Abstract
Recent studies of anaerobic toluene catabolism have demonstrated a novel reaction for anaerobic hydrocarbon activation: the addition of the methyl carbon of toluene to fumarate to form benzylsuccinate. In vitro studies of the anaerobic benzylsuccinate synthase reaction indicate that the H atom abstracted from the toluene methyl group during addition to fumarate is retained in the succinyl moiety of benzylsuccinate. Based on structural studies of benzylsuccinate formed during anaerobic, in vitro assays with denitrifying, toluene-mineralizing strain T, we now report the following characteristics of the benzylsuccinate synthase reaction: (i) it is highly stereospecific, resulting in >95% formation of the (+)-benzylsuccinic acid enantiomer [(R)-2-benzyl-3-carboxypropionic acid], and (ii) active benzylsuccinate synthase does not contain an abstracted methyl H atom from toluene at the beginning or at the end of a catalytic cycle.
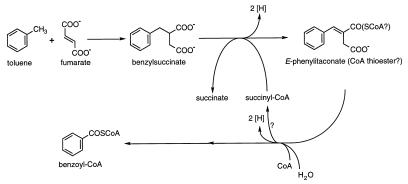
A novel enzymatic reaction for anaerobic toluene activation has been reported recently that involves the addition of the methyl carbon of toluene to the double bond of fumarate to form benzylsuccinate (Fig. 1). This reaction is of considerable biochemical interest not only as a novel means of aromatic hydrocarbon activation but also as a novel means of enzymatic carbon-carbon bond formation. The benzylsuccinate synthase reaction, which has thus far been documented in three anaerobic, toluene-degrading bacteria (denitrifying strain T [2] and Thauera aromatica [5] and sulfate-reducing strain PRTOL1 [3]), is clearly distinguished from the only class of reactions previously known to activate aromatic hydrocarbons, oxygenase reactions, which require molecular oxygen as a cosubstrate (e.g., see reference 11).
FIG. 1.
Proposed reactions involved in anaerobic toluene oxidation to benzoyl-CoA. This pathway is based on research conducted with denitrifying strain T (2) and T. aromatica (5) and sulfate-reducing strain PRTOL1 (3).
In vitro studies with strain T (2) and T. aromatica (5) suggest that benzylsuccinate formation is the first step of toluene mineralization to carbon dioxide, based in part on the observed conversion of benzylsuccinate to benzoyl-coenzyme A (CoA) (a known intermediate of anaerobic toluene mineralization; e.g., see references 1, 5, 10, and 14) in the presence of nitrate and a source of CoA. Although all reactions of an anaerobic toluene mineralization pathway have not been demonstrated, there exists strong evidence for several reactions depicted in Fig. 1, namely, the toluene-fumarate addition reaction (2, 3, 5, 12) and the CoA-dependent conversion of benzylsuccinate to E-phenylitaconate or its CoA thioester (2) and subsequently to benzoyl-CoA (2, 5). Intervening reactions in the pathway outlined in Fig. 1 have been proposed (2, 5).
The reaction mechanism of benzylsuccinate synthase has not yet been elucidated. However, studies with two Thauera strains suggest that the reaction may be radical, based largely on a high level of homology between the predicted amino acid sequence of the carboxy-terminal region of benzylsuccinate synthase in these Thauera strains and conserved amino acid residues that have been shown to be essential for the radical mechanism of pyruvate formate-lyase in Escherichia coli (8, 12). In order to develop a mechanistic understanding of the toluene-fumarate addition reaction, we have concentrated on structural analysis of the reaction product, benzylsuccinate. In previous studies, we discovered that the H atom abstracted from the toluene methyl group during addition to fumarate is retained in the succinyl moiety of benzylsuccinate (2, 3). In this article, we present evidence that this transferred H atom and the benzyl moiety of a benzylsuccinate molecule derive from the same parent toluene molecule and that the toluene-fumarate addition reaction is highly stereospecific, forming predominantly, if not exclusively, (+)-benzylsuccinic acid [or (R)-2-benzyl-3-carboxypropionic acid]. The data reported in this article were generated from anaerobic assays conducted with permeabilized cells of toluene-grown, denitrifying strain T (2, 9).
Stereospecificity of benzylsuccinate synthase.
Chiral high-performance liquid chromatography (HPLC) analyses of benzylsuccinate formed anaerobically in vitro from toluene and fumarate indicate that only the (+) enantiomer was produced. For these analyses, benzylsuccinate was solvent extracted from permeabilized-cell assays (1-ml total volume in 20 mM MOPS [morpholinopropanesulfonic acid] buffer, pH 7.2 [2]) that were amended with toluene (400 nmol), fumarate (500 nmol), permeabilized strain T cells (∼4 mg of protein), and titanium(III) chloride as a reductant (0.2 mM). The solvent (diethyl ether) extracts were exchanged into HPLC eluent and analyzed with a Hewlett-Packard Series 1050 liquid chromatograph. The mobile phase was a 93:7:0.02 (vol/vol/vol) mixture of hexane, ethanol, and trifluoroacetic acid, respectively, flowing isocratically at 1 ml/min through a CHIRALPAK AD column (particle size, 10 μm; 250 mm [length] by 4.6 mm [inner diameter]) (Chiral Technologies, Inc., Exton, Pa.). The injection loop volume was 100 μl. Benzylsuccinate enantiomers were detected with a wavelength of 254 nm. Authentic (+)-benzylsuccinic acid [or (R)-2-benzyl-3-carboxypropionic acid] and (−)-benzylsuccinic acid [or (S)-2-benzyl-3-carboxypropionic acid] standards (99% purity) were purchased from Radian International (Austin, Tex.).
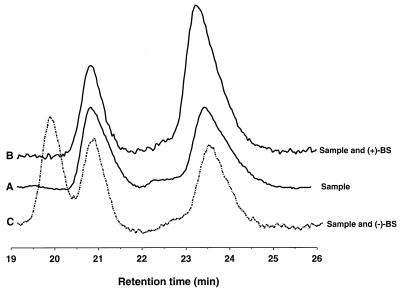
In Fig. 2, an HPLC chromatogram of an ether extract pooled from six permeabilized-cell assays is shown along with chromatograms of the same extract coinjected with either a (+)- or a (−)-benzylsuccinate standard (0.8 mM). The chiral HPLC chromatograms demonstrate that a compound was present in the sample extract that coeluted with a (+)-benzylsuccinate standard and that there was no detectable compound in the extract that eluted at the retention time of a (−)-benzylsuccinate standard. Since gas chromatography-mass spectrometry (GC-MS) analysis confirmed that benzylsuccinate was a predominant component of this sample extract (data not shown), the chiral HPLC results can be interpreted to indicate that (+)-benzylsuccinate was produced from toluene and fumarate. If it were assumed that (−)-benzylsuccinate was present at just below its detection limit, then (−)-benzylsuccinate would constitute less than 5% of the total benzylsuccinate present in the extract.
FIG. 2.
Chiral HPLC analysis of benzylsuccinate formed in vitro from toluene and fumarate. (A) Sample containing benzylsuccinate produced from toluene and fumarate by permeabilized strain T cells (see text for experimental conditions); (B) the sample coinjected with a (+)-benzylsuccinate standard (0.8 mM); (C) the sample coinjected with a (−)-benzylsuccinate standard (0.8 mM). The identity of the compound eluting at ∼21 min is unknown.
As an alternative approach to investigating the stereospecificity of benzylsuccinate synthesis from toluene and fumarate, the ability of permeabilized strain T cells to metabolize (+)- and (−)-benzylsuccinate standards was assessed. Previous studies have shown that in vitro oxidation of benzylsuccinate by toluene-degrading, denitrifying bacteria (strain T and T. aromatica) requires the presence of a source of CoA and an electron acceptor (2, 5), as indicated in Fig. 1. Preliminary experiments intended to optimize benzylsuccinate oxidation in in vitro assays determined that succinyl-CoA was a better CoA source than free CoA. In these preliminary experiments, assay mixtures (1-ml total volume in 20 mM MOPS buffer; pH 7.2) containing racemic commercial benzylsuccinate (150 nmol), nitrate (2 mM), permeabilized cells (3 mg of protein), titanium(III) chloride (0.2 mM), and either CoA (0.3 mM) or succinyl-CoA (0.3 mM) were incubated for 1 h, subjected to alkaline hydrolysis to cleave CoA thioesters that may have formed, and then extracted, derivatized with diazomethane, and analyzed by GC-MS using methods described previously (2, 4). In these experiments, the yields of observed benzylsuccinate oxidation products (E-phenylitaconate and benzoyl-CoA) were approximately three times greater in the assays amended with succinyl-CoA than in the assays amended with free CoA (data not shown). Furthermore, in analogous assays amended with toluene (400 nmol) and fumarate (500 nmol) rather than with benzylsuccinate, E-phenylitaconate and benzoyl-CoA, yields were approximately four to six times greater in the assays amended with succinyl-CoA than in the assays amended with free CoA. On the basis of these results, assays used to investigate (+)- and (−)-benzylsuccinate oxidation were amended with succinyl-CoA rather than with free CoA.
The results of the assays used to investigate (+)- and (−)-benzylsuccinate oxidation, expressed as nanomoles of benzylsuccinate, E-phenylitaconate, and benzoate (or benzoyl-CoA) detected after incubation, are presented in Table 1. The tabulated values are the averages of duplicate assays (except for the control without benzylsuccinate, which was not replicated). After incubation, assay mixtures amended with (+)-benzylsuccinate contained no detectable benzylsuccinate (detection limit, ∼0.2 nmol). As evidence that the (+)-benzylsuccinate was metabolized, most of the initial mass of benzylsuccinate was recovered as the oxidation products E-phenylitaconate and benzoate (accounting for the observed analytical recovery of ∼50%; Table 1). In contrast, in assay mixtures amended with (−)-benzylsuccinate, the mass of benzylsuccinate recovered after incubation (50 nmol) was virtually identical to the amount recovered in benzylsuccinate-amended controls that were not amended with permeabilized cells (52 nmol; data not shown in Table 1). Furthermore, the amounts of E-phenylitaconate and benzoate found in assay mixtures amended with (−)-benzylsuccinate were very low and were similar to the amounts found in a control to which no benzylsuccinate had been added (Table 1); the source of the small amounts of E-phenylitaconate and benzoate observed in the assays amended with (−)-benzylsuccinate and in the control without amended benzylsuccinate was probably residual benzylsuccinate present in the toluene-grown, permeabilized strain T cells.
TABLE 1.
Metabolism of (+)- and (−)-benzylsuccinate by permeabilized strain T cellsa
| Benzylsuccinate isomer added | Amt detected after incubation (nmol)
|
||
|---|---|---|---|
| Benzylsuccinate | E-phenylitaconate | Benzoate | |
| (+) | <DLb | 37 | 3 |
| (−) | 50 | 0.4 | 0.9 |
| None | <DL | 0.3 | 0.7 |
The anaerobic assay mixtures (total volume, 1 ml; incubation time, 1 h) were amended with (+)- or (−)-benzylsuccinate (100 nmol, except in the control), succinyl-CoA (0.35 mM), nitrate (2 mM), titanium(III) chloride (0.2 mM), and ∼3 mg of protein from permeabilized, toluene-grown strain T cells. Analytical recovery of benzylsuccinate in these experiments was approximately 51% ± 3.9% (mean ± standard deviation) based on benzylsuccinate-amended control experiments without permeabilized cells (data not shown) and the (−)-benzylsuccinate-amended assays represented in the table.
<DL, less than the detection limit of approximately 0.2 nmol.
Fate of the methyl H atom abstracted from toluene.
As reported previously for strains T and PRTOL1, the H atom abstracted from the toluene methyl group during addition to fumarate is retained in the succinyl moiety of benzylsuccinate (2, 3). This was substantiated by comparing the electron impact mass spectra of deuterium-labeled and unlabeled benzylsuccinate formed in vitro from fumarate and either labeled or unlabeled toluene (2, 3).
We further investigated whether both the H atom abstracted from toluene and the benzyl portion of its parent toluene molecule are retained in the same benzylsuccinate molecule (i.e., whether or not the benzyl [C6H5CH2−] moiety of benzylsuccinate and the abstracted methyl H atom retained in the succinyl moiety of benzylsuccinate derive from the exact same toluene molecule). To examine this possibility, an anaerobic, 1-h assay was conducted with an equimolar mixture of labeled and unlabeled toluene (∼200 nmol each of toluene-α,α,α-d3 and unlabeled toluene), fumarate (500 nmol), permeabilized cells (∼3 mg of protein), and titanium(III) chloride (0.2 mM). After incubation and diethyl ether extraction of the assay mixture, the extract was derivatized with diazomethane (to form dimethyl benzylsuccinate) and analyzed by GC-MS using methods described elsewhere (2, 4). The concept underlying this experiment is as follows. If the abstracted methyl H (or D) atom remains with its parent toluene molecule, then there should be a bimodal (1:1) distribution of dimethyl benzylsuccinates with molecular weights of 236 (C13H16O4; from unlabeled toluene) and 239 (C13D3H13O4; from toluene-α,α,α-d3). If instead the abstracted H (or D) atom does not remain with its parent toluene molecule, then there should be a 50% chance of adding either a D or an H atom to the succinyl moiety of a benzylsuccinate molecule containing a benzyl group from either toluene or toluene-α,α,α-d3 (i.e., C6H5CH2- or C6H5CD2-), resulting in a tetramodal (1:1:1:1) distribution of dimethyl benzylsuccinates with molecular weights of 236, 237, 238, and 239.
The results of this experiment are presented in Table 2. The distribution of benzylsuccinate was largely bimodal, with a predominance of molecular weights of 236 and 239. The relative distribution of 237-Da dimethyl benzylsuccinate in the sample was similar to that in an unlabeled dimethyl benzylsuccinate standard (Table 2). The occurrence of 237-Da dimethyl benzylsuccinate is consistent with the natural isotopic abundance of 13C (13), which can be used to predict a relative abundance for 237-Da benzylsuccinate of 14.3% (close to the observed 15 to 16%). An analogous explanation can be used for the relative abundance of 240-Da benzylsuccinate, which is probably a 13C-labeled version of the 239-Da benzylsuccinate and cannot be explained by deuterium labeling alone.
TABLE 2.
Molecular weight distributions of benzylsuccinate molecules in an unlabeled benzylsuccinate standard and formed during an in vitro assay
| Benzylsuccinate source | Relative distributiona (%) of benzylsuccinates with a molecular wt of:
|
||||
|---|---|---|---|---|---|
| 236 | 237 | 238 | 239 | 240 | |
| Assay (equimolar toluene and toluene-d3)b | 100 | 16 | ≤1 | 70 | 9.0 |
| Unlabeled benzylsuccinate (dimethyl ester) standard | 100 | 15 | 2 | <DLc | <DL |
Areas of dimethyl benzylsuccinate peaks integrated with quantification ions of m/z 236, 237, 238, 239, and 240 normalized to the area for m/z 236.
The total amount of benzylsuccinate formed in this assay amended with ∼400 nmol of toluene was 250 to 300 nmol. Assay conditions are given in the text.
<DL, less than the detection limit of approximately 1.
Concluding remarks.
This article describes salient characteristics of the unique benzylsuccinate synthase reaction. Enzymatic addition of toluene to fumarate to yield benzylsuccinate is a unique reaction with respect to carbon-carbon bond formation as well as aromatic hydrocarbon activation. Benzylsuccinate synthase differs from known enzymes that catalyze the formation of new carbon-carbon bonds in that it catalyzes the addition of a carbon atom to a carbon-carbon double bond rather than to a carbon-oxygen double bond or to CO2. Benzylsuccinate synthase also differs from the well-characterized mono- and dioxygenases, which use molecular oxygen to oxidatively activate aromatic hydrocarbons by hydroxylation. In contrast, benzylsuccinate synthase activates toluene by catalyzing its addition to a carboxylated substrate, fumarate. As a result of the benzylsuccinate synthase reaction, the methyl carbon of toluene is transformed to a methylene carbon that is in a beta position to a carboxyl group.
This study, which relies on structural analysis of benzylsuccinate formed in vitro, reports two observations that provide insight into the mechanism of benzylsuccinate synthase. First, the benzylsuccinate synthase reaction is highly stereospecific, resulting in >95% formation of the (+)-benzylsuccinate enantiomer [(R)-2-benzyl-3-carboxypropionic acid]. This suggests that toluene adds to the re face of a C-2 carbon of fumarate, which is an sp2-hybridized center. Second, the benzyl moiety of benzylsuccinate and the abstracted methyl H atom retained in the succinyl moiety of benzylsuccinate derive from the exact same toluene molecule (Table 2). This observation suggests that active benzylsuccinate synthase does not contain an abstracted methyl H atom at the beginning or at the end of a catalytic cycle. In contrast, examples of carbon-carbon bond cleaving enzymes that do contain some fragment of a substrate molecule before and after a catalytic cycle include citrate lyase from Klebsiella aerogenes and Streptococcus diacetilactis (7, 15, 16) as well as citramalate lyase from Clostridium tetanomorphum (6); both enzymes are acetylated in the unbound state.
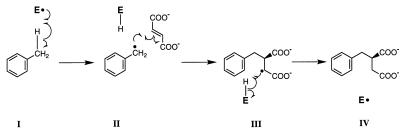
The proposed reaction mechanism depicted in Fig. 3 is consistent with findings presented in this study and with suggestions of other researchers regarding the possible radical nature of the benzylsuccinate synthase reaction (8, 12). Initially, activated benzylsuccinate synthase containing a free radical could abstract a hydrogen atom from the methyl carbon of toluene to yield a benzyl radical (I). The benzyl radical could then add to the double bond of fumarate to yield a (+)-benzylsuccinyl radical (II). The abstracted H atom retained by the enzyme could then react with the (+)-benzylsuccinyl radical (III) to yield (+)-benzylsuccinate and the activated enzyme radical (IV). In vitro studies with purified benzylsuccinate synthase will be required to determine the validity of the proposed radical mechanism.
FIG. 3.
Proposed reaction mechanism for benzylsuccinate synthase. E represents the enzyme. Note that the exact same H atom is bound to the enzyme in II and III.
Acknowledgments
Funding for this study was provided by the National Science Foundation (MCB-9723312) and by the Office of Research and Development, U.S. Environmental Protection Agency, under grant R-815738 through the Western Region Hazardous Substance Research Center. Additional support was provided through an OTL Research Incentive Fund (Stanford University) and a Terman Fellowship to A.M.S.
We thank John Brauman (Stanford University) for helpful discussions.
REFERENCES
- 1.Beller H R, Reinhard M, Grbić-Galić D. Metabolic by-products of anaerobic toluene degradation by sulfate-reducing enrichment cultures. Appl Environ Microbiol. 1992;58:3192–3195. doi: 10.1128/aem.58.9.3192-3195.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beller H R, Spormann A M. Anaerobic activation of toluene and o-xylene by addition to fumarate in denitrifying strain T. J Bacteriol. 1997;179:670–676. doi: 10.1128/jb.179.3.670-676.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beller H R, Spormann A M. Benzylsuccinate formation as a means of anaerobic toluene activation by sulfate-reducing strain PRTOL1. Appl Environ Microbiol. 1997;63:3729–3731. doi: 10.1128/aem.63.9.3729-3731.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beller H R, Spormann A M, Sharma P K, Cole J R, Reinhard M. Isolation and characterization of a novel toluene-degrading, sulfate-reducing bacterium. Appl Environ Microbiol. 1996;62:1188–1196. doi: 10.1128/aem.62.4.1188-1196.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Biegert T, Fuchs G, Heider J. Evidence that anaerobic oxidation of toluene in the denitrifying bacterium Thauera aromatica is initiated by formation of benzylsuccinate from toluene and fumarate. Eur J Biochem. 1996;238:661–668. doi: 10.1111/j.1432-1033.1996.0661w.x. [DOI] [PubMed] [Google Scholar]
- 6.Buckel W, Bobi A. Enzyme complex citramalate lyase from Clostridium tetanomorphum. Eur J Biochem. 1976;64:255–262. doi: 10.1111/j.1432-1033.1976.tb10295.x. [DOI] [PubMed] [Google Scholar]
- 7.Buckel W, Buschmeier V, Eggerer H. Mechanism of action of citrate-lyase from Klebsiella aerogenes. Hoppe-Seyler’s Z Physiol Chem. 1971;352:1195–1205. [PubMed] [Google Scholar]
- 8.Coschigano P W, Wehrman T S, Young L Y. Identification and analysis of genes involved in anaerobic toluene metabolism by strain T1: putative role of a glycine free radical. Appl Environ Microbiol. 1998;64:1650–1656. doi: 10.1128/aem.64.5.1650-1656.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dolfing J, Zeyer J, Binder-Eicher P, Schwarzenbach R P. Isolation and characterization of a bacterium that mineralizes toluene in the absence of molecular oxygen. Arch Microbiol. 1990;154:336–341. doi: 10.1007/BF00276528. [DOI] [PubMed] [Google Scholar]
- 10.Frazer A C, Ling W, Young L Y. Substrate induction and metabolite accumulation during anaerobic toluene utilization by the denitrifying strain T1. Appl Environ Microbiol. 1993;59:3157–3160. doi: 10.1128/aem.59.9.3157-3160.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gibson D T, Subramanian V. Microbial degradation of aromatic hydrocarbons. In: Gibson D T, editor. Microbial degradation of organic compounds. New York, N.Y: Marcel Dekker, Inc.; 1984. pp. 181–252. [Google Scholar]
- 12.Leuthner B, Leutwein C, Schulz H, Hörth P, Haehnel W, Schiltz E, Schägger H, Heider J. Biochemical and genetic characterization of benzylsuccinate synthase from Thauera aromatica: a new glycyl radical enzyme catalysing the first step in anaerobic toluene metabolism. Mol Microbiol. 1998;28:615–628. doi: 10.1046/j.1365-2958.1998.00826.x. [DOI] [PubMed] [Google Scholar]
- 13.McLafferty F W. Interpretation of mass spectra. 3rd ed. Mill Valley, Calif: University Science Books; 1980. [Google Scholar]
- 14.Seyfried B, Glod G, Schocher R, Tschech A, Zeyer J. Initial reactions in the anaerobic oxidation of toluene and m-xylene by denitrifying bacteria. Appl Environ Microbiol. 1994;60:4047–4052. doi: 10.1128/aem.60.11.4047-4052.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Singh M, Srere P A. Purification and properties of citrate lyase from Streptococcus diacetilactis: physicochemical and immunological studies of an acetyl enzyme. J Biol Chem. 1975;250:5818–5825. [PubMed] [Google Scholar]
- 16.Srere P A, Boettger B, Brooks G C. Citrate lyase: a pantothenate-containing enzyme. Proc Natl Acad Sci USA. 1972;69:1201–1202. doi: 10.1073/pnas.69.5.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]