ABSTRACT
Gestational diabetes mellitus (GDM) is a pathological condition during pregnancy characterized by impaired glucose tolerance, and the failure of pancreatic beta‐cells to respond appropriately to an increased insulin demand. However, while the majority of women with GDM will return to normoglycemia after delivery, they have up to a seven times higher risk of developing type 2 diabetes during midlife, compared with those with no history of GDM. Gestational diabetes mellitus also increases the risk of multiple metabolic disorders, including non‐alcoholic fatty liver disease, obesity, and cardiovascular diseases. Lipid metabolism undergoes significant changes throughout the gestational period, and lipid dysregulation is strongly associated with GDM and the progression to future type 2 diabetes. In addition to common lipid variables, discovery‐based omics techniques, such as metabolomics and lipidomics, have identified lipid biomarkers that correlate with GDM. These lipid species also show considerable potential in predicting the onset of GDM and subsequent type 2 diabetes post‐delivery. This review aims to update the current knowledge of the role that lipids play in the onset of GDM, with a focus on potential lipid biomarkers or metabolic pathways. These biomarkers may be useful in establishing predictive models to accurately predict the future onset of GDM and type 2 diabetes, and early intervention may help to reduce the complications associated with GDM.
Keywords: Biomarkers, Gestational diabetes, Lipid metabolism
Lipid dysregulation exhibits a robust association with gestational diabetes (GDM), and the identification of lipid biomarkers has enabled their application in the early prediction of both GDM and the subsequent onset of type 2 diabetes following delivery.
INTRODUCTION
Gestational diabetes mellitus (GDM) is diagnosed mostly during the second and third trimester of pregnancy, with a failure of pancreatic beta‐cells to respond appropriately to the insulin requirements during gestation, leading to impaired glucose tolerance or hyperglycemia 1 , 2 . Depending on the diagnostic criteria used, it occurs in approximately 7–8% of pregnant women, and ranges up to 20% 3 , 4 , 5 . These diagnostic criteria encompass various standards, such as the National Diabetes Data Group (NDDG) criteria 6 , the Carpenter and Coustan criteria 7 , the International Association of Diabetes and Pregnancy Study Groups (IADPSG) criteria 8 , the World Health Organization (WHO) criteria 9 , and the National Institute for Health and Care Excellence (NICE) criteria 10 , among others.
The complications of GDM include short‐term (pre‐eclampsia, cesarean section in mothers, and hypoglycemia and jaundice in infants) and long‐term (type 2 diabetes, non‐alcoholic fatty liver disease, cardiovascular, and renal diseases in mothers and obesity in new‐born children) 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 . The majority of women with a history of GDM return to normoglycemia post‐delivery, however, up to 35% of them develop glucose intolerance within the first 2 months postpartum 21 . It has been reported that women with a history of GDM have a higher risk of developing future type 2 diabetes compared with those who have a normoglycaemic pregnancy 22 , 23 , 24 . In fact, up to 50% women with gestational diabetes mellitus will progress to future type 2 diabetes within 10 years post‐delivery 22 , 25 . Compared with the general population, the women who progress to type 2 diabetes at a younger age show a higher risk of developing renal and cardiovascular diseases, as well as non‐alcoholic fatty liver disease (NAFLD), which may lead to early mortality 4 , 13 , 15 , 17 , 18 , 26 .
There are several risk factors linked to the onset of GDM, such as a higher body mass index (BMI), increased maternal age, family history of GDM, and ethnicity 27 , 28 . Besides these risk factors, lipids also play vital roles in the development of GDM. Lipids exhibit various cellular functions such as energy support, cellular structure component, and cell signaling 29 , 30 , 31 , 32 . GDM‐related traditional lipid profiles have been identified, such as triacylglycerols (TAGs), total cholesterol (TC), low‐density lipoprotein cholesterol (LDL‐C), very low‐density lipoprotein cholesterol (VLDL‐C), and high‐density lipoprotein cholesterol (HDL‐C) 33 . However, these lipids can not reflect the comprehensive lipid metabolism status, both in physiological and pathological conditions. To further explore the GDM‐related lipid profiles, discovery‐based omics techniques have been developed and applied to identify potential metabolic biomarkers or pathways that are correlated with diseases such as GDM 34 , 35 , 36 , 37 .
This study provides a review of the relationships between lipid metabolism and GDM, aiming to enhance the awareness of the importance of lipid dysregulation in the onset of GDM. Besides, we aim to highlight several plasma biomarkers that can be used to predict GDM as early as the first trimester. Identifying biomarkers can provide a molecular rationale to further explore the regulation of lipid to avoid the onset of pregnancy disorders and further metabolic disorders at an early stage.
MATERNAL LIPID CHANGES DURING NORMAL PREGNANCY
Maternal lipid metabolism changes dramatically throughout the pregnancy and can be divided into two phases: anabolic and catabolic 38 , 39 . In the early stages of pregnancy (1st and 2nd trimester), the maternal pancreatic beta‐cell mass increases to enhance insulin secretion, resulting in enhanced de novo lipogenesis (DNL) and leads to lipid storage 38 , 40 , 41 , 42 . During this period, the activity of adipose tissue lipoprotein lipase (LPL) can also be increased or unchanged 43 , 44 , leading to enhanced hydrolysis of circulating triacylglycerols and the production of lipid products such as non‐esterified fatty acids (NEFAs), 2‐monoacylglycerol, and glycerol 45 , 46 . These products are then taken up and used for re‐synthesis of TAGs. Whereas in late pregnancy (3rd trimester), it has been reported that insulin sensitivity may gradually decline to 40–50% of the normal range, both in women with normal glucose tolerance and in women with GDM 47 , 48 , 49 . Increased insulin resistance (IR) leads to enhanced lipolysis, decreased LPL activity, and biosynthesis of fatty acid, facilitating the process of fat breakdown in the late trimester of gestation 38 , 40 , 41 , 42 , 43 , 50 , 51 , 52 .
CIRCULATING LIPID PROFILES AND GDM
Cholesterol metabolism was reported to be involved in the development of GDM 33 , 53 , 54 , 55 , 56 , 57 , 58 . Longitudinal studies have evaluated the lipid profile changes throughout the normal pregnancy, revealing that TC, LDL‐C, and the TAG/HDL‐C ratio increase progressively during pregnancy, while HDL‐C increases from the 1st to the 2nd trimester along with a slight decrease in the 3rd trimester 54 , 55 . However, the ratio of LDL‐C to HDL‐C remains unchanged 56 . In contrast, women with GDM usually show increased insulin resistance and exhibit significantly higher levels of TC, LDL‐C, and VLDL‐C, as well as lower HDL‐C levels than those with a normal pregnancy 33 , 54 , 56 , 57 , 58 .
The adipose tissue secretes multiple adipokines which are mostly pro‐inflammatory 59 and are associated with various metabolic diseases, such as GDM, type 2 diabetes, and obesity 60 . Adiponectins have been shown to be negatively associated with GDM 60 , whereas leptin was linked with a higher risk of GDM 61 . An inverse association between the adiponectin/leptin ratio and the GDM risk was found in mild to moderate obese women (BMI < 35 kg/m2) 62 . This finding was further supported by Ye et al. 61 who discovered that the leptin/adiponectin ratio was positively associated with GDM.
Despite these lipid species, discovery‐based omics techniques have been used for efficient biological system investigations, including metabolic profiling 63 . Lipidomics comprise a major portion of metabolomics, which allows a large proportion of the lipidome to be analyzed. The workflow of lipidomics study usually includes the following steps: sample collection, sample preparation, identification and quantification of metabolites and lipids, data pre‐processing, statistical analysis, biomarker discovery as well as clinical diagnosis, and early‐stage prediction (Figure 1).
Figure 1.
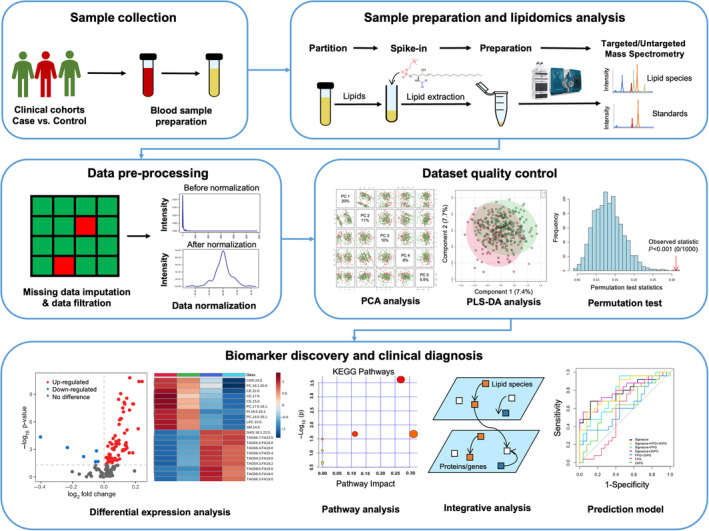
Schematic representation of the lipidomics study workflow. This figure illustrates the comprehensive workflow of lipidomics studies, encompassing key stages such as sample collection, sample preparation, acquisition of lipidomics data, data pre‐processing, data quality assessment, and the identification of top candidates to serve as potential biomarkers and signaling pathways.
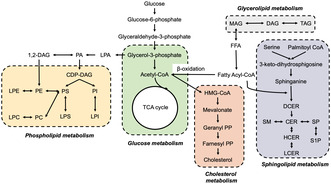
Lipid metabolism has been found to be involved in the progression of GDM, and consists of different types of lipid species, such as diacylglycerols (DAGs), TAGs, phospholipids, sphingolipids, fatty acids, and other metabolic substances that are converted to each other (Figure 2). Rahman et al. 64 discovered that women at a higher risk of developing GDM had elevated plasma DAGs and short, saturated/low unsaturated TAGs at 10–14 weeks gestation. Liu et al. 65 also reported fasting lipids including TAGs were positively associated with GDM. Similarly, GDM in obese women was also associated with elevated DAGs and TAGs 66 , 67 . Increased TAGs were correlated with impaired glucose metabolism in muscle tissue and inhibited insulin signaling pathway, leading to insulin resistance 68 . These findings indicate that increased de novo lipogenesis might be involved in the pathogenesis of GDM by affecting glucose homeostasis.
Figure 2.
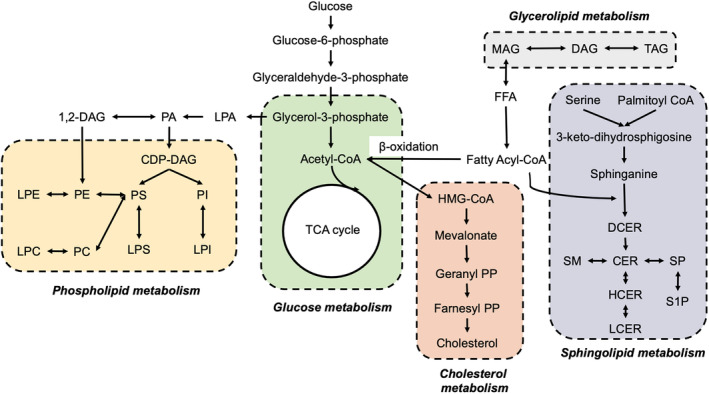
Lipid metabolism pathways involved in the onset of gestational diabetes mellitus (GDM). The interconnected metabolic pathways of lipid biosynthesis, encompassing fatty acids, neutral lipids, phospholipids, sphingolipids, and cholesterol metabolism, all of which play pivotal roles in the development of GDM.
The metabolism of phospholipids and sphingolipids are also found to be associated with a risk of GDM. Zhan et al. 69 showed that glycerophospholipids were the most prevalent altered lipid species at the second and third trimesters, when compared women with GDM with healthy pregnant women. Similarly, Rahman et al. 64 discovered that plasma sphingomyelins (SMs) and phosphatidylcholines (PCs) were negatively correlated with GDM risk in the early trimester. In another study, the plasma lipid profile was measured in the early trimester, and a lipid score consisting of 10 lipid species (mainly glycerophospholipids and glycerolipids) was established, which was linked with increased GDM risk 70 . Dudzik et al. 71 found that lysoglycerophospholipids (LPCs) had a close association with the glycemic state of women. Wang et al. discovered ten lipids that were significantly associated with GDM independent of confounding factors, five of them (phosphatidylinositol 40:6, alkylphosphatidylcholine 36:1, phosphatidylethanolamine plasmalogen 38:6, DAG 18:0/18:1, and alkylphosphatidylethanolamine 40:5) were positively correlated and five of them (sphingomyelin 34:1, dihexosyl‐ceramide 24:0, mono hexosyl ceramide 18:0, dihexosyl ceramide 24:1, and PC 40:7) were negatively correlated with GDM 72 . These findings were further supported by Liu et al. 73 who showed that disturbances of glycerophospholipid and sphingolipid metabolism were associated with GDM, and may contribute to the onset of GDM through the dysregulation of glucose homeostasis and beta‐cell function.
As GDM progresses, the circulating metabolic profile including amino acids (AAs) also undergoes corresponding changes. By comparing the metabolome of pre‐GDM and GDM, Walejko et al. 74 discovered that pre‐GDM women had increased branched‐chain amino acids (BCAAs) and sugars, whereas women with GDM showed increased lipids and decreased AAs. A longitudinal study revealed that polyunsaturated phospholipids rather than saturated phospholipids were significantly lower in women with GDM throughout the pregnancy, even before the onset of GDM 75 . Another longitudinal study showed the fold change (2nd trimester/1st trimester ratio) of lysophosphatidylcholine (LysoPC(20:4)), uric acid, and six AAs strongly differed between the GDM and control groups 76 .
Furthermore, the association between fatty acids and GDM onset has been explored, but the results have not shown consistent agreement across various studies. Short‐chain fatty acids (SCFAs), such as alpha‐hydroxybutyric acid (alpha‐HB), myristic acid (beta‐HB), palmitic acid, and butyric acid were strongly associated with the risk of GDM 53 , 77 , 78 , 79 . Lower levels of long‐chain fatty acids, including three saturated fatty acids (SFAs) and one unsaturated fatty acid (UFA) were found in women with GDM, compared with healthy pregnant women 80 . In contrast, Pan et al. 81 showed that SFAs were positively correlated with GDM risk. Zhu et al. 82 discovered that increased levels of serum even‐chain SFAs and decreased levels of serum odd‐chain SFA enhanced the risk of GDM in pregnant women. Pan et al. 81 indicated omega‐6 polyunsaturated fatty acids (PUFAs) were found to be negatively associated with GDM onset. However, on the contrary, another study showed no difference in omega‐6 PUFA and arachidonic acid between the GDM and non‐GDM groups 83 . The fatty acid composition of cholesteryl esters and SFAs were found to be related to GDM as well 84 , 85 . These inconsistent results may result from a different sample size and type of lipid profiling, and further studies with more participants are strongly warranted.
BIOMARKERS USED FOR THE EARLY PREDICTION OF GDM
A standard test for GDM diagnosis is the 75 g 2 h oral glucose tolerance test (OGTT) performed at 24–28 weeks of gestation, as recommended by the International Association of Diabetes and Pregnancy Study Groups 8 . While the National Institutes of Health (NIH) recommends a ‘two‐step approach’ which includes a 50 g 1 h OGTT followed by a 100 g 3 h OGTT 86 . The American Diabetes Association (ADA) recommends both options to diagnose GDM 87 . However, both tests are usually performed in late second trimester, which is too late to incorporate effective interventions and to prevent potential GDM‐related complications both in mothers and infants. Hence, there is a pressing need to develop non‐invasive and precise predictive models capable of identifying high‐risk GDM populations at early gestation, to facilitate early intervention.
Lipidomics acts as an effective tool to screen potential biomarkers and to establish a predictive model to identify women with high risk of developing GDM at early gestation, superior to common clinical variables. Recent studies focused on establishing predictive models to predict GDM onset are summarized in Table 1. Briefly, these studies employed metabolomics or lipidomics to analyze the pre‐onset metabolome in GDM, and successfully identified lipid species with a strong predictive capability for the onset of GDM, compared with traditional risk factors of GDM. Nonetheless, the effectiveness of these predictive models should be confirmed through validation in additional clinical cohorts, ensuring their applicability in real‐world scenarios.
Table 1.
Summary of predictive lipid biomarkers of GDM.
| Year | Author | Technique | Sample | GW | Lipid biomarkers | Predictive power |
|---|---|---|---|---|---|---|
| 2015 88 | Enquobahrie et al. | GC–MS | Serum | 16 weeks | Linoleic acid, oleic acid, myristic acid, d‐galactose, d‐sorbitol, o‐phosphocolamine, l‐alanine, l‐valine, 5‐hydroxy‐l‐tryptophan, l‐serine, sarcosine, l‐pyroglutamic acid, l‐mimosine, l‐lactic acid, glycolic acid, fumaric acid, and urea | AUC 0.871 |
| 2015 89 | Pinto et al. | NMR | Plasma | 2–21 weeks | 26 resonances | Q2 0.6 |
| 2016 90 | Lu et al. | MS | Serum | 15.2 ± 0.07 weeks | TAG(51.1), TAG(48:1), PC(32:1) and PCae(40:3) | AUC 0.71 |
| 2016 91 | Nevalainen et al. | MS | Serum | 1st trimester | Arginine, glycine, and 3‐hydroxy‐isovalerylcarnitine | AUC 0.72 |
| 2018 92 | Hou et al. | LC–MS, GC, NMR | Serum | 12 weeks | BMI, retinol binding protein 4 (RBP4), n‐acetylaspartic acid, and C16:1 (cis‐7) | AUC 0.751 |
| 2020 93 | McBride et al. | NMR | Serum | BiB cohort: 24–28 weeks, UPBEAT cohort: 27–28 + 6 weeks | Monounsaturated fatty acids (MUFA), ratios of MUFA to omega 3 fatty acids and total fatty acids, ratio of apolipoprotein B to apolipoprotein A‐1 (APOA:APOB1) | AUC 0.69 |
| 2021 73 | Liu et al. | LC–MS | Serum | 10 weeks | PC (40:6), LPCs (16:0, 17:0, 18:0, 18:1), LPEs (16:0, 18:0), Cer (36:2), FA (20:0), GCA, GDCA, GCDCA, GUDCA, TDCA, TCDCA, TMA and L‐carnitine | AUC 0.91 |
| 2021 94 | Lu et al. | GC–MS | Serum | 2nd and 3rd trimesters |
Group 1: methyl‐2‐oxovaleric acid, d‐gluconolactone, d‐glucose, hydroxybutyric acid, alpha‐hydroxyisobutyric acid, isobutyric acid, isovaleric acid, octanoic acid, glycocholic acid, nonanoic acid, myristic acid, DHA, and palmitic acid. Group 2: glycylproline, alpha‐ketoisovaleric acid, ketoleucine, acetic acid, hydroxybutyric acid, isobutyric acid, isovaleric acid, caproic acid, heptanoic acid, pyruvic acid, arachidonic acid, adrenic acid, and citramalic acid |
AUC 0.807 (at 2nd trimester) AUC 0.81 (at 3rd trimester) |
| 2021 78 | Raczkowska et al. | GC–MS | Serum | 8–14 weeks | Myristic acid, alpha‐hydroxybutyric acid and beta‐hydroxybutyric acid | AUC 0.791 |
| 2021 95 | McMichael et al. | UPLC–MS | Plasma | 10–16 weeks | SM 14:0, hypoxanthine, alpha‐hydroxybutyrate, and xanthine | AUC 0.833 |
| 2021 72 | Wang et al. | HPLC–MS | Plasma | 6–15 weeks | Phosphatidylinositol 40:6, alkylphosphatidylcholine 36:1, phosphatidylethanolamine plasmalogen 38:6, diacylglyceride 18:0/18:1, alkylphosphatidylethanolamine 40:5, sphingomyelin 34:1, dihexosyl‐ceramide 24:0, mono hexosyl ceramide 18:0, dihexosyl ceramide 24:1, and phosphatidylcholine 40:7 | AUC 0.801 |
| 2021 69 | Zhan et al. | UPLC/Q‐TOF‐MS | Serum | 2nd and 3rd trimesters | Phosphatidylcholine (22:6(4Z,7Z,10Z,13Z,16Z,19Z)/P‐18:1(11Z)), phosphatidylethanolamine (22:2(13Z,16Z)/P‐18:1(11Z)), monoacylglycerol (15:0/0:0/0:0), lysophosphatidylethanolamine (0:0/18:0), and cyclic phosphatidic acid (16:0/0:0). | AUC 0.701–0.873 (2nd trimester); AUC 0.702–0.889 (3rd trimester) |
| 2021 96 | Zhang et al. | LC–MS | Plasma | 12–16 weeks and 24–28 weeks | 17(S)‐HDoHE and sebacic acid | AUC 0.71 (at 12–16 weeks); AUC 0.78 (at 24–28 weeks) |
| 2022 80 | He et al. | GC–MS | Plasma and urine | 11–14 weeks | Cysteine, malonic acid, alanine, 11,14‐eicosadienoic acid, stearic acid, arachidic acid, and 2‐methyloctadecanoic acid | AUC 0.928 |
| 2022 97 | Peng et al. | UPLC‐MS | Serum | 3rd trimester | 27 metabolic peaks; 5‐metabolite panel: l‐valine, hypoxanthine, eicosapentaenoic acid, 2‐amino‐1,3,4‐octadecanotriol, and choline. | AUC 0.90–0.93 (27 metabolic peaks); AUC 0.769 (5‐metabolite panel) |
| 2022 98 | Zhu et al. | GC–MS | Serum | 10–13 weeks, and 17–19 weeks |
(GW10‐13) 17‐metabolite panel: 3 amino acids, 4 lipids, 5 purine and pyrimidine metabolites, and 8 carbohydrate metabolites. (GW17‐19) 13‐metabolite panel: 3 amino acids, 3 lipids, 2 purine and pyrimidine metabolites, and 5 carbohydrate metabolites |
AUC 0.832 (at 10–13 weeks); AUC 0.797 (at 17–19 weeks) |
AUC, area under the curve; BMI, body mass index; GC–MS, gas chromatography/ mass spectrometry; GW, gestational week; HPLC, high‐performance liquid chromatography; LC–MS, liquid chromatography‐mass spectrometry; MS, mass spectrometry; NMR, nuclear magnetic resonance; Q‐TOF, quadrupole time‐of‐flight; Sens, sensitivity; Spec, specificity; UPLC, ultra‐performance liquid chromatography.
LIPID CHANGES IN THE TRANSITION FROM GDM TO FUTURE TYPE 2 DIABETES POST‐DELIVERY
Several studies have investigated the role of lipid dysregulation in the transition from GDM to the future incidence of type 2 diabetes after delivery. The Study of Women, Infant Feeding, and Type 2 Diabetes after GDM Pregnancy (SWIFT) is a well‐established, ethnically diverse prospective cohort that enrolled 1,035 women with GDM pregnancy who delivered a singleton, live‐born infant from 2008 to 2011. Participants have been followed up for the onset of type 2 diabetes for up to 10 years 99 . By using this clinical cohort, Allalou et al. 34 conducted targeted metabolomics on a subset of the SWIFT cohort (N = 244), and identified 22 metabolites significantly differentiating women who developed type 2 diabetes after a GDM pregnancy from those who did not. These metabolites include 8 amino acids, 6 sphingolipids, 3 phospholipids, 3 biogenic amines, and 1 fatty acid 34 . Lai et al. 35 also applied targeted metabolomics in a larger subset (n = 658) of the SWIFT study, and showed an overall increase in diacyl‐PCs, as well as a decrease in sphingolipids and acyl‐alkyl‐PCs among women with a history of GDM who progressed to future type 2 diabetes. To further investigate the lipid profiles associated with the transition from GDM to type 2 diabetes, Lai et al. 36 used targeted lipidomics to detect up to 1,008 lipid species, and found that upregulation of glycerolipid metabolism (including DAGs and TAGs) as well as impaired sphingolipid metabolism (including sphingomyelins, hexosylceramide, and lactosylceramide) were associated with future type 2 diabetes risk, and these changes were present years prior to the onset of diabetes and were revealed during the early postpartum period. Lappas et al. 100 reinforced these findings by highlighting that the cholesteryl ester species, alkenyl phosphatidylethanolamine species, and phosphatidylserine species exhibited the strongest associations with the risk of type 2 diabetes following GDM pregnancy. Khan et al. 101 provided additional evidence supporting the previous findings that diminished sphingolipid metabolism was linked to the progression from GDM to type 2 diabetes. Additionally, their study demonstrated that blocking sphingolipid metabolism impaired pancreatic beta‐cell function in a mouse model 101 .
Besides phospholipid and sphingolipid dysmetabolism, there are other metabolites involved in the development of type 2 diabetes after GDM pregnancy. In a pilot study, significant alterations were observed in the levels of 2‐hydroxybutyrate, 3‐hydroxybutyrate, and stearic acid when comparing women with GDM who subsequently developed type 2 diabetes after delivery with those who did not 102 . Liu et al. 65 discovered a group of metabolites (such as TAGs, glycerol, long‐chain acylcarnitines, 3‐hydroxybutyrate, NEFA) that mediated the relationship between GDM and postpartum abnormal glucose metabolism (AGM) postpartum. Batchuluun et al. 103 also found that short‐chain acylcarnitines were associated with the onset of type 2 diabetes following a GDM pregnancy. Additionally, in a Singapore cohort, Wang et al. 104 identified 23 metabolites that were associated with postpartum abnormal glucose metabolism.
Despite lipid dysregulation, amino acid metabolism was also found to be involved in the transition from GDM to type 2 diabetes. This was highlighted by studies conducted by Lai et al. 35 and Allalou et al., 34 where they found that activation of amino acid metabolism was strongly associated with the development of type 2 diabetes in women with a recent history of GDM. Similarly, Andersson‐Hall et al. 105 discovered that in women with GDM with future incident type 2 diabetes, levels of BCAAs and 3‐hydroxyisobutyrate were elevated, which were linked with insulin resistance and lipid metabolism (Table 2).
Table 2.
Summary of lipid changes in the transition from GDM to future type 2 diabetes.
| Year | Author | Technique | Sample | Lipid biomarkers |
|---|---|---|---|---|
| 2015 100 | Lappas et al. | HPLC–MS | Plasma | CE 20:4, PE(P‐36:2) and PS 38:4 |
| 2016 34 | Allalou et al. | LC–MS/MS | Plasma | 2‐AAA, Gly, Ile, Leu, Thr, Trp, Tyr, Val. xLeu+, Hexoses, SM(OH)C16:1, SM(OH)C22:2, SM C18:0, SM C18:1, SM C20:2, SM C24:1, PC ae C40:5, PC ae C42:5, PC ae C44:5, AC10, AC3, Palmitoleic acid (C16:1 n9) |
| 2018 105 | Andersson‐Hall et al. | NMR | Plasma | BCAAs and 3‐hydroxyisobyturate |
| 2019 101 | Khan et al. | LC–MS/MS | Plasma | Increased TAG and decreased CE, Cer, NEFA, LCer, LPC, LPE, PE, and SM in women with future type 2 diabetes |
| 2020 35 | Lai et al. | LC–MS/MS | Plasma | Increased AAs as well as diacyl‐glycerophospholipids and decreased sphingolipids and acyl‐alkyl‐glycerophospholipids among women with future type 2 diabetes |
| 2020 36 | Lai et al. | LC–MS/MS | Plasma | Increased TAG, DAG, and decreased SM, HCer, and LCer among women with future type 2 diabetes |
| 2021 104 | Wang et al. | LC–MS | Serum | P‐cresol sulfate, linoleic acid, glycocholic acid, lysoPC(16:1) and lysoPC(20:3) |
2‐AAA, 2‐aminoadipic acid; AA, amino acid; AC, acylcarnitine; AGM, abnormal glucose metabolism; BCAA, branched‐chain amino acid; CE, cholesteryl ester; Cer, ceramide; DAG, diacylglycerol; GDM, gestational diabetes mellitus; Gly, glycine; HCer, hexosylceramide; HPLC, high‐performance liquid chromatography; Ile, isoleucine; LCer, lactosylceramide; LC‐MS, liquid chromatography‐mass spectrometry; Leu, leucine; LPC, lysophosphatidylcholine; LPE, lysophosphatidylethanolamine; MS, mass spectrometry; NEFA, non‐esterified fatty acid; NMR, nuclear magnetic resonance; PE, phosphatidylethanolamine; PS, phosphatidylserine; SM, sphingomyelin; TAG, triacylglycerol; Thr, threonine; Trp, tryptophan; Tyr, tyrosine; Val, valine.
To predict the future transition from GDM to type 2 diabetes, Lai et al. 35 , 36 established two predictive models using metabolites and lipid species, respectively, which both achieved superior performance compared with common clinical variables such as fasting plasma glucose and 2 h plasma glucose. Similarly, one study also established a predictive model including three lipid species (CE 20:4, the alkenylphosphatidylethanolamine species PE(P‐36:2) and the phosphatidylserine species PS 38:4) which could accurately predict the onset of type 2 diabetes in women with previous GDM 100 . Liu et al. 65 found that the addition of leucine/isoleucine, valine, 3‐hydroxybutyrate, and acetylcarnitine (AC C2) to clinical factors improved the prediction of later glucose dysregulation following GDM pregnancy, with the area under the curves (AUCs) ranging from 0.707–0.725. Wang et al. 104 identified five metabolites [p‐cresol sulfate, linoleic acid, glycocholic acid, lysoPC(16:1), and lysoPC(20:3)] that predicted postpartum abnormal glucose metabolism with an AUC value of 0.92–0.94, along with traditional risk factors.
Overall, these findings suggest that lipid and AAs dysregulation both contribute to the progression from GDM to type 2 diabetes, and the shift from glycerolipid to phospholipid and sphingolipid metabolism appears to be a possible mechanism involved in this transition, as illustrated in Figure 2. However, additional research conducted in well‐established clinical cohorts is warranted to identify the type 2 diabetes‐related metabolome at the early stage.
DISCUSSION AND CONCLUSION
Human maternal lipid metabolism during normal pregnancy is well understood, but there are still questions regarding the pathogenesis of GDM and the transition to future type 2 diabetes. Several studies have attempted to use different methodologies to measure lipid profiles and have identified lipid biomarkers or metabolic pathways associated with GDM and type 2 diabetes. These lipid biomarkers include certain types of fatty acids, glycerolipids, phospholipids, sphingolipids, cholesterol, and lipoproteins. However, the findings are inconsistent across studies and quite inconclusive, which could be due to the heterogeneities in cohort and study design, as well as study populations (race/ethnicity), sample sizes, diagnostic criteria, potential confounding factors, and statistical methods used during the analysis. Additionally, the regulation and interactions among the lipid metabolism pathways are quite complex and not entirely known. Functional studies that interfere with specific target proteins/genes to regulate lipid metabolism and to prevent the onset of GDM and type 2 diabetes are strongly recommended. However, while predictive models consisting of different lipids have been established and are superior to the common clinical variables, further verification in other cohorts is necessary for clinical translation. With the rapid development of artificial intelligence, more methods can be used to build predictive models and to facilitate early recognition of high‐risk populations for early intervention to prevent severe diabetic complications.
Overall, this review has summarized recent findings over the past few decades, and has identified the role that lipids play in the pathogenesis of GDM and progression to type 2 diabetes. Additional research on the liver/pancreatic cell/muscle cell/adipocyte function and related molecular biology would provide a better understanding of lipid metabolism under diabetic conditions.
DISCLOSURE
The authors declare no conflict of interest.
Approval of the research protocol: N/A.
Informed consent: N/A.
Registry and the registration no. of the study/trial: N/A.
Animal studies: N/A.
ACKNOWLEDGMENTS
This study is supported by National Natural Science Foundation of China: 82100702 (ZZ), http://www.nsfc.gov.cn. Medical Health Science and Technology Project of Zhejiang Provincial Health Commission: 2023RC036 (ZZ), http://kj.wsjkw.zj.gov.cn. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
REFERENCES
- 1. Johns EC, Denison FC, Norman JE, et al. Gestational diabetes mellitus: Mechanisms, treatment, and complications. Trends Endocrinol Metab 2018; 29: 743–754. [DOI] [PubMed] [Google Scholar]
- 2. American DA. Diagnosis and classification of diabetes mellitus. Diabetes Care 2004; 27(Suppl 1): S5–S10. [DOI] [PubMed] [Google Scholar]
- 3. Melchior H, Kurch‐Bek D, Mund M. The prevalence of gestational diabetes. Dtsch Arztebl Int 2017; 114: 412–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Magee MS, Walden CE, Benedetti TJ, et al. Influence of diagnostic criteria on the incidence of gestational diabetes and perinatal morbidity. JAMA 1993; 269: 609–615. [PubMed] [Google Scholar]
- 5. Zhu Y, Zhang C. Prevalence of gestational diabetes and risk of progression to type 2 diabetes: A global perspective. Curr Diab Rep 2016; 16: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. National Diabetes Data Group. Diabetes 1979; 28: 1039–1057. [DOI] [PubMed] [Google Scholar]
- 7. Carpenter MW, Coustan DR. Criteria for screening tests for gestational diabetes. Am J Obstet Gynecol 1982; 144: 768–773. [DOI] [PubMed] [Google Scholar]
- 8. International Association of Diabetes and Pregnancy Study Groups Consensus Panel , Metzger BE, Gabbe SG, et al. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care 2010; 33: 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Diagnostic criteria and classification of hyperglycaemia first detected in pregnancy: A World Health Organization guideline. Diabetes Res Clin Pract 2014; 103: 341–363. [DOI] [PubMed] [Google Scholar]
- 10. National Collaborating Centre for Women's and Children's Health . Diabetes in Pregnancy: Management of Diabetes and its Complications from Preconception to the Postnatal Period. London: National Institute for Health and Care Excellence, 2015. [PubMed] [Google Scholar]
- 11. Mack LR, Tomich PG. Gestational diabetes: Diagnosis, classification, and clinical care. Obstet Gynecol Clin North Am 2017; 44: 207–217. [DOI] [PubMed] [Google Scholar]
- 12. Kennelly MA, McAuliffe FM. Prediction and prevention of gestational diabetes: An update of recent literature. Eur J Obstet Gynecol Reprod Biol 2016; 202: 92–98. [DOI] [PubMed] [Google Scholar]
- 13. Beharier O, Shoham‐Vardi I, Pariente G, et al. Gestational diabetes mellitus is a significant risk factor for long‐term maternal renal disease. J Clin Endocrinol Metab 2015; 100: 1412–1416. [DOI] [PubMed] [Google Scholar]
- 14. Shah BR, Retnakaran R, Booth GL. Increased risk of cardiovascular disease in young women following gestational diabetes mellitus. Diabetes Care 2008; 31: 1668–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Retnakaran R, Shah BR. Role of type 2 diabetes in determining retinal, renal, and cardiovascular outcomes in women with previous gestational diabetes mellitus. Diabetes Care 2017; 40: 101–108. [DOI] [PubMed] [Google Scholar]
- 16. Fadl H, Magnuson A, Ostlund I, et al. Gestational diabetes mellitus and later cardiovascular disease: A Swedish population based case‐control study. BJOG 2014; 121: 1530–1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tobias DK, Stuart JJ, Li S, et al. Association of History of gestational diabetes with long‐term cardiovascular disease risk in a large prospective cohort of US women. JAMA Intern Med 2017; 177: 1735–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ajmera VH, Gunderson EP, VanWagner LB, et al. Gestational diabetes mellitus is strongly associated with non‐alcoholic fatty liver disease. Am J Gastroenterol 2016; 111: 658–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lowe WL Jr, Scholtens DM, Kuang A, et al. Hyperglycemia and adverse pregnancy outcome follow‐up study (HAPO FUS): Maternal gestational diabetes mellitus and childhood glucose metabolism. Diabetes Care 2019; 42: 372–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wu Q, Chen Y, Ma H, et al. The heterogeneous associations between gestational weight gain and adverse pregnancy outcomes in gestational diabetes mellitus according to abnormal glucose metabolism. Nutr Diabetes 2023; 13: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gunderson EP, Hurston SR, Ning X, et al. Lactation and progression to type 2 diabetes mellitus after gestational diabetes mellitus: A prospective cohort study. Ann Intern Med 2015; 163: 889–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bellamy L, Casas JP, Hingorani AD, et al. Type 2 diabetes mellitus after gestational diabetes: A systematic review and meta‐analysis. Lancet 2009; 373: 1773–1779. [DOI] [PubMed] [Google Scholar]
- 23. Chen Q, Francis E, Hu G, et al. Metabolomic profiling of women with gestational diabetes mellitus and their offspring: Review of metabolomics studies. J Diabetes Complications 2018; 32: 512–523. [DOI] [PubMed] [Google Scholar]
- 24. Gunderson EP, Lewis CE, Tsai AL, et al. A 20‐year prospective study of childbearing and incidence of diabetes in young women, controlling for glycemia before conception: The Coronary Artery Risk Development in young Adults (CARDIA) Study. Diabetes 2007; 56: 2990–2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tobias DK. Prediction and prevention of type 2 diabetes in women with a history of GDM. Curr Diab Rep 2018; 18: 78. [DOI] [PubMed] [Google Scholar]
- 26. Gunderson EP, Jaffe MG. Pregnancy and subsequent glucose intolerance in women of childbearing age: Heeding the early warning signs for primary prevention of cardiovascular disease in women. JAMA Intern Med 2017; 177: 1742–1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Plows JF, Stanley JL, Baker PN, et al. The pathophysiology of gestational diabetes mellitus. Int J Mol Sci 2018; 19: 3342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yuen L, Wong VW, Simmons D. Ethnic disparities in gestational diabetes. Curr Diab Rep 2018; 18: 68. [DOI] [PubMed] [Google Scholar]
- 29. Meikle PJ, Wong G, Barlow CK, et al. Lipidomics: Potential role in risk prediction and therapeutic monitoring for diabetes and cardiovascular disease. Pharmacol Ther 2014; 143: 12–23. [DOI] [PubMed] [Google Scholar]
- 30. Rhee EP, Cheng S, Larson MG, et al. Lipid profiling identifies a triacylglycerol signature of insulin resistance and improves diabetes prediction in humans. J Clin Invest 2011; 121: 1402–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hu C, Kong H, Qu F, et al. Application of plasma lipidomics in studying the response of patients with essential hypertension to antihypertensive drug therapy. Mol Biosyst 2011; 7: 3271–3279. [DOI] [PubMed] [Google Scholar]
- 32. Yetukuri L, Katajamaa M, Medina‐Gomez G, et al. Bioinformatics strategies for lipidomics analysis: Characterization of obesity related hepatic steatosis. BMC Syst Biol 2007; 1: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rahnemaei FA, Pakzad R, Amirian A, et al. Effect of gestational diabetes mellitus on lipid profile: A systematic review and meta‐analysis. Open Med 2022; 17: 70–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Allalou A, Nalla A, Prentice KJ, et al. A predictive metabolic signature for the transition from gestational diabetes mellitus to type 2 diabetes. Diabetes 2016; 65: 2529–2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lai M, Liu Y, Ronnett GV, et al. Amino acid and lipid metabolism in post‐gestational diabetes and progression to type 2 diabetes: A metabolic profiling study. PLoS Med 2020; 17: e1003112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lai M, Al Rijjal D, Rost HL, et al. Underlying dyslipidemia postpartum in women with a recent GDM pregnancy who develop type 2 diabetes. Elife 2020; 9: e59153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhang Z, Lai M, Piro AL, et al. Intensive lactation among women with recent gestational diabetes significantly alters the early postpartum circulating lipid profile: The SWIFT study. BMC Med 2021; 19: 241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Herrera E. Lipid metabolism in pregnancy and its consequences in the fetus and newborn. Endocrine 2002; 19: 43–55. [DOI] [PubMed] [Google Scholar]
- 39. Wang Q, Liu C, Zhang Z. Transthyretin and Normal human pregnancy: Mini review. Crit Rev Eukaryot Gene Expr 2016; 26: 273–277. [DOI] [PubMed] [Google Scholar]
- 40. Villar J, Cogswell M, Kestler E, et al. Effect of fat and fat‐free mass deposition during pregnancy on birth weight. Am J Obstet Gynecol 1992; 167: 1344–1352. [DOI] [PubMed] [Google Scholar]
- 41. Pujol E, Proenza A, Llado I, et al. Pregnancy effects on rat adipose tissue lipolytic capacity are dependent on anatomical location. Cell Physiol Biochem 2005; 16: 229–236. [DOI] [PubMed] [Google Scholar]
- 42. Martineau MG, Raker C, Dixon PH, et al. The metabolic profile of intrahepatic cholestasis of pregnancy is associated with impaired glucose tolerance, dyslipidemia, and increased fetal growth. Diabetes Care 2015; 38: 243–248. [DOI] [PubMed] [Google Scholar]
- 43. Alvarez JJ, Montelongo A, Iglesias A, et al. Longitudinal study on lipoprotein profile, high density lipoprotein subclass, and postheparin lipases during gestation in women. J Lipid Res 1996; 37: 299–308. [PubMed] [Google Scholar]
- 44. Rebuffe‐Scrive M, Enk L, Crona N, et al. Fat cell metabolism in different regions in women. Effect of menstrual cycle, pregnancy, and lactation. J Clin Invest 1985; 75: 1973–1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lasuncion MA, Herrera E. “In vitro” utilization of labelled esterified fatty acids and glyceride glycerol from triglyceride‐rich lipoproteins in rat adipose tissue. Horm Metab Res 1981; 13: 335–339. [DOI] [PubMed] [Google Scholar]
- 46. Lasuncion MA, Herrera E. Changes with starvation in the rat of the lipoprotein lipase activity and hydrolysis of triacylglycerols from triacylglycerol‐rich lipoproteins in adipose tissue preparations. Biochem J 1983; 210: 639–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sonagra AD, Biradar SM, Dattatreya K, et al. Normal pregnancy‐A state of insulin resistance. J Clin Diagn Res 2014; 8: CC01–CC03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Catalano PM. Trying to understand gestational diabetes. Diabet Med 2014; 31: 273–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sivan E, Homko CJ, Chen X, et al. Effect of insulin on fat metabolism during and after normal pregnancy. Diabetes 1999; 48: 834–838. [DOI] [PubMed] [Google Scholar]
- 50. Ramos MP, Crespo‐Solans MD, del Campo S, et al. Fat accumulation in the rat during early pregnancy is modulated by enhanced insulin responsiveness. Am J Physiol Endocrinol Metab 2003; 285: E318–E328. [DOI] [PubMed] [Google Scholar]
- 51. Dahlgren J. Pregnancy and insulin resistance. Metab Syndr Relat Disord 2006; 4: 149–152. [DOI] [PubMed] [Google Scholar]
- 52. Barbour LA, McCurdy CE, Hernandez TL, et al. Cellular mechanisms for insulin resistance in normal pregnancy and gestational diabetes. Diabetes Care 2007; 30(Suppl 2): S112–S119. [DOI] [PubMed] [Google Scholar]
- 53. Zietek M, Celewicz Z, Szczuko M. Short‐chain fatty acids, maternal microbiota and metabolism in pregnancy. Nutrients 2021; 13: 1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hu J, Gillies CL, Lin S, et al. Association of maternal lipid profile and gestational diabetes mellitus: A systematic review and meta‐analysis of 292 studies and 97,880 women. EClinicalMedicine 2021; 34: 100830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wang J, Li Z, Lin L. Maternal lipid profiles in women with and without gestational diabetes mellitus. Medicine 2019; 98: e15320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Cibickova L, Schovanek J, Karasek D. Changes in serum lipid levels during pregnancy in women with gestational diabetes. A narrative review. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 2021; 165: 8–12. [DOI] [PubMed] [Google Scholar]
- 57. Ryckman KK, Spracklen CN, Smith CJ, et al. Maternal lipid levels during pregnancy and gestational diabetes: A systematic review and meta‐analysis. BJOG 2015; 122: 643–651. [DOI] [PubMed] [Google Scholar]
- 58. Layton J, Powe C, Allard C, et al. Maternal lipid profile differs by gestational diabetes physiologic subtype. Metabolism 2019; 91: 39–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ramachandrayya SA, D'Cunha P, Rebeiro C. Maternal circulating levels of adipocytokines and insulin resistance as predictors of gestational diabetes mellitus: Preliminary findings of a longitudinal descriptive study. J Diabetes Metab Disord 2020; 19: 1447–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Xu J, Zhao YH, Chen YP, et al. Maternal circulating concentrations of tumor necrosis factor‐alpha, leptin, and adiponectin in gestational diabetes mellitus: A systematic review and meta‐analysis. ScientificWorldJournal 2014; 2014: 926932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ye Y, Wu P, Wang Y, et al. Adiponectin, leptin, and leptin/adiponectin ratio with risk of gestational diabetes mellitus: A prospective nested case‐control study among Chinese women. Diabetes Res Clin Pract 2022; 191: 110039. [DOI] [PubMed] [Google Scholar]
- 62. Thagaard IN, Krebs L, Holm JC, et al. Adiponectin and leptin as first trimester markers for gestational diabetes mellitus: A cohort study. Clin Chem Lab Med 2017; 55: 1805–1812. [DOI] [PubMed] [Google Scholar]
- 63. Dai X, Shen L. Advances and trends in omics technology development. Front Med 2022; 9: 911861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Rahman ML, Feng YA, Fiehn O, et al. Plasma lipidomics profile in pregnancy and gestational diabetes risk: A prospective study in a multiracial/ethnic cohort. BMJ Open Diabetes Res Care 2021; 9: e001551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Liu Y, Kuang A, Bain JR, et al. Maternal metabolites associated with gestational diabetes mellitus and a postpartum disorder of glucose metabolism. J Clin Endocrinol Metab 2021; 106: 3283–3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Furse S, Koulman A, Ozanne SE, et al. Altered lipid metabolism in obese women with gestational diabetes and associations with offspring adiposity. J Clin Endocrinol Metab 2022; 107: e2825–e2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Furse S, White SL, Meek CL, et al. Altered triglyceride and phospholipid metabolism predates the diagnosis of gestational diabetes in obese pregnancy. Mol Omics 2019; 15: 420–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Yaribeygi H, Farrokhi FR, Butler AE, et al. Insulin resistance: Review of the underlying molecular mechanisms. J Cell Physiol 2019; 234: 8152–8161. [DOI] [PubMed] [Google Scholar]
- 69. Zhan Y, Wang J, He X, et al. Plasma metabolites, especially lipid metabolites, are altered in pregnant women with gestational diabetes mellitus. Clin Chim Acta 2021; 517: 139–148. [DOI] [PubMed] [Google Scholar]
- 70. Wang Y, Wu P, Huang Y, et al. BMI and lipidomic biomarkers with risk of gestational diabetes in pregnant women. Obesity 2022; 30: 2044–2054. [DOI] [PubMed] [Google Scholar]
- 71. Dudzik D, Zorawski M, Skotnicki M, et al. Metabolic fingerprint of gestational diabetes mellitus. J Proteomics 2014; 103: 57–71. [DOI] [PubMed] [Google Scholar]
- 72. Wang Y, Huang Y, Wu P, et al. Plasma lipidomics in early pregnancy and risk of gestational diabetes mellitus: A prospective nested case‐control study in Chinese women. Am J Clin Nutr 2021; 114: 1763–1773. [DOI] [PubMed] [Google Scholar]
- 73. Liu J, Li J, Li W, et al. Predictive values of serum metabolites in early pregnancy and their possible pathways for gestational diabetes: A nested case‐control study in Tianjin, China. J Diabetes Complications 2021; 35: 108048. [DOI] [PubMed] [Google Scholar]
- 74. Walejko JM, Chelliah A, Keller‐Wood M, et al. Diabetes leads to alterations in Normal metabolic transitions of pregnancy as revealed by time‐course metabolomics. Metabolites 2020; 10: 350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Law KP, Mao X, Han TL, et al. Unsaturated plasma phospholipids are consistently lower in the patients diagnosed with gestational diabetes mellitus throughout pregnancy: A longitudinal metabolomics study of Chinese pregnant women part 1. Clin Chim Acta 2017; 465: 53–71. [DOI] [PubMed] [Google Scholar]
- 76. Zhao H, Li H, Chung ACK, et al. Large‐scale longitudinal metabolomics study reveals different trimester‐specific alterations of metabolites in relation to gestational diabetes mellitus. J Proteome Res 2019; 18: 292–300. [DOI] [PubMed] [Google Scholar]
- 77. Pappa KI, Anagnou NP, Salamalekis E, et al. Gestational diabetes exhibits lack of carnitine deficiency despite relatively low carnitine levels and alterations in ketogenesis. J Matern Fetal Neonatal Med 2005; 17: 63–68. [DOI] [PubMed] [Google Scholar]
- 78. Raczkowska BA, Mojsak P, Rojo D, et al. Gas chromatography‐mass spectroscopy‐based metabolomics analysis reveals potential biochemical markers for diagnosis of gestational diabetes mellitus. Front Pharmacol 2021; 12: 770240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Mokkala K, Vahlberg T, Houttu N, et al. Distinct metabolomic profile because of gestational diabetes and its treatment mode in women with overweight and obesity. Obesity 2020; 28: 1637–1644. [DOI] [PubMed] [Google Scholar]
- 80. He XL, Hu XJ, Luo BY, et al. The effects of gestational diabetes mellitus with maternal age between 35 and 40 years on the metabolite profiles of plasma and urine. BMC Pregnancy Childbirth 2022; 22: 174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Pan XF, Huang Y, Li X, et al. Circulating fatty acids and risk of gestational diabetes mellitus: Prospective analyses in China. Eur J Endocrinol 2021; 185: 87–97. [DOI] [PubMed] [Google Scholar]
- 82. Zhu Y, Tsai MY, Sun Q, et al. A prospective and longitudinal study of plasma phospholipid saturated fatty acid profile in relation to cardiometabolic biomarkers and the risk of gestational diabetes. Am J Clin Nutr 2018; 107: 1017–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Taschereau‐Charron A, Bilodeau JF, Larose J, et al. F2‐isoprostanes and fatty acids profile in early pregnancy complicated by pre‐existing diabetes. Prostaglandins Leukot Essent Fatty Acids 2018; 135: 115–120. [DOI] [PubMed] [Google Scholar]
- 84. Burzynska‐Pedziwiatr I, Dudzik D, Sansone A, et al. Targeted and untargeted metabolomic approach for GDM diagnosis. Front Mol Biosci 2022; 9: 997436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Wang S, Liu Y, Qin S, et al. Composition of maternal circulating short‐chain fatty acids in gestational diabetes mellitus and their associations with placental metabolism. Nutrients 2022; 14: 3727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Vandorsten JP, Dodson WC, Espeland MA, et al. NIH consensus development conference: Diagnosing gestational diabetes mellitus. NIH Consens State Sci Statements 2013; 29: 1–31. [PubMed] [Google Scholar]
- 87. American Diabetes Association Professional Practice Committee . 2. Classification and diagnosis of diabetes: Standards of medical Care in Diabetes‐2022. Diabetes Care 2022; 45(Suppl 1): S17–S38. [DOI] [PubMed] [Google Scholar]
- 88. Enquobahrie DA, Denis M, Tadesse MG, et al. Maternal early pregnancy serum metabolites and risk of gestational diabetes mellitus. J Clin Endocrinol Metab 2015; 100: 4348–4356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Pinto J, Almeida LM, Martins AS, et al. Prediction of gestational diabetes through NMR metabolomics of maternal blood. J Proteome Res 2015; 14: 2696–2706. [DOI] [PubMed] [Google Scholar]
- 90. Lu L, Koulman A, Petry CJ, et al. An unbiased Lipidomics approach identifies early second trimester lipids predictive of maternal glycemic traits and gestational diabetes mellitus. Diabetes Care 2016; 39: 2232–2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Nevalainen J, Sairanen M, Appelblom H, et al. First‐trimester maternal serum amino acids and acylcarnitines are significant predictors of gestational diabetes. Rev Diabet Stud 2016; 13: 236–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Hou W, Meng X, Zhao A, et al. Development of multimarker diagnostic models from metabolomics analysis for gestational diabetes mellitus (GDM). Mol Cell Proteomics 2018; 17: 431–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. McBride N, Yousefi P, White SL, et al. Do nuclear magnetic resonance (NMR)‐based metabolomics improve the prediction of pregnancy‐related disorders? Findings from a UK birth cohort with independent validation. BMC Med 2020; 18: 366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Lu W, Luo M, Fang X, et al. Discovery of metabolic biomarkers for gestational diabetes mellitus in a Chinese population. Nutr Metab 2021; 18: 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. McMichael LE, Heath H, Johnson CM, et al. Metabolites involved in purine degradation, insulin resistance, and fatty acid oxidation are associated with prediction of gestational diabetes in plasma. Metabolomics 2021; 17: 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Zhang H, Zhao Y, Zhao D, et al. Potential biomarkers identified in plasma of patients with gestational diabetes mellitus. Metabolomics 2021; 17: 99. [DOI] [PubMed] [Google Scholar]
- 97. Peng ML, Zhang Z, Zhou M, et al. Identification of differential metabolites using untargeted metabolomics between gestational diabetes and normal pregnant women. Int J Gynaecol Obstet 2022; 159: 903–911. [DOI] [PubMed] [Google Scholar]
- 98. Zhu Y, Barupal DK, Ngo AL, et al. Predictive metabolomic markers in early to mid‐pregnancy for gestational diabetes mellitus: A prospective test and validation study. Diabetes 2022; 71: 1807–1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Gunderson EP, Matias SL, Hurston SR, et al. Study of women, infant feeding, and type 2 diabetes mellitus after GDM pregnancy (SWIFT), a prospective cohort study: Methodology and design. BMC Public Health 2011; 11: 952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Lappas M, Mundra PA, Wong G, et al. The prediction of type 2 diabetes in women with previous gestational diabetes mellitus using lipidomics. Diabetologia 2015; 58: 1436–1442. [DOI] [PubMed] [Google Scholar]
- 101. Khan SR, Manialawy Y, Obersterescu A, et al. Diminished sphingolipid metabolism, a hallmark of future type 2 diabetes pathogenesis, is linked to pancreatic beta cell dysfunction. iScience 2020; 23: 101566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Dudzik D, Zorawski M, Skotnicki M, et al. GC‐MS based gestational diabetes mellitus longitudinal study: Identification of 2‐and 3‐hydroxybutyrate as potential prognostic biomarkers. J Pharm Biomed Anal 2017; 10: 90–98. [DOI] [PubMed] [Google Scholar]
- 103. Batchuluun B, Al Rijjal D, Prentice KJ, et al. Elevated medium‐chain acylcarnitines are associated with gestational diabetes mellitus and early progression to type 2 diabetes and induce pancreatic beta‐cell dysfunction. Diabetes 2018; 67: 885–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Wang XM, Gao Y, Eriksson JG, et al. Metabolic signatures in the conversion from gestational diabetes mellitus to postpartum abnormal glucose metabolism: A pilot study in Asian women. Sci Rep 2021; 11(1): 16435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Andersson‐Hall U, Gustavsson C, Pedersen A, et al. Higher concentrations of BCAAs and 3‐HIB are associated with insulin resistance in the transition from gestational diabetes to type 2 diabetes. J Diabetes Res 2018; 2018: 4207067. [DOI] [PMC free article] [PubMed] [Google Scholar]


