Abstract
Aims/Introduction
Coronavirus disease 2019 (COVID‐19) vaccinations have been proven to be generally safe in healthy populations. However, the data on vaccine safety in patients with type 1 diabetes are scarce. This study aimed to evaluate the frequency and severity of short‐term (<7‐day) adverse vaccination events (AEs) and their risk factors among type 1 diabetes patients.
Materials and Methods
This study analyzed data from the COVID‐19 vaccination in Autoimmune Diseases (COVAD) survey database (May to December 2021; 110 collaborators, 94 countries), comparing <7‐day COVID‐19 vaccine AE among type 1 diabetes patients and healthy controls (HCs). Descriptive statistics; propensity score matching (1:4) using the variables age, sex and ethnicity; and multivariate analyses were carried out.
Results
This study analyzed 5,480 completed survey responses. Of all responses, 5,408 were HCs, 72 were type 1 diabetes patients (43 females, 48.0% white European ancestry) and Pfizer was the most administered vaccine (39%). A total of 4,052 (73.9%) respondents had received two vaccine doses. Patients with type 1 diabetes had a comparable risk of injection site pain, minor and major vaccine AEs, as well as associated hospitalizations to HCs. However, type 1 diabetes patients had a higher risk of severe rashes (3% vs 0.4%, OR 8.0, 95% confidence interval 1.7–36), P = 0.007), although reassuringly, these were rare (n = 2 among type 1 diabetes patients).
Conclusions
COVID‐19 vaccination was safe and well tolerated in patients with type 1 diabetes with similar AE profiles compared with HCs, although severe rashes were more common in type 1 diabetes patients.
Keywords: COVID‐19, Type 1 diabetes mellitus, Vaccine
We evaluated short‐term <7‐day COVID‐19 adverse vaccination events in a global sample of patients with type 1 diabetes and healthy controls from the COVAD cross‐sectional patient self‐reported e‐survey. COVID‐19 vaccination was safe and well tolerated in patients with type 1 diabetes, with a comparable risk of minor and major adverse vaccination events, and hospitalizations to healthy controls, although severe rashes were more frequent in type 1 diabetes patients.
INTRODUCTION
Patients with diabetes mellitus have been identified as a high‐risk group for severe acute respiratory syndrome‐associated coronavirus‐2 (SARS‐CoV‐2) infection and poor coronavirus disease 2019 (COVID‐19) outcomes. A possible pathophysiological basis might lie in the bidirectional relationship between diabetes and SARS‐CoV‐2 infection. Poor glycemic control in patients with diabetes might lead to alterations in innate cell‐mediated immunity, low leukocyte recruitment, decreased macrocytic phagocytosis and poor cytokine response, which are believed to facilitate the progression of SARS‐CoV‐2 infection to severe COVID‐19 1 . In contrast, the hyperinflammatory state associated with COVID‐19 can exacerbate insulin resistance, thus worsening glycemic control 1 .
COVID‐19‐associated mortality is significantly high, especially among black and Asian type 1 diabetes patients, with patients with additional comorbidities, including hypertension and dyslipidemia, at a greater risk 1 , 2 . In 2020, a study reported that >50% of type 1 diabetes patients with COVID‐19 infection developed hyperglycemia; one‐third of whom experienced diabetic ketoacidosis 3 . COVID‐19 is also believed to accelerate the cardiovascular and renal complications of diabetes 2 .
Despite the higher susceptibility to infection and higher risk of COVID‐19‐associated morbidity and mortality, vaccine hesitancy remains a prevalent problem in patients with type 1 diabetes 4 , 5 , 6 . Isolated anecdotal reports of the rapid development of diabetic ketoacidosis or severe hyperglycemia in type 1 diabetes patients after the administration of messenger ribonucleic acid vaccines have further precipitated this hesitancy 7 , 8 .
The safety and efficacy of COVID‐19 vaccines have been extensively studied and reported in the general population. However, substantial gaps remain in vaccine safety data among patients with autoimmune diseases, including type 1 diabetes 9 . This propagates uncertainty, and thus, vaccine hesitancy. Given the increased risks of morbidity and mortality, COVID‐19 vaccination might provide an effective recourse to reduce these severe outcomes.
Here, we explore the frequency and severity of COVID‐19 vaccine side‐effects among type 1 diabetes patients compared with healthy controls (HCs) using data from the COVID‐19 vaccination in autoimmune diseases (COVAD) international survey.
MATERIALS AND METHODS
Study design
The COVAD study is a global multicentric patient self‐reported e‐survey to assess post‐COVID‐19 vaccination adverse events (AEs) in patients with autoimmune diseases carried out in early 2021 10 . At the time of data collection for this study, the survey questionnaire was disseminated in healthcare centers in 94 countries targeting patients with rheumatic or non‐rheumatic autoimmune disorders, as well as healthy controls through social media platforms and patient support groups. The Checklist for Reporting Results of the Internet E‐Surveys (CHERRIES) was adhered to for survey design, validation, pilot testing and extensive vetting by experts 11 , 12 .
Data collection
The COVAD study was designed to study different adverse events in patients with different autoimmune diseases. This was a comprehensive dataset involving participants diagnosed with different systemic autoimmune diseases, including type 1 diabetes, inflammatory bowel disease, autoimmune thyroid diseases and the entire spectrum of autoimmune rheumatic diseases. The detailed study protocol is available online in a separate publication 10 . The study questionnaire consisted of 36 questions with a core item set of demographics, autoimmune rheumatic diseases details, COVID‐19 infection history and course, vaccination details, 7‐day post‐vaccination adverse events, and outcome measures using the Patient‐Reported Outcomes Measurement Information System (PROMIS) tool 13 . We used the Centers for Disease Control and Prevention website for the major and minor adverse events of COVID vaccines within 7 days of vaccination 14 .
A validated questionnaire was hosted on an online platform – surveymonkey.com – following pilot testing and translations, and extensively circulated by the international COVAD study group (110 collaborators, 94 countries) in their clinics, patient support groups and social media platforms. Convenience sampling, snowball and targeted approaches were used to include any interested respondent aged >18 years.
Data extraction
Among the survey respondents, a subgroup of individuals with type 1 diabetes was identified for analysis for this manuscript. Data were extracted from the COVAD study database on 1 January 2022. Respondents who had completed the survey in full and had received at least a single dose of a COVID‐19 vaccine were included in the analysis. Out of a total of 18,883 respondents, 10,479 completed the survey. After removing 4,999 respondents with autoimmune rheumatic diseases, 5,408 healthy controls and 72 respondents with type 1 diabetes were identified. Relevant outcome measures: 7‐day vaccine AEs, as well as demographic details and the type of COVID‐19 vaccine received were retrieved. Vaccine AEs were categorized as minor and major adverse events.
Minor AEs included injection site (arm) pain and soreness, muscle pain in all arms and legs, body ache, fever, chills, nausea/vomiting, diarrhea, headache, rash, fatigue, abdominal pain, high pulse rate or palpitations, rise in blood pressure, fainting, difficulty in breathing, dizziness, chest pain, and other unlisted specified by the respondent as a response to an open‐ended question. Major AEs included anaphylaxis (shock), marked difficulty in breathing, tongue swelling or throat closure, severe diffuse body rash (hives), hospitalization and others specified by the respondent.
Ethical considerations
Ethical approval was obtained from the Institutional Ethics Committee of Sanjay Gandhi Postgraduate Institute of Medical Sciences, Raebareli Road, Lucknow, Uttar Pradesh, India, and all participants consented electronically as per local guidelines.
Statistical analysis
Descriptive statistics were carried out, and categorical variables are presented in frequencies (n) and percentages (%). Continuous variables are presented as mean (range). The χ2‐test and Mann–Whitney U‐test were used to compare type 1 diabetes patients with HCs for categorical and continuous variables, respectively (Table 1).
Table 1.
Population characteristics of the whole unmatched cohort
| Variable | Total (n = 5,480) | Type 1 diabetes patients (n = 72) | HCs (n = 5,408) | P‐value |
|---|---|---|---|---|
| Mean age, years (range) | 35 (26–48) | 45 (34–56) | 35 (26–48) | <0.001* |
| Sex (M : F) | 1,830:3,616 (1:1.9) | 29:43 (1:1.5) | 1,801:3,573 (1:2) | 0.227 |
| Ethnicity | ||||
| White | 2,431 (44) | 35 (48) | 2,396 (44) | 0.147 |
| African American/African origin | 36 (0.7) | 0 (0) | 36 (0.7) | |
| Asian | 1,473 (27) | 27 (37) | 1,446 (27) | |
| Hispanic | 836 (15) | 5 (7) | 836 (15) | |
| Native American/Indigenous/Pacific Islander | 29 (0.5) | 0 (0) | 29 (0.5) | |
| Do not wish to disclose | 369 (6.7) | 2 (3) | 367 (7) | |
| Other | 301 (5.5) | 3 (4) | 298 (5.5) | |
| Vaccine taken | ||||
| Pfizer‐BioNTech | 1965 (36) | 28 (39) | 1937 (36) | <0.001* |
| Oxford/AstraZeneca | 571 (10) | 6 (8) | 565 (10) | |
| Johnson & Johnson (J&J) | 46 (0.8) | 2 (3) | 44 (1) | |
| Moderna | 247 (4.5) | 1 (1.4) | 246 (4.5) | |
| Novavax | 3 (0.1) | 1 (1.4) | 2 (0) | |
| Covishield (serum institute India) | 731 (13) | 21 (29) | 710 (13) | |
| Covaxin (Bharat Biotech) | 130 (2.4) | 1 (1.4) | 129 (2.4) | |
| Sputnik | 147 (2.7) | 2 (3) | 145 (2.7) | |
| Sinopharm | 1,308 (24) | 7 (10) | 1,301 (24) | |
| I am not sure | 36 (0.7) | 1 (1.4) | 35 (0.6) | |
| Others | 294 (4.4) | 2 (3) | 294 (5.4) | |
The χ2‐test and Mann–Whitney U‐test were carried out.
P < 0.05 significant.
F, female; HCs, healthy controls; M, male.
To address bias arising out of a small number of type 1 diabetes patients and the multifactorial nature of perceived side‐effects, we carried out propensity score matching (PSM). PSM 1:4 match was carried out using the variables age, sex and ethnicity, with a tolerance cut‐off of 0 to obtain a matched population for type 1 diabetes patients from the cohort of HCs.
Although PSM is a robust analysis method, it cannot control for the unmeasured confounding, and causes a reduction in sample size, because matches for all patients cannot be found, whereas multivariate regression allows balances of all covariates 15 . Hence, results were further compared using logistic regression. Binary logistic regression adjusted for age, sex, ethnicity and stratified by country of origin was also carried out between type 1 diabetes patients and HCs for the vaccine‐related AEs. A P‐value <0.05 was considered statistically significant. Statistics were carried out using IBM SPSS version 28 (IBM, Armonk, NY, USA).
RESULTS
Population characteristics
Among 18,883 respondents to the survey at the time of data extraction, those with autoimmune disorders other than type 1 diabetes were excluded. Of these, 5,408 HCs and 72 type 1 diabetes patients were included in the final analysis (Figure 1), with baseline characteristics detailed as Table 1. All respondents included in the final analysis had received at least a single dose of the vaccine at the time of survey completion, and 73.9% had received two primary doses. Most respondents among HCs (36%, n = 1937) and type 1 diabetes patients (39%, n = 28) had received the Pfizer vaccine (P < 0.001).
Figure 1.
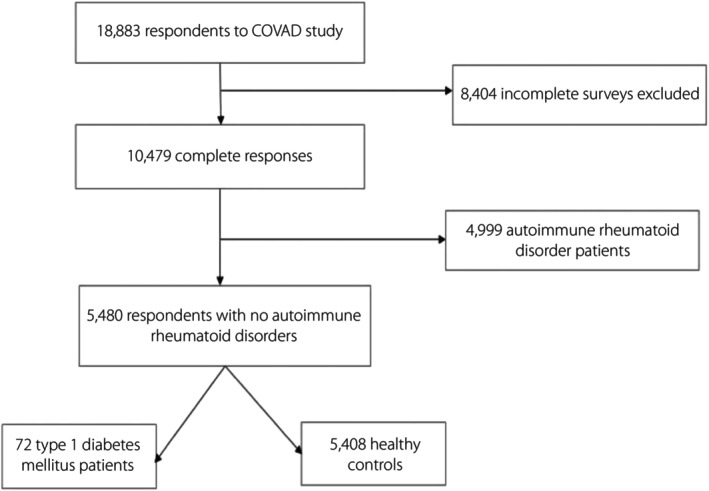
Flowchart of study participants. COVAD, COVID‐19 Vaccination in Autoimmune Diseases.
Propensity score matching analysis
A total of 72 type 1 diabetes patients and 280 HCs were PSM matched by the covariates mentioned prior (Table 2). Patients with type 1 diabetes had a higher frequency of the minor AEs of fever (odds ratio [OR 2.0], 95% confidence interval [CI] 1.1–4.0; P = 0.023), diarrhea (OR 5.4, 95% CI 1.1–24.8; P = 0.015) and dizziness (OR 3.5, 95% CI 1.1–10.9; P = 0.019) after vaccination compared with matched HCs. However, overall minor AEs, major AEs and hospitalization frequency were reassuringly comparable between the two groups (all P > 0.05; Table 3).
Table 2.
Population characteristics of the matched cohort (1:4).
| Variable | Total (n = 352) | Type 1 diabetes patients (n = 72) | HCs (n = 280) | P‐value |
|---|---|---|---|---|
| Age (years) | 44 (34–55) | 45 (34–56) | 47 (34–56) | 0.662 |
| Sex (M : F) | 132:220 (1:1.6) | 29:43 (1:1.5) | 103:177 (1:1.7) | 0.585 |
| Ethnicity, n (%) | ||||
| African American or of African origin | – | – | – | 0.970 |
| Asian | 137 (38.9) | 27 (37.5) | 110 (39.3) | |
| Caucasian | 159 (45.2) | 35 (48.6) | 124 (44.3) | |
| Hispanic | 31 (8.8) | 5 (6.9) | 26 (9.3) | |
| Native American/Indigenous/Pacific Islander | 1 (0.3) | 0 (0) | 1 (0.4) | |
| Do not wish to disclose (4) | 10 (2.8) | 2. (2.8) | 8 (2.9) | |
| Other | 14 (4.0) | 3 (4.2) | 11 (3.9) | |
| Vaccine taken | ||||
| Pfizer‐BioNTech | 113 (32.1) | 28 (38.9) | 85 (75.2) | <0.001 |
| Oxford/AstraZeneca | 22 (6.3) | 6 (8.3) | 16 (5.7) | |
| Johnson & Johnson (J&J) | 2 (0.6) | 0 (0) | 2 (2.8) | |
| Moderna | 6 (1.7) | 1 (1.4) | 5 (1.8) | |
| Novavax | 1 (0.3) | 0 (0) | 1 (1.4) | |
| Covishield (serum institute India) | 38 (10.8) | 21 (29.2) | 17 (6.1) | |
| Covaxin (Bharat Biotech) | 4 (1.1) | 1 (1.4) | 3 (1.1) | |
| Sputnik | 48 (13.6) | 2 (9.7) | 46 (34.6) | |
| Sinopharm | 104 (29.5) | 7 (9.7) | 97 (34.6) | |
| I am not sure | 3 (0.9) | 1 (1.4) | 3 (1.1) | |
| Others | 11 (3.1) | 2 (2.8) | 9 (3.2) | |
Propensity score matching (1:4) carried out with age, sex and ethnicity with tolerance cut‐off of 0.1. Eight of the type 1 diabetes patients had only three appropriate matches.
F, female; HCs, healthy controls; M, male.
Table 3.
Comparison of vaccine adverse events between type 1 diabetes and healthy controls of the matched cohort: results from propensity score matching analysis
| Type 1 diabetes patients (n = 72) | HCs (n = 280) | OR (95% CI) | P‐value | |
|---|---|---|---|---|
| n (%) | n (%) | |||
| Any AE | 54 (75.0) | 203 (72.5) | – | 0.670 |
| Injection site pain | 37 (51.4) | 139 (49.6) | – | 0.792 |
| Minor adverse reaction to vaccine | ||||
| Any minor AE | 54 (75.0) | 203 (72.5) | – | 0.670 |
| Myalgia | 9 (12.5) | 37 (80.4) | – | 0.873 |
| Body ache | 12 (16.7) | 40 (14.3) | – | 0.612 |
| Fever | 17 (23.6) | 36 (12.9) | 2.0 (1.1–4.0) | 0.023 |
| Chills | 8 (11.1) | 20 (7.1) | – | 0.267 |
| Nausea and vomiting | 5 (6.9) | 9 (3.2) | – | 0.149 |
| Headache | 13 (18.1) | 51 (18.2) | – | 0.975 |
| Rashes | 1 (1.4) | 3 (1.1) | – | 0.821 |
| Fatigue | 17 (23.6) | 63 (22.5) | – | 0.841 |
| Diarrhea | 4 (5.6) | 3 (1.1) | 5.4 (1.1–24.8) | 0.015 |
| Abdominal pain | 1 (1.4) | 2 (0.7) | – | 0.579 |
| Rise in blood pressure | 0 (0) | 1 (0.4) | – | 0.612 |
| Difficulty in breathing | 1 (1.4) | 2 (0.7) | – | 0.579 |
| Dizziness | 6 (8.3) | 7 (2.5) | 3.5 (1.1–10.9) | 0.019 |
| Chest pain | 1 (1.4) | 3 (1.1) | – | 0.821 |
| Others | 2 (2.7) | 19 (6.7) | – | 0.299 |
| Major AEs | ||||
| Any major AEs | 2 (2.8) | 7 (2.5) | – | 0.894 |
| Anaphylaxis | 0 (0) | 0 (0) | – | – |
| Marked difficulty in breathing | 1 (1.4) | 3 (1.1) | – | 0.821 |
| Throat closure | 0 (0) | 0 (0) | – | – |
| Severe rashes | 2 (4) | 2 (0.7) | – | 0.141 |
| Others | 0 (0) | 0 (0) | – | – |
| Hospitalization | 1 (1.4) | 1 (0.4) | – | 0.299 |
AE, adverse effect; CI, confidence interval; HC, healthy control; OR, odds ratio.
Logistic regression analysis
Nearly three‐quarters (76%) of type 1 diabetes patients and HCs (77%) reported some form of vaccine AEs, including both minor and major AEs (OR 0.9, 95% CI 0.5–1.6; P = 0.868), with injection site pain being the most reported event. The patient‐reported frequency of injection site pain was lower in type 1 diabetes patients as compared with HCs, although the significance was lost in the adjusted analysis (Table 4). However, patients with type 1 diabetes had a significantly higher risk of severe rash (diffuse body rash or hives) than HCs (OR 8.0, 95% CI 1.7–36.0; P = 0.007), possibly arising out of bias due to limited numbers (n = 2). These included a 67‐year‐old white woman from Australia who received ChAdOx1‐S (recombinant) vaccine and a 58‐year‐old Hispanic man from Mexico who received Ad5‐nCoV (CanSino) vaccine. Both these patients reassuringly reported no other complications.
Table 4.
Comparison of vaccine adverse events between type1 diabetes patients and healthy controls of unmatched cohort (univariate and multivariate)
| Type 1 diabetes patients (n = 72) | HCs (n = 5,408) | Univariate | Multivariate † | |||
|---|---|---|---|---|---|---|
| OR (CI) | P‐value | OR (CI) | P‐value | |||
| n (%) | n (%) | |||||
| Any AE | 55 (76) | 4,176 (77) | 0.9 (0.5–1.6) | 0.868 | ||
| Injection site pain | 37 (51) | 3,401 (63) | 0.6 (0.3–0.9) | 0.045* | 0.6 (0.4–1.1) | 0.113 |
| Minor AEs to vaccine | ||||||
| Any minor AE | 54 (75) | 4,175 (77) | 0.8 (0.5–1.5) | 0.659 | ||
| Myalgia | 9 (12) | 829 (15) | 0.7 (0.3–1.5) | 0.508 | ||
| Body ache | 12 (16) | 1,149 (21) | 0.7 (0.3–1.3) | 0.345 | ||
| Fever | 17 (23) | 1,050 (19) | 1.2 (0.7–2.2) | 0.372 | ||
| Chills | 8 (11) | 694 (13) | 0.8 (0.4–1.7) | 0.664 | ||
| Nausea and vomiting | 5 (7) | 256 (5) | 1.5 (0.6–3.7) | 0.382 | ||
| Headache | 13 (18) | 1,266 (23) | 0.7 (0.3–1.3) | 0.286 | ||
| Rashes | 1 (1.4) | 64 (1) | 1.1 (0.1–8.5) | 0.873 | ||
| Fatigue | 17 (23) | 1,491 (27) | 0.8 (0.4–1.4) | 0.455 | ||
| Diarrhea | 4 (5) | 132 (2) | 2.3 (0.8–6.5) | 0.091 | ||
| Abdominal pain | 1 (1.4) | 83 (1.5) | 0.9 (0.1–6.5) | 0.920 | ||
| Rise in pulse rate | 0 (0) | 143 (3) | – | 0.162 | ||
| Rise in blood pressure | 0 (0) | 51 (1) | – | 0.408 | ||
| Fainting | 0 (0) | 22 (0.5) | – | 0.588 | ||
| Difficulty in breathing | 1 (1.4) | 65 (1) | 1.9 (0.8–4.5) | 0.885 | ||
| Dizziness | 6 (8) | 242 (4) | 1.9 (0.8–4.5) | 0.118 | ||
| Chest pain | 1 (1.4) | 69 (1) | 1.0 (0.1–7.9) | 0.932 | ||
| Others | 2 (3) | 354 (6) | 0.4 (0.1–1.6) | 0.212 | ||
| Major AEs | ||||||
| Any major AEs | 2 (3) | 128 (2) | 1.1 (0.2–4.8) | 0.820 | ||
| Anaphylaxis | 0 (0) | 5 (0.1) | – | 0.796 | ||
| Marked difficulty in breathing | 1 (1.4) | 34 (0.6) | 2.2 (0.3–16.4) | 0.421 | ||
| Throat closure | 0 (0) | 9 (0.2) | – | 0.729 | ||
| Severe rashes | 2 (3) | 20 (0.4) | 7.6 (1.7–33.5) | 0.001* | 7.4 (1.6–33.8) | 0.009* |
| Others | 0 (0) | 88 (1.6) | – | 0.536 | ||
| Hospitalization | 1 (1.4) | 12 (0.2) | 6.3 (0.8–49.3) | 0.158 | ||
AE, adverse effect; CI, confidence interval; HCs, healthy controls; OR, odds ratio.
Statistically significant.
Binary logistic regression adjusted for age, gender, ethnicity, vaccine type and stratified by country of origin.
DISCUSSION
COVID‐19 has been a significant cause of global morbidity and mortality 14 . People with chronic diseases, including diabetes, represent a high‐risk group for poor COVID‐19 outcomes, including an increased risk of long COVID‐19 or post‐COVID‐19 syndrome, myocardial infarction and cerebrovascular accidents 16 , 17 , 18 , 19 . Achieving global vaccination and reducing vaccine hesitancy is a priority for these individuals 20 , 21 . Unfortunately, safety data on adults living with type 1 diabetes are limited, and most studies are physician reported, whereas patient‐reported data are lacking in the literature 16 .
We reassuringly found a high rate of vaccine uptake among patients with type 1 diabetes, and a favorable vaccine safety profile comparable with HCs, except for a higher frequency of fever, diarrhea and dizziness, with the majority of reported AEs being minor and not requiring hospitalization, mirroring previous studies 10 , 22 . The results of logistic regression did show a significantly increased risk for severe rashes in type 1 diabetes patients as compared with HCs, but the absolute number of type 1 diabetes patients reporting severe rash was only two. These findings were also consistent with that of other systemic autoimmune diseases 9 , 23 .
Despite data on COVID‐19 vaccine side‐effects in type 1 diabetes patients, reports of severe vaccine AEs are scarce and anecdotal 7 , 20 . Furthermore, in most of these cases, the onset of hyperglycemic symptoms was 15 h to 6 days post‐vaccination, raising the possibility that these post‐vaccination hyperglycemic episodes were likely immune‐mediated 20 . The possible role of adjuvants in inducing aberrant immune responses in these patients with altered immune status cannot be excluded 24 . A few cases of diabetic ketoacidosis and hyperosmolar hyperglycemic state have been reported among type 2 diabetes patients with glycated hemoglobin >12% 25 , 26 , indicating poor glycemic control. Therefore, the addition of oxidative stress from vaccinations to poor glycemic control might be associated with post‐vaccination complications 20 . The isolated reports should not deter COVID‐19 vaccination in this patient group. Furthermore, a recent study showed the overall short‐term safety of SARS‐CoV‐2 vaccination on glycemic control in autoimmune diabetes patients 27 .
The limitations of the present study include the self‐reported nature of AEs, which could not be verified, with a relatively small number of type 1 diabetes patients despite a large control group. We did not assess glycemic control. The survey did not collect data on other comorbidities, especially type 2 diabetes. The COVAD survey was designed to outline patient experience and embody patient voice; therefore, complications, such as type 1 diabetes‐related ketoacidosis and other laboratory‐based AEs, were omitted from the survey questionnaire. Due to the small cohort of type 1 diabetes patients, the differences in AEs among different vaccine categories were not analyzed. We recommend that the identification of AEs in specific vaccine groups might be a focused area of interest in future studies. A follow‐up study addressing these lacunae is underway in the form of a second COVAD survey 24 .
A plethora of studies related to physician‐reported vaccine AEs; however, a substantial gap exists between physician‐defined AEs and what the patient experiences as an AE, which could potentially contribute to continued vaccine hesitancy. It is crucial to bridge this gap and identify the patient's experience of vaccine AEs.
Nevertheless, the present study provides unique sights into the effects of COVID‐19 vaccination in this rare and understudied disease, with a geographically and ethnically diverse global sample of patients, giving generalizability and reliability to our findings, and might aid physicians in making informed decisions regarding vaccination in these patients.
The present study reiterates that COVID vaccination is safe in adults living with type 1 diabetes, and adds to the growing body of evidence that the benefits of COVID‐19 vaccination in reducing severe COVID‐19 outcomes outweigh the risk of small potential AEs in patients with chronic diseases, such as type 1 diabetes.
DISCLOSURE
ALT has received honoraria for advisory boards and speaking for Abbvie, Gilead, Janssen, Lilly, Novartis, Pfizer, and UCB. IP has received research funding and/or honoraria from Amgen, AstraZeneca, Aurinia Pharmaceuticals, Elli Lilly and Company, Gilead Sciences, GlaxoSmithKline, Janssen Pharmaceuticals, Novartis, and F. Hoffmann‐La Roche AG. RA has a consultancy relationship with and/or has received research funding from Bristol Myers‐Squibb, Pfizer, Genentech, Octapharma, CSL Behring, Mallinckrodt, AstraZeneca, Corbus, Kezar, Abbvie, Janssen, Kyverna Alexion, Argenx, Q32, EMD‐Serono, Boehringer Ingelheim, Roivant, Merck, Galapagos, Actigraph, Scipher, Horizon Therepeutics, Teva, Beigene, ANI Pharmaceuticals, Biogen, Nuvig, Capella Bioscience, and CabalettaBio. TV has received speaker honoraria from Pfizer and AstraZeneca. The other authors declare no conflict of interest.
Approval of the research protocol: Ethical approval was obtained from the Institutional Ethics Committee of Sanjay Gandhi Postgraduate Institute of Medical Sciences, Raebareli Road, Lucknow, 226,014.
Informed consent: All participants consented electronically.
Registry and registration no. of the study/trial: IEC Code: 2021‐143‐IP‐EXP‐39.
Animal studies: N/A.
FUNDING
No specific funding was obtained for this manuscript.
DISCLAIMER
No part of this manuscript is copied or published elsewhere in whole or in part.
Supporting information
Appendix S1 | COVID‐19 Vaccination in Autoimmune Diseases (COVAD) Study Group Author List and Affiliations.
ACKNOWLEDGMENTS
The authors are grateful to all respondents for completing the questionnaire. The authors also thank the Myositis Association, Myositis India, Myositis UK, Myositis Support and Understanding, the Myositis Global Network, Deutsche Gesellschaft für Muskelkranke e.V. (DGM), Dutch and Swedish Myositis patient support groups, Cure JM, Cure IBM, Sjögren's India Foundation, Patients Engage, Scleroderma India, Lupus UK, Lupus Sweden, Emirates Arthritis Foundation, EULAR PARE, ArLAR research group, AAAA patient group, Myositis Association of Australia, APLAR myositis special interest group, Thai Rheumatism association, PANLAR, AFLAR NRAS, Anti‐Synthetase Syndrome support group, and various other patient support groups and organizations for their contribution to the dissemination of this survey. Finally, the authors thank all members of the COVAD study group for their invaluable role in the data collection.
COVAD Study Group Authors: Elena Nikiphorou, James B Lilleker, Hector Chinoy, Jessica Day, Nelly Ziade, Babur Salim, Miguel A Saavedra, Lorenzo Cavagna, Marcin Milchert, Johannes Knitza, Masataka Kuwana, Oliver Distler, Sinan Kardes, Minchul Kim, Tamer A Gheita, Yogesh Preet Singh, Rajiv Ranjan, Avinash Jain, Sapan C Pandya, Rakesh Kumar Pilania, Aman Sharma, Manesh Manoj M, Vikas Gupta, Chengappa G Kavadichanda, Pradeepta Sekhar Patro, Sajal Ajmani, Sanat Phatak, Rudra Prosad Goswami, Abhra Chandra Chowdhury, Ashish Jacob Mathew, Padnamabha Shenoy, Ajay Asranna, Keerthi Talari Bommakanti, Anuj Shukla, Kunal Chandwar, Vishwesh Agarwal, Kshitij Jagtap, Döndü Üsküdar Cansu, John D Pauling, Chris Wincup, Ashima Makol, Nicoletta Del Papa, Gianluca Sambataro, Atzeni Fabiola, Marcello Govoni, Simone Parisi, Elena Bartoloni Bocci, Gian Domenico Sebastiani, Enrico Fusaro, Marco Sebastiani, Luca Quartuccio, Franco Franceschini, Pier Paolo Sainaghi, Giovanni Orsolini, Rossella De Angelis, Maria Giovanna Danielli, Vincenzo Venerito, Lisa S Traboco, Suryo Anggoro Kusumo Wibowo, Jorge Rojas Serrano, Ignacio García‐De La Torre, Erick Adrian Zamora Tehozol, Jesús Loarce‐Martos, Sergio Prieto‐González, Raquel Aranega Gonzalez, Akira Yoshida, Ran Nakashima, Shinji Sato, Naoki Kimura, Yuko Kaneko, Stylianos Tomaras, Margarita Aleksandrovna Gromova, Or Aharonov, Ihsane Hmamouchi, Leonardo Santos Hoff, Margherita Giannini, François Maurier, Julien Campagne, Alain Meyer, Melinda Nagy‐Vincze, Daman Langguth, Vidya Limaye, Merrilee Needham, Nilesh Srivastav, Marie Hudson, Océane Landon‐Cardinal, Syahrul Sazliyana Shaharir, Wilmer Gerardo Rojas Zuleta, José António Pereira Silva, João Eurico Fonseca and Olena Zimba.
Contributor Information
Tulika Chatterjee, Email: tulika5@uic.edu.
COVAD Study Group:
Elena Nikiphorou, James B Lilleker, Hector Chinoy, Jessica Day, Nelly Ziade, Babur Salim, Miguel A Saavedra, Lorenzo Cavagna, Marcin Milchert, Johannes Knitza, Masataka Kuwana, Oliver Distler, Sinan Kardes, Minchul Kim, Tamer A Gheita, Yogesh Preet Singh, Rajiv Ranjan, Avinash Jain, Sapan C Pandya, Rakesh Kumar Pilania, Aman Sharma, Manesh Manoj M, Vikas Gupta, Chengappa G Kavadichanda, Pradeepta Sekhar Patro, Sajal Ajmani, Sanat Phatak, Rudra Prosad Goswami, Abhra Chandra Chowdhury, Ashish Jacob Mathew, Padnamabha Shenoy, Ajay Asranna, Keerthi Talari Bommakanti, Anuj Shukla, Kunal Chandwar, Vishwesh Agarwal, Kshitij Jagtap, Döndü Üsküdar Cansu, John D Pauling, Chris Wincup, Ashima Makol, Nicoletta Del Papa, Gianluca Sambataro, Atzeni Fabiola, Marcello Govoni, Simone Parisi, Elena Bartoloni Bocci, Gian Domenico Sebastiani, Enrico Fusaro, Marco Sebastiani, Luca Quartuccio, Franco Franceschini, Pier Paolo Sainaghi, Giovanni Orsolini, Rossella De Angelis, Maria Giovanna Danielli, Vincenzo Venerito, Lisa S Traboco, Suryo Anggoro Kusumo Wibowo, Jorge Rojas Serrano, Ignacio García‐De La Torre, Erick Adrian Zamora Tehozol, Jesús Loarce‐Martos, Sergio Prieto‐González, Raquel Aranega Gonzalez, Akira Yoshida, Ran Nakashima, Shinji Sato, Naoki Kimura, Yuko Kaneko, Stylianos Tomaras, Margarita Aleksandrovna Gromova, Or Aharonov, Ihsane Hmamouchi, Leonardo Santos Hoff, Margherita Giannini, François Maurier, Julien Campagne, Alain Meyer, Melinda Nagy‐Vincze, Daman Langguth, Vidya Limaye, Merrilee Needham, Nilesh Srivastav, Marie Hudson, Océane Landon‐Cardinal, Syahrul Sazliyana Shaharir, Wilmer Gerardo Rojas Zuleta, José António Pereira Silva, João Eurico Fonseca, and Olena Zimba
DATA AVAILABILITY STATEMENT
The datasets generated and/or analyzed during the current study are not publicly available, but are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Ahmed AS, Alotaibi WS, Aldubayan MA, et al. Factors affecting the incidence, progression, and severity of COVID‐19 in type 1 diabetes mellitus. Biomed Res Int 2021; 2021: 1676914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Holman N, Knighton P, Kar P, et al. Risk factor or COVID‐19‐related mortality in people with type 1 and type 2 diabetes in England: a population‐based cohort study. Lancet Diabetes Endocrinol 2020; 8: 823–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ebekozien OA, Noor N, Gallagher MP, et al. Type 1 diabetes and COVID‐19: preliminary findings from a multicenter surveillance study in the U.S. Diabetes Care 2020; 43: e83–e85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wang Y, Duan L, Li M, et al. COVID‐19 vaccine hesitancy and associated factors among diabetes patients: a cross‐sectional survey in Changzhi, Shanxi, China. Vaccines 2022; 10: 129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Aldossari KK, Alharbi MB, Alkahtani SM, et al. COVID‐19 vaccine hesitancy among patients with diabetes in Saudi Arabia. Diabetes Metab Syndr 2021; 15: 102271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Scoccimarro D, Panichi L, Ragghianti B, et al. Sars‐CoV2 vaccine hesitancy in Italy: a survey on subjects with diabetes. Nutr Metab Cardiovasc Dis 2021; 31: 3243–3246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zilbermint M, Demidowich AP. Severe diabetic ketoacidosis after the second dose of mRNA‐1273 COVID‐19 vaccine. J Diabetes Sci Technol 2022; 16: 248–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wilson C. Vaccine side effects. New Sci 2021; 250: 10. [Google Scholar]
- 9. Gil‐Vila A, Ravichandran N, Selva‐O'Callaghan A, et al. COVID‐19 vaccination in autoimmune diseases (COVAD) study: vaccine safety in idiopathic inflammatory myopathies. Muscle Nerve 2022; 66: 426–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sen P, Gupta L, Lilleker JB, et al. COVID‐19 vaccination in autoimmune disease (COVAD) survey protocol. Rheumatol Int 2022; 42: 23–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Eysenbach G. Improving the quality of web surveys: the checklist for reporting results of internet E‐surveys (CHERRIES). J Med Internet Res 2004; 6: e34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gaur PS, Zimba O, Agarwal V, et al. Reporting survey based studies – a primer for authors. J Korean Med Sci 2020; 35: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. PROMIS , n.d. Available from: https://www.healthmeasures.net/score‐and‐interpret/interpret‐scores/promis Accessed October 20, 2022.
- 14. US Centers for Disease Control and Prevention . Possible side effects after getting a COVID‐19 vaccine. Published September 28, 2021. Available from: https://www.cdc.gov/coronavirus/2019‐ncov/vaccines/expect/after.html Accessed September 29, 2021.
- 15. Benedetto U, Head SJ, Angelini GD, et al. Statistical primer: propensity score matching and its alternatives. Eur J Cardiothorac Surg 2018; 53: 1112–1117. [DOI] [PubMed] [Google Scholar]
- 16. Meo SA, Bukhari IA, Akram J, et al. COVID‐19 vaccines: comparison of biological, pharmacological characteristics and adverse effects of Pfizer/BioNTech and Moderna vaccines. Eur Rev Med Pharmacol Sci 2021; 25: 1663–1669. [DOI] [PubMed] [Google Scholar]
- 17. Modin D, Claggett B, Køber L, et al. Influenza vaccination is associated with reduced cardiovascular mortality in adults with diabetes: a nationwide cohort study. Diabetes Care 2020; 43: 2226–2233. [DOI] [PubMed] [Google Scholar]
- 18. Gomez CAG, Cosatti M, Coello VVC, et al. Ab1101 prevalence of long covid in rheumatic disease patients: analysis of SAR covid registry. Ann Rheum Dis 2022; 81: 1668–1669. [Google Scholar]
- 19. DiIorio M, Kennedy K, Liew JW, et al. Prolonged COVID‐19 symptom duration in people with systemic autoimmune rheumatic diseases: results from the COVID‐19 global rheumatology alliance vaccine survey. RMD Open 2022; 8: e002587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ganakumar V, Jethwani P, Roy A, et al. Diabetic ketoacidosis (DKA) in type 1 diabetes mellitus (T1DM) temporally related to COVID‐19 vaccination. Diabetes Metab Syndr Clin Res Rev 2022; 16: 102371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Singh A, Khillan R, Mishra Y, et al. The safety profile of COVID‐19 vaccinations in the United States. Am J Infect Control 2022; 50: 15–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dicembrini I, Vitale V, Cosentino C, et al. Interstitial glucose monitoring, type 1 diabetes and COVID‐19 vaccine: the patient‐reported outcomes and vaccine‐associated changes in glucose and side effects (PRO‐VACS). Acta Diabetol 2022; 59: 435–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sen P, Ravichandran N, Nune A, et al. COVID‐19 vaccination‐related adverse events among autoimmune disease patients: results from the COVAD study. Rheumatology (Oxford) 2022; 62: 65–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fazal ZZ, Sen P, Joshi M, et al. COVAD survey 2 long‐term outcomes: unmet need and protocol. Rheumatol Int 2022; 42: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Edwards AE, Vathenen R, Henson SM, et al. Acute hyperglycaemic crisis after vaccination against COVID‐19: a case series. Diabet Med 2021; 38: e14631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lee HJ, Sajan A, Tomer Y. Hyperglycemic emergencies associated with COVID‐19 vaccination: a case series and discussion. J Endocr Soc 2021; 5: bvab141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. D'Onofrio L, Coraggio L, Zurru A, et al. Short‐term safety profile of Sars‐Cov2 vaccination on glucose control: continuous glucose monitoring data in people with autoimmune diabetes. Diabetes Res Clin Pract 2021; 179: 109022. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 | COVID‐19 Vaccination in Autoimmune Diseases (COVAD) Study Group Author List and Affiliations.
Data Availability Statement
The datasets generated and/or analyzed during the current study are not publicly available, but are available from the corresponding author upon reasonable request.


