Abstract
Dental caries (DC)-induced pulp infections usually undergo the common endodontic treatment, root canal therapy (RCT). Endodontically treated teeth are devitalized, become brittle and susceptible for re-infection which eventually results in dental loss. These complications arise because the devitalized pulp losses its ability for innate homeostasis, repair and regeneration. Therefore, restoring the vitality, structure and function of the inflamed pulp and compromised dentin have become the focal points in regenerative endodontics. There are very few evidences, so far, that connect methylenetetrahydrofolate reductase single nucleotide polymorphisms (MTHFR-SNPs) and dental disorders. However, the primary consequences of MTHFR-SNPs, in terms of excessive homocysteine and folate deficiency, are well-known contributors to dental diseases. This article identifies the possible mechanisms by which MTHFR-SNP-carriers are susceptible for DC-induced pulp inflammation (PI); and discusses a cell-homing based strategy for in vivo transplantation in an orthotopic model to regenerate the functional dentine-pulp complex which includes dentinogenesis, neurogenesis and vasculogenesis, in the SNP-carriers.
Keywords: Dental PI, Methylenetetrahydrofolate reductase, Single nucleotide polymorphism, Cell-homing, Orthotopic model, Regenerative endodontics
1. Introduction
Methylenetetrahydrofolate-reductase (MTHFR), the rate-limiting enzyme in the methylation cycle, is a regulatory enzyme that links the methylation- and folate-cycles in the one-carbon-metabolism. MTHFR converts 5,10-methylenetetrahydrofolate to 5-methyltetrahydrofolate (Fig. 1), which is the active folate and a co-substrate for recycling homocysteine (HCY) to methionine (Wan et al., 2018). MTHFR single-nucleotide-polymorphisms (SNPs) produce thermolabile enzyme with reduced enzymatic activity. Therefore, compromised enzyme activity or MTHFR deficiency is associated with impaired methylation, excess HCY and folate deficiency. Two MTHFR-SNPs, C667T and A1298C are clinically relevant (Rozen, 2000). Degree of enzyme activity depends on the nature of SNPs (homozygous or heterozygous or compound heterozygous) and the genotype (C677T or A1298C).
Fig. 1.
Folate cycle, methylation and remethylation pathways. ADMA-asymmetric dimethylarginine; Bet-betaine; BHMT-betaine homocysteine methyltransferase; DHF-dihydrofolate; DMG-dimethylglycine; HCY-homocysteine; L-arg-L-arginine; MTases-methyltransferases; MTHFR-methylenetetrahydrofolate reductase; MTHF-methyltetrahydrofolate; MS-methionine synthase; SAM-s-adenosylmethionine; SAH-s-adenosylhomocysteine; THF-tetrahydrofolate.
MTHFR-SNPs are associated with several disorders including cardiovascular-disease, cancers, neural tube defects, Alzheimer’s-disease and dental complications (Senghore et al., 2018, Rozen, 2000, Stover et al., 2015). So far, the relationship between MTHFR-SNPs and dental disorders, specifically, tooth decay (DC and pulpitis) and periodontal disease are understudied. We have gathered the very few direct and indirect evidences that connect SNPs and their primary consequences with DC and PI. Further, we have derived the possible mechanisms that are associated with systemic and local cellular events which link the SNPs and their consequences with DC and PI.
The WHO-2022 report shows that between 1990 and 2019, there was a modest decrease in the prevalence of tooth loss only in the low-income countries, while all other country income groups had increasing number of cases for tooth loss (WHO, 2022). This data suggests that novel-yet-affordable dental healthcare strategies, to address DC, pulpitis and tooth loss, have to be identified, translated and implemented into clinical practice, so that there is relevance between mainstream research and dental health needs of the population. With regard to RCT, the existing clinical practice to prevent tooth loss, dental loss is higher for root canal (RC)-treated teeth than the non-treated teeth because of secondary caries and the associated complex restoration processes.
This review focuses on i) the potential mechanisms by which MTHFR-SNPs and their primary consequences could contribute to DC and PI, and ii) how an experimental regeneration strategy can assist in regeneration of a complete functional dentine-pulp complex with dentinogenesis, vasculogenesis and neurogenesis, in SNP-carriers. Evidence show that HCY accumulation and folate deficiency (primary consequences of MTHFR-SNPs) are associated with pathological consequences in dental disorders. However, only a very few evidences connect MTHFR-SNPs with dental complications. Lack of rich evidences need not be considered for SNPs as either insignificant contributors for dental complications or that SNPs-mediated oral manifestations are a rare occurrence. Rather, there is a need to prioritize evaluation and monitoring of circulating HCY and folate concentrations in dental-diseases and -clinical practice.
2. Evidences connecting MTHFR-SNPs, consequences of SNPs and dental disorders
-
i)
In Saudi children who were receiving dental therapy (Bagher et al., 2021), a high frequency of A1298C than C667T has been observed. This suggests that MTHFR-SNPs influence dental complications and the precise role of MTHFR-SNPs in oral manifestations should be established.
-
ii)
A case report demonstrating the detailed oral manifestations in a MTHFR-SNP carrier shows that certain dental issues are associated with hyper-homocysteinemia (Bernabe et al., 2020), wherein the patient has C667T SNP, mild hyper-homocysteinemia, osteoclastic activity and uneven oral tissue remodeling.
-
iii)
A Japanese study (Taguchi et al., 2022) identified the association between plasma HCY concentration and tooth loss. Postmenopausal Japanese women, with higher circulating HCY had fewer teeth at baseline, suggesting that HCY could trigger tissue protein degeneration in the oral cavity.
-
iv)
Folate deficiency is a risk factor in early childhood. There is increased DC and gingival problems in children with systemic vitamin-B12 deficiency (Mistry et al., 2017). In support, folic-acid has prevented early childhood DC (Alpan & Karakan, 2018). Besides, lower folate consumption leads to excess HCY to result in increased salivary oxidative markers and thus to increased caries activity. Prevalence of DC and gingival complications in children with systemic vitamin-B12 deficiency is higher than the vitamin-B12-sufficient children (Mistry et al., 2017).
3. Possible mechanisms, mediated by MTHFR-SNPs, excess HCY and folate deficiency, which contribute to the pathogenesis of DC and PIs
In response to bacterial infection, several molecules and mechanisms regulate dental pulp defence and repair processes, (Farges et al., 2015). Biological markers in circulation or diseased pulp tissue in PI are well-established (Rechenberg et al., 2016). However, few studies report on the mechanisms by which systemic events, such as, MTHFR-SNPs-mediated HCY accumulation and/or folate deficiency, contribute to DC or impair the dental reparative processes in the inflamed pulp
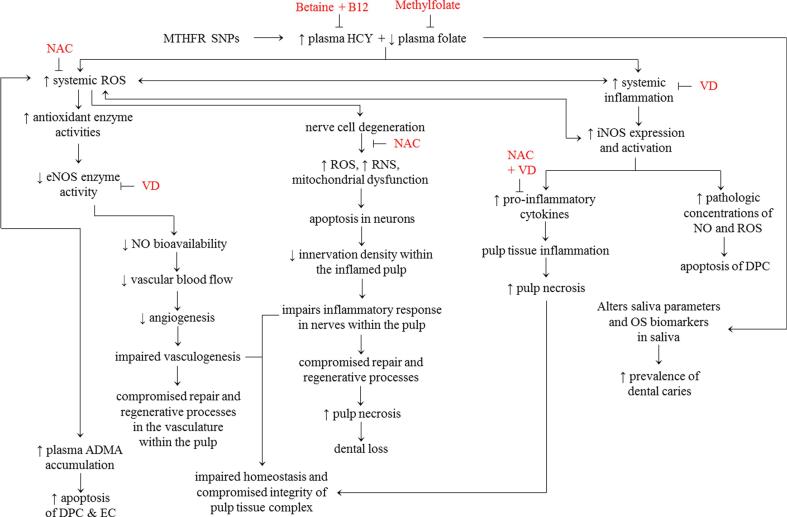
The two principal adverse implications of MTHFR-SNPs, plasma HCY accumulation and folate deficiency (Fig. 2), lead to elevated biomarkers of oxidative stress (OS) and inflammation, systemically and locally.
Fig. 2.
Proposed Mechanisms. Potential mechanisms by which circulating excess homocysteine and folate deficiency contribute to the development of dental caries, PI and impaired reparative and regenerative processes. ADMA-asymmetric dimethylarginine; B12-vitamin B12; DPC-dental pulp cells; EC-endothelial cells; eNOS-endothelial nitric oxide; HCY-homocysteine; iNOS-inflammatory nitric oxide synthase; NAC-N-acetylcysteine; OS-oxidative stress; RNS-reactive nitrogen species; ROS-reactive oxygen species; VD-vitamin D.
Clinical research shows that circulating HCY is a potent pro-oxidant molecule, which triggers OS through excess reactive-oxygen-species (ROS) production (Nanetti et al., 2013). Excessive ROS, eventually triggers several events including i) an imbalance in the salivary pro- and anti-oxidants, as indicated by the presence of biomarkers of oxidative damage in the saliva of children with caries than in children without caries (de Sousa et al., 2022), ii) activation of inducible-nitric-oxide-synthase (iNOS) activity (pro-inflammatory molecule), iii) reduction in endothelial-nitric-oxide-synthase (eNOS) activity and the consequent reduction in endothelial nitric-oxide-bioavailability (which decreases the blood flow and angiogenic processes), iv) causes plasma asymmetric-dimethylarginine (ADMA) accumulation (pro-oxidant and pro-inflammatory molecule), iv) augments nicotinamide-adenine-dinucleotide-phosphate (NADPH)-oxidase and impairs thioredoxin which cumulatively enhance ROS production, thus the vicious cycle for OS gets reinforced in the presence of accumulated HCY (Tyagi et al., 2005). Pathological concentration of nitric-oxide, which is produced by ROS-induced iNOS, induces apoptosis of human dental pulp cells (Park et al., 2014). In support of the regulatory role of excess systemic OS in dental disorders, it is evidenced that in endodontic pathologies (pulpal inflammation and periodontitis) OS is a critical pathological mechanism, because biomarkers of OS are evident at the local RC contents and systemic (saliva) level (Vengerfeldt et al., 2017). Besides these HCY-induced pathological events, excess HCY has neurodegenerative effect (Chen et al., 2017), in which neurons are damaged via OS, apoptosis, mitochondrial dysfunction, DNA damage in nerve cells (Fan et al., 2020), mitochondrial damage and generation of excess ROS and reactive-nitrogen-species (RNS). Damaged nerve cells then loses the ability to repair hard and soft dental tissue (Zhan et al., 2021). To counteract the neurodegenerative effect, N-acetylcysteine (NAC) supplementation has been suggested as an effective therapy for its role in producing glutathione, an efficient antioxidant (Tardiolo et al., 2018). Furthermore, excess HCY triggers pro-inflammatory responses in clinical studies (Durga et al., 2005), by altering the biosynthesis of chemokines/cytokines, including tumor necrosis factor-a (TNFa), interleukins (IL-1b, −2, −6, −8) and nuclear factor kappa-B (NFkB) (Gokkusu et al., 2010). Eventually, these pro-inflammatory signals promote rapid pulp tissue degeneration and necrosis (Park et al., 2015).
Besides, the excess HCY-induced pathological mechanisms, folate deficiency is associated with DC through up-regulated OS biomarkers in saliva. Systemically, folate deficiency augments OS, to result in endothelial-dysfunction, compromised DNA repair and enhanced apoptosis. Deficiencies of vitamin-B12, vitamin-B6 and folate, which are required for HCY metabolism, can lead to circulatory HCY accumulation (Alpan & Karakan, 2018).
In MTHFR-SNPs, reduced MTHFR activity leads to decreased folate biosynthesis, thus, SNPs could be one of the underlying factors in DC. Saliva has antibacterial and antioxidant molecules, which exert a protective role, against the development of caries. Insufficient folate concentration up-regulates the salivary HCY through impaired HCY metabolism. Folate deficiency, via up-regulated HCY, augments the pro-inflammatory signals, in terms of up-regulated RNA and protein levels of inflammatory mediators (IL1b, IL6, TNFa and monocyte chemoattractant protein-1 (MCP1, (Kolb & Petrie, 2013). In support, supplementation of folic-acid showed improvement in certain inflammatory biomarkers (Asbaghi et al., 2021). Epidemiological studies show that folate deficiency and the resultant HCY accumulation regulate the pathogenic processes in neurodegenerative conditions (Kronenberg et al., 2008), trigger oxidative DNA damage and impair vascular function (Endres et al., 2005).
Based on these evidences, in dental tissue, it is possible that more HCY and folate deficiency, result in excess systemic OS and inflammation, which could cause an imbalance in the i) homeostasis and compromise the integrity and ii) repair and regenerative mechanisms. Furthermore, in MTHFR-SNP-carriers, when DC sets in, PI and eventual pulp necrosis occurs, which results in dental loss, even with the routine anti-caries therapy. This possibility arises because of the persistent systemic changes activated by excess HCY- and folate deficiency-mediated augmented OS and pro-inflammatory milieu. Additionally, the routine anti-caries therapy may be insufficient to address these persistent underlying pathological events. Hence, future clinical studies can evaluate the effects of antioxidants and anti-inflammatory oral supplements in the SNP-carriers with early stage DC, to assess whether or not the supplements prevent the progression of early stage caries to PI and pulp necrosis.
4. Biomarkers of pulp inflammation
Dental pulp consists of connective tissue, blood vessels, nerve fibers, extracellular matrix, growth factors and cells (odontoblasts, fibroblasts, dental pulp stem cells, immune cells, neurons and endothelial cells) (Gaje & Ceausu, 2020). These cells are redox-sensitive, capable of responding to pro-inflammatory stimuli, and produce diverse stress response-molecules that are associated with the natural repair mechanisms in the pulp.
When the rigid shell covering the dental pulp, loses its structural integrity, pulp is exposed to adverse stimuli from the oral environment such as caries, cracks etc. These adversities permit microbes and other secreted molecules into the pulp. Initially, Pulp responds to stimuli by becoming inflamed, which progresses to pulp necrosis and infection (Yu & Abbott, 2007). The inflamed pulp initiates stress response as pulp defence mechanisms, which are carried out by several factors (inflammatory mediators, cytokines, growth factors, proteases and antimicrobial peptides)(Rechenberg et al., 2016), and these molecules are the biomarkers that indicate the pulp health status.
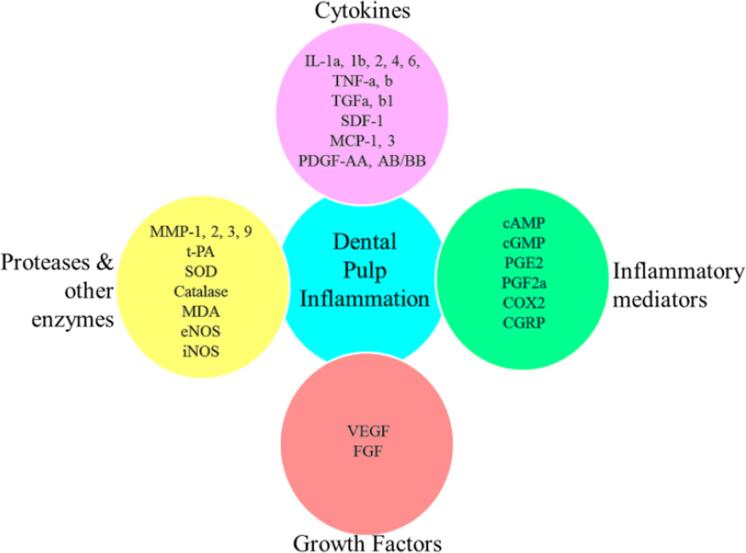
In PI, there are two critical events, microcirculation and sensory nerve activity (Olgart, 1992), which are mediated by molecular factors. Generally, PI is caused by infection of the pulp by oral microorganisms (Hahn et al., 1991). Microbes enter into the pulp tissue through DC. Post-pulp infection, cells in the pulp send immune responses in different forms (cell differentiation, form mechanical barriers, transmit sensations) to contain infection, promote repair and signal injury (Rechenberg et al., 2016). These signaling events trigger the inflamed pulp tissue to release several molecules which indicates inflammation. These immune mediators in-turn lead to series of inflammatory events and reparative processes (Rechenberg et al., 2016). Biomarkers, as listed in Fig. 3, associated with the pathogenesis of PI and the change in their concentrations, indicate that excess OS and/or inflammation could have altered their expressions.
Fig. 3.
Biomarkers of dental pulp inflammation. Presence of these biomarkers, among the others, indicates dental pulp inflammation. cAMP-cyclic adenosine monophosphate; cGMP-cyclic guanosine monophosphate; CGRP-calcitonin gene related peptide; COX2-cyclooxygenase2; eNOS-endothelial nitric oxide synthase; FGF-fibroblast growth factor; IL-interleukin; iNOS-inflammatory nitric oxide synthase; MDA-malondialdehyde; MMP-matrix metallopeptidase; MCP1-monocyte chemoattractant protein1; PDGF-platelet derived growth factor; PGE-prostaglandin E2; PGF2-prostaglandin F2; SDF1-stromal cell derived factor1; SOD-superoxide dismutase; t-PA-tissue plasminogen activator; TNF-tumor necrosis factor; TGF-transforming growth factor; VEGF-vascular endothelial growth factor.
Among the biomarkers of PI, several molecules such as eNOS, iNOS, antioxidants [superoxide dismutase (SOD), catalase] and inflammatory molecules [cytokines: ILs, TNFa, transforming growth factor beta1 (TGFb) (Gómez-García et al., 2022), inflammatory mediators: cyclic adenosine monophosphate (cAMP), prostaglandin E2(PGE2), cyclooxygenase2 (COX2)], are regulated by excess HCY and folate deficiency. In the inflamed pulp, as a part of defence process, these molecules are either up- or down-regulated as immune response depending on the intensity of the stressors. Similar trend has been observed in the way these molecules respond to HCY accumulation and folate deficiency. OS and inflammation are prevalent in excessive HCY and folate deficiency and in inflamed pulp, hence, the uniform stress responses.
5. Potential strategies to address the MTHFR-SNPs-, excess HCY- or folate deficiency-mediated dental disorders
Previously, folic-acid supplementation has been considered as HCY reducing therapy (Zhang et al., 2022). However, clinical studies show that of folic-acid supplementation did not render protection to individuals who are predisposed to the ill-effects of HCY accumulation (Huang et al., 2018). Besides this, folic-acid supplementation led to only a slight reduction in the accumulated HCY and it did not alter the inflammatory responses in terms of inflammatory mediatory molecules (C-reactive protein (CRP) and oxidized low density lipoprotein (LDL)) (Durga et al., 2005).
Parallely, betaine or trimethylglycine supplementation reduces plasma HCY (Olthof et al., 2003). Betaine has anti-inflammatory effect, it reduces OS, increases autophagy, remodels gut microbiome and regulates epigenetic modification (Wang et al., 2021). Betaine inhibits NFkB activity, thus, it regulates the concentrations of cytokines including IL1B, IL6 and TNFa (Kim et al., 2017). Besides, betaine regulates the one-carbon-metabolism, which is a direct support for carriers. Betaine, an endogenous molecule, is a supplementary methyl donor for HCY remethylation reaction, which reduces HCY concentration (Owens et al., 2016). Therefore, betaine-mediated HCY methylation is an alternative route to reduce HCY and to render protection from HCY accumulation in MTHFR-SNP-carriers (Owens et al., 2016).
Folate is one of the nutritional requirements for MTHFR-SNP-carriers; hence, folate supplementation has independent benefits to address the consequences of SNPs (Malinow et al., 1997). Although not much is known about the role of vitamin-B12 and folate on the hard tissues of teeth, a protective effect against tooth decay and periodontal disease has been reported (Mistry et al., 2017). Folate, through anti-inflammatory effect, reverses the excess HCY-induced NFkB and IL-6 (L. Zhang et al., 2021).
Observational studies (Clarke & Armitage, 2000) have raised the possibility that up-regulated HCY level may be associated with other underlying fctors, such as systemic OS and/or inflammation. Thus, we propose that an efficient therapeutic strategy to reduce HCY and address the underlying consequences of MTHFR-SNPs could include antioxidants and anti-inflammatory molecules, such as NAC (antioxidant), vitamin-D (antioxidant and anti-inflammatory), besides the inclusion of B-vitamins and betaine. In support of this proposition, studies have demonstrated the benefits of including independent molecules (methylfolate, vitamin-B12, NAC and betaine) as a therapeutic cocktail, for HCY reduction, antioxidant or anti-inflammatory effects (Leon et al., 2018).
A recent study (Liu et al., 2021) assessed the effect of oral daily essential nutrients (DEN) supplements as Ocufolin, in MTHFR-SNP-carriers to reverse retinal microvascular damage. Six months Ocufolin oral supplementation reversed microangiopathy in diabetic retinopathy, suggesting that MTHFR-SNP-carriers, who have multiple nutrient deficiencies, could significantly benefit from oral DEN supplementation to address the impairments associated with the SNPs, which needs to be evaluated for several MTHFR-SNP-associated pathologies.
Based on this information, we suggest that large scale clinical trial can evaluate the effect of combined multiple oral supplements including betaine, NAC, vitamin-B12, folate, vitamin-D and age-appropriate DEN, to restore the MTHFR-SNP consequences, specifically in dental complications such as DC and PI, along with the routine dental therapy.
6. Dentine-pulp complex regeneration in MTHFR-SNP-carriers, a future perspective
The anatomy of dental pulp, with its complex connective tissue and neurovascular network, makes it difficult to restore its physiologically functional status, post-bacterial infection or a trauma of the immature or mature permanent teeth. The most effective and routine clinical practice, so far, for pulp disease is RCT, which retains the diseased tooth, however, there is a possibility of reinfection (Kwack & Lee, 2022). Besides this, a tooth without the vital pulp after RCT loses the regenerative capacity which leads to brittle tooth and extraction (Su et al., 2011). Thus, there is a need to identify strategies for clinical interventions that will promote regeneration of the functional dentine-pulp complex in the devitalized tooth.
Considerable percentage of children (Bagher et al., 2021) who are MTHFR-SNP-carriers are receiving dental therapy. Loss of vital pulp in immature permanent teeth leads to added complications which include termination of dentin formation, root development, apical closure and eventual tooth maturation (Moussa & Aparicio, 2019). Hence, identification of strategies to preserve or regenerate the pulp vitality in younger population who are susceptible for repeated dental complications, due to the consequences of SNPs deserves to be investigated in future. Moreover, MTHFR-SNPs continuously generate stress stimuli, either at elevated concentrations or mitigated levels, in terms of several signaling molecules, including folate deficiency, excessive HCY, ROS and pro-inflammatory cytokines, which lead to the pro-oxidant and pro-inflammatory status at the local tissue level and systemically. Such sustained stress compromises the tissue healing and repair processes. Therefore, next generation dental therapies for SNP-carriers can determine the strategies to continuously reduce excessive stress or maintain the physiological status, rather than inhibition of the stress stimuli so that functional repair mechanisms are retained (Hozhabri et al., 2015).
Ideally, for SNP-carriers, treatment for an immature and mature permanent teeth (with the infected and inflamed, necrotic pulp tissue) that requires pulpotomy or pulpectomy, can be a therapeutic option that regenerates the complete and functional dentine-pulp complex, with neurovascular properties. This future therapy, in order to address the persistent excessive OS and inflammation at the local and systemic levels, needs to include oral interventions (as antioxidants and anti-inflammatories) to address the systemic ill-effects and interventions for within the tooth cavity to facilitate the local regenerative process in carriers. To address these future requirements, we hypothesize that MTHFR-SNP-carriers need two-prong approach, which includes i) oral multi-nutrients supplementation (DEN, including betaine) for systemic benefits and ii) chemotaxis induced cell-homing strategy that uses multiple bioactive molecules (betaine, vitamin-D, NAC, vitamin-B12 and methylfolate) to facilitate efficient regeneration of the dentine-pulp complex with new dentine formation, neurogenic and vasculogenic properties. In support of the utilization of the selected bioactive molecules for the proposed regenerative model, vitamins (vitamin-B, -D and -E), NAC and betaine have been reported to enhance the dental regeneration process by eliciting cytoprotective, anti-oxidant and anti-inflammatory effects, (Escobar et al., 2020, Fernández-Villa et al., 2018, Kornsuthisopon et al., 2022, Meng et al., 2022, Pei et al., 2018, Woo et al., 2015).
To test our hypothesis, an experimental mice model (consisting of carriers or non-carriers of MTHFR-SNP) can be utilized for the chemotaxis-induced cell-homing strategy (J. Y. Kim et al., 2010). In this approach, multiple morphogens [vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF), platelet-derived growth factor (PDGF), nerve growth factor (NGF) and bone morphogenetic protein-7 (BMP7)] and multiple bioactive molecules (betaine, methylfolate, vitamin-B12, vitamin-D, NAC) can be delivered simultaneously within the tooth cavity, where-in the delivery process can be in a controlled manner and as an extended release, so as to facilitate the growth of the different types of cells within the tooth cavity. Based on this cell-homing concept, an ectopic in vivo implantation has been carried out successfully (Kim et al., 2010). Our model will have the next upgraded version of the existing strategy. Instead of ectopic in vivo implantation, our mice would undergo orthotopic transplantation. The recipient mice for the donor teeth will be genetically modified to have MTHFR-SNPs. Non-SNP carrier mice can provide the donor teeth. This will create the SNP-relevant environment, to assess whether or not i) the regeneration of the dentine-pulp complex is a possibility in carriers, ii) the multiple bioactive molecules have positive influence on the regeneration process in carriers, and iii) it is beneficial to parallely address the systemic and tissue-level changes, in order to restore the physiological levels of OS and inflammation and regrow the complete and functional dentine-pulp complex in carriers.
A suitable drug delivery system can be identified as described (Makvandi et al., 2021), for the injectable bioactive cues consisting of different biomolecules, as suggested in the proposed strategy. The two key focal points to identify a suitable drug delivery system for the required application can include i) the mechanism of action of the bioactive molecules loaded nanoparticles system, in which the molecules are either entrapped or surface adsorbed and ii) the release profile of the bioactive molecules, among the others.
The proposed dentine-pulp regeneration model (Fig. 4), in principle, works similar to the model reported by Lee et al., (Meng et al., 2022), except for the involvement of MTHFR-SNP carrier mice. From the normal healthy, non-SNP carrier mice, entire permanent maxillary and mandibular incisors and cuspids can be freshly extracted. Residual tissues (periodontal and peri-apical) can be removed, cleaned and disinfected. Tooth can be subjected to the routine endodontic treatment. Through a small access, pulp tissue gets removed; and RC can be cleaned and shaped. All teeth can be autoclaved to prepare the biological tissue-free teeth. Into the pulp chambers and RCs of the endodontically treated teeth, morphogens- and bioactive molecules-eluting collagen gel (Nica et al., 2020) can be injected. The collagen gel packed teeth can then be implanted subcutaneously to the recipient, SNP carrier mice. Post implantation, carrier mice will receive the oral multi-nutrient supplementation, until a new dentine-pulp complex with neurovascular property regenerates or for 3 weeks (Kim et al., 2010). Retrieved implants will be utilized to establish the extent of new cellularization, dentinogenesis, vasculogenesis and neurogenesis. For the control, non-carrier mice group for transplantation and the SNP carrier mice groups which do not receive either oral supplements or the bioactive molecules can be considered. Mice with MTHFR-SNPs (heterozygous and homozygous knockout) can be generated as described (Chen et al., 2001, Macklin, 2020). The described knockout mice model has shown that betaine supplementation, reduces the excess HCY concentrations (Schwahn et al., 2003). Should the proposed model result in the regeneration of a complete and functional dentine-pulp complex in carriers, then, it could be a clinically translatable and sustainable strategy for routine clinical practice for the SNP-carriers. The proposed cell-homing strategy with multiple bioactive molecules and morphogens can be one of the next-generation therapies, wherein the proposed model can be followed after the routine RCT, instead of the current dental filling, without the need for implantation or transplantation.
Fig. 4.
Proposed regeneration strategies. Prospective chemotaxis-induced cell-homing strategy for MTHFR-SNP-carriers using an orthotopic model for regeneration of dentine-pulp complex with neurovascular properties.
7. Outlook
Taken together, there is a possibility for MTHFR-SNPs to be an underlying reason for the repeated dental PI because excess HCY and folate deficiency systemically contributes to the pathogenic processes. Therefore, restoring normalcy in systemic concentrations of HCY and folate through oral supplements could have reparative effect in SNP-carriers. The proposed chemotaxis-induced cell-homing strategy in the orthotopic model could result in regeneration of functional dentine-pulp complex with dentinogenesis, neurogenesis and vasculogenesis. These propositions need to be validated experimentally, in future.
CRediT authorship contribution statement
G. Uma Maheswari: Writing – original draft, Writing – review & editing. B. Yamini: Writing – original draft, Writing – review & editing. V.E. Dhandapani: Writing – review & editing. Bader O. Almutairi: Writing – review & editing. Selvaraj Arokiyaraj: Writing – review & editing. Kanchana M. Karuppiah: Conceptualization, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Peer review under responsibility of King Saud University.
References
- Alpan, A.L., Karakan, N.C., 2018. Folate in Dentistry. In: B Group Vitamins - Current Uses and Perspectives. 10.5772/intechopen.74055. [DOI]
- Asbaghi O., Ashtary-Larky D., Bagheri R., Moosavian P., Nazarian B., Afrisham R., Kelishadi M.R., Wong A., Dutheil F., Suzuki K., Naeini A.A. Effects of folic acid supplementation on inflammatory markers: a grade-assessed systematic review and dose–response meta-analysis of randomized controlled trials. Nutrients. 2021;13(7) doi: 10.3390/nu13072327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagher A.M., Young A.P., Neamatallah T., Al-Amoudi R.M., Bagher S.M., Denovan-Wright E.M. Prevalence of methylenetetrahydrofolate reductase gene polymorphisms (C677T, and A1298C) among Saudi children receiving dental treatment. Ann. Saudi Med. 2021;41(1):1–7. doi: 10.5144/0256-4947.2021.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernabe E., Marcenes W., Hernandez C.R., Bailey J., Abreu L.G., Alipour V., Amini S., Arabloo J., Arefi Z., Arora A., Ayanore M.A., Bärnighausen T.W., Bijani A., Cho D.Y., Chu D.T., Crowe C.S., Demoz G.T., Demsie D.G., Dibaji Forooshani Z.S., Kassebaum N.J. Global, Regional, and national levels and trends in burden of oral conditions from 1990 to 2017: a systematic analysis for the global burden of disease 2017 study. J. Dent. Res. 2020;99(4):362–373. doi: 10.1177/0022034520908533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S., Dong Z., Zhao Y., Sai N., Wang X., Liu H., Huang G., Zhang X. Homocysteine induces mitochondrial dysfunction involving the crosstalk between oxidative stress and mitochondrial pSTAT3 in rat ischemic brain. Sci. Rep. 2017;7(1):1–12. doi: 10.1038/s41598-017-07112-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z., Karaplis A.C., Ackerman S.L., Pogribny I.P., Melnyk S., Lussier-Cacan S., Chen M.F., Pai A., John S.W.M., Smith R.S., Bottiglieri T., Bagley P., Selhub J., Rudnicki M.A., James S.J., Rozen R. Mice deficient in methylenetetrahydrofolate reductase exhibit hyperhomocysteinemia and decreased methylation capacity, with neuropathology and aortic lipid deposition. Hum. Mol. Genet. 2001;10(5):433–443. doi: 10.1093/hmg/10.5.433. [DOI] [PubMed] [Google Scholar]
- Clarke R., Armitage J. Vitamin supplements and cardiovascular risk: Review of the randomized trials of homocysteine-lowering vitamin supplements. Semin. Thromb. Hemost. 2000;26(3):341–348. doi: 10.1055/s-2000-8101. [DOI] [PubMed] [Google Scholar]
- de Sousa Né Y.G., Frazão D.R., Bittencourt L.O., Fagundes N.C.F., Marañón-Vásquez G., Crespo-Lopez M.E., Maia L.C., Lima R.R. Are dental caries associated with oxidative stress in saliva in children and adolescents? A systematic review. Metabolites. 2022;12(9) doi: 10.3390/metabo12090858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durga J., Van Tits L.J.H., Schouten E.G., Kok F.J., Verhoef P. Effect of lowering of homocysteine levels on inflammatory markers: a randomized controlled trial. Arch. Intern. Med. 2005;165(12):1388–1394. doi: 10.1001/archinte.165.12.1388. [DOI] [PubMed] [Google Scholar]
- Endres M., Ahmadi M., Kruman I., Biniszkiewicz D., Meisel A., Gertz K. Folate deficiency increases postischemic brain injury. Stroke. 2005;36(2):321–325. doi: 10.1161/01.STR.0000153008.60517.ab. [DOI] [PubMed] [Google Scholar]
- Escobar L.M., Bendahan Z., Bayona A., Castellanos J.E., González M.C. Effect of vitamins D and E on the proliferation, viability, and differentiation of human dental pulp stem cells: an in vitro study. Int. J. Dentistry. 2020;2020 doi: 10.1155/2020/8860840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan X., Zhang L., Li H., Chen G., Qi G., Ma X., Jin Y. Role of homocysteine in the development and progression of Parkinson’s disease. Ann. Clin. Transl. Neurol. 2020;7(11):2332–2338. doi: 10.1002/acn3.51227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farges J.C., Alliot-Licht B., Renard E., Ducret M., Gaudin A., Smith A.J., Cooper P.R. Dental pulp defence and repair mechanisms in dental caries. Mediators Inflamm. 2015;2015 doi: 10.1155/2015/230251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Villa D., Gómez-Lavín M.J., Abradelo C., Román J.S., Rojo L. Tissue engineering therapies based on folic acid and other vitamin B derivatives. Functional mechanisms and current applications in regenerative medicine. Int. J. Mol. Sci. 2018;19(12) doi: 10.3390/ijms19124068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaje P.N., Ceausu R.A. Cell types of the dental pulp behind the odontoblast. Res. Clin. Med. 2020;4(2):16–18. [Google Scholar]
- Gokkusu C., Tulubas F., Unlucerci Y., Ozkok E., Umman B., Aydin M. Homocysteine and pro-inflammatory cytokine concentrations in acute heart disease. Cytokine. 2010;50(1):15–18. doi: 10.1016/j.cyto.2009.12.015. [DOI] [PubMed] [Google Scholar]
- Gómez-García A.P., López-Vidal Y., Pinto-Cardoso S., Aguirre-García M.M. Overexpression of proinflammatory cytokines in dental pulp tissue and distinct bacterial microbiota in carious teeth of Mexican Individuals. Front. Cell. Infect. Microbiol. 2022;12(December):1–15. doi: 10.3389/fcimb.2022.958722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn C.L., Falkler W.A., Minah G.E. Microbiological studies of carious dentine from human teeth with irreversible pulpitis. Arch. Oral Biol. 1991;36(2):147–153. doi: 10.1016/0003-9969(91)90077-8. [DOI] [PubMed] [Google Scholar]
- Hozhabri N.S.T., Benson M.D., Vu M.D., Patel R.H., Martinez R.M., Nakhaie F.N., Kim H.K.W., Varanasi V.G. Decreasing NF-κB expression enhances odontoblastic differentiation and collagen expression in dental pulp stem cells exposed to inflammatory cytokines. PLoS One. 2015;10(1):1–16. doi: 10.1371/journal.pone.0113334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X., Qin X., Yang W., Liu L., Jiang C., Zhang X., Jiang S., Bao H., Su H., Li P., He M., Song Y., Zhao M., Yin D., Wang Y., Zhang Y., Li J., Yang R., Wu Y., Cheng X. MTHFR gene and serum folate interaction on serum homocysteine lowering prospect for precision folic acid treatment. Arterioscler. Thromb. Vasc. Biol. 2018;38(3):679–685. doi: 10.1161/ATVBAHA.117.310211. [DOI] [PubMed] [Google Scholar]
- Kim D.H., Kim S.M., Lee B., Lee E.K., Chung K.W., Moon K.M., An H.J., Kim K.M., Yu B.P., Chung H.Y. Effect of betaine on hepatic insulin resistance through FOXO1-induced NLRP3 inflammasome. J. Nutr. Biochem. 2017;45:104–114. doi: 10.1016/j.jnutbio.2017.04.014. [DOI] [PubMed] [Google Scholar]
- Kim J.Y., Xin X., Moioli E.K., Chung J., Lee C.H., Chen M., Fu S.Y., Koch P.D., Mao J.J. Regeneration of dental-pulp-like tissue by chemotaxis-induced cell homing. Tissue Eng. - Part A. 2010;16(10):3023–3031. doi: 10.1089/ten.tea.2010.0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb A.F., Petrie L. Folate deficiency enhances the inflammatory response of macrophages. Mol. Immunol. 2013;54(2):164–172. doi: 10.1016/j.molimm.2012.11.012. [DOI] [PubMed] [Google Scholar]
- Kornsuthisopon C., Nantanapiboon D., Rochanavibhata S., Nowwarote N., Namangkalakul W., Osathanon T. Betaine promotes osteogenic differentiation in immortalized human dental pulp-derived cells. BDJ Open. 2022;8(1) doi: 10.1038/s41405-022-00123-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronenberg G., Harms C., Sobol R.W., Cardozo-Pelaez F., Linhart H., Winter B., Balkaya M., Gertz K., Gay S.B., Cox D., Eckart S., Ahmadi M., Juckel G., Kempermann G., Hellweg R., Sohr R., Hörtnagl H., Wilson S.H., Jaenisch R., Endres M. Folate deficiency induces neurodegeneration and brain dysfunction in mice lacking uracil DNA glycosylase. J. Neurosci. 2008;28(28):7219–7230. doi: 10.1523/JNEUROSCI.0940-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwack K.H., Lee H.W. Clinical potential of dental pulp stem cells in pulp regeneration: current endodontic progress and future perspectives. Front. Cell Dev. Biol. 2022;10(April):1–18. doi: 10.3389/fcell.2022.857066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leon M., Sawmiller D., Shytle R.D., Tan J. Therapeutic cocktail approach for treatment of hyperhomocysteinemia in Alzheimer’s disease. Cell Med. 2018;10 doi: 10.1177/2155179017722280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z., Jiang H., Townsend J.H., Wang J. Effects of Ocufolin on retinal microvasculature in patients with mild non-proliferative diabetic retinopathy carrying polymorphisms of the MTHFR gene. BMJ Open Diabetes Res. Care. 2021;9(1):1–9. doi: 10.1136/bmjdrc-2021-002327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macklin, C., 2020. UC Berkeley UC Berkeley Electronic Theses and Dissertations. DNA Mediated Assembly of Protein Heterodimers on Membrane Surfaces, 67. https://escholarship.org/uc/item/98384265.
- Makvandi P., Josic U., Delfi M., Pinelli F., Jahed V., Kaya E., Ashrafizadeh M., Zarepour A., Rossi F., Zarrabi A., Agarwal T., Zare E.N., Ghomi M., Kumar Maiti T., Breschi L., Tay F.R. Drug delivery (Nano)Platforms for oral and dental applications: tissue regeneration, infection control, and cancer management. Adv. Sci. 2021;8(8) doi: 10.1002/advs.202004014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinow M.R., Nieto F.J., Kruger W.D., Duell P.B., Hess D.L., Gluckman R.A., Block P.C., Holzgang C.R., Anderson P.H., Seltzer D., Upson B., Lin Q.R. The effects of folic acid supplementation on plasma total homocysteine are modulated by multivitamin use and methylenetetrahydrofolate reductase genotypes. Arterioscler. Thromb. Vasc. Biol. 1997;17(6):1157–1162. doi: 10.1161/01.ATV.17.6.1157. [DOI] [PubMed] [Google Scholar]
- Meng Z., Liu J., Feng Z., Guo S., Wang M., Wang Z., Li Z., Li H., Sui L. N-acetylcysteine regulates dental follicle stem cell osteogenesis and alveolar bone repair via ROS scavenging. Stem Cell Res Ther. 2022;13(1):1–16. doi: 10.1186/s13287-022-03161-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mistry L., Dhariwal N.S., Majeed A., Badakar C. Assessment of Vitamin B12 and its correlation with dental caries and gingival diseases in 10- to 14-year-old children: a cross-sectional study. Int. J. Clin. Pediatric Dent. 2017;10(2):142–146. doi: 10.5005/jp-journals-10005-1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moussa D.G., Aparicio C. Present and future of tissue engineering scaffolds for dentin-pulp complex regeneration. J. Tissue Eng. Regen. Med. 2019;13(1):58–75. doi: 10.1002/term.2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanetti L., Vignini A., Raffaelli F., Giulietti A., Bartolini M., Perozzi C., Silvestrini M., Provinciali L., Mazzanti L. Homocysteine and oxidative stress in acute stroke. Adv. Biosci. Biotechnol. 2013;04(11):15–23. doi: 10.4236/abb.2013.411a2003. [DOI] [Google Scholar]
- Nica C., Lin Z., Sculean A., Asparuhova M.B. Adsorption and release of growth factors from four different porcine-derived collagen matrices. Materials. 2020;13(11):1–20. doi: 10.3390/ma13112635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olgart, L.M., 1992. Involvement of sensory nerves in hemodynamic reactions. In: Proceedings of the Finnish Dental Society. Suomen Hammaslääkäriseuran Toimituksia, 88 Suppl 1, 403–410. [PubMed]
- Olthof, M. R., Vliet, T. Van, Boelsma, E., Verhoef, P., 2003. Human Nutrition and Metabolism Leads to Immediate and Long Term Lowering of Plasma Homocysteine in Healthy Men and Women 1, 2. March, pp. 4135–4138. [DOI] [PubMed]
- Owens G.D., Smith P.D., Jadavji N.M. Neurogenesis unchanged by MTHFR deficiency in three-week-old mice. J. Young Investigators. 2016;31(6):39–43. doi: 10.22186/jyi.31.6.39-44.2007. [DOI] [Google Scholar]
- Park M.Y., Jeong Y.J., Kang G.C., Kim M.H., Kim S.H., Chung H.J., Jung J.Y., Kim W.J. Nitric oxide-induced apoptosis of human dental pulp cells is mediated by the mitochondria-dependent pathway. Korean J. Physiol. Pharmacol. 2014;18(1):25–32. doi: 10.4196/kjpp.2014.18.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S.H., Ye L., Love R.M., Farges J.C., Yumoto H. Inflammation of the dental pulp. Mediators Inflamm. 2015;2015 doi: 10.1155/2015/980196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei Y., Liu H., Yang Y., Yang Y., Jiao Y., Tay F.R., Chen J. Biological activities and potential oral applications of N-acetylcysteine: Progress and prospects. Oxid. Med. Cell. Longev. 2018;2018 doi: 10.1155/2018/2835787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rechenberg D.K., Galicia J.C., Peters O.A. Biological markers for pulpal inflammation: a systematic review. PLoS One. 2016;11(11):1–24. doi: 10.1371/journal.pone.0167289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozen R. Molecular Biology of Methylenetetrahydrofolate Reductase (MTHFR): interrelationships with folic acid. Homocysteine Vascular Dis. 2000;271–289 doi: 10.1007/978-94-017-1789-2_16. [DOI] [Google Scholar]
- Schwahn B.C., Chen Z., Laryea M.D., Wendel U., Lussier-Cacan S., Genest J., Mar M.H., Zeisel S.H., Castro C., Garrow T., Rozen R. Homocysteine-betaine interactions in a murine model of 5,10-methylenetetrahydrofolate reductase deficiency. FASEB J.: Official Publication Federation Am. Societies Experimental Biol. 2003;17(3):512–514. doi: 10.1096/fj.02-0456fje. [DOI] [PubMed] [Google Scholar]
- Senghore T., Li Y.F., Sung F.C., Tsai M.H., Hua C.H., Liu C.S., Hung M.F., Yeh C.C. Associations between MTHFR polymorphisms and the risk of potentially malignant oral disorders. Anticancer Res. 2018;38(7):4021–4026. doi: 10.21873/anticanres.12690. [DOI] [PubMed] [Google Scholar]
- Stover P.J., MacFarlane A.J., Field M.S. Bringing clarity to the role of MTHFR variants in neural tube defect prevention. Am. J. Clin. Nutr. 2015;101(6):1111–1112. doi: 10.3945/ajcn.115.111088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Y., Wang C., Ye L. Healing rate and post-obturation pain of single- versus multiple-visit endodontic treatment for infected root canals: a systematic review. J. Endod. 2011;37(2):125–132. doi: 10.1016/j.joen.2010.09.005. [DOI] [PubMed] [Google Scholar]
- Taguchi A., Saito M., Shiraki M. Association of pentosidine and homocysteine levels with number of teeth present in Japanese postmenopausal women. J. Bone Miner. Metab. 2022;1–18 doi: 10.1007/s00774-022-01343-5. [DOI] [PubMed] [Google Scholar]
- Tardiolo G., Bramanti P., Mazzon E. Overview on the effects of N-acetylcysteine in neurodegenerative diseases. Molecules. 2018;23(12) doi: 10.3390/molecules23123305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyagi N., Sedoris K.C., Steed M., Ovechkin A.V., Moshal K.S., Tyagi S.C. Mechanisms of homocysteine-induced oxidative stress. Am. J. Physiol. Heart Circ. Physiol. 2005;289(6 58–6):2649–2656. doi: 10.1152/ajpheart.00548.2005. [DOI] [PubMed] [Google Scholar]
- Vengerfeldt V., Mändar R., Saag M., Piir A., Kullisaar T. Oxidative stress in patients with endodontic pathologies. J. Pain Res. 2017;10:2031–2040. doi: 10.2147/JPR.S141366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan L., Li Y., Zhang Z., Sun Z., He Y., Li R. Methylenetetrahydrofolate reductase and psychiatric diseases. Translational Psychiatry. 2018;8(1) doi: 10.1038/s41398-018-0276-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Ma C., Gong L., Dai S., Li Y. Preventive and therapeutic role of betaine in liver disease: a review on molecular mechanisms. Eur. J. Pharmacol. 2021;912(December):1–11. doi: 10.1016/j.ejphar.2021.174604. [DOI] [PubMed] [Google Scholar]
- WHO, 2022. Global oral health status report. In: Dental Abstracts, Vol. 57, Issue 2.
- Woo S.M., Lim H.S., Jeong K.Y., Kim S.M., Kim W.J. Vitamin D promotes odontogenic differentiation of human dental pulp cells via ERK activation. Mol. Cells. 2015;38(7):604–609. doi: 10.14348/molcells.2015.2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu C., Abbott P.V. An overview of the dental pulp: Its functions and responses to injury. Aust. Dent. J. 2007;52(1 SUPPL.):S4–S6. doi: 10.1111/j.1834-7819.2007.tb00525.x. [DOI] [PubMed] [Google Scholar]
- Zhan C., Huang M., Yang X., Hou J. Dental nerves: a neglected mediator of pulpitis. Int. Endod. J. 2021;54(1):85–99. doi: 10.1111/iej.13400. [DOI] [PubMed] [Google Scholar]
- Zhang L., Li Z., Xing C., Gao N., Xu R. Folate Reverses NF-κB p65/Rela/IL-6 Level Induced by Hyperhomocysteinemia in Spontaneously Hypertensive Rats. Front. Pharmacol. 2021;12(September):1–11. doi: 10.3389/fphar.2021.651582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S., Wang T., Wang H., Tang J., Hou A., Yan X., Yu B., Ran S., Luo M., Tang Y., Yang R., Song D., He H. Effects of individualized administration of folic acid on prothrombotic state and vascular endothelial function with H-type hypertension A double-blinded, randomized clinical cohort study. Medicine (United States) 2022;101(3):3–8. doi: 10.1097/MD.0000000000028628. [DOI] [PMC free article] [PubMed] [Google Scholar]