ABSTRACT
Introduction
Previous studies have shown that the serum uric acid‐to‐high‐density lipoprotein cholesterol ratio (UHR) is related to metabolic syndrome. However, no existing study has examined the relationship between UHR and insulin resistance (IR). Therefore, this study aims to explore the association between the UHR and IR in patients with type 2 diabetes mellitus (T2DM).
Methods
Patients with type 2 diabetes mellitus (1,532 males and 1,013 females) were enrolled. Insulin resistance was measured by homeostatic model assessment of insulin resistance (HOMA‐IR) and was defined as HOMI‐IR ≥ 2.69. Pearson correlation, multiple logistic regression, ROC analysis, and subgroup analysis were used to evaluate the association between UHR and IR.
Results
UHR was associated with HOMA‐IR in patients with type 2 diabetes mellitus (pearson's correlation coefficient = 0.274 in males and 0.337 in females, P < 0.001). Multiple logistic regression analysis showed that UHR was significantly correlated with insulin resistance (OR = 1.06, 95%CI = 1.03–1.08 in males and OR = 1.11, 95%CI = 1.08–1.15 in females). The area under the ROC curve (AUC) of UHR (AUC = 0.665 for males and 0.717 for females, all P < 0.01) was the largest compared with that of UA and HDL‐C in insulin resistance. Subgroup analysis showed that there was a more significantly positive correlation among subjects with BMI ≥ 24 kg/m2, age < 60 years old, HbA1c < 7%, non‐hypertension, or in female subjects.
Conclusion
Elevated UHR is significantly correlated with insulin resistance, which can be used as an indicator of insulin resistance in patients with type 2 diabetes mellitus.
Keywords: High density lipoprotein cholesterol, Uric acid, Uric acid‐to‐high‐density lipoprotein cholesterol ratio
UHR is positively correlated with an increase in HOMA‐IR and the risk of insulin resistance (IR) in a mass of patients with type 2 diabetes mellitus. UHR is more effective in detecting IR compared with uric acid or HDL‐C alone.
INTRODUCTION
Insulin resistance (IR) is regarded as a significant factor in various pathological conditions, such as atherosclerosis, metabolic syndrome (MetS), diabetes mellitus, and hypertension. Therefore, it is crucial to accurately measure insulin resistance. The gold standard for insulin resistance measurement is the hyperinsulinemic‐euglycemic clamp 1 , but it is not suitable for routine clinical use due to accessibility, cost, replicability, and reproducibility issues 1 , 2 , 3 , 4 , 5 . As an alternative, the homeostasis model assessment for insulin resistance (HOMA‐IR) index is used widely in adults 6 . However, HOMA calculations require the measurement of fasting plasma insulin, which is not done routinely in clinical settings. Therefore, there is a need for a diagnostic test that is precise, easy, and cost‐effective in predicting insulin resistance.
Uric acid can cause atherosclerosis and insulin resistance by reducing nitric oxide production, promoting vascular smooth muscle proliferation, and resulting in endothelial dysfunction 7 . Additionally, low levels of HDL‐C play a role in the development of metabolic syndrome and insulin resistance 8 , 9 , 10 , 11 , 12 . More recently, the uric acid‐to‐HDL ratio (UHR) has been identified as a marker that increases in inflammatory conditions 13 . Kocak et al. (2019) proposed the use of UHR as an effective diagnostic tool for identifying metabolic syndrome in individuals with type 2 diabetes 14 . Furthermore, UHR has been found to be significantly correlated with fasting plasma glucose and HbA1c levels, making it a useful marker for assessing the control of type 2 diabetes mellitus in males 15 , as well as in hepatic steatosis 16 , non‐alcoholic fatty liver disease 17 , and Hashimoto's thyroiditis 13 .
Despite the findings outlined above, the association between the UHA and insulin resistance in patients with type 2 diabetes mellitus remains unclear. In this study, a large cross‐sectional study was conducted to investigate the association between UHR and insulin resistance and to determine whether UHR could serve as a practical and novel biomarker for diagnosing insulin resistance.
METHODS
Subjects and study design
In this cross‐sectional study, a total of 2,545 patients with type 2 diabetes mellitus who were admitted to the Department of Endocrinology of the Second Affiliated Hospital and Yuying Children's Hospital of Wenzhou Medical University between January 2020 and August 2022 were included. The study was approved by the hospital's ethical review committee (approval number: LCKY2020‐01), and written consent was obtained from all patients with type 2 diabetes mellitus. The inclusion criteria were as follows: a diagnosis of type 2 diabetes mellitus according to WHO criteria, age ≥ 20 years old, complete biochemical parameters, and clinical data. Patients with an alcohol intake of 70 g/week or more (females) and 140 g/week or more (males), severe kidney dysfunction, other infectious or systemic diseases, and those who underwent uric acid‐reducing therapy were excluded.
Biochemical and anthropometric measurements
The following data were collected at admission: history of hypertension, smoking habits, alcohol intake, application of lipid‐lowering drugs (LLDs), and physical measurements including height, blood pressure, waist circumference, and weight. Specifically, the definitions of alcohol status, hypertension, BMI, and smoking were described in our previous study 18 .
Blood samples were collected after overnight fasting and 2 h after breakfast on the second day of admission. Low‐density lipoprotein cholesterol (LDL‐C), glycosylated hemoglobin (HbA1c), serum uric acid (UA), total cholesterol (TC), fasting C‐peptide (FCP), triglycerides (TG), albumin, 2 h postprandial C‐peptide (2 h PCP), alanine aminotransferase (ALT), creatinine, high‐density lipoprotein cholesterol (HDL‐C), aspartate transaminase (AST), 2 h postprandial plasma glucose (2 h PPG) and fasting plasma glucose (FPG) were determined as described previously 18 .
Assessment of IR
Insulin resistance was assessed using the homeostatic model assessment of insulin resistance (HOMA‐IR) formula: HOMA‐IR = 1.5 + FPG [mmol/L] × FCP [pmol/L]/2,800 19 . Insulin resistance was defined as HOMI‐IR ≥ 2.69 20 .
Statistical analysis
UHR (%) was calculated by UA (mg/dL)/HDL (mg/dL) *100. The data were analyzed using SPSS 22.0 (SPSS Inc., Chicago, IL, USA). The UA, HDL, and UHR were different between genders; as a result, males and females were analyzed independently. The normality of continuous variables was assessed, and were expressed as median and interquartile range or mean ± SD. In order to assess the distinctions between the two groups, the Mann–Whitney U test or the t‐test were adopted for continuous variables, and chi‐square tests were adopted for categorical variables. In addition, Pearson correlation was utilized to examine the associations between UHR and metabolic risk factors. The patients were divided into quartiles based on their UHA levels (≤8.95, 8.95–12.59, 12.59–16.90, ≥16.90 in the female group, ≤12.56, 12.56–16.70, 16.70–22.09, ≥22.09 in the male group). Subgroup analysis was used to stratify the patients according to gender, age, BMI, HbA1c levels, and hypertension. Binary logistic regression was used to assess the association between UHA levels, quartiles, and the presence of insulin resistance. Receiver operating characteristic (ROC) curve analysis was carried out to assess the diagnostic effectiveness of UHR in detecting IR. P < 0.05 was considered as a significant difference.
RESULTS
Characteristics of participants with IR and non‐IR
As presented in Table 1, the prevalence of insulin resistance reached 15.9% in males and 17.5% in females, respectively. The HOMA‐IR, proportion individuals with hypertension, the percentages of the subjects taking LLD, weight, WC, SBP, HbA1c, FPG, FCP, 2 h PCP, creatinine, uric acid, TG, and UHR levels were all higher in patients with IR than in individuals without IR for both genders (P < 0.001). Female subjects with insulin resistance were older than non‐IR subjects (P < 0.001). Furthermore, LDL levels were lower in patients with insulin resistance than in individuals without insulin resistance for both genders.
Table 1.
Baseline characteristics of the patients with type 2 diabetes mellitus stratified by insulin resistance and gender
| Male | P‐value | Female | P‐value | |||
|---|---|---|---|---|---|---|
| IR positive | IR negative | IR positive | IR negative | |||
| N | 244 | 1,288 | 177 | 836 | ||
| Age, years | 55.3 ± 16.5 | 55.7 ± 14.1 | 0.737 | 66.5 ± 13.8 | 61.8 ± 13.6 | <0.001 |
| Duration of diabetes, year | 4.5 ± 5.9 | 5.3 ± 6.2 | 0.483 | 7.5 ± 6.2 | 9.0 ± 6.5 | 0.285 |
| Hypertension, n (%) | 60.1 | 42.3 | <0.001 | 65.5 | 50.1 | 0.004 |
| Height, cm | 169.7 ± 6.2 | 169.0 ± 7.8 | 0.167 | 156.1 ± 5.9 | 156.6 ± 7.7 | 0.439 |
| Weight, cm | 76.4 ± 18.1 | 69.1 ± 12.7 | <0.001 | 61.9 ± 12.2 | 58.1 ± 12.0 | <0.001 |
| Body mass index, kg/m2 | 26.3 ± 5.2 | 24.4 ± 11.2 | 0.013 | 25.3 ± 4.4 | 24.0 ± 9.9 | 0.096 |
| Waist circumference, cm | 92.9 ± 12.7 | 88.1 ± 22.9 | <0.001 | 88.3 ± 10.1 | 84.5 ± 11.1 | <0.001 |
| Systolic blood pressure, mmHg | 147.0 ± 23.7 | 138.5 ± 26.5 | <0.001 | 149.0 ± 25.8 | 142.6 ± 27.8 | 0.005 |
| Diastolic blood pressure, mmHg | 87.3 ± 7.3 | 84.1 ± 24.9 | 0.045 | 82.5 ± 7.9 | 82.0 ± 7.9 | 0.407 |
| Current smoking, % | 40.2 | 41.8 | 1.000 | 0 | 1.3 | 0.601 |
| Current drinking, % | 11.3 | 10.2 | 0.726 | 1.8 | 0 | 0.504 |
| Hemoglobin A1c, mmol/L | 8.8 ± 2.1 | 9.5 ± 2.3 | <0.001 | 8.6 ± 1.7 | 9.4 ± 2.2 | <0.001 |
| FPG, mmol/L | 8.1 ± 2.4 | 6.3 ± 1.7 | <0.001 | 8.4 ± 2.2 | 6.7 ± 1.9 | <0.001 |
| 2 h PPG, mmol/L | 16.1 ± 3.8 | 16.3 ± 3.8 | 0.357 | 17.1 ± 4.0 | 17.2 ± 3.9 | 0.744 |
| FCP, ng/mL | 2.04 ± 0.98 | 0.66 ± 0.37 | <0.001 | 1.96 ± 0.76 | 0.65 ± 0.37 | <0.001 |
| 2 h PCP, ng/mL | 4.67 ± 2.58 | 2.54 ± 1.84 | <0.001 | 4.78 ± 2.69 | 2.41 ± 1.79 | <0.001 |
| HOMA‐IR | 3.45 ± 1.43 | 2.00 ± 0.29 | <0.001 | 3.37 ± 0.72 | 2.02 ± 0.30 | <0.001 |
| Albumin, g/dL | 40.9 ± 5.4 | 40.4 ± 4.2 | 0.144 | 41.3 ± 6.13 | 39.7 ± 3.74 | <0.001 |
| Creatinine, μmol/L | 102.0 ± 76.9 | 75.3 ± 28.7 | <0.001 | 74.1 ± 40.9 | 58.2 ± 33.7 | <0.001 |
| Uric acid, μmol/L | 421.9 ± 122.1 | 358.6 ± 101.6 | <0.001 | 376.4 ± 121.6 | 300.8 ± 92.1 | <0.001 |
| Total cholesterol, mmol/L | 4.52 ± 1.48 | 4.49 ± 1.29 | 0.712 | 4.52 ± 1.32 | 4.64 ± 1.20 | 0.262 |
| Triglycerides, mmol/L | 2.88 ± 3.21 | 1.88 ± 1.71 | <0.001 | 2.53 ± 1.87 | 1.72 ± 1.13 | <0.001 |
| HDL‐cholesterol, mmol/L | 0.93 ± 0.30 | 0.99 ± 0.29 | 0.003 | 1.00 ± 0.30 | 1.14 ± 0.31 | <0.001 |
| LDL‐cholesterol, mmol/L | 2.57 ± 1.02 | 2.82 ± 1.08 | 0.001 | 2.61 ± 1.13 | 2.87 ± 1.05 | 0.005 |
| UHR (%) | 21.7 ± 8.8 | 17.4 ± 7.5 | <0.001 | 17.9 ± 7.9 | 12.7 ± 6.0 | <0.001 |
Values are mean ± SD or number (%). P < 0.05 was deemed significant (comparison between IR positive and IR negative). 2 h PPG, 2 h postprandial plasma glucose; BMI, body mass index; FBG, fasting blood glucose; HbA1c, glycosylated hemoglobin; HDL‐c, high density lipoprotein cholesterol; LDL‐c, low density lipoprotein cholesterol; LLD, lipid‐lowering drugs; TC, total cholesterol; TG, triglyceride; UHR, serum uric acid‐to‐high‐density lipoprotein cholesterol ratio.
Correlation between UHR and clinical and biochemical parameters
Pearson's correlation of UHR with the metabolic parameters can be found in Table 2. It could be observed that in males, UHR was positively correlated with BMI (r = 0.247, P < 0.001), WC (r = 0.199, P < 0.001), SBP (r = 0.046, P = 0.050), DBP (r = 0.088, P < 0.001), TG (r = 0.408, P < 0.001), FPG (r = 0.067, P = 0.009), and FCP (r = 0.277, P < 0.001). In females, BMI (r = 0.0.174, P < 0.001), WC (r = 0.190, P < 0.001), SBP (r = 0.057, P = 0.043), DBP (r = 0.091, P = 0.001), HbA1c (r = −0.072, P = 0.020), TG (r = 0.398, P < 0.001), FPG (r = 0.094, P = 0.003), FCP (r = 0.373, P < 0.001) were correlated with UHR (Table 2).
Table 2.
Pearson correlation of UHR levels with clinical and biochemical parameters
| Variable | Male | Female | ||
|---|---|---|---|---|
| r | P | r | P | |
| BMI | 0.247 | <0.001 | 0.174 | <0.001 |
| WC | 0.199 | <0.001 | 0.190 | <0.001 |
| SBP | 0.046 | 0.050 | 0.057 | 0.043 |
| DBP | 0.088 | <0.001 | 0.091 | 0.001 |
| HbA1c | 0.017 | 0.524 | −0.072 | 0.020 |
| TG | 0.408 | <0.001 | 0.398 | <0.001 |
| LDL‐C | −0.123 | <0.001 | −0.194 | <0.001 |
| FPG | 0.067 | 0.009 | 0.094 | 0.003 |
| FCP | 0.277 | <0.001 | 0.373 | <0.001 |
Correlation between UHA and IR
The level of UHR was significantly positively correlated with the HOMA‐IR level (r = 0.274, P < 0.001 in males, r = 0.337, P < 0.001 in females; Figure 1). Table 3 shows binary logistic analysis for the association between UHA with insulin resistance in patients with type 2 diabetes mellitus. Before (Model 1) and after adjustment for age, BMI (Model 2), a higher UHA level was associated with an increased risk of insulin resistance (P < 0.001). After further correction for LLDs, BMI, WC, SBP, DBP, HbA1c, serum creatinine, serum albumin, UHR, drinking, and smoking (Model 3), the UHA level continued to be positively correlated with the presence of insulin resistance regardless of gender (all P < 0.001).
Figure 1.
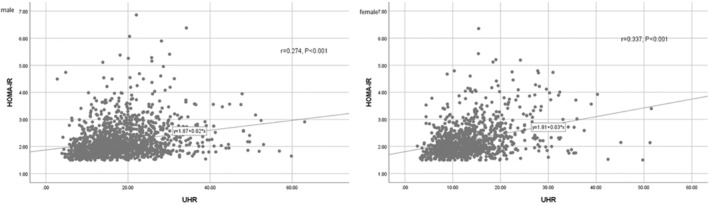
Scatter diagrams showing the correlation between the UHR and HOMA‐IR.
Table 3.
Association of the insulin resistance with UHR levels
| Crude model | Model I | Model II | ||||
|---|---|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | |
| Male | ||||||
| UHR | 1.06 (1.05–1.08) | <0.001 | 1.06 (1.05–1.08) | <0.001 | 1.06 (1.03–1.08) | <0.001 |
| UHR (quartile) | ||||||
| Q1 | Ref | Ref | Ref | |||
| Q2 | 1.53 (0.94–2.49) | 0.088 | 1.52 (0.93–2.49) | 0.096 | 1.43 (0.79–2.57) | 0.236 |
| Q3 | 2.48 (1.57–3.90) | <0.001 | 2.49 (1.58–3.94) | <0.001 | 2.46 (1.43–4.25) | 0.001 |
| Q4 | 4.14 (2.68–6.41) | <0.001 | 4.19 (2.70–6.52) | <0.001 | 3.64 (2.12–6.25) | <0.001 |
| Female | ||||||
| UHR | 1.11 (1.08–1.14) | <0.001 | 1.11 (1.08–1.14) | <0.001 | 1.11(1.08–1.15) | <0.001 |
| UHR (quartile) | ||||||
| Q1 | Ref | Ref | Ref | |||
| Q2 | 1.00 (0.52–1.91) | 1.000 | 1.00 (0.52–1.92) | 1.000 | 0.79 (0.37–1.65) | 0.523 |
| Q3 | 2.99 (1.73–5.17) | <0.001 | 2.90 (1.66–5.05) | <0.001 | 2.27 (1.20–4.28) | 0.011 |
| Q4 | 6.40 (3.77–10.84) | <0.001 | 6.43 (3.75–11.02) | <0.001 | 4.16 (2.20–7.88) | <0.001 |
Crude model: adjusted for none. Model I: adjusted for age and BMI. Model II: adjusted for age, BMI, DD, WC, SBP, DBP, HbA1c, serum creatinine, serum albumin, UHR, drinking, smoking.
Subgroup analysis to assess the relationship between UHR and insulin resistance
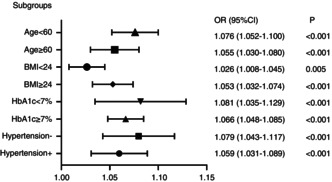
In order to evaluate the effects of subgroups in modifying the association between UHR and insulin resistance, subgroup analyses were used by age (<60 or 60 years old), BMI (<24 or ≥ 24 kg/m2), HbA1c (<7% or ≥7%), and history of hypertension (Figure 2). It was found that the P values for the subgroups were less than 0.005. UHR was independently correlated with insulin resistance, and this independent association was more obvious in patients with type 2 diabetes mellitus with a BMI ≥ 24 kg/m2, age < 60 years old, HbA1c < 7%, and no hypertension.
Figure 2.
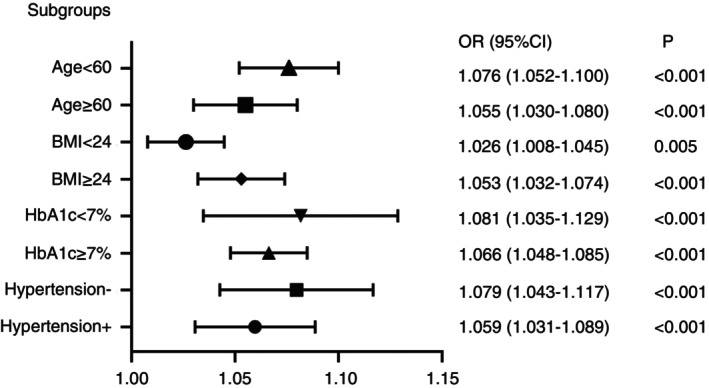
Subgroup analysis based on the multivariate logistic regression analysis of the association between UHR and insulin resistance.
The predictive value of UHR for IR
The receiver operating characteristic curve (ROC) of UHR, UA, and HDL‐C to diagnosing insulin resistance is illustrated in Figure 3. Table 4 shows that the area under the curve (AUC) for UHR in the ROC analysis was 0.665 (95% CI: 0.627–0.703) in males, 0.717 (95% CI: 0.674–0.760) in females, which was considerably higher than that of UA and HDL‐C (P < 0.001), suggesting that UHR may be a better indicator for insulin resistance than just UA or HDL‐C alone, although its diagnostic accuracy is still somewhat limited.
Figure 3.
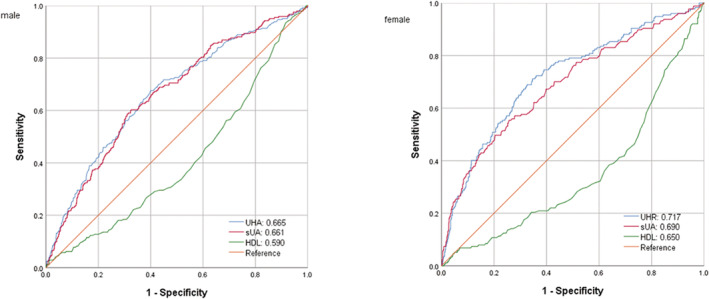
ROC analysis of UHR, uric acid, and HDL‐C to insulin resistance among patients with type 2 diabetes mellitus.
Table 4.
The results of ROC analysis of UHR for the diagnosis of insulin resistance
| Nutritional indices | Cut‐off | Sensitivity (%) | Specificity (%) | Youden's index | AUC | 95% CI |
|---|---|---|---|---|---|---|
| Male | 19.0 | 60.2 | 67.5 | 0.277 | 0.665 | 0.627–0.703 |
| Female | 13.6 | 71.2 | 65.3 | 0.365 | 0.717 | 0.674–0.760 |
DISCUSSION
Our study provides strong evidence that UHR is positively correlated with an increase in HOMA‐IR and the risk of insulin resistance (IR) in a mass of patients with type 2 diabetes mellitus. This relationship remains consistent regardless of gender, BMI, age, HbA1c, and history of hypertension. Notably, our ROC analysis demonstrates that UHR is more effective in detecting insulin resistance compared with UA or HDL‐C alone, indicating that UHR is a specific and sensitive marker for insulin resistance.
Recent prospective studies in an adult population have shown that hyperuricemia is a predictor of insulin resistance and type 2 diabetes mellitus 21 , 22 , 23 . After a 15‐year follow‐up, Krishnan et al. 22 found that hyperuricemia increases the risk of developing type 2 diabetes mellitus by 1.87‐times and insulin resistance by 1.36‐times. Another meta‐analysis, Kodama et al. 23 revealed that serum uric acid levels increase the risk of type 2 diabetes mellitus by 17% for every 1 mg/dL increase. Additionally, an increase in HDL‐C is considered to be a protective factor against insulin resistance 24 . Given that uric acid or HDL‐C alone can serve as biomarkers for insulin resistance, we investigated whether the combination of these two indicators, known as UHR, could better identify insulin resistance in patients with type 2 diabetes mellitus. As shown in Figure 3, our results showed that UHR had the largest AUC compared with uric acid and HDL‐C alone, indicating its superior performance in detecting insulin resistance.
Previous studies have reported the usefulness of UHR in predicting metabolic syndrome. Kocak et al. found that serum UHR could effectively predict metabolic syndrome in individuals with diabetes mellitus in Turkey 14 . Similarly, Yazdi et al. discovered that UHR could be used to screen for and to diagnose metabolic syndrome risks in Iranians without diabetes mellitus 25 . Furthermore, Kocak et al. demonstrated that UHR outperformed other established criteria, including uric acid, as a marker for metabolic syndrome 15 . In addition, Kosekli et al. conducted a study within a single institution, revealing a connection between UHR and nonalcoholic liver disease 16 . Furthermore, a cross‐sectional study conducted among lean Chinese individuals also identified a relationship between UHR and nonalcoholic liver disease 17 . Moreover, a recent epidemiological study demonstrated that UHR can contribute to an increased inflammatory burden 13 . However, this study is the first to investigate the association between UHR and elevated HOMA‐IR or the risk of insulin resistance in patients with type 2 diabetes mellitus. This association may be attributed to the accumulation of metabolic or inflammatory changes.
In addition, it was found that UHR is associated with various metabolic‐inflammatory diseases. Studies conducted by Aktas et al. suggested that elevated UHR is an independent risk factor for poor blood pressure control in individuals with hypertension 26 . Lee et al. found that high UHR values were positively associated with incident ischemic heart disease in Koreans without diabetes mellitus 27 . Aktas et al. proposed that UHR can be utilized in the evaluation of diabetes mellitus control in males with diabetes mellitus 15 . Aktas et al. reported that UHR has an independent predictive role in diabetic kidney injury, and it has a significant correlation with other markers of kidney function 28 . Zhang et al. found a significant correlation between UHR and baPWV existed in females but not in males 29 . Another study by Ozge demonstrated a significant positive correlation between UHR and thyroid stimulating hormone (TSH), as well as a negative correlation with free T4 (FT4) 13 . indicating that UHR can serve as a reliable and valuable marker for Hashimoto's thyroiditis. Our results were consistent with the above studies. It was observed that there was a significant correlation between UHR and various factors including BMI, WC, SBP, DBP, HbA1c, and FPG in patients with type 2 diabetes mellitus. These results highlight the potential value of UHR in future clinical applications and warrant further promotion.
Subgroup analysis on age, BMI, HbA1c, and a history of hypertension was further explored. The correlation between UHR and insulin resistance was more obvious in patients with type 2 diabetes mellitus and a BMI ≥ 24 kg/m2, age < 60 years old, HbA1c < 7%, and non‐hypertension. Importantly, for the above population, the insulin resistance is often neglected. Accordingly, UHR should be regarded as an important factor to identify insulin resistance, especially for the above population.
Uric acid and HDL‐c are used very widely as indicators in clinical practice. UHR usage is simple and low cost, which has a strong correlation with insulin resistance and has some predictive power. This allows clinicians to find insulin resistance in a timely manner in clinical work to delay or even to prevent the development of diabetes mellitus. It will improve the type 2 diabetes mellitus patient's life and treatment and save economic costs.
There are potential mechanistic explanations for the association between UHR and insulin resistance. Elevated levels of uric acid can increase oxidative stress in adipocytes by upregulating monocyte chemotactic protein‐1 and downregulating adiponectin 30 . This pro‐oxidative effect may promote the accumulation of adipose tissue 31 , 32 , thus resulting in insulin resistance 33 . Furthermore, uric acid‐induced reduction in nitric oxide levels can impair glucose uptake by skeletal muscle, further exacerbating insulin resistance 31 . Studies have demonstrated that a reduction of uric acid levels through xanthine oxidase inhibitors and uricosuric agents can reverse insulin resistance in conditions such as fructose‐induced metabolic syndrome and leptin receptor‐mediated obesity 30 , 33 , 34 , 35 . HDL‐C has the effects of reverse transport of cholesterol, which can reduce atherosclerosis, anti‐thrombosis, anti‐inflammation, vasodilation, and antiapoptosis 36 . Given that UHR is a fusion of the inflammatory response and lipid metabolism, the findings from our study suggest that UHR can potentially serve as a possible indicator for insulin resistance.
The advantage of this study is that the subjects were well characterized on the basis of a large population and different indicators were corrected in the model, thus improving the reliability of the results. However, this study also has some limitations. First of all, the causal relationship between UHR and insulin resistance cannot be determined through cross‐sectional studies. Secondly, we recommend using HOMA‐IR to evaluate insulin resistance. However, HOMA‐IR has been associated with FPG, which is closely linked to liver IR, but not to muscle IR 37 . Further research is required to examine the relationship between UHR and insulin resistance by taking advantage of gold standard hyperinsulinemic‐euglycemic clamp. Thirdly, the study population is limited to patients with type 2 diabetes mellitus. Therefore, a further prospective cohort study is necessary to confirm and promote the current findings in a larger population, including those without diabetes.
In conclusion, our large‐scale cross‐sectional study shows that UHR is a novel and practical biomarker for systemic inflammation, which is independently and positively correlated with insulin resistance, and appears to have higher insulin resistance AUC values than UA or HDL‐C alone.
DISCLOSURE
The authors declare that they have no conflict of interest.
Approval of the research protocol: This study has been approved by the Ethics Committee of the Second Affiliated Hospital of Wenzhou Medical University.
Informed Consent: The written informed consent of all subjects was obtained following the Declaration of Helsinki.
Approval date of Registry and the Registration No. of the study /trial: LCKY2020‐01.
Animal Studies: N/A.
ACKNOWLEDGMENTS
The authors thank the staff at the Department of Endocrinology, the Second Affiliated Hospital and Yuying Children's Hospital of Wenzhou Medical University, and all the patients who participated in the study.
REFERENCES
- 1. Tam CS, Xie W, Johnson WD, et al. Defining insulin resistance from hyperinsulinemic‐euglycemic clamps. Diabetes Care 2012; 35: 1605–1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care 2004; 27: 1487–1495. [DOI] [PubMed] [Google Scholar]
- 3. Espinel‐Bermudez MC, Robles‐Cervantes JA, del Sagrario Villarreal‐Hernandez L, et al. Insulin resistance in adult primary care patients with a surrogate index, Guadalajara, Mexico, 2012. J Invest Med 2015; 63: 247–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Borai A, Livingstone C, Ferns GA. The biochemical assessment of insulin resistance. Ann Clin Biochem 2007; 44: 324–342. [DOI] [PubMed] [Google Scholar]
- 5. Rudvik A, Mansson M. Evaluation of surrogate measures of insulin sensitivity ‐ correlation with gold standard is not enough. BMC Med Res Methodol 2018; 18: 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Burke JP, Hale DE, Hazuda HP, et al. A quantitative scale of acanthosis nigricans. Diabetes Care 1999; 22: 1655–1659. [DOI] [PubMed] [Google Scholar]
- 7. Meshkani R, Zargari M, Larijani B. The relationship between uric acid and metabolic syndrome in normal glucose tolerance and normal fasting glucose subjects. Acta Diabetol 2011; 48: 79–88. [DOI] [PubMed] [Google Scholar]
- 8. Leavens KF, Birnbaum MJ. Insulin signaling to hepatic lipid metabolism in health and disease. Crit Rev Biochem Mol Biol 2011; 46: 200–215. [DOI] [PubMed] [Google Scholar]
- 9. von Eckardstein A, Sibler RA. Possible contributions of lipoproteins and cholesterol to the pathogenesis of diabetes mellitus type 2. Curr Opin Lipidol 2011; 22: 26–32. [DOI] [PubMed] [Google Scholar]
- 10. Han T, Cheng Y, Tian S, et al. Changes in triglycerides and high‐density lipoprotein cholesterol may precede peripheral insulin resistance, with 2‐h insulin partially mediating this unidirectional relationship: A prospective cohort study. Cardiovasc Diabetol 2016; 15: 154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rachek LI. Free fatty acids and skeletal muscle insulin resistance. Prog Mol Biol Transl Sci 2014; 121: 267–292. [DOI] [PubMed] [Google Scholar]
- 12. Karhapaa P, Malkki M, Laakso M. Isolated low HDL cholesterol. An insulin‐resistant state. Diabetes 1994; 43: 411–417. [DOI] [PubMed] [Google Scholar]
- 13. Kurtkulagi O, Tel BMA, Kahveci G, et al. Hashimoto's thyroiditis is associated with elevated serum uric acid to high density lipoprotein‐cholesterol ratio. Rom J Intern Med 2021; 59: 403–408. [DOI] [PubMed] [Google Scholar]
- 14. Kocak MZ, Aktas G, Erkus E, et al. Serum uric acid to HDL‐cholesterol ratio is a strong predictor of metabolic syndrome in type 2 diabetes mellitus. Rev Assoc Med Bras 1992; 65: 9–15. [DOI] [PubMed] [Google Scholar]
- 15. Aktas G, Kocak MZ, Bilgin S, et al. Uric acid to HDL cholesterol ratio is a strong predictor of diabetic control in men with type 2 diabetes mellitus. Aging Male 2020; 23: 1098–1102. [DOI] [PubMed] [Google Scholar]
- 16. Kosekli MA, Kurtkulagii O, Kahveci G, et al. The association between serum uric acid to high density lipoprotein‐cholesterol ratio and non‐alcoholic fatty liver disease: The abund study. Rev Assoc Med Bras 1992; 67: 549–554. [DOI] [PubMed] [Google Scholar]
- 17. Zhang YN, Wang QQ, Chen YS, et al. Association between serum uric acid to HDL‐cholesterol ratio and nonalcoholic fatty liver disease in lean Chinese adults. Int J Endocrinol 2020; 2020: 5953461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Su X, Xu J, Zheng C. The relationship between non‐alcoholic fatty liver and skeletal muscle mass to visceral fat area ratio in women with type 2 diabetes. BMC Endocr Disord 2019; 19: 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fan H, Pan Q, Zhang P, et al. Influence of islet function on typing and prognosis of new‐onset diabetes after intensive insulin therapy. Med Sci Monit 2013; 19: 787–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ascaso JF, Pardo S, Real JT, et al. Diagnosing insulin resistance by simple quantitative methods in subjects with normal glucose metabolism. Diabetes Care 2003; 26: 3320–3325. [DOI] [PubMed] [Google Scholar]
- 21. Juraschek SP, McAdams‐Demarco M, Miller ER, et al. Temporal relationship between uric acid concentration and risk of diabetes in a community‐based study population. Am J Epidemiol 2014; 179: 684–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Krishnan E, Pandya BJ, Chung L, et al. Hyperuricemia in young adults and risk of insulin resistance, prediabetes, and diabetes: A 15‐year follow‐up study. Am J Epidemiol 2012; 176: 108–116. [DOI] [PubMed] [Google Scholar]
- 23. Kodama S, Saito K, Yachi Y, et al. Association between serum uric acid and development of type 2 diabetes. Diabetes Care 2009; 32: 1737–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Anan F, Yonemochi H, Masaki T, et al. High‐density lipoprotein cholesterol and insulin resistance are independent and additive markers of left ventricular hypertrophy in essential hypertension. Hypertens Res 2007; 30: 125–131. [DOI] [PubMed] [Google Scholar]
- 25. Yazdi F, Baghaei MH, Baniasad A, et al. Investigating the relationship between serum uric acid to high‐density lipoprotein ratio and metabolic syndrome. Endocrinol Diabetes Metab 2022; 5: e00311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Aktas G, Khalid A, Kurtkulagi O, et al. Poorly controlled hypertension is associated with elevated serum uric acid to HDL‐cholesterol ratio: A cross‐sectional cohort study. Postgrad Med 2022; 134: 297–302. [DOI] [PubMed] [Google Scholar]
- 27. Park B, Jung DH, Lee YJ. Predictive value of serum uric acid to HDL cholesterol ratio for incident ischemic heart disease in non‐diabetic Koreans. Biomedicine 2022; 10: 1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hernaez R, Lazo M, Bonekamp S, et al. Diagnostic accuracy and reliability of ultrasonography for the detection of fatty liver: A meta‐analysis. Hepatology 2011; 54: 1082–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wang H, Ba Y, Gao X, et al. Association between serum uric acid to high density lipoprotein‐cholesterol ratio and arterial stiffness in a Japanese population. Medicine (Baltimore) 2023; 102: e34182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Baldwin W, McRae S, Marek G, et al. Hyperuricemia as a mediator of the proinflammatory endocrine imbalance in the adipose tissue in a murine model of the metabolic syndrome. Diabetes 2011; 60: 1258–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Johnson RJ, Lanaspa MA, Gaucher EA. Uric acid: A danger signal from the RNA world that may have a role in the epidemic of obesity, metabolic syndrome, and cardiorenal disease: Evolutionary considerations. Semin Nephrol 2011; 31: 394–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lee H, Lee YJ, Choi H, et al. Reactive oxygen species facilitate adipocyte differentiation by accelerating mitotic clonal expansion. J Biol Chem 2009; 284: 10601–10609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Furukawa S, Fujita T, Shimabukuro M, et al. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest 2004; 114: 1752–1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nakagawa T, Hu H, Zharikov S, et al. A causal role for uric acid in fructose‐induced metabolic syndrome. Am J Physiol Renal Physiol 2006; 290: F625–F631. [DOI] [PubMed] [Google Scholar]
- 35. Sanchez‐Lozada LG, Tapia E, Bautista‐Garcia P, et al. Effects of febuxostat on metabolic and renal alterations in rats with fructose‐induced metabolic syndrome. Am J Physiol Renal Physiol 2008; 294: F710–F718. [DOI] [PubMed] [Google Scholar]
- 36. Nagao M, Nakajima H, Toh R, et al. Cardioprotective effects of high‐density lipoprotein beyond its anti‐atherogenic action. J Atheroscler Thromb 2018; 25: 985–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Matthews DR, Hosker JP, Rudenski AS, et al. Homeostasis model assessment: Insulin resistance and beta‐cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985; 28: 412–419. [DOI] [PubMed] [Google Scholar]


