94)90353-0. [DOI] [PubMed] [Google Scholar]
- 22.MacWilliams M P, Gumport R I, Gardner J F. Genetic analysis of the bacteriophage λ attL nucleoprotein complex. Genetics. 1996;143:1069–1079. doi: 10.1093/genetics/143.3.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nunes-Düby S E, Kwon H J, Tirumalai R S, Ellenberger T, Landy A. Similarities and differences among 105 members of the Int family of site-specific recombinases. Nucleic Acids Res. 1998;26:391–406. doi: 10.1093/nar/26.2.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ouellette M, Roy P H. Homology of ORFs from Tn2603 and from R46 to site-specific recombinases. Nucleic Acids Res. 1987;15:10055. doi: 10.1093/nar/15.23.10055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pan G, Luetke K, Sadowski P D. Mechanism of cleavage and ligation by FLP recombinase: classification of mutations in FLP protein by in vitro complementation analysis. Mol Cell Biol. 1993;13:3167–3175. doi: 10.1128/mcb.13.6.3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pargellis C A, Nunes-Düby S E, Moitoso de Vargas L, Landy A. Suicide recombination substrates yield covalent λ integrase-DNA complexes and lead to identification of the active site tyrosine. J Biol Chem. 1988;263:7678–7685. [PubMed] [Google Scholar]
- 27.Parsons R L, Evans B R, Zheng L, Jayaram M. Functional analysis of Arg-308 mutants of Flp recombinase. J Biol Chem. 1990;265:4527–4533. [PubMed] [Google Scholar]
- 28.Paulsen I T, Littlejohn T G, Rådström P, Sundström L, Sköld O, Swedberg G, Skurray R A. The 3′ conserved segment of integrons contains a gene associated with multidrug resistance to antiseptics and disinfectants. Antimicrob Agents Chemother. 1993;37:761–768. doi: 10.1128/aac.37.4.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Prasad P V, Young L-J, Jayaram M. Mutations in the 2-μm circle site-specific recombinase that abolish recombination without affecting substrate recognition. Proc Natl Acad Sci USA. 1987;84:2189–2193. doi: 10.1073/pnas.84.8.2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Recchia G D, Hall R M. Gene cassettes: a new class of mobile elements. Microbiology (Reading) 1995;141:3015–3027. doi: 10.1099/13500872-141-12-3015. [DOI] [PubMed] [Google Scholar]
- 31.Recchia G D, Stokes H W, Hall R M. Characterization of specific and secondary recombination sites recognised by the integron DNA integrase. Nucleic Acids Res. 1994;22:2071–2078. doi: 10.1093/nar/22.11.2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stokes H W, Hall R M. A novel family of potentially mobile DNA elements encoding site-specific gene-integration functions: integrons. Mol Microbiol. 1989;3:1669–1683. doi: 10.1111/j.1365-2958.1989.tb00153.x. [DOI] [PubMed] [Google Scholar]
- 33.Stokes H W, O’Gorman D B, Recchia G D, Parsekhian M, Hall R M. Structure and function of 59-base element recombination sites associated with mobile gene cassettes. Mol Microbiol. 1997;26:731–745. doi: 10.1046/j.1365-2958.1997.6091980.x. [DOI] [PubMed] [Google Scholar]