Abstract
Background
Bacterial colonization is an essential aspect of bronchiectasis. Although Haemophilus influenzae is a frequent colonizer in some regions, its clinical impacts are poorly understood. This study aimed to elucidate the impact of H. influenzae colonization in patients with bronchiectasis.
Methods
This retrospective study screened adult patients diagnosed with bronchiectasis at a tertiary referral center between April 1, 2003, and May 16, 2021, in South Korea. Propensity score matching was used to match patients with and without H. influenzae colonization. We assessed the severity of bronchiectasis as per the bronchiectasis severity index, the incidence of exacerbation, differences in lung function, and all-cause mortality.
Results
Out of the 4,500 patients with bronchiectasis, 79 (1.8%) were colonized by H. influenzae. After 1:2 propensity score matching, 78 and 154 patients were selected from the H. influenzae colonizer and non-colonizer groups, respectively. Although there were no significant differences between the groups regarding baseline demographics, patients colonized with H. influenzae had a higher bronchiectasis severity index (median 6 [interquartile range 4–8] vs. 4 [2–7], p = 0.002), associated with extensive radiographic involvement (52.2% vs. 37.2%, p = 0.045) and mild exacerbation without hospitalization (adjusted incidence rate ratio 0.15; 95% confidence interval 0.12–0.24). Lung function and mortality rates did not reveal significant differences, regardless of H. influenzae colonization.
Conclusion
H. influenzae colonization in bronchiectasis was associated with more severe disease and greater incidence of mild exacerbation, but not lung function and mortality. Attention should be paid to patients with bronchiectasis with H. influenzae colonization.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12890-023-02823-8.
Keywords: Bronchiectasis, Haemophilus influenzae, Pseudomonas, Propensity scores, Prognostic factor
Background
Bronchiectasis is characterized by structural abnormalities of the airways, accompanied by bronchial tissue destruction [1]. Symptoms of bronchiectasis include recurrent respiratory infections that require antibiotics, cough with phlegm, shortness of breath, and intermittent hemoptysis [2]. Chronic inflammation and infection of the bronchi and airways lead to chronic bacterial colonization, which further causes inflammation and structural damage to the airways, creating the so-called “vicious cycle” of bronchiectasis [3]. There are only a few evidence-based treatments for bronchiectasis, and they are all primarily aimed at treating bacterial colonization [4].
The predominant colonizers in bronchiectasis are Pseudomonas aeruginosa and Haemophilus influenzae [5]. The microbiome shows regional variation: P. aeruginosa is the most prevalent species in the U.S., whereas, in Europe, it is H. influenzae [6]. Both bacteria can form biofilms that protect the bacteria from the body’s immune system or reduce systemic delivery of antibiotics, increasing antibiotic resistance and causing an inflammatory response, leading to additional airway damage, thereby creating a vicious cycle [1, 7].
It is well known that P. aeruginosa colonization contributes to pulmonary dysfunction and frequent exacerbations and is associated with high mortality risk [8]. H. influenzae was repeatedly cultured in stable chronic obstructive pulmonary disease (COPD) patients with bronchiectasis [9]. Chronic H. influenzae colonizers show increased inflammation and disease activity [10]. Considering that both bronchiectasis and COPD are respiratory diseases of a chronic nature, we hypothesized that H. influenzae colonization could be of clinical importance.
Despite the high prevalence of H. influenzae in bronchiectasis in some regions, the clinical impact of this colonization is not well known [5]. We aimed to evaluate the effects of H. influenzae colonization on the characteristics and clinical outcomes of patients diagnosed with bronchiectasis.
Methods
Study design and patient selection
This was a single-center, retrospective cohort study of patients with bronchiectasis at Seoul National University Bundang Hospital, Republic of Korea. Adult (> 18 years) patients were screened according to their International Classification of Diseases-10 codes in the electronic health records system. We reviewed medical records and selected patients with a diagnosis of bronchiectasis who had chest computed tomography (CT) results and sputum cultures. To rule out the influence of nontuberculous mycobacteria, the importance of which has already been well established, patients in whom these bacteria were identified were excluded. Patients included in this study were selected from those who submitted sputum for further testing. The method of sputum collection involved patients expectorating sputum spontaneously, without adherence to a pre-defined protocol. The day of bronchiectasis diagnosis was defined as the date on which the diagnosis of bronchiectasis was entered into the electronic medical record system, and the date of the most recent outpatient visit was determined as the date of the last follow-up. Patients were divided into two groups according to the presence of H. influenzae from sputum culture during their stable status [11].
Propensity score matching and data collection
Due to the largely imbalanced data, we used a propensity score-based method to reduce the effects of disturbances in observational studies. The propensity score matching process was performed using the following variables to ensure comparability between groups: age, sex, colonization of P. aeruginosa or other bacteria, follow-up duration, and the number of pulmonary function tests [12, 13].
After matching, demographics, comorbidities, radiographic, laboratory, and microbiological findings were reviewed, and the bronchiectasis severity index (BSI) was calculated [14]. The BSI consists of age, body mass index (BMI), forced expiratory volume in 1 s (FEV1), hospitalization history, exacerbation frequency, degree of breathlessness assessed by the modified Medical Research Council dyspnea scale, bacterial colonization status, and radiographic findings [14]. Comorbidities included chronic lung diseases such as COPD and asthma and systemic diseases possibly associated with bronchiectasis such as rheumatoid arthritis and inflammatory bowel disease. The cause of bronchiectasis was defined as infectious if there was a past or childhood infection such as tuberculosis, pertussis, or measles, and idiopathic if there was no specific history [5]. Bacterial colonization was defined as the detection of bacteria at least once from the sputum sample during a stable status [11].
The Seoul National University Bundang Hospital Institutional Review Board approved this study (protocol number B-2106-689-106) and waived the need for informed consent owing to the study’s observational nature and the use of anonymized data. This study was conducted in accordance with the Declaration of Helsinki.
Outcome measures
Exacerbation of bronchiectasis was defined as acute aggravation of respiratory symptoms such as changes in sputum nature, shortness of breath, increased cough or fatigue, and hemoptysis. Mild exacerbation was defined as that requiring an outpatient prescription of oral antibiotics, and severe exacerbation was defined as a worsening course and hospitalization or embolization [14]. Incidence rate was used to effectively report the frequency of acute exacerbations in patients with bronchiectasis, and negative binomial regression analysis was performed to compare exacerbation incidence rates between groups.
Pulmonary function indicators, including FEV1, forced vital capacity (FVC), and FEV1/FVC ratio, and the results obtained at an outpatient clinic within two years before and after diagnosis were adopted as the baseline pulmonary function. The date of death was obtained from data requested by the Ministry of Public Administration and Security. Linear mixed regression analyses were performed to compare the repeated measures of pulmonary function.
Other statistical considerations
A standard (unconditional) analysis was performed and considered valid [15]. Simple descriptive statistics of the mean with standard deviation were used for continuous parametric data, median with interquartile range (IQR) was used for continuous nonparametric data, and frequencies and percentages for categorical data. Subgroup comparisons were performed using the chi-squared test, Fisher’s exact test, and Mann-Whitney U Test, depending on data distribution. Kaplan-Meier curves and log-rank tests were used for survival analyses [16]. The Division of Statistics in the Medical Research Collaborating Centre reviewed and approved the statistical analyses at Seoul National University Bundang Hospital. The REporting of studies Conducted using Observational Routinely-collected Data checklist is available in the online supplement [see Additional file 1].
Results
Propensity score matching and patient characteristics
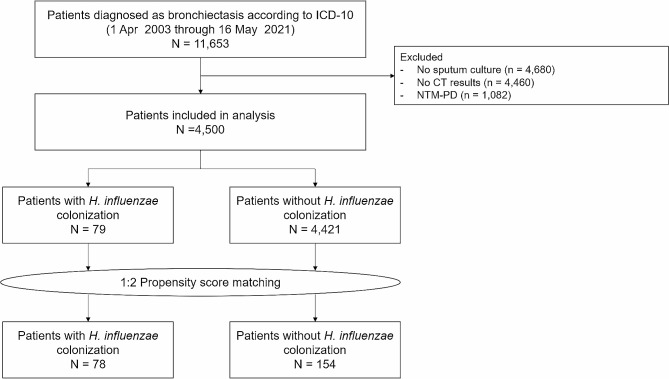
Among the patients who visited the outpatient department of pulmonology from April 1, 2003, to May 16, 2021, 11,653 patients were screened to be diagnosed with bronchiectasis according to the International Classification of Diseases-10 in our electronic health records system. After excluding 4,680 patients without sputum culture results, 4,460 patients without CT scans, and 1,082 patients with nontuberculous mycobacteria, 4,500 adult patients were included in the analysis (Fig. 1).
Fig. 1.
Flowchart of the patient selection process
Out of the 4,500 patients, 79 (1.8%) were colonized by H. influenzae. After 2:1 propensity score matching of the remaining 4,421 patients, 156 were selected. H. influenzae colonizer (n = 78) and non-colonizer (n = 156) groups showed significant differences in terms of P. aeruginosa colonization (p < 0.001), colonization with organisms other than H. influenzae and P. aeruginosa (p = 0.001), duration of follow-up (p < 0.001), and the number of pulmonary function tests performed (p = 0.005). The significant differences observed between the two groups are listed in Table 1.
Table 1.
Patient characteristics before and after matching
| Variables | Before matching | After matching | ||||
|---|---|---|---|---|---|---|
|
H. Influenzae
colonizers |
H. Influenzae
non-colonizers |
P |
H. Influenzae
colonizers |
H. Influenzae
non-colonizers |
P | |
| n = 79 | n = 4,421 | n = 78 | n = 154 | |||
| Age | 59.0 [51.5–65.0] | 60.0 [52.0–67.0] | 0.298 | 59.0 [52.0–65.0] | 58.5 [51.0–66.0] | 0.977 |
| Sex, male | 32 (40.5) | 1859 (42.0) | 0.873 | 32 (41.0) | 64 (41.6) | > 0.999 |
| Colonization of Pseudomonas aeruginosa | 24 (30.4) | 549 (12.4) | < 0.001 | 23 (29.5) | 45 (29.2) | > 0.999 |
| Colonization of any other bacteria | 37 (46.8) | 1293 (29.2) | 0.001 | 36 (46.2) | 65 (42.2) | 0.665 |
| Follow-up duration, years | 7.90 [2.56–13.39] | 4.02 [1.14–8.40] | < 0.001 | 7.73 [2.52–13.37] | 9.10 [2.68–13.90] | 0.919 |
| Number of PFTs, counts | 2.00 [1.00–5.00] | 1.00 [1.00–3.00] | 0.050 | 2.00 [1.00–5.00] | 2.00 [1.00–5.00] | 0.486 |
Data are shown as count (percentage) or median [interquartile range]. Abbreviations: PFT, pulmonary function test
In the overall patient group, the median age was 59 (IQR, 51–66) years, and there was a female predominance (58.6%). The most common comorbidities were a history of tuberculosis (34.4%), hypertension (21.6%), and COPD (19.1%). There were no significant differences between the two groups regarding BMI (p = 0.233), smoking history (p = 0.497), underlying comorbidities, or possible causes of bronchiectasis. The patient characteristics of the two groups are described in detail in Table 2.
Table 2.
Baseline characteristics of bronchiectasis patients selected after propensity score matching
| Variables | Total |
H. Influenzae
colonizers |
H. Influenzae
non-colonizers |
P |
|---|---|---|---|---|
| N = 232 | n = 78 | n = 154 | ||
| Age, years | 59.00 [51.00–66.00] | 58.50 [51.00–66.25] | 59.00 [51.75–65.00] | 0.977 |
| Male sex | 96 (41.4) | 32 (41.0) | 64 (41.6) | > 0.999 |
| BMI, kg/m 2 | 21.55 [19.80–25.29] | 22.41 [19.41–25.26] | 22.65 [20.63–25.40] | 0.233 |
| Smoking | 0.497 | |||
| Never smoker | 179 (74.3) | 60 (76.9) | 119 (77.3) | |
| Former smoker | 25 (10.4) | 6 (7.7) | 19 (12.3) | |
| Current smoker | 27 (11.2) | 12 (15.4) | 15 (9.7) | |
| Comorbidities | ||||
| TB history | 83 (34.4) | 25 (32.1) | 58 (37.7) | 0.382 |
| Hypertension | 52 (21.6) | 18 (23.1) | 34 (22.1) | 0.884 |
| COPD | 46 (19.1) | 21 (26.9) | 25 (16.2) | 0.074 |
| Asthma | 38 (15.8) | 18 (23.1) | 20 (13.0) | 0.073 |
| Possible cause of bronchiectasis | ||||
| Idiopathic | 142 (58.9) | 49 (62.8) | 93 (60.4) | 0.765 |
| Infectious | 88 (36.5) | 29 (37.2) | 59 (37.7) | 0.769 |
Data are shown as count (percentage) or median [interquartile range]. Abbreviations: BMI, Body Mass Index; TB, Tuberculosis; COPD, Chronic obstructive pulmonary disease
Comparison of BSI score
The total BSI score was significantly higher in the H. influenzae colonizer group (median 6 [IQR, 4–8] vs. 4 [IQR, 2–7], p = 0.002). Patients colonized by H. Influenzae had more frequent exacerbations (40.6% vs. 18.6%, p = 0.002) and more extensive radiographic involvement (52.2% vs. 37.2%, p = 0.045). However, age (p = 0.676), BMI (p = 0.898), FEV1 (p = 0.204), modified Medical Research Council dyspnea scale (p = 0.321), and P. aeuruginosa colonization rate (p = 0.725) were not significantly different between the two groups (Table 3).
Table 3.
Comparison of bronchiectasis severity index according to the colonization of Haemophilus influenzae
| Variables | Score points | Total |
H. Influenzae
colonizers |
H. Influenzae
non-colonizers |
P |
|---|---|---|---|---|---|
| N = 198 | n = 69 | n = 129 | |||
| Age, years | 0.676 | ||||
| <50 | 0 | 42 (21.2) | 14 (20.3) | 28 (21.7) | |
| 50–69 | 2 | 129 (65.2) | 46 (66.7) | 83 (64.3) | |
| 70–79 | 4 | 21 (10.6) | 9 (13.0) | 12 (9.3) | |
| 80+ | 6 | 6 (3.0) | 0 (0.0) | 6 (4.7) | |
| BMI < 18.5 kg/m2 | 2 | 25(12.6) | 16 (12.4) | 9 (13.0) | 0.898 |
| FEV1, % predicted | 0.204 | ||||
| >80 | 0 | 108 (54.5) | 34 (49.3) | 74 (57.4) | |
| 50–80 | 1 | 70 (35.4) | 27 (39.1) | 43 (33.3) | |
| 30–49 | 2 | 18 (9.1) | 6 (8.7) | 12 (9.3) | |
| <30 | 3 | 2 (1.0) | 2 (2.9) | 0 (0.0) | |
| Hospital admission before study within two years | 5 | 16 (8.1) | 7 (10.1) | 9 (7.0) | 0.438 |
| Exacerbations before study within two years ≥ 3 | 2 | 52 (26.3) | 28 (40.6) | 24 (18.6) | 0.002 |
| MRC dyspnea score | 0.321 | ||||
| 0 | 0 | 197 (99.5) | 68 (98.6) | 129(100.0) | |
| 1–2 | 2 | 1 (0.5) | 1 (1.4) | 0 (0.0) | |
| ≥3 | 3 | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Pseudomonas colonization | 3 | 60 (30.3) | 22 (31.9) | 38 (29.5) | 0.725 |
| Colonization with other organisms | 1 | 137 (69.2) | 69 (100.0) | 68 (52.7) | < 0.001 |
| ≥ 3 lobes involved or cystic bronchiectasis | 1 | 84 (42.4) | 36 (52.2) | 48 (37.2) | 0.045 |
| Total BSI score | 26 | 5 [3–8] | 6 [4–8] | 4 [2–7] | 0.002 |
Data are shown as count (percentile) or median [interquartile range] unless specified otherwise. Abbreviations: BMI, Body Mass Index; FEV1, forced expiratory volume in one second; mMRC, modified Medical Research Council dyspnea scale; BSI, bronchiectasis severity index
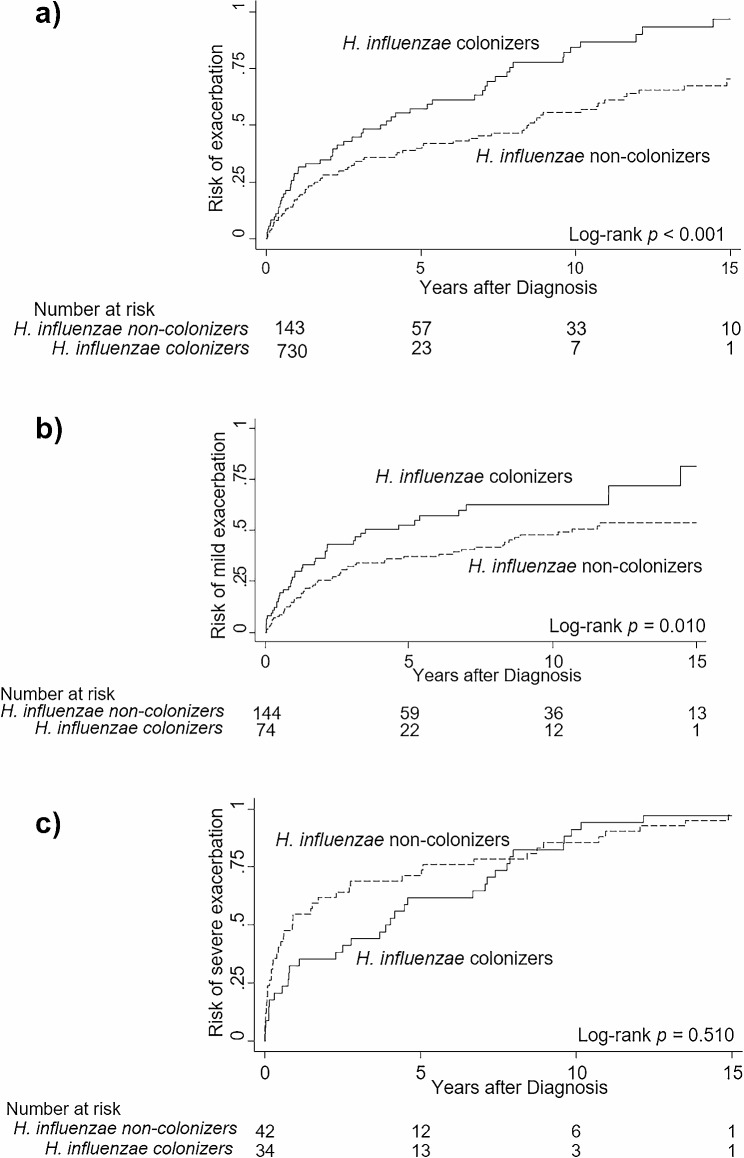
Risk of exacerbations
Patients with H. influenzae colonization had a shorter time to overall exacerbation than those without H. influenzae colonization (log-rank test, p < 0.001); when exacerbation was split into two categories, the difference was maintained for mild exacerbation (log-rank test, p = 0.010) but not for severe exacerbation. (Log-rank test, p = 0.510) (Fig. 2). After multivariate adjustment, H. influenzae colonization (incidence rate ratio [IRR] 1.54, 95% confidence interval [CI] 1.06–2.24, p = 0.023), P. aeruginosa colonization (adjusted IRR 1.70, 95% CI 1.12–2.56, p = 0.012), underlying asthma (adjusted IRR 1.77, 95% CI 1.10–2.85, p = 0.019), and involvement of more than three lobes (adjusted IRR 1.72, 95% CI 1.14–2.57, p = 0.009) were revealed to have a significant association with the incidence rate of mild exacerbation (Table 4).
Fig. 2.
Effect of colonization of Haemophilus influenzae on the risk of exacerbation of bronchiectasis. The lines indicate Kaplan-Meier curves assessing the risk of exacerbation after diagnosis. Solid lines indicate patients with Haemophilus influenzae colonization, while dashed lines indicate patients without colonization. Compared to patients without colonization, patients with Haemophilus influenzae colonization had higher risk of (a) any exacerbation, defined as worsening of respiratory symptoms such as cough, increased sputum, or requiring antibiotics or hospitalization (p < 0.001) and (b) mild exacerbation requiring prescription of oral antibiotics from an outpatient clinic (p = 0.010). However, no difference was observed regarding (c) severe exacerbation requiring hospitalization or emergency room visits (p = 0.510)
Table 4.
Factors associated with the incidence rate of mild exacerbation* in bronchiectasis patients
| Variables | Unadjusted IRR | P | Adjusted IRR | P |
|---|---|---|---|---|
| H. influenzae colonization† | 1.63 [1.08–2.44] | 0.019 | 1.54 [1.06–2.24] | 0.023 |
| P. aeruginosa colonization† | 2.04 [1.38–3.03] | < 0.001 | 1.70 [1.12–2.56] | 0.012 |
| Bacteria other than H. influenzae and P. aeruginosa colonization | 1.90 [1.27–2.84] | 0.002 | 1.31 [0.86–1.99] | 0.208 |
| BMI (kg/m2) | 0.93 [0.88–0.99] | 0.023 | 0.96 [0.91–1.02] | 0.190 |
| Chronic obstructive pulmonary disease | 1.85 [1.18–2.91] | 0.008 | 1.03 [0.67–1.58] | 0.896 |
| Asthma | 1.71 [1.02–2.86] | 0.041 | 1.77 [1.10–2.85] | 0.019 |
| Hospitalization history within two years | 2.25 [1.22–4.14] | 0.009 | 1.52 [0.86–2.66] | 0.148 |
| Involvement ≥ 3 lobes | 2.50 [1.69–3.68] | < 0.001 | 1.72 [1.14–2.57] | 0.009 |
*Mild exacerbation refers to an outpatient prescription of oral antibiotics due to exacerbation. †Colonization was defined as when any type of bacteria was detected at least once from respiratory specimen culture during routine practice. Data are shown as median [interquartile range] unless specified otherwise. Abbreviations: IRR, incidence rate ratio; BMI, Body Mass Index
Abbreviations: ICD, International Classification of Diseases; CT, computed tomography; NTM-PD, Non-tuberculous mycobacterial pulmonary disease
Comparison of lung function and overall survival
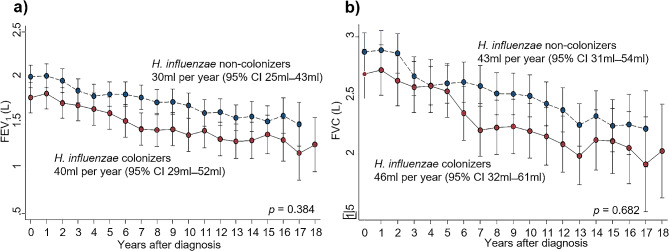
The baseline and annual changes in FEV1 and FVC revealed similar findings regardless of H. influenzae colonization status. The baseline FEV1 was 1.70 L (IQR 1.25–2.30 L) vs. 1.98 L (IQR 1.50–2.44) (p = 0.082), and FVC was 2.65 L (IQR 2.06–3.27 L) vs. 2.81 L (IQR 2.30–3.33 L) (p = 0.170) for H. influenzae colonizers and non-colonizers, respectively. The annual decline in FEV1 was 40 ml (95% CI 29–52 ml) for H. influenzae colonizers, while it was 30 ml (95% CI 25–43 ml) for non-colonizers, which was not a statistically significant difference (p = 0.384). The decline in FVC was 46 ml (95% CI 32–61 ml) per year for H. influenzae colonizers and 43 ml (95% CI 31–54 ml) per year for non-colonizers (p = 0.682) (Fig. 3).
Fig. 3.
Decline of pulmonary function according to the colonization of Haemophilus influenzae in bronchiectasis patients. The annual decline of (a) FEV1 (p = 0.382) and (b) FVC (p = 0.628) did not show significant differences regardless of Haemophilus influenzae colonization in bronchiectasis patients. Solid lines indicate patients with Haemophilus influenzae colonization, while dashed lines indicate patients without colonization. Abbreviations: FEV1, forced expiratory volume in one second; FVC, forced vital capacity; CI, confidence interval
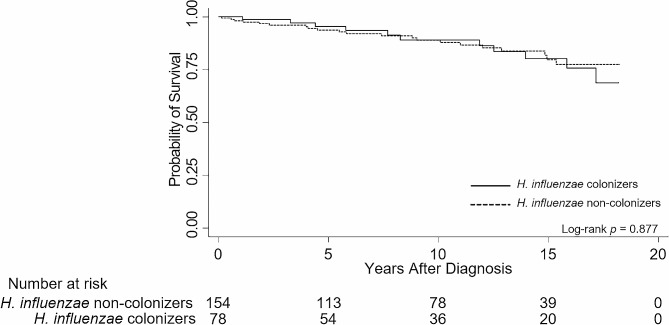
The probability of survival did not differ according to the colonization status of H. influenzae (log-rank test, p = 0.877). The Kaplan-Meier survival curve is shown in Fig. 4.
Fig. 4.
Impact of Haemophilus influenzae colonization on the all-cause mortality of bronchiectasis patients. Colonization of Haemophilus influenzae did not have a significant impact on all-cause mortality of bronchiectasis patients (log-rank p = 0.877). Solid lines indicate patients with Haemophilus influenzae colonization, while dashed lines indicate patients without colonization
Discussion
This study evaluated the impact of H. influenzae colonization on the clinical features and prognosis of patients with bronchiectasis. Patients with bronchiectasis colonized by H. influenzae had higher BSI scores, attributed to more extensive radiological involvement and a higher frequency of mild exacerbations. Lung function and mortality rates did not differ significantly according to the presence of H. influenzae.
This is the first study to evaluate the impact of H. influenzae colonization on bronchiectasis. According to our results, H. influenzae colonization did not affect lung function or mortality; however, there was a difference in the frequency of exacerbations. In particular, a higher incidence of mild exacerbation in the H. influenzae colonization group implies a lower quality of life and frequent hospital visits, although hospitalization was not required. The grave impact of H. influenzae colonization can be inferred from previous studies on patients with COPD. COPD patients colonized with H. influenzae had increased airway inflammation and decreased lung volume compared to non-colonizers [11]. Also, it is known to be common in patients with moderate-to-severe COPD [17]. Taken together, H. influenzae colonization in patients with bronchiectasis is likely to induce more airway inflammatory responses and contribute to a poor prognosis. Although the effects of other respiratory diseases or coinfection were not studied in depth in this study, we have tried to outline the significant impact of H. influenzae colonization. In addition, considering that H. influenzae is often not cultured during disease exacerbation [9], this study indicates the importance of a culture study to acquire colonization information, especially that of H. influenzae, in the initial treatment of patients with bronchiectasis.
In this study, H. influenzae colonization did not affect severe exacerbation, overall mortality, or changes in lung function. In individuals with intact immunity, H. influenzae usually causes upper respiratory tract diseases. Lower respiratory tract infections are rare because of host immune responses that prevent transmission to the lower respiratory tract. When the mucosal host immune mechanism is compromised, lower respiratory tract infections can occur via the immune evasion mechanism of H. influenzae [18]. Indeed, the high rates of H. influenzae infection in conditions such as cystic fibrosis or immotile ciliary syndrome, characterized by abnormal mucociliary function, further emphasize the importance of these host defense mechanisms [19, 20]. In our study, most patients were followed up at the outpatient clinic, implying normal immunity rather than an immune-compromised situation. Therefore, the probability of identifying H. influenzae may have been lower, and there may not have been a significant difference owing to the small number of patients. However, identifying H. influenzae may indicate an abnormal immune system inside the bronchi, making it necessary to pay more attention while devising a treatment plan.
A common feature of P. aeruginosa and H. influenzae is their ability to form biofilms, facilitating antibiotic resistance, but they are not equally virulent. This may be due to the wide range of virulence factors and proinflammatory properties of P. aeruginosa [21]. In addition, H. influenzae is a common resident of the upper respiratory tract, which may mean that its presence is less destructive to the lower respiratory tract environment [22].
Despite these meaningful findings, our study has several limitations. First, this was a retrospective study. Regular follow-ups and detailed evaluations regarding the etiology of bronchiectasis could not be performed. Second, patients who did not undergo sputum culture were excluded, leading to a possible selection bias and limited generalizability. Third, the detection rate of H. influenzae was lower than expected. Based on previous studies, the most commonly observed bacterial pathogens in patients with bronchiectasis were Haemophilus spp. (19–55%) Pseudomonas spp. (26–58%) and Streptococcus pneumoniae (12%) [7, 23]. This may be due to the difficult culture process of H. influenzae. Usually, a standard method using chocolate agar medium is used for H. influenzae culture [24], which has high sensitivity, but a low specificity due to possible contamination with other bacterial species. Our study adopted a selective culture method using a medium supplemented with vancomycin, bacitracin, and clindamycin. Therefore, the specificity is higher, but the culture sensitivity may be lower [25]. Finally, our study did not consider the use of inhaled corticosteroids in patients with bronchiectasis. Future research efforts may benefit from incorporating inhaled corticosteroids as a covariate to further elucidate its specific impact on bronchiectasis severity and exacerbation risk.
Conclusions
In conclusion, colonization of H. influenzae in bronchiectasis was associated with a higher risk of mild exacerbation but not severe exacerbation, lung function decline, or all-cause mortality. Although further evaluations are necessary, colonization with H. influenzae may have a harmful impact on patients with bronchiectasis.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank the Division of Statistics in Medical Research Collaborating Center at Seoul National University Bundang Hospital for statistical analysis.
Abbreviations
- COPD
Chronic obstructive pulmonary disease
- BSI
Bronchiectasis severity index
- BMI
Body mass index
- FEV1
Forced expiratory volume in 1 s
- IRR
Incidence rate ratio
- FVC
Forced vital capacity
- IQR
Interquartile range
- CI
Confidence interval
Author contributions
SHY and HJK contributed to the conception and design of the work. SHY, MJS, BSK, YWK, SYL, YJL, JSP, YJC, JHL, CTL, and HJK acquired the data, while SHY and HJK analyzed it. MJS, BSK, YWK, SYL, YJL, JSP, YJC, JHL, and CTL interpreted the data. SHY drafted the work, and MJS, BSK, YWK, SYL, YJL, JSP, YJC, JHL, CTL, and HJK revised it critically for important intellectual content. All authors approved the final version of the manuscript and agreed to be accountable for all aspects of the work.
Funding
This work was supported by grant no 06-2021-0345 from the SNUBH Research Fund. The funder did not have any role in the study.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The Institutional Review Board approved this study of the Seoul National University Bundang Hospital (protocol number B-2106-689-106). The need for informed consent was waived owing to the study’s observational nature and the use of anonymized data.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
2/9/2024
This article has been corrected since original publication; please see the linked erratum for further details.
Change history
2/13/2024
A Correction to this paper has been published: 10.1186/s12890-024-02881-6
References
- 1.McShane PJ, Naureckas ET, Tino G, Strek ME. Non-cystic fibrosis bronchiectasis. Am J Respir Crit Care Med. 2013;188(6):647–56. doi: 10.1164/rccm.201303-0411CI. [DOI] [PubMed] [Google Scholar]
- 2.Barker AF, Bronchiectasis N Engl J Med. 2002;346(18):1383–93. doi: 10.1056/NEJMra012519. [DOI] [PubMed] [Google Scholar]
- 3.PJ C. Inflammation: a two-edged sword–the model of bronchiectasis. Eur J Respiratory Dis. 1986;147(6). [PubMed]
- 4.Finch S, McDonnell MJ, Abo-Leyah H, Aliberti S, Chalmers JD. A comprehensive analysis of the impact of Pseudomonas aeruginosa colonization on prognosis in Adult Bronchiectasis. Ann Am Thorac Soc. 2015;12(11):1602–11. doi: 10.1513/AnnalsATS.201506-333OC. [DOI] [PubMed] [Google Scholar]
- 5.Chandrasekaran R, Mac Aogain M, Chalmers JD, Elborn SJ, Chotirmall SH. Geographic variation in the etiology, epidemiology, and microbiology of bronchiectasis. BMC Pulm Med. 2018;18(1):83. doi: 10.1186/s12890-018-0638-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Richardson H, Dicker AJ, Barclay H, Chalmers JD. The microbiome in bronchiectasis. Eur Respir Rev. 2019;28(153). [DOI] [PMC free article] [PubMed]
- 7.Angrill J, R de Celis CA, Rañó A, Gonzalez J, Solé T, Xaubet A, et al. Bacterial colonisation in patients with bronchiectasis: microbiological pattern and risk factors. Thorax. 2002;57:15–9. doi: 10.1136/thorax.57.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weaver D, Gago S, Bromley M, Bowyer P. The human lung mycobiome in chronic Respiratory Disease: limitations of methods and our current understanding. Curr Fungal Infect Rep. 2019;13(3):109–19. doi: 10.1007/s12281-019-00347-5. [DOI] [Google Scholar]
- 9.Tufvesson E, Bjermer L, Ekberg M. Patients with Chronic Obstructive Pulmonary Disease and chronically colonized with Haemophilus influenzae during stable Disease phase have increased airway inflammation. Int J Chron Obstruct Pulmon Dis. 2015;10:881–9. doi: 10.2147/COPD.S78748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tufvesson E, Markstad H, Bozovic G, Ekberg M, Bjermer L. Inflammation and chronic colonization of Haemophilus influenzae in sputum in COPD patients related to the degree of Emphysema and bronchiectasis in high-resolution computed tomography. Int J Chron Obstruct Pulmon Dis. 2017;12:3211–9. doi: 10.2147/COPD.S137578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murphy TF, Brauer AL, Schiffmacher AT, Sethi S. Persistent colonization by Haemophilus influenzae in Chronic Obstructive Pulmonary Disease. Am J Respir Crit Care Med. 2004;170(3):266–72. doi: 10.1164/rccm.200403-354OC. [DOI] [PubMed] [Google Scholar]
- 12.Austin PC. An introduction to Propensity score methods for reducing the effects of confounding in Observational studies. Multivar Behav Res. 2011;46(3):399–424. doi: 10.1080/00273171.2011.568786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ho DE, Imai K, King G, Stuart EA. MatchIt: nonparametric preprocessing for Parametric Causal Inference. J Stat Softw. 2011;42(8):28. doi: 10.18637/jss.v042.i08. [DOI] [Google Scholar]
- 14.Hill AT, Sullivan AL, Chalmers JD, De Soyza A, Elborn SJ, Floto AR, et al. British thoracic Society Guideline for bronchiectasis in adults. Thorax. 2019;74(Suppl 1):1–69. doi: 10.1136/thoraxjnl-2018-212463. [DOI] [PubMed] [Google Scholar]
- 15.Pearce N. Analysis of matched case-control studies. BMJ. 2016;352:i969. doi: 10.1136/bmj.i969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Araujo D, Shteinberg M, Aliberti S, Goeminne PC, Hill AT, Fardon TC et al. The Independent contribution of Pseudomonas aeruginosa Infection to long-term clinical outcomes in bronchiectasis. Eur Respir J. 2018;51(2). [DOI] [PubMed]
- 17.Martinez-Garcia MA, Soler-Cataluna JJ, Donat Sanz Y, Catalan Serra P, Agramunt Lerma M, Ballestin Vicente J, et al. Factors associated with bronchiectasis in patients with COPD. Chest. 2011;140(5):1130–7. doi: 10.1378/chest.10-1758. [DOI] [PubMed] [Google Scholar]
- 18.Finney LJ, Ritchie A, Pollard E, Johnston SL, Mallia P. Lower airway colonization and inflammatory response in COPD: a focus on Haemophilus influenzae. Int J Chron Obstruct Pulmon Dis. 2014;9:1119–32. doi: 10.2147/COPD.S54477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alikhan MM, Lee FE. Understanding nontypeable Haemophilus influenzae and Chronic Obstructive Pulmonary Disease. Curr Opin Pulm Med. 2014;20(2):159–64. doi: 10.1097/MCP.0000000000000023. [DOI] [PubMed] [Google Scholar]
- 20.King P. Haemophilus influenzae and the lung. Clin Translational Med. 2012;1:1–10. doi: 10.1186/2001-1326-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sadikot RT, Blackwell TS, Christman JW, Prince AS. Pathogen-host interactions in Pseudomonas aeruginosa Pneumonia. Am J Respir Crit Care Med. 2005;171(11):1209–23. doi: 10.1164/rccm.200408-1044SO. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rogers GB, van der Gast CJ, Serisier DJ. Predominant pathogen competition and core microbiota divergence in chronic airway Infection. ISME J. 2015;9(1):217–25. doi: 10.1038/ismej.2014.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Borekci S, Halis AN, Aygun G, Musellim B. Bacterial colonization and associated factors in patients with bronchiectasis. Ann Thorac Med. 2016;11(1):55–9. doi: 10.4103/1817-1737.172297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Howard AJ, Ison CA. Mackie and McCartney practical medical Microbiology. 14th ed. Churchill Livingstone; 2006.
- 25.Murray PR, Baron EJ, Jorgensen JH, Landry ML. Manual of Clinical Microbiology. ASM Press; 2007.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.