Abstract
Background
Prostate cancer is the second leading cause of cancer death in men. Long standing observations have found prostate cancer responsive to androgen suppression. The primary approach to androgen suppression for men with advanced disease (cancer that has spread outside the prostate gland) has been castration. However, medical or surgical castration eliminates only 90% to 95% of the daily testosterone production. The remainder is produced in the adrenal glands. In the 1980s Labrie hypothesized that counteracting adrenal androgens would further inhibit tumor growth and possibly improve symptoms and survival beyond the response achieved with monotherapy. In response to this hypothesis a number of anti‐androgen agents were identified and used in combination with medical or surgical castration to obtain maximal androgen blockade (MAB). Despite multiple clinical trials and several meta‐analyses the clinical efficacy and cost effectiveness of MAB compared with monotherapy has not been clearly established.
Objectives
This systematic review assessed the effect of maximal androgen blockade (MAB) on survival when compared to castration (medical or surgical) alone for patients with advanced prostate cancer.
Search methods
Randomized controlled trials were searched in general and specialized databases (MEDLINE, EMBASE, Cancerlit, Cochrane Library, VA Cochrane Prostate Disease register) and by reviewing bibliographies.
Selection criteria
All published randomized trials were eligible for inclusion provided they (1) randomized men with advanced prostate cancer to receive a non‐steroidal anti‐androgen (NSAA) medication in addition to castration (medical or surgical) or to castration alone, and (2) reported overall survival, progression‐free survival, cancer‐specific survival, and/or adverse events. Eligibility was assessed by two independent reviewers.
Data collection and analysis
Information on patients, interventions, and outcomes were extracted by two independent reviewers using a standardized form. The main outcome measure for comparing effectiveness was overall survival at one, two, and five years. Secondary outcome measures included progression‐free survival and cancer‐specific survival. The relationship of specific NSAA on outcome was evaluated. Additionally, the incidence of adverse effects was measured.
Main results
Twenty trials enrolling 6320 patients were included. The pooled OR for overall survival was 1.03 (95% CI:0.85 to 1.25), 1.16 (95% CI:1.00 to 1.33), and 1.29 (95% CI:1.11 to 1.50) at 1, 2, and 5 years respectively. Overall survival was only significant at five years. The risk difference at 5 years was 0.048 (95% CI:0.02 to 0.077) and NNT at 5 years 20.8. Progression‐free survival was improved only at 1‐year follow up (OR = 1.38) and cancer‐free survival was improved only at 5 years (OR = 1.22). Adverse events occurred more frequently in those assigned to MAB and resulted in withdrawal in 10%. Quality of life was measured in only one study favored orchiectomy alone (less diarrhea and better emotional functioning in the first six months).
Authors' conclusions
MAB produces a modest overall and cancer‐specific survival at five years but is associated with increased adverse events and reduced quality of life.
Plain language summary
Maximal androgen (hormone) blockade therapy may improve chances of longer survival in men with advanced prostate cancer, but may not be suitable for all men.
The prostate gland is a common site of cancer in older men. Treatments for prostate cancer include surgery and radiation therapy. Male hormones (androgens) stimulate prostate cancer growth. Hormone suppression therapy, which decreases hormone levels, is therefore also used to try to treat the cancer. Maximal androgen blockade (MAB) uses drugs to completely block male hormones. The review found that there is modest evidence that MAB improves the chances of longer survival for men with advanced prostate cancer. However, there are also adverse effects of MAB treatment that may mean that it is not a suitable treatment for all men.
Background
An estimated 180,000 new cases of prostate cancer are expected in the United States in 1999. Prostate cancer is the second leading cause of cancer death in men. This year alone it is expected to cause almost 40,000 deaths (Landis 1999). The 1994 United States Medicare expenditures for the treatment of prostate cancer were almost 1.5 billion dollars. A large proportion of this expense was associated with the use of anti‐androgen interventions. Their justification hinges on long standing observations about the responsiveness of prostate cancer to androgen suppression. The primary approach to androgen suppression for men with advanced disease (cancer that has spread outside the prostate gland) has been castration. Surgical castration, achieved by the use of bilateral orchiectomy, almost completely eliminates serum testosterone and will have a short‐term symptomatic and objective tumor response in 70% to 80% (Crawford 1989). Medical castration, by the use of luteinizing hormone releasing hormone (LHRH) inhibitors or anti‐androgens that block testosterone effect at the cellular level, produces an equivalent effect (Conn 1991). However, medical or surgical castration eliminates only 90% to 95% of the daily testosterone production. The remainder is produced in the adrenal glands. In the 1980s Labrie hypothesized that counteracting adrenal androgens would further inhibit tumor growth and possibly improve symptoms and survival beyond the response achieved with monotherapy (Denis 1993a). In response to this hypothesis a number of anti‐androgen agents were identified and used in combination with medical or surgical castration to obtain maximal androgen blockade (MAB). Despite multiple clinical trials and several meta‐analyses the clinical efficacy and cost effectiveness of MAB compared with monotherapy has not been clearly established (Denis 1993b;PCTOG 1995;Bennett 1999).
This lack of clarity may reflect clinical considerations such as inter‐trial differences in the approach to maximal androgen blockade, the type of castration used, the stage of disease, and the timing of treatment. For example, the approach to maximal androgen blockade may include a non‐steroidal anti‐androgen drug (flutamide, nilutamide, or bicalutamide) in addition to castration (orchiectomy or LHRH agonist) or a steroidal drug (cyproterone acetate) may be used instead of a non‐steroidal anti‐androgen (NSAA). Because of the potential health impact and the tremendous expense associated with MAB, it is important that we know if this approach improves overall, disease‐specific and progression‐free survival. This systematic review and meta‐analysis utilized the methods and data set developed by the Blue Cross and Blue Shield Technology Evaluation Center (TEC) and sponsored by the Agency of Health Care Policy and Research (AHCPR). In response to concerns about the comparability of MAB trials, we focused only on the RCTs that compared MAB including an NSAA to castration (medical or surgical) alone. The primary endpoint of the review was overall survival. Secondary endpoints included progression‐free survival and cancer specific survival. Information regarding quality of life and adverse events was also summarized.
This systematic review had two main purposes. First, the review summarized the available evidence across NSAAs to determine if MAB therapy compared to castration alone (surgical or medical) had a beneficial effect on survival, progression‐free survival, and cancer‐specific survival in men with advanced prostate cancer. Second, the review organized and summarized the available evidence by specific type of NSAA used for MAB in order to determine if there were any differences in outcome related to the type of NSAA utilized for MAB.
Objectives
Primary To evaluate the relative efficacy of maximal androgen blockade on overall survival using any NSAA compared to castration alone (surgical or medical) for men with advanced prostate cancer.
Secondary To evaluate the relative efficacy of maximal androgen blockade on progression‐free survival and/or cancer‐specific survival using any NSAA compared to castration alone (surgical or medical) for men with advanced prostate cancer.
To evaluate the relative efficacy of maximal androgen blockade using specific NSAAs (flutamide, nilutamide, bicalutamide) compared to castration alone (surgical or medical) on overall survival, progression‐free survival, and/or cancer‐specific survival for men with advanced prostate cancer.
To determine the incidence of adverse effects from maximal androgen blockade using any NSAA when compared to castration alone (surgical or medical).
Methods
Criteria for considering studies for this review
Types of studies
For efficacy outcomes the review included only published randomized controlled trials of NSAA therapy in addition to castration (medical or surgical) compared to castration alone (medical or surgical). For adverse events, randomized trials were included and nonrandomized phase II studies were included if they reported the frequency of patients withdrawing from therapy due to adverse events.
Types of participants
Trials were included if they enrolled men with advanced prostate cancer who were not previously treated with hormonal therapy for prostate cancer and reported survival outcomes. For this review advanced prostate cancer was defined to include the following groups:
those with disseminated and/or symptomatic metastases (defined as stage D1/D2, N+ or M1 disease); and
those with asymptomatic metastatic or minimally advanced disease (defined as stage C or T3/T4Nx or N0 disease).
Wherever possible, data for subgroups were analyzed separately. In addition, for purposes of the meta‐analysis, sensitivity analyses were restricted to the studies that provided separate data for stage D2 (M1) patients.
Types of interventions
Eligible trials were those that evaluated maximal androgen blockade (castration plus NSAA) compared to castration alone (surgical or medical). Specifically, randomized controlled trials that reported the outcomes of interest were selected if they evaluated survival according to any one of the following four comparisons:
orchiectomy compared with orchiectomy plus a NSAA;
LHRH agonist compared with a LHRH agonist plus a NSAA;
orchiectomy compared with a LHRH agonist plus a NSAA; or
either orchiectomy or a LHRH agonist alone compared with either orchiectomy or a LHRH agonist plus a NSAA.
Types of outcome measures
Eligible trials measured overall survival, progression‐free survival, cancer‐specific survival, and/or adverse effects of treatment. Quality of life measures were included only for studies evaluating survival. Each of these measures were compared and analyzed separately. To determine the validity of data synthesis across separate studies, the reviewers abstracted the definitions used by each study to describe cancer‐specific survival and progression‐free survival.
Search methods for identification of studies
The TEC/AHCPR methodology was used (BCBSA 1998). The review was based only on published literature. A comprehensive literature search was performed to identify all publications of relevant randomized controlled trials. The search process used the MEDLINE, Cancerlit, and Embase databases. The search of these databases focused on all randomized controlled trials published since 1966 that included any of the following terms in the title, abstract, or in their keyword list: leuprolide, goserelin, buserelin, flutamide, nilutamide, bicalutamide, and orchiectomy (castration, orchidectomy). The search results were limited to include only those articles that are indexed under the MeSH heading "prostatic neoplasms;" and addressed studies on human subjects. The UK Cochrane Center search strategy for identifying randomized controlled trials was used to further limit the search results. In addition, the Cochrane Controlled Trials Register, the CENTRAL register, and the VA Cochrane Prostate Disease register were searched for trials focused on any of these agents in men with prostate cancer. The yield from this search strategy was matched against the table of contents/list of trials compiled by the Prostate Cancer Trialists Collaborative Group (1995) and the trials cited in the MetaWorks meta‐analysis to determine if any relevant trials were omitted. To supplement this strategy, issues of Current Contents on Diskette was searched to identify recently published articles that were not indexed by the online databases.
Data collection and analysis
The titles and abstracts identified by the electronic search were screened by two independent reviewers and evaluated against inclusion and exclusion criteria. Any reference sorted into the "include" category by either reviewer was retrieved. Any reference excluded by one reviewer was referred to the second reviewer for evaluation. Any reference sorted as "exclude" that was subsequently sorted as "include" by the alternate reviewer was retrieved. Any reference sorted as "uncertain" by both reviewers was reconsidered with a bias toward inclusion. After article retrieval, each reviewer evaluated the articles against the inclusion and exclusion criteria. Disagreements were resolved by consensus.
The two reviewers independently abstracted data for each eligible study using a standardized electronic data abstraction form. Data elements included the following: trial identifiers; study methods (including enrollment and withdrawal numbers); patient characteristics; outcomes; and comments. Reviewers sought information on five types of actuarial outcomes: overall survival; progression‐free survival; cancer‐specific survival; the time to hormone refractory status; and the time‐to‐treatment failure. The proportion of patients surviving 1 year, 2 years, 5 years, and 10 years was sought in addition to median survival duration. When not reported, these elements were estimated from survival curves available in the published reports. After data abstraction, the data was compared and disagreements were resolved by consensus.
The following potential parameters of methodological quality were assessed: success in concealing randomization (selection bias), successful randomization or equivalent baseline susceptibility to the outcomes of interest (confounding/selection bias), completeness of follow up (attrition bias), equivalent performance of outcome assessment (detection bias), and blinded assessment of outcomes (detection bias). A simple approach to assessing quality differences was incorporated into the review by comparing how each included study performed against each criterion. The effect of inter‐study quality differences on outcomes was determined by sensitivity analysis.
Quantitative analyses of outcomes were based on intention to treat. Approximate chi‐square tests for heterogeneity were used to assess outcome data for comparability with the assumption of a uniform risk ratio (P>0.10). The pooled odds ratio and the pooled risk difference were calculated to determine both the direction and magnitude of treatment effect. To test the robustness of the results several sensitivity analyses were be performed. Data were analyzed with the random‐effects model. If sensitivity analyses indicated that smaller or less well designed studies changed the results the studies were analyzed separately.
Results
Description of studies
The literature search and selection process identified 27 randomized controlled trials that compared survival with monotherapy to the outcomes of maximal androgen blockade. Seven of these trials were excluded (de Voogt 1990; Di Silverio 1990; Jorgensen 1993; Klosterhalfen 1987; Robinson 1995; Thorpe 1996; Williams 1990) because a steroidal anti‐androgen, cyproterone, was used for maximal androgen blockade. One additional (28th) trial (Schellhamer 1997) compared 4 separate regimens for maximal androgen blockade and was excluded because there was no comparison to monotherapy. Consequently, 20 trials that enrolled 6320 patients were eligible for this review (see Table).
Most patients (n = 6095) studied had distant metastases (D2 or M1). Eight studies (n = 3271) included only patients with distant metastases (Beland 1990; Crawford 1989; Crawford 1990; Denis 1993; Eisenberger 1997; Ferrari 1993; Ferrari 1996; Navratil 1987). Two studies (Periti 1995; Schulze 1988) did not provide detailed information about percentage with M1 disease (n = 225). In the remaining trials most patients (mean weighted percent = 78%) had M1 disease (n = 2824).
Twelve trials (n = 4672) used flutamide, 8 (n = 1648) used nilutamide, and none used bicalutamide. Of the 6320 enrolled patients, 5523 were actually randomized to treatment or control (n = 2768 to MAB), and 5432 (98.4%) were analyzable (n = 2726 assigned to MAB). Nineteen trials were classified as two‐arm trials. However, one of these trials was a two‐by‐two factorial design [Schulze 1988]. For this review, the 2 monotherapy arms were pooled together as were the 2 maximal androgen blockade arms. One trial was a three‐arm study (Brisset 1987). This trial compared monotherapy to two combined androgen blockade arms, each with a different dose of nilutamide. Among the 20 eligible trials comparing maximal androgen blockade with monotherapy, the monotherapy was orchiectomy in 9 studies (n = 2671 received orchiectomy), a LHRH agonist in 10 studies (n = 2959 received medical castration); and orchiectomy or a LHRH agonist (n = 690) in 1 study (Schulze 1988). All trials with flutamide used the same dose (250 mg 3 times daily). All but one of the trials with nilutamide used a dose of 300 mg daily. In one study (Dijkman 1997) and in one of the three arms in a second trial (Brisset 1987) a dose of 150 mg daily was used.
Data were available to calculate overall survival at 1, 2, and 5 years in 13, 14, and 7 studies, respectively. With respect to progression‐free survival at one, two and five years, data were available from seven, five, and two studies, respectively. For cancer‐specific survival at one, two and five years, data were obtained from five, four, and two studies, respectively.
Risk of bias in included studies
Of the 20 studies included, only 8 specified the method of randomization (Beland 1990; Boccardo 1993; Bono 1998; Crawford 1989; Denis 1993; Dijkman 1997; Iversen 1990; Namer 1990). Allocation was adequately concealed in six (Beland 1990; Boccardo 1993; Bono 1998; Crawford 1989; Denis 1993; Dijkman 1997). In this meta‐analysis studies that blinded patients and investigators to group assignment and that used intent‐to‐treat analysis of outcomes were classified as higher quality studies. Blinding was not considered applicable for studies of maximal androgen blockade when any arm received orchiectomy. There were eight higher quality studies identified (Beland 1990; Brisset 1987; Crawford 1989; Dijkman 1997; Fourcade 1990; Navratil 1987; Schulze 1988; Zalcberg 1996). Another potential systematic difference between studies was noted. Of the 11 studies that used a LHRH agonist for monotherapy, only 3 added a brief initial treatment with an anti‐androgen to control the tumor flare reaction (Ferrari 1993; Ferrari 1996; Bono 1998). Consequently, the eight studies not using an anti‐androgen to control the flare reaction may be biased against the monotherapy arm. For this reason the nine trials that used orchiectomy as the monotherapy may be a better comparison for MAB.
Effects of interventions
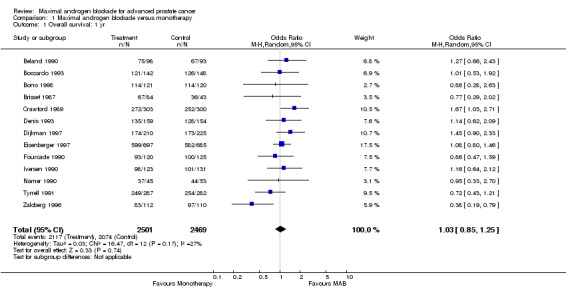
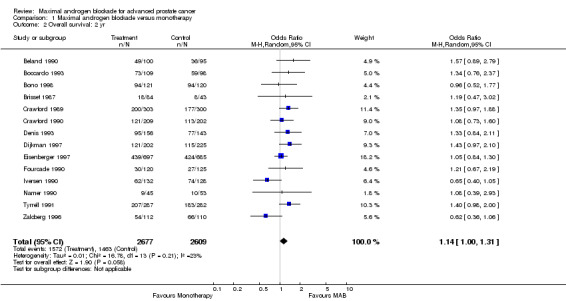
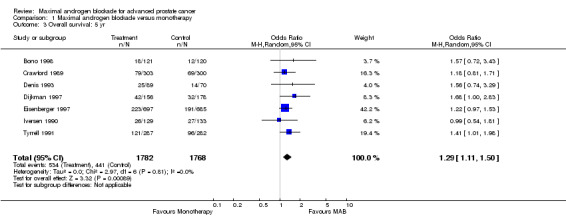
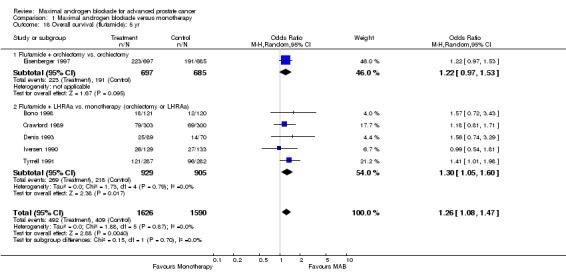
Overall survival: Three studies (Crawford 1989Dijkman 1997;Denis 1993) reported a statistically significant survival benefit that favored MAB with a five‐year survival advantage ranging from 3% to 9%. The remaining trials reported no significant difference. The pooled estimate of the OR for overall survival progressively increased over time (see Tables): OR = 1.03 (95% CI: 0.85 to 1.25) at 1 year, OR=1.16 (95% CI: 1.00 to 1.33) at 2 years, and OR=1.29 (95% CI: 1.11 to 1.50) at 5 years. The pooled risk difference and the numbers of patients that needed treatment (NNT) for one additional patient to survive to a given follow‐up time were calculated to provide an estimate of the magnitude of treatment effect. The pooled estimate of risk difference (RD) increased over time favoring MAB: RD = 0.003 (95% CI: ‐0.022 to 0.028) at 1 year, RD = 0.032 (95% CI: 0.000 to 0.064) at 2 years, and RD=0.048 (95% CI: 0.02 to 0.077) at 5 years. The NNT progressively decreased over time. The NNT was 333.3 (95% CI: na to 35.7) at 1 year, 31.3 (95% CI: na to 15.6) at 2 years, and 20.8 (95% CI: 50 to 12.9) at 5 years.
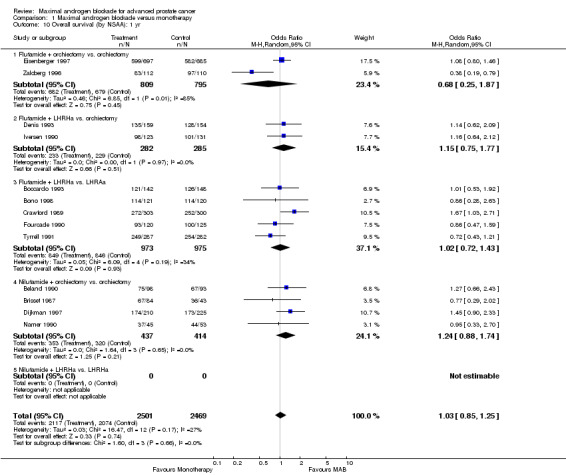
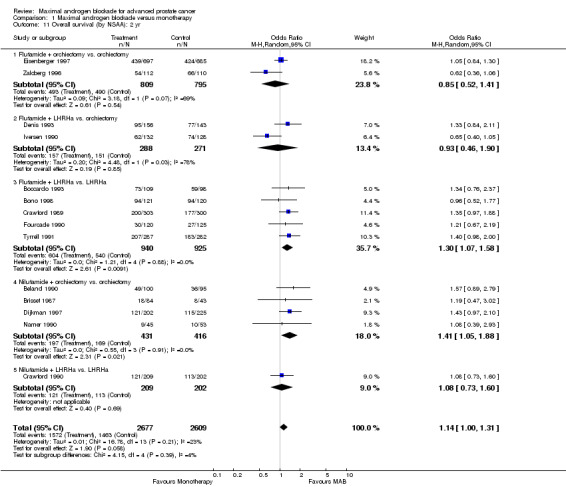
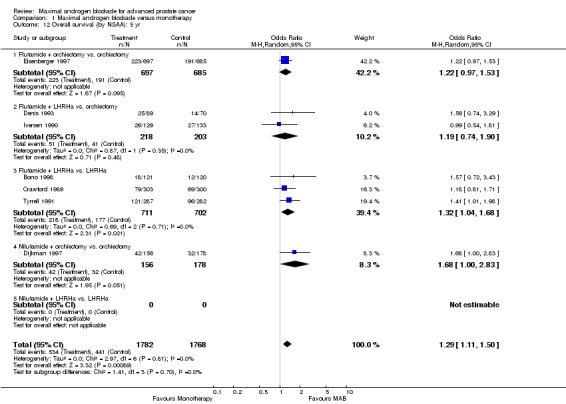
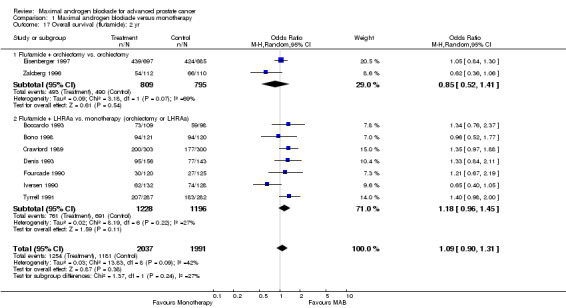
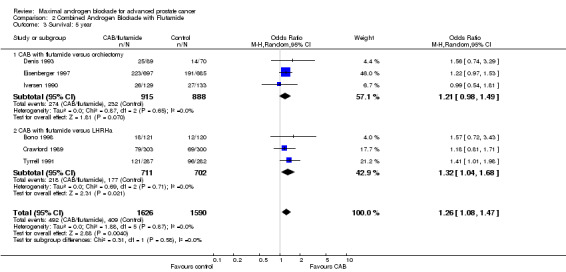
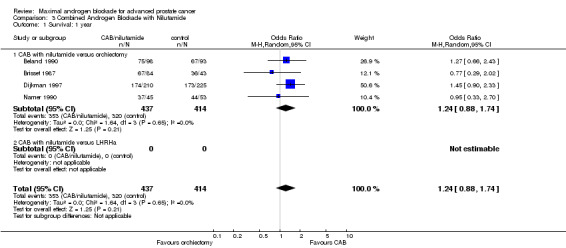
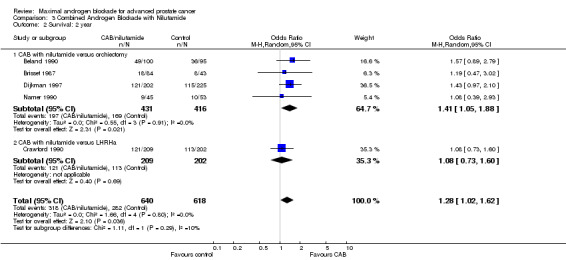
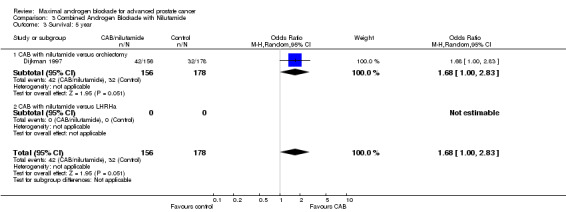
Subgroup analysis was performed to determine if the type of monotherapy used in the control group was important and to examine possible differences between NSAAs used for MAB. When flutamide in combination with orchiectomy was compared to orchiectomy alone, the pooled OR was 0.68 (95% CI: 0.25 to 1.87) at 1 year, 0.85 (95% CI: 0.52 to 1.41) at 2 years, and 1.22 (95% CI: 0.97 to 1.53) at 5 years. When flutamide was used in combination with a LHRH agonist compared to monotherapy, the pooled OR for overall survival was 1.07 (95% CI: 0.84 to 1.35) at 1 year, 1.21 (95% CI: 0.97 to 1.50) at 2 years, and 1.30 (95% CI: 1.05 to 1.60) at 5 years. When nilutamide in combination with orchiectomy was compared to orchiectomy alone, the pooled OR was 1.24 (95% CI: 0.88 to 1.74) at 1 year, 1.28 (95% CI: 1.02 to 1.62) at 2 years, and 1.68 (95% CI: 1.00 to 2.83) at 5 years.
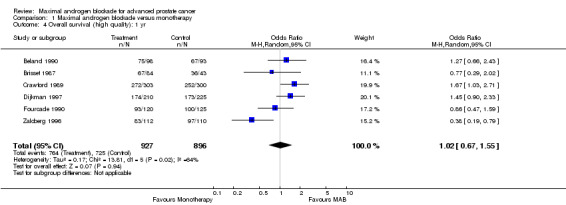
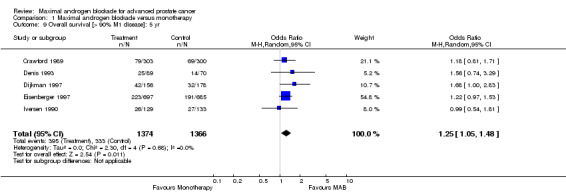
Additional subgroup analyses were performed to examine the relationship between the extent of disease and overall survival as well as the relationship of study quality to overall survival. When only studies with more than 90% M1 disease were included, the point estimate of the OR for overall survival was significant only at 5 years. The OR was 1.10 (95% CI: 0.86 to 1.41) at 1 year, 1.10 (95% CI: 0.92 to 1.32) at 2 years, and 1.25 (95% CI: 1.05 to 1.48) at 5 years. When analysis was limited to studies identified as being of high quality, the pooled OR for overall survival was only significant at 5 years. The OR was 1.02 (95% CI: 0.67 to 1.55) at 1 year, 1.21 (95% CI: 0.93 to 1.32) at 2 years, and 1.34 (95% CI: 0.96 to 1.87) at 5 years.
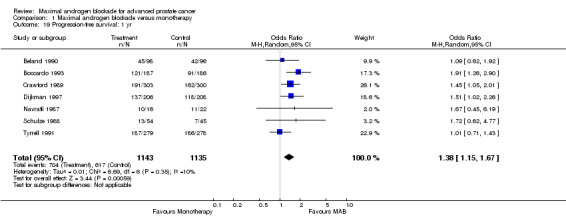
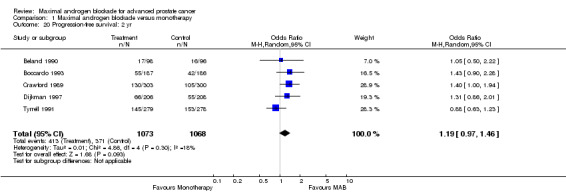
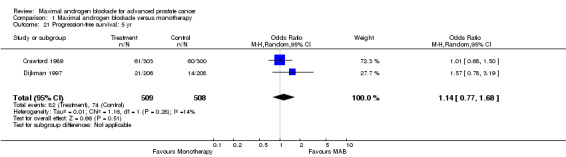
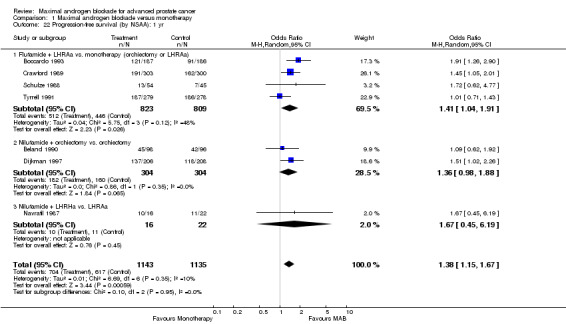
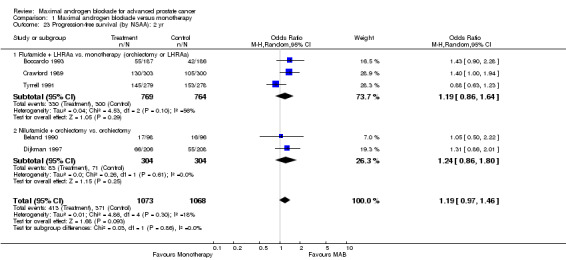
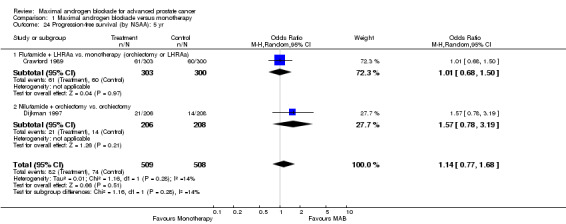
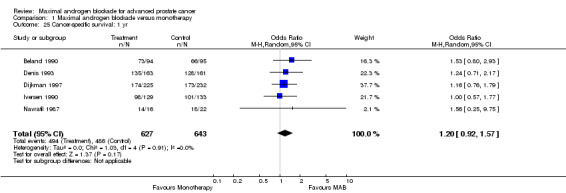
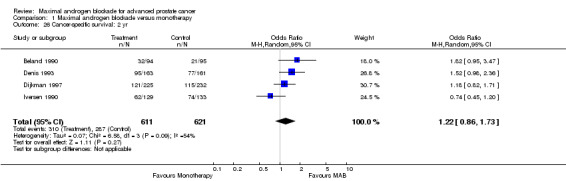
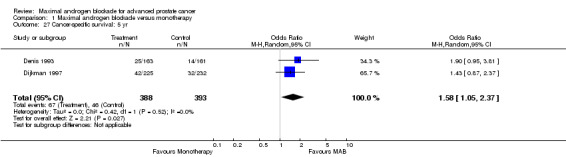
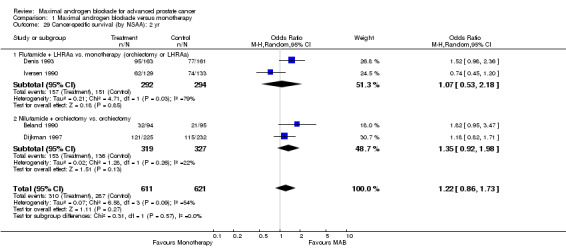
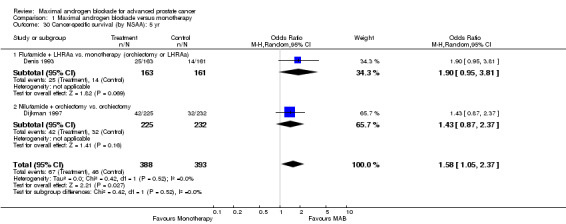
Progression‐free and cancer‐specific survival The pooled OR for progression‐free survival was determined for specific follow‐up time intervals and progressively decreased. The pooled OR at 1 year was 1.38 (95% CI: 1.15 to 1.67), 1.19 (95% CI: 0.97 to 1.46) at 2 years and 1.14 (95% CI: 0.77 to 1.68) at 5 years. Cancer‐specific survival progressively increased over time. The pooled OR of cancer‐specific survival was 1.20 (95% CI: 0.92 to 1.57) at 1 year, 1.22 (95% CI: 0.86 to 1.73) at 2 years and 1.58 (95% CI: 1.05 to 2.37) at 5 years.
Adverse events and quality of life Adverse events were categorized by system and pooled to estimate the frequency of event occurrence. These have been reported elsewhere (AHCPR report). The major differences between those assigned to monotherapy with medical or surgical castration compared to those assigned to MAB included diarrhea (1.8% vs 9.7%), GI pain (1.6% vs 7.4%), and non‐specific ophthalmologic events (5.4% vs 29%). The occurrence of adverse events was more frequent with MAB than with monotherapy resulting in a withdrawal rate of = 10% for those receiving MAB. Only one study assessed the effect of MAB on quality of life (QOL) compared to orchiectomy alone (Moinpour 1998). After a 3‐month follow up patients receiving flutamide and orchiectomy reported more diarrhea (8.6% vs 2.7%) and at both the 3‐month and 6‐month follow‐up points worse emotional functioning.
Discussion
This systematic review and meta‐analysis evaluated the available evidence regarding the effect of MAB on overall survival of patients with advanced prostate cancer. The pooled estimates of the OR and RD increased in favor of MAB over time. However, the pooled OR and RD at 1 and 2 years were not statistically significant. Only the 5‐year follow‐up observation significantly favored MAB (OR=1.29). At 5 years the pooled risk difference increased to an absolute difference of approximately 5%. The number of patients that needed treatment in order to save one life decreased from 31.3 at two years to 20.8 at five years. Cancer‐specific survival was also improved at 5 years. Consequently, interpretation of the pooled evidence supports the existence of a modest clinical benefit that is statistically significant only at five years of follow‐up. Interestingly, progression‐free survival was significantly improved with MAB at the end of the first year but not at later follow‐up intervals. At the present time the available evidence suggests that progression‐free survival, but not overall survival, is improved with MAB during the first year and that overall survival and cancer‐specific survival are moderately improved with MAB at five years follow up.
The conclusion that MAB has a beneficial effect in comparison to monotherapy must be cautiously interpreted for several reasons. Only a small number of studies provided five year follow‐up data for overall and cancer‐specific survival raising the possibility of publication bias. Further, the apparent benefit of MAB may be dependent upon the type of monotherapy used for comparison. At two years, studies that used orchiectomy as the monotherapy were not significantly different than MAB while studies that used a LHRH agonist revealed a benefit that favored MAB. With additional subgroup analysis, possible NSAA class differences were also noted. For the studies of MAB that utilized flutamide as the NSAA in comparison to orchiectomy the point estimate of the OR at two years favored orchiectomy over flutamide although the difference was not statistically significant. For those studies of MAB that incorporated nilutamide as the NSAA in comparison to orchiectomy there was a statistically significant difference favoring MAB with nilutamide over orchiectomy. The 95% confidence intervals for the pooled class effects overlap. It was not possible to determine if there were NSAA class differences in overall survival.
When the analysis was repeated for studies that included predominantly patients with > 90% M1 disease the same outcomes were identified. When higher quality studies were analyzed, the same progressive increase in OR for overall survival was noted with longer periods of observation. However, the results were not statistically significant.
Although there was a small overall survival benefit at 5 years, adverse events were more frequent in those receiving MAB and resulted in withdrawal of therapy in more than 10% of the patients. Only 4% of those receiving monotherapy withdrew. Additionally, there was a reduction in the QOL in the first 6 months of MAB. However, fatal or permanent disabling adverse events with MAB are rare.
This systematic review and meta‐analysis utilized the odds ratio as the effect measure for summation across studies and the pooled risk difference was calculated in order to provide a clinically meaningful estimate of the magnitude of treatment benefit. One methodological problem resulted from the variety of therapies available and the fact that they were not all compared against the same control. A method for handling this problem was described by Hasselblad (Hasselblad 1998) and applied in the AHCPR report. Survival rates for prostate cancer are poor, which implies a large value for the hazard rate (the rate of death across time). Although these values may not be exactly constant over time, in general, it was assumed that the hazard was constant. Based on these assumptions the object of the AHCPR report was to obtain estimates of the hazard rate for each arm of each study, or to obtain the estimate of the proportional hazards term and its standard error. For studies that did not provide this, estimates from other statistics were obtained. For the meta‐analyses a random effects model was utilized.
For the Cochrane Review, odds ratios and risk differences for overall, cancer‐specific and progression‐free survival at one, two, and five years were derived from two sources of data available in the AHCPR report. First, data was directly abstracted from individual studies providing the "number of surviving patients" at each time period. The "percent survival" listed in the report (and generally calculated from the hazard rate) was then used to calculate the "number at risk" for each time period. Second, the "number of patients at risk" and the "number reaching each endpoint" at one, two, or five years were obtained based on the provided "number at risk at baseline" and the "percent survival" at the corresponding time interval as calculated from the individual study hazard rates. We assumed that the number at risk at one, two, and five years were equal to the number at risk at baseline.
When available the raw data was utilized preferentially. To test the validity of our assumptions we conducted a sensitivity analysis to test the variation in odds ratios and risk differences in studies that provided data as listed above. Odds ratios for these studies did not vary by more by more than 10%. Additionally, the effect size and statistical significance of our results were compared to the AHCPR report.
In the AHCPR report a formal cost‐effectiveness (CE) analysis was conducted. At five years, the only time point that favored MAB, the hazard ratio was 0.86 (< 1 supports treatment benefit). Using this effect measure the CE of NSAA plus orchiectomy compared to orchiectomy alone was $94,300 per quality adjusted life year (QALY) gained. When they analyzed treatment with a NSAA in addition to a LHRH agonist compared to orchiectomy alone, the CE was $207,500/QALY. In a sensitivity analysis, they assumed a hazard ratio of 0.78 (lower 95% CI limit) and recalculated both comparisons. The CE was $56,500/QALY and $123,600/QALY, respectively.
Authors' conclusions
Implications for practice.
There is no clear overall survival benefit for MAB at one and two year follow up. Progression‐free survival is better with MAB after one year of follow up but not at two years or five years. MAB may provide a modest overall survival benefit at five years. The occurrence of adverse events is greater with MAB than with monotherapy and results more frequently in withdrawal of therapy. There is also a reduction in quality of life. Consequently, the routine use of MAB for advanced prostate cancer should be re‐evaluated. The modest overall survival benefit attributed to MAB at 5 years must be weighed against the increased risk of adverse events, the reductions in quality of life and the associated costs.
Implications for research.
Additional research to study class differences in overall survival for NSAAs compared to orchiectomy would be helpful in determining the magnitude of additional benefit that may be achieved by the type of NSAA used for MAB. Specifically, it is important to determine if there is a difference between flutamide and nilutamide on overall survival when compared to orchiectomy. Additional research on the efficacy of MAB using bicalutamide is needed before it can be recommended as a component of MAB therapy.
Feedback
Incomplete data in this Cochrane review
Summary
The first 3 sentences of the abstract for this review are potentially misleading. They seem to imply that the results given come from an analyses of 20 trials (6320 men). However, the full dataset does not contribute to any of the pooled results, since some trials are missing from each of the analyses. The 5‐year result is actually based on only 7 trials (3450 men). There is inadequate discussion of the possibility that the data used for the analyses in this review are importantly different from those that were identified but not available; and any that were not judged to be eligible because they had not been published. Because of the potential for bias due to the exclusion of such a large proportion of the data, the results and conclusions of the review may give a false impression of the benefits of the treatments under investigation. A more complete analyses of these trials is available in a systematic review of individual patient data from the trials, which was published in April 2000 by the Prostate Cancer Trialists' Collaborative Group (PCTCG 2000). The results of the Cochrane Review should be discussed in the light of the findings of the updated IPD review, rather than the findings of the 1995 report of the Prostate Cancer Trialists' Collaborative Group (note: the reference for which should be to the PCTCG, not PCTOG).
Reference PCTCG 2000. Prostate Cancer Trialists' Collaborative Group. Maximum androgen blockade in advanced prostate cancer: an overview of the randomised trials. Lancet 2000;355:1491‐8.
Contributors
Mike Clarke
I am one of the principal investigators in the IPD review mentioned in my comment.
Adverse effects of the treatments compared
Summary
1. This important review deals only very cursorily with the adverse effects of NSAA and castration. It does not help the reader to weigh the benefits of the treatments against their harms. To say that adverse effects have been reported in the AHCPR report is disappointing and unhelpful. That report is not easily available; an address for obtaining it could be added to the reference.
2. Nothing is said in the section on methodological quality or in the table of characteristics of included trials about the ways in which adverse events were ascertained, documented and assessed in the trials.
3. The last para of Results mentions that only one study (Moinpour 1988) assessed the effects of the treatments on quality of life, but this was apparently not one of the included trials: the reference is listed under additional references, and is not in the table of characteristics of included trials. Why was the study not included? Please correct or explain this, and describe clearly what was done and what was found in this study.
4. Have either of the two consumers working with the Review Group seen and commented on this review? I suggest that they be asked specifically to do so, and be shown my criticisms.
Contributors
A. Herxheimer
I certify that I have no affiliations with or involvement in any organisation or entity with a direct financial interest in the subject matter of my criticisms.
Incomplete data in this Cochrane review
Summary
Further to my earlier comment, The Cochrane Library (Issue 1, 2002) contains a new item in the Cochrane Controlled Trials Register (CENTRAL) for a publication of this review in a paper journal (Schmitt B, Wilt TJ, Schellhammer PF, DeMasi V, Sartor O, Crawford ED, Bennett CL. Combined androgen blockade with nonsteroidal antiandrogens for advanced prostate cancer: a systematic review. Urology 2001;57:727‐32). The abstract for this publication contains the clarifications requested in my comment relating to the number of patients and trials in the analyses reported in the Results section of the abstract. It would be helpful if the abstract for the Cochrane review could be edited so that it also shows this information. The reference and discussion in the Cochrane review relating to the PCTCG overview (as mentioned in my earlier comment) also needs to be updated.
Contributors
Mike Clarke
What's new
| Date | Event | Description |
|---|---|---|
| 4 April 2011 | Amended | Review is out of date and has been withdrawn. The editor has been unable to contact the primary author. |
History
Protocol first published: Issue 2, 1999 Review first published: Issue 2, 2000
| Date | Event | Description |
|---|---|---|
| 2 June 2008 | Amended | Converted to new review format. |
| 14 January 1999 | New citation required and conclusions have changed | Substantive amendment |
Notes
Review is out of date and has been withdrawn. The editor has been unable to contact the primary author.
Acknowledgements
Thanks to Indulis Rutks and Rod Mac Donald for their assistance.
Data and analyses
Comparison 1. Maximal androgen blockade versus monotherapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall survival: 1 yr | 13 | 4970 | Odds Ratio (M‐H, Random, 95% CI) | 1.03 [0.85, 1.25] |
| 2 Overall survival: 2 yr | 14 | 5286 | Odds Ratio (M‐H, Random, 95% CI) | 1.14 [1.00, 1.31] |
| 3 Overall survival: 5 yr | 7 | 3550 | Odds Ratio (M‐H, Random, 95% CI) | 1.29 [1.11, 1.50] |
| 4 Overall survival (high quality): 1 yr | 6 | 1823 | Odds Ratio (M‐H, Random, 95% CI) | 1.02 [0.67, 1.55] |
| 5 Overall survival (high quality): 2 yr | 6 | 1819 | Odds Ratio (M‐H, Random, 95% CI) | 1.21 [0.93, 1.57] |
| 6 Overall survival (high quality): 5 yr | 2 | 937 | Odds Ratio (M‐H, Random, 95% CI) | 1.34 [0.96, 1.87] |
| 7 Overall survival [> 90% M1 disease]: 1 yr | 9 | 3625 | Odds Ratio (M‐H, Random, 95% CI) | 1.10 [0.86, 1.41] |
| 8 Overall survival [> 90% M1 disease]: 2 yr | 10 | 4024 | Odds Ratio (M‐H, Random, 95% CI) | 1.10 [0.92, 1.32] |
| 9 Overall survival [> 90% M1 disease]: 5 yr | 5 | 2740 | Odds Ratio (M‐H, Random, 95% CI) | 1.25 [1.05, 1.48] |
| 10 Overall survival (by NSAA): 1 yr | 13 | 4970 | Odds Ratio (M‐H, Random, 95% CI) | 1.03 [0.85, 1.25] |
| 10.1 Flutamide + orchiectomy vs. orchiectomy | 2 | 1604 | Odds Ratio (M‐H, Random, 95% CI) | 0.68 [0.25, 1.87] |
| 10.2 Flutamide + LHRHa vs. orchiectomy | 2 | 567 | Odds Ratio (M‐H, Random, 95% CI) | 1.15 [0.75, 1.77] |
| 10.3 Flutamide + LHRHa vs. LHRAa | 5 | 1948 | Odds Ratio (M‐H, Random, 95% CI) | 1.02 [0.72, 1.43] |
| 10.4 Nilutamide + orchiectomy vs. orchiectomy | 4 | 851 | Odds Ratio (M‐H, Random, 95% CI) | 1.24 [0.88, 1.74] |
| 10.5 Nilutamide + LHRHa vs. LHRHa | 0 | 0 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Overall survival (by NSAA): 2 yr | 14 | 5286 | Odds Ratio (M‐H, Random, 95% CI) | 1.14 [1.00, 1.31] |
| 11.1 Flutamide + orchiectomy vs. orchiectomy | 2 | 1604 | Odds Ratio (M‐H, Random, 95% CI) | 0.85 [0.52, 1.41] |
| 11.2 Flutamide + LHRHa vs. orchiectomy | 2 | 559 | Odds Ratio (M‐H, Random, 95% CI) | 0.93 [0.46, 1.90] |
| 11.3 Flutamide + LHRHa vs. LHRHa | 5 | 1865 | Odds Ratio (M‐H, Random, 95% CI) | 1.30 [1.07, 1.58] |
| 11.4 Nilutamide + orchiectomy vs. orchiectomy | 4 | 847 | Odds Ratio (M‐H, Random, 95% CI) | 1.41 [1.05, 1.88] |
| 11.5 Nilutamide + LHRHa vs. LHRHa | 1 | 411 | Odds Ratio (M‐H, Random, 95% CI) | 1.08 [0.73, 1.60] |
| 12 Overall survival (by NSAA): 5 yr | 7 | 3550 | Odds Ratio (M‐H, Random, 95% CI) | 1.29 [1.11, 1.50] |
| 12.1 Flutamide + orchiectomy vs. orchiectomy | 1 | 1382 | Odds Ratio (M‐H, Random, 95% CI) | 1.22 [0.97, 1.53] |
| 12.2 Flutamide + LHRHa vs. orchiectomy | 2 | 421 | Odds Ratio (M‐H, Random, 95% CI) | 1.19 [0.74, 1.90] |
| 12.3 Flutamide + LHRHa vs. LHRHa | 3 | 1413 | Odds Ratio (M‐H, Random, 95% CI) | 1.32 [1.04, 1.68] |
| 12.4 Nilutamide + orchiectomy vs. orchiectomy | 1 | 334 | Odds Ratio (M‐H, Random, 95% CI) | 1.68 [1.00, 2.83] |
| 12.5 Nilutamide + LHRHa vs. LHRHa | 0 | 0 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
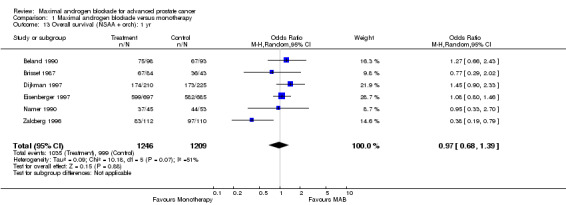
| 13 Overall survival (NSAA + orch): 1 yr | 6 | 2455 | Odds Ratio (M‐H, Random, 95% CI) | 0.97 [0.68, 1.39] |
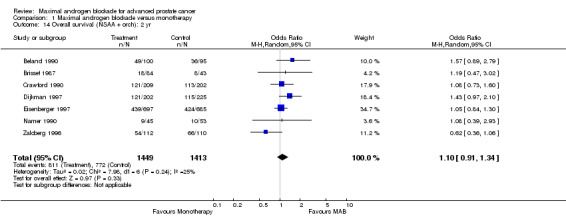
| 14 Overall survival (NSAA + orch): 2 yr | 7 | 2862 | Odds Ratio (M‐H, Random, 95% CI) | 1.10 [0.91, 1.34] |
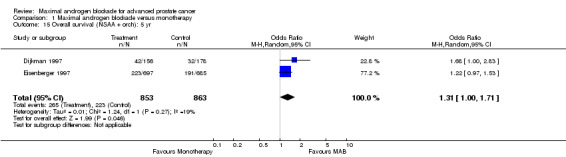
| 15 Overall survival (NSAA + orch): 5 yr | 2 | 1716 | Odds Ratio (M‐H, Random, 95% CI) | 1.31 [1.00, 1.71] |
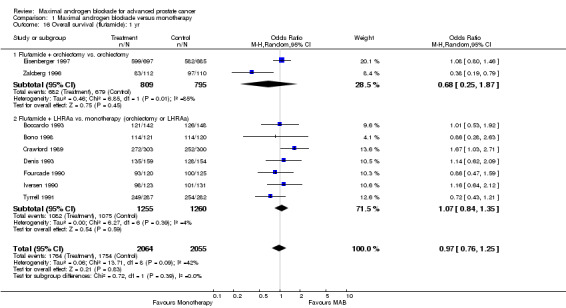
| 16 Overall survival (flutamide): 1 yr | 9 | 4119 | Odds Ratio (M‐H, Random, 95% CI) | 0.97 [0.76, 1.25] |
| 16.1 Flutamide + orchiectomy vs. orchiectomy | 2 | 1604 | Odds Ratio (M‐H, Random, 95% CI) | 0.68 [0.25, 1.87] |
| 16.2 Flutamide + LHRAa vs. monotherapy (orchiectomy or LHRAa) | 7 | 2515 | Odds Ratio (M‐H, Random, 95% CI) | 1.07 [0.84, 1.35] |
| 17 Overall survival (flutamide): 2 yr | 9 | 4028 | Odds Ratio (M‐H, Random, 95% CI) | 1.09 [0.90, 1.31] |
| 17.1 Flutamide + orchiectomy vs. orchiectomy | 2 | 1604 | Odds Ratio (M‐H, Random, 95% CI) | 0.85 [0.52, 1.41] |
| 17.2 Flutamide + LHRAa vs. monotherapy (orchiectomy or LHRAa) | 7 | 2424 | Odds Ratio (M‐H, Random, 95% CI) | 1.18 [0.96, 1.45] |
| 18 Overall survival (flutamide): 5 yr | 6 | 3216 | Odds Ratio (M‐H, Random, 95% CI) | 1.26 [1.08, 1.47] |
| 18.1 Flutamide + orchiectomy vs. orchiectomy | 1 | 1382 | Odds Ratio (M‐H, Random, 95% CI) | 1.22 [0.97, 1.53] |
| 18.2 Flutamide + LHRAa vs. monotherapy (orchiectomy or LHRAa) | 5 | 1834 | Odds Ratio (M‐H, Random, 95% CI) | 1.30 [1.05, 1.60] |
| 19 Progression‐free survival: 1 yr | 7 | 2278 | Odds Ratio (M‐H, Random, 95% CI) | 1.38 [1.15, 1.67] |
| 20 Progression‐free survival: 2 yr | 5 | 2141 | Odds Ratio (M‐H, Random, 95% CI) | 1.19 [0.97, 1.46] |
| 21 Progression‐free survival: 5 yr | 2 | 1017 | Odds Ratio (M‐H, Random, 95% CI) | 1.14 [0.77, 1.68] |
| 22 Progression‐free survival (by NSAA): 1 yr | 7 | 2278 | Odds Ratio (M‐H, Random, 95% CI) | 1.38 [1.15, 1.67] |
| 22.1 Flutamide + LHRAa vs. monotherapy (orchiectomy or LHRAa) | 4 | 1632 | Odds Ratio (M‐H, Random, 95% CI) | 1.41 [1.04, 1.91] |
| 22.2 Nilutamide + orchiectomy vs. orchiectomy | 2 | 608 | Odds Ratio (M‐H, Random, 95% CI) | 1.36 [0.98, 1.88] |
| 22.3 Nilutamide + LHRHa vs. LHRAa | 1 | 38 | Odds Ratio (M‐H, Random, 95% CI) | 1.67 [0.45, 6.19] |
| 23 Progression‐free survival (by NSAA): 2 yr | 5 | 2141 | Odds Ratio (M‐H, Random, 95% CI) | 1.19 [0.97, 1.46] |
| 23.1 Flutamide + LHRAa vs. monotherapy (orchiectomy or LHRAa) | 3 | 1533 | Odds Ratio (M‐H, Random, 95% CI) | 1.19 [0.86, 1.64] |
| 23.2 Nilutamide + orchiectomy vs. orchiectomy | 2 | 608 | Odds Ratio (M‐H, Random, 95% CI) | 1.24 [0.86, 1.80] |
| 24 Progression‐free survival (by NSAA): 5 yr | 2 | 1017 | Odds Ratio (M‐H, Random, 95% CI) | 1.14 [0.77, 1.68] |
| 24.1 Flutamide + LHRAa vs. monotherapy (orchiectomy or LHRAa) | 1 | 603 | Odds Ratio (M‐H, Random, 95% CI) | 1.01 [0.68, 1.50] |
| 24.2 Nilutamide + orchiectomy vs. orchiectomy | 1 | 414 | Odds Ratio (M‐H, Random, 95% CI) | 1.57 [0.78, 3.19] |
| 25 Cancer‐specific survival: 1 yr | 5 | 1270 | Odds Ratio (M‐H, Random, 95% CI) | 1.20 [0.92, 1.57] |
| 26 Cancer‐specific survival: 2 yr | 4 | 1232 | Odds Ratio (M‐H, Random, 95% CI) | 1.22 [0.86, 1.73] |
| 27 Cancer‐specific survival: 5 yr | 2 | 781 | Odds Ratio (M‐H, Random, 95% CI) | 1.58 [1.05, 2.37] |
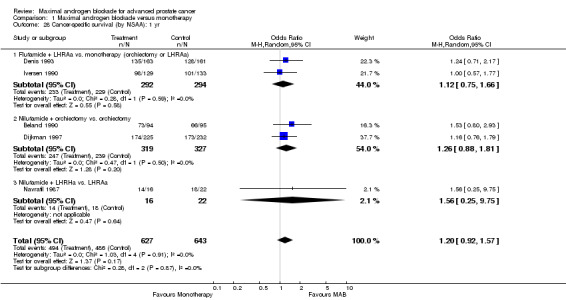
| 28 Cancer‐specific survival (by NSAA): 1 yr | 5 | 1270 | Odds Ratio (M‐H, Random, 95% CI) | 1.20 [0.92, 1.57] |
| 28.1 Flutamide + LHRAa vs. monotherapy (orchiectomy or LHRAa) | 2 | 586 | Odds Ratio (M‐H, Random, 95% CI) | 1.12 [0.75, 1.66] |
| 28.2 Nilutamide + orchiectomy vs. orchiectomy | 2 | 646 | Odds Ratio (M‐H, Random, 95% CI) | 1.26 [0.88, 1.81] |
| 28.3 Nilutamide + LHRHa vs. LHRAa | 1 | 38 | Odds Ratio (M‐H, Random, 95% CI) | 1.56 [0.25, 9.75] |
| 29 Cancer‐specific survival (by NSAA): 2 yr | 4 | 1232 | Odds Ratio (M‐H, Random, 95% CI) | 1.22 [0.86, 1.73] |
| 29.1 Flutamide + LHRAa vs. monotherapy (orchiectomy or LHRAa) | 2 | 586 | Odds Ratio (M‐H, Random, 95% CI) | 1.07 [0.53, 2.18] |
| 29.2 Nilutamide + orchiectomy vs. orchiectomy | 2 | 646 | Odds Ratio (M‐H, Random, 95% CI) | 1.35 [0.92, 1.98] |
| 30 Cancer‐specific survival (by NSAA): 5 yr | 2 | 781 | Odds Ratio (M‐H, Random, 95% CI) | 1.58 [1.05, 2.37] |
| 30.1 Flutamide + LHRAa vs. monotherapy (orchiectomy or LHRAa) | 1 | 324 | Odds Ratio (M‐H, Random, 95% CI) | 1.90 [0.95, 3.81] |
| 30.2 Nilutamide + orchiectomy vs. orchiectomy | 1 | 457 | Odds Ratio (M‐H, Random, 95% CI) | 1.43 [0.87, 2.37] |
1.1. Analysis.
Comparison 1 Maximal androgen blockade versus monotherapy, Outcome 1 Overall survival: 1 yr.
1.2. Analysis.
Comparison 1 Maximal androgen blockade versus monotherapy, Outcome 2 Overall survival: 2 yr.
1.3. Analysis.
Comparison 1 Maximal androgen blockade versus monotherapy, Outcome 3 Overall survival: 5 yr.
1.4. Analysis.
Comparison 1 Maximal androgen blockade versus monotherapy, Outcome 4 Overall survival (high quality): 1 yr.
1.5. Analysis.
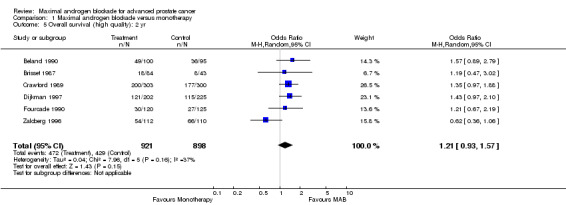
Comparison 1 Maximal androgen blockade versus monotherapy, Outcome 5 Overall survival (high quality): 2 yr.
1.6. Analysis.
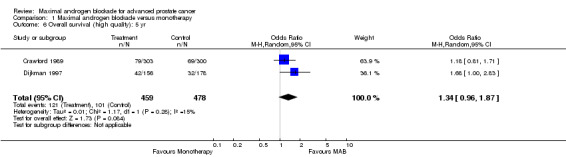
Comparison 1 Maximal androgen blockade versus monotherapy, Outcome 6 Overall survival (high quality): 5 yr.
1.7. Analysis.
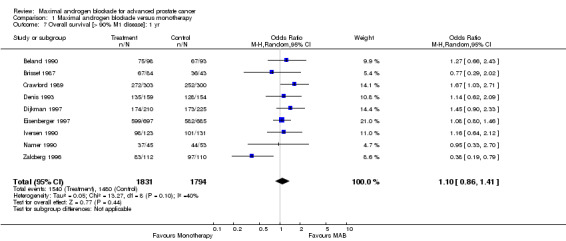
Comparison 1 Maximal androgen blockade versus monotherapy, Outcome 7 Overall survival [> 90% M1 disease]: 1 yr.
1.8. Analysis.
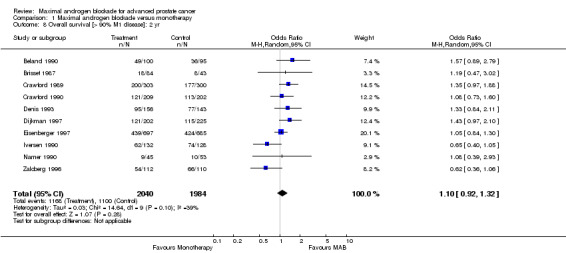
Comparison 1 Maximal androgen blockade versus monotherapy, Outcome 8 Overall survival [> 90% M1 disease]: 2 yr.
1.9. Analysis.
Comparison 1 Maximal androgen blockade versus monotherapy, Outcome 9 Overall survival [> 90% M1 disease]: 5 yr.
1.10. Analysis.
Comparison 1 Maximal androgen blockade versus monotherapy, Outcome 10 Overall survival (by NSAA): 1 yr.
1.11. Analysis.
Comparison 1 Maximal androgen blockade versus monotherapy, Outcome 11 Overall survival (by NSAA): 2 yr.
1.12. Analysis.
Comparison 1 Maximal androgen blockade versus monotherapy, Outcome 12 Overall survival (by NSAA): 5 yr.
1.13. Analysis.
Comparison 1 Maximal androgen blockade versus monotherapy, Outcome 13 Overall survival (NSAA + orch): 1 yr.
1.14. Analysis.
Comparison 1 Maximal androgen blockade versus monotherapy, Outcome 14 Overall survival (NSAA + orch): 2 yr.
1.15. Analysis.
Comparison 1 Maximal androgen blockade versus monotherapy, Outcome 15 Overall survival (NSAA + orch): 5 yr.
1.16. Analysis.
Comparison 1 Maximal androgen blockade versus monotherapy, Outcome 16 Overall survival (flutamide): 1 yr.
1.17. Analysis.
Comparison 1 Maximal androgen blockade versus monotherapy, Outcome 17 Overall survival (flutamide): 2 yr.
1.18. Analysis.
Comparison 1 Maximal androgen blockade versus monotherapy, Outcome 18 Overall survival (flutamide): 5 yr.
1.19. Analysis.
Comparison 1 Maximal androgen blockade versus monotherapy, Outcome 19 Progression‐free survival: 1 yr.
1.20. Analysis.
Comparison 1 Maximal androgen blockade versus monotherapy, Outcome 20 Progression‐free survival: 2 yr.
1.21. Analysis.
Comparison 1 Maximal androgen blockade versus monotherapy, Outcome 21 Progression‐free survival: 5 yr.
1.22. Analysis.
Comparison 1 Maximal androgen blockade versus monotherapy, Outcome 22 Progression‐free survival (by NSAA): 1 yr.
1.23. Analysis.
Comparison 1 Maximal androgen blockade versus monotherapy, Outcome 23 Progression‐free survival (by NSAA): 2 yr.
1.24. Analysis.
Comparison 1 Maximal androgen blockade versus monotherapy, Outcome 24 Progression‐free survival (by NSAA): 5 yr.
1.25. Analysis.
Comparison 1 Maximal androgen blockade versus monotherapy, Outcome 25 Cancer‐specific survival: 1 yr.
1.26. Analysis.
Comparison 1 Maximal androgen blockade versus monotherapy, Outcome 26 Cancer‐specific survival: 2 yr.
1.27. Analysis.
Comparison 1 Maximal androgen blockade versus monotherapy, Outcome 27 Cancer‐specific survival: 5 yr.
1.28. Analysis.
Comparison 1 Maximal androgen blockade versus monotherapy, Outcome 28 Cancer‐specific survival (by NSAA): 1 yr.
1.29. Analysis.
Comparison 1 Maximal androgen blockade versus monotherapy, Outcome 29 Cancer‐specific survival (by NSAA): 2 yr.
1.30. Analysis.
Comparison 1 Maximal androgen blockade versus monotherapy, Outcome 30 Cancer‐specific survival (by NSAA): 5 yr.
Comparison 2. Combined Androgen Blockade with Flutamide.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
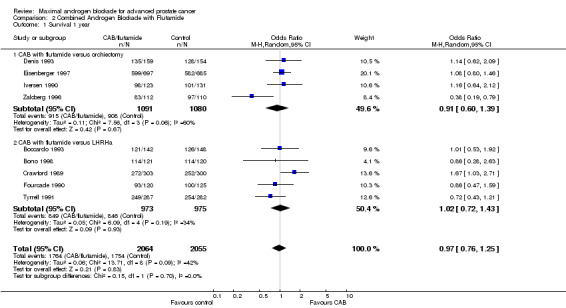
| 1 Survival 1 year | 9 | 4119 | Odds Ratio (M‐H, Random, 95% CI) | 0.97 [0.76, 1.25] |
| 1.1 CAB with flutamide versus orchiectomy | 4 | 2171 | Odds Ratio (M‐H, Random, 95% CI) | 0.91 [0.60, 1.39] |
| 1.2 CAB with flutamide versus LHRHa | 5 | 1948 | Odds Ratio (M‐H, Random, 95% CI) | 1.02 [0.72, 1.43] |
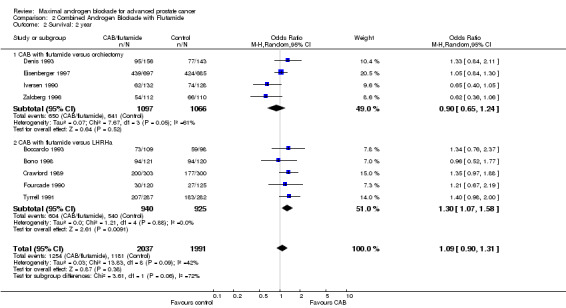
| 2 Survival: 2 year | 9 | 4028 | Odds Ratio (M‐H, Random, 95% CI) | 1.09 [0.90, 1.31] |
| 2.1 CAB with flutamide versus orchiectomy | 4 | 2163 | Odds Ratio (M‐H, Random, 95% CI) | 0.90 [0.65, 1.24] |
| 2.2 CAB with flutamide versus LHRHa | 5 | 1865 | Odds Ratio (M‐H, Random, 95% CI) | 1.30 [1.07, 1.58] |
| 3 Survival: 5 year | 6 | 3216 | Odds Ratio (M‐H, Random, 95% CI) | 1.26 [1.08, 1.47] |
| 3.1 CAB with flutamide versus orchiectomy | 3 | 1803 | Odds Ratio (M‐H, Random, 95% CI) | 1.21 [0.98, 1.49] |
| 3.2 CAB with flutamide versus LHRHa | 3 | 1413 | Odds Ratio (M‐H, Random, 95% CI) | 1.32 [1.04, 1.68] |
2.1. Analysis.
Comparison 2 Combined Androgen Blockade with Flutamide, Outcome 1 Survival 1 year.
2.2. Analysis.
Comparison 2 Combined Androgen Blockade with Flutamide, Outcome 2 Survival: 2 year.
2.3. Analysis.
Comparison 2 Combined Androgen Blockade with Flutamide, Outcome 3 Survival: 5 year.
Comparison 3. Combined Androgen Blockade with Nilutamide.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Survival: 1 year | 4 | 851 | Odds Ratio (M‐H, Random, 95% CI) | 1.24 [0.88, 1.74] |
| 1.1 CAB with nilutamide versus orchiectomy | 4 | 851 | Odds Ratio (M‐H, Random, 95% CI) | 1.24 [0.88, 1.74] |
| 1.2 CAB with nilutamide versus LHRHa | 0 | 0 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Survival: 2 year | 5 | 1258 | Odds Ratio (M‐H, Random, 95% CI) | 1.28 [1.02, 1.62] |
| 2.1 CAB with nilutamide versus orchiectomy | 4 | 847 | Odds Ratio (M‐H, Random, 95% CI) | 1.41 [1.05, 1.88] |
| 2.2 CAB with nilutamide versus LHRHa | 1 | 411 | Odds Ratio (M‐H, Random, 95% CI) | 1.08 [0.73, 1.60] |
| 3 Survival: 5 year | 1 | 334 | Odds Ratio (M‐H, Random, 95% CI) | 1.68 [1.00, 2.83] |
| 3.1 CAB with nilutamide versus orchiectomy | 1 | 334 | Odds Ratio (M‐H, Random, 95% CI) | 1.68 [1.00, 2.83] |
| 3.2 CAB with nilutamide versus LHRHa | 0 | 0 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
3.1. Analysis.
Comparison 3 Combined Androgen Blockade with Nilutamide, Outcome 1 Survival: 1 year.
3.2. Analysis.
Comparison 3 Combined Androgen Blockade with Nilutamide, Outcome 2 Survival: 2 year.
3.3. Analysis.
Comparison 3 Combined Androgen Blockade with Nilutamide, Outcome 3 Survival: 5 year.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Beland 1990.
| Methods | Multicenter Randomization: computer‐generated by center, balanced by bloc Double‐blinded: yes Withdrawals documented: yes Intention‐to‐treat analysis (ITT): modified | |
| Participants | Geographic setting: Canada Number enrolled: 208 Number randomized, control: 96 Number analyzed, control: 96 Number randomized, treatment: 98 Number analyzed, treatment: 98 Median/mean age control group: 70 Median/mean age treatment group: 69 | |
| Interventions | Control: bilateral orchiectomy plus placebo Treatment: bilateral orchiectomy plus nilutamide 300 mg Median time to follow‐up: NA Lost to follow‐up: NA | |
| Outcomes | Overall survival Cancer‐specific survival Progression‐related outcomes Time to treatment failure Adverse events | |
| Notes | Percentage M1 disease, control: 100% Percentage M1 disease, treatment: 100% Percentage poorly differentiated tumor, control: NA Percentage poorly differentiated tumor, treatment: NA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Boccardo 1993.
| Methods | Multicenter Randomization: central randomization by telephone contact Double‐blinded: no Withdrawals documented: yes Intention‐to‐treat analysis (ITT): modified | |
| Participants | Geographic setting: Italy Number enrolled: 373 Number randomized, control: 186 Number analyzed, control: 186 Number randomized, treatment: 187 Number analyzed, treatment: 187 Median/mean age control group: 74 Median/mean age treatment group: 73 | |
| Interventions | Control: goserelin 3.6 mg sc Treatment: goserelin 3.6 mg sc plus flutamide 750 mg Median time to follow‐up: 24 months Lost to follow‐up: NA | |
| Outcomes | Overall survival Cancer‐specific survival Progression‐related outcomes Time to treatment failure Adverse events | |
| Notes | Percentage M1 disease, control: 68% Percentage M1 disease, treatment: 62% Percentage poorly differentiated tumor, control: 30% Percentage poorly differentiated tumor, treatment: 25% | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Bono 1998.
| Methods | Multicenter Randomization: Centrally registered and randomized Double‐blinded: no Withdrawals documented: yes Intention‐to‐treat analysis (ITT): yes | |
| Participants | Geographic setting: Italy Number enrolled: 277 Number randomized, control: 120 Number analyzed, control: 120 Number randomized, treatment: 121 Number analyzed, treatment: 121 Median/mean age control group: 70 Median/mean age treatment group: 67 | |
| Interventions | Control: leuprolide 3.75 mg sc Treatment: leuprolide 3.75 mg sc plus flutamide 750 mg Median time to follow‐up: Lost to follow‐up: NA | |
| Outcomes | Overall survival Cancer‐specific survival Progression‐related outcomes Time to treatment failure Adverse events | |
| Notes | Percentage M1 disease, control: 49% Percentage M1 disease, treatment: 54% Percentage poorly differentiated tumor, control: 31% Percentage poorly differentiated tumor, treatment: 39% | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Brisset 1987.
| Methods | Multicenter Randomization: Not specified Double‐blinded: yes Withdrawals documented: partial Intention‐to‐treat analysis (ITT): none | |
| Participants | Geographic setting: France Number enrolled: 195 Number randomized, control: 43 Number analyzed, control: 43 Number randomized, treatment: 46 Number analyzed, treatment: 46 Median/mean age control group: 72 Median/mean age treatment group: 73 | |
| Interventions | Control: bilateral orchiectomy plus placebo Treatment: bilateral orchiectomy plus nilutamide 150 mg Median time to follow‐up: 20 months Lost to follow‐up: NA | |
| Outcomes | Overall survival Cancer‐specific survival Progression‐related outcomes Time to treatment failure Adverse events | |
| Notes | Percentage M1 disease, control: 88% Percentage M1 disease, treatment: 91% Percentage poorly differentiated tumor, control: NA Percentage poorly differentiated tumor, treatment: NA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Crawford 1989.
| Methods | Randomization: Central dynamic centralization Double‐blinded: yes Withdrawals documented: partial Intention‐to‐treat analysis (ITT): modified | |
| Participants | Geographic setting: US Number enrolled: 617 Number randomized, control: 300 Number analyzed, control: 300 Number randomized, treatment: 303 Number analyzed, treatment: 303 Median/mean age control group: 67 Median/mean age treatment group: 67 | |
| Interventions | Control: leuprolide 1 mg sc q.d. plus placebo Treatment: leuprolide 1 mg sc q.d. plus flutamide 750 mg Median time to follow‐up: NA Lost to follow‐up: NA | |
| Outcomes | Overall survival Cancer‐specific survival Progression‐related outcomes Time to treatment failure Adverse events | |
| Notes | Percentage M1 disease, control: 100% Percentage M1 disease, treatment: 100% Percentage poorly differentiated tumor, control: NA Percentage poorly differentiated tumor, treatment: NA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Crawford 1990.
| Methods | Multicenter Randomization: Not specified Double‐blinded: yes Withdrawals documented: no Intention‐to‐treat analysis (ITT): none | |
| Participants | Geographic setting: North America Number enrolled: 411 Number randomized, control: NA Number randomized, treatment: NA Number analyzed, treatment: 209 Number analyzed, control: 202 Median/mean age control group: NA Median/mean age treatment group: NA | |
| Interventions | Control: leuprolide plus placebo Treatment: leuprolide plus nilutamide Median time to follow‐up: NA Lost to follow‐up: NA | |
| Outcomes | Overall survival Cancer‐specific survival Progression‐related outcomes Time to treatment failure Adverse events | |
| Notes | Percentage M1 disease, control: 100% Percentage M1 disease, treatment: 100% Percentage poorly differentiated tumor, control: NA Percentage poorly differentiated tumor, treatment: NA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Denis 1993.
| Methods | Multicenter Randomization: centrally randomized by the EORTC data center by telephone or email Double‐blinded: no Withdrawals documented: partial Intention‐to‐treat analysis (ITT): none | |
| Participants | Geographic setting: Europe Number enrolled: 327 Number randomized, control: 155 Number analyzed, control: 148 Number randomized, treatment: 155 Number analyzed, treatment: 149 Median/mean age control group: Median/mean age treatment group: | |
| Interventions | Control: bilateral orchiectomy Treatment: goserelin 3.6 mg sc plus flutamide 750 mg Median time to follow‐up: 60 months Lost to follow‐up: NA # not completed: Control 7; Treatment 6 | |
| Outcomes | Overall survival Cancer‐specific survival Progression‐related outcomes Time to treatment failure Adverse events | |
| Notes | Percentage M1 disease, control: 100% Percentage M1 disease, treatment: 100% Percentage poorly differentiated tumor, control: 33% Percentage poorly differentiated tumor, treatment: 30% | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Dijkman 1997.
| Methods | Multicenter Randomization: centralized, stratified by center, balanced by bloc Double‐blinded: yes Withdrawals documented: yes Intention‐to‐treat analysis (ITT): modified | |
| Participants | Geographic setting: Number enrolled: 457 Number randomized, control: 232 Number analyzed, control: 232 Number randomized, treatment: 225 Number analyzed, treatment: 225 Median/mean age control group: 72 Median/mean age treatment group: 71 | |
| Interventions | Control: bilateral orchiectomy plus placebo Treatment: bilateral orchiectomy plus nilutamide 300 mg 1 month/ then 150 mg Median time to follow‐up: NA Lost to follow‐up (protocol deviation): Control 16; Treatment 18 | |
| Outcomes | Overall survival Cancer‐specific survival Progression‐related outcomes Time to treatment failure Adverse events | |
| Notes | Percentage M1 disease, control: 100% Percentage M1 disease, treatment: 98% Percentage poorly differentiated tumor, control: 39% Percentage poorly differentiated tumor, treatment: 38% | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Eisenberger 1997.
| Methods | center Randomization: Not specified Double‐blinded: yes Withdrawals documented: no Intention‐to‐treat analysis (ITT): unknown | |
| Participants | Geographic setting: United States Number enrolled: 1387 Number randomized, control: 681 Number analyzed, control: 681 Number randomized, treatment: 690 Number analyzed, treatment: 690 Median/mean age control group: Median/mean age treatment group: | |
| Interventions | Control: bilateral orchiectomy Treatment: bilateral orchiectomy plus flutamide 750 mg Median time to follow‐up: NA Lost to follow‐up: NA | |
| Outcomes | Overall survival Cancer‐specific survival Progression‐related outcomes Time to treatment failure Adverse events | |
| Notes | Percentage M1 disease, control: 100% Percentage M1 disease, treatment: 100% Percentage poorly differentiated tumor, control: NA Percentage poorly differentiated tumor, treatment: NA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Ferrari 1993.
| Methods | Randomization: Not specified Double‐blinded: No Withdrawals documented: partial Intention‐to‐treat analysis (ITT): none | |
| Participants | Geographic setting: Italy Number enrolled: 122 Number randomized, control: 62 Number analyzed, control: 46 Number randomized, treatment: 60 Number analyzed, treatment: 62 Median/mean age control group: 69 Median/mean age treatment group: 72 | |
| Interventions | Control: buserelin 1.5 mg sc x 7 days then 1.2 mg IN Treatment: buserelin 1.5 mg sc x 7 days then 1.2 mg IN plus flutamide 750 mg Median time to follow‐up: 19.77 months (Tx); 20.33 months (Cx) Lost to follow‐up: Control 14; Treatment 11 | |
| Outcomes | Overall survival Cancer‐specific survival Progression‐related outcomes Time to treatment failure Adverse events | |
| Notes | Percentage M1 disease, control: 100% Percentage M1 disease, treatment: 100% Percentage poorly differentiated tumor, control: NA Percentage poorly differentiated tumor, treatment: NA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Ferrari 1996.
| Methods | Randomization: Not specified Double‐blinded: No Withdrawals documented: partial Intention‐to‐treat analysis (ITT): none | |
| Participants | Geographic setting: Italy Number enrolled: 150 Number randomized, control: 76 Number analyzed, control: 62 Number randomized, treatment: 74 Number analyzed, treatment: 63 Median/mean age control group: 72 Median/mean age treatment group: 69 | |
| Interventions | Control: leuprolide IM (no dose given) x 28d; cyproterone 150 mg given for 3 weeks. Treatment: leuprolide IM (no dose given) plus flutamide 750 mg Median time to follow‐up: 30 months Lost to follow‐up: NA | |
| Outcomes | Overall survival Cancer‐specific survival Progression‐related outcomes Time to treatment failure Adverse events | |
| Notes | Percentage M1 disease, control: 100% Percentage M1 disease, treatment: 100% Percentage poorly differentiated tumor, control: NA Percentage poorly differentiated tumor, treatment: NA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | D ‐ Not used |
Fourcade 1990.
| Methods | Multicenter Randomization: Not specified Double‐blinded: yes Withdrawals documented: no Intention‐to‐treat analysis (ITT): yes | |
| Participants | Geographic setting: France Number enrolled: 245 Number randomized, control: 125 Number analyzed, control: 125 Number randomized, treatment: 120 Number analyzed, treatment: 120 Median/mean age control group: 74 Median/mean age treatment group: 74 | |
| Interventions | Control: goserelin 3.6 mg sc Treatment: goserelin 3.6 mg sc plus flutamide 750 mg Median time to follow‐up: NA Lost to follow‐up: NA | |
| Outcomes | Overall survival Cancer‐specific survival Progression‐related outcomes Time to treatment failure Adverse events | |
| Notes | Percentage M1 disease, control: 84% Percentage M1 disease, treatment: 81% Percentage poorly differentiated tumor, control: 39% Percentage poorly differentiated tumor, treatment: 40% | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Iversen 1990.
| Methods | Multicenter Randomization: Stratified by participating center, local block randomization Double‐blinded: no Withdrawals documented: partial Intention‐to‐treat analysis (ITT): yes | |
| Participants | Geographic setting: Denmark Number enrolled: 264 Number randomized, control: 133 Number analyzed,control: 133 Number randomized, treatment: 129 Number analyzed, treatment: 129 Median/mean age control group: 72 Median/mean age treatment group: 72 | |
| Interventions | Control: total or subcapsular bilateral orchiectomy Treatment: goserelin 3.6 mg sc plus flutamide 750 mg Median time to follow‐up: 57 months Lost to follow‐up: NA | |
| Outcomes | Overall survival Cancer‐specific survival Progression‐related outcomes Time to treatment failure Adverse events | |
| Notes | Percentage M1 disease, control:89% Percentage M1 disease, treatment: 93% Percentage poorly differentiated tumor, control: 63% Percentage poorly differentiated tumor, treatment: 50% | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Knonagel 1989.
| Methods | Multicenter Randomization: No details Double‐blinded: yes Withdrawals documented: no Intention‐to‐treat analysis (ITT): none | |
| Participants | Geographic setting: Switzerland Number enrolled: 51 Number randomized, control: 25 Number analyzed, control: 20 Number randomized, treatment: 26 Number analyzed, treatment: 19 Median/mean age control group: 74 Median/mean age treatment group: 74 | |
| Interventions | Control: bilateral orchiectomy plus placebo Treatment: bilateral orchiectomy plus nilutamide 300 mg Median time to follow‐up: NA Lost to follow‐up: NA | |
| Outcomes | Overall survival Cancer‐specific survival Progression‐related outcomes Time to treatment failure Adverse events | |
| Notes | Percentage M1 disease, control: 96% Percentage M1 disease, treatment: 92% Percentage poorly differentiated tumor, control: NS Percentage poorly differentiated tumor, treatment: NS | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Namer 1990.
| Methods | Multicenter Randomization: Balanced by treating institution Double‐blinded: yes Withdrawals documented: partial Intention‐to‐treat analysis (ITT): none | |
| Participants | Geographic setting: France Number enrolled: 151 Number randomized, control: 60 Number analyzed, control: 53 Number randomized, treatment: 65 Number analyzed, treatment: 45 Median/mean age control group: 72 Median/mean age treatment group: 72 | |
| Interventions | Control: bilateral orchiectomy plus placebo Treatment: bilateral orchiectomy plus nilutamide 300 mg Median time to follow‐up: 23.4 months Lost to follow‐up: NA # not completed: Control 4; Treatment 8 | |
| Outcomes | Overall survival Cancer‐specific survival Progression‐related outcomes Time to treatment failure Adverse events | |
| Notes | Percentage M1 disease, control: 100% Percentage M1 disease, treatment: 97% Percentage poorly differentiated tumor, control: 34% Percentage poorly differentiated tumor, treatment: 53% | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Navratil 1987.
| Methods | Multicenter Randomization: Not specified Double‐blinded: yes Withdrawals documented: partial Intention‐to‐treat analysis (ITT): modified | |
| Participants | Geographic setting: France Number enrolled: 49 Number randomized, control: 22 Number analyzed, control: 22 Number randomized, treatment: 16 Number analyzed, treatment: 16 Median/mean age control group: 75 Median/mean age treatment group: 72 | |
| Interventions | Control: buserelin 0.5 mg sc plus placebo Treatment: buserelin 0.5 mg sc plus nilutamide 300 mg Median time to follow‐up: NA Lost to follow‐up: NA | |
| Outcomes | Overall survival Cancer‐specific survival Progression‐related outcomes Time to treatment failure Adverse events | |
| Notes | Percentage M1 disease, control: 100% Percentage M1 disease, treatment: 100% Percentage poorly differentiated tumor, control: NS Percentage poorly differentiated tumor, treatment: NS | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Periti 1995.
| Methods | Multicenter Randomization: Not specified Double‐blinded: yes Withdrawals documented: no Intention‐to‐treat analysis (ITT): none | |
| Participants | Geographic setting: Italy Number enrolled: 126 Number randomized, control: No details Number analyzed, control: Number randomized, treatment: Number analyzed, treatment: Median/mean age control group: Median/mean age treatment group: | |
| Interventions | Control: leuprolide 3.75 mg sc Treatment: leuprolide 3.75 mg sc plus nilutamide 300 mg 1st month then 150 mg Median time to follow‐up: 30 months Lost to follow‐up: NA | |
| Outcomes | Overall survival Cancer‐specific survival Progression‐related outcomes Time to treatment failure Adverse events | |
| Notes | Percentage M1 disease, control: No details Percentage M1 disease, treatment: No details Percentage poorly differentiated tumor, control: No details Percentage poorly differentiated tumor, treatment: No details | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Schulze 1988.
| Methods | Multicenter Randomization: Not specified Double‐blinded: NA Withdrawals documented: partial Intention‐to‐treat analysis (ITT): modified | |
| Participants | Geographic setting: Germany Number enrolled: 99 Number randomized, control: 45 Number analyzed, control: 45 Number randomized, treatment: 54 Number analyzed, treatment: 54 Median/mean age control group: No details Median/mean age treatment group: No details | |
| Interventions | Control: bilateral orchiectomy or goserelin 3.6 mg sc Treatment: bilateral orchiectomy or goserelin 3.6 mg sc plus flutamide 750 mg Median time to follow‐up: NA Lost to follow‐up: Control 5; Treatment 5 # not completed: Control 5; Treatment 5 | |
| Outcomes | Overall survival Cancer‐specific survival Progression‐related outcomes Time to treatment failure Adverse events | |
| Notes | Percentage M1 disease, control: NS Percentage M1 disease, treatment: NS Percentage poorly differentiated tumor, control: NS Percentage poorly differentiated tumor, treatment: NS | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Tyrrell 1991.
| Methods | Multicenter Randomization: Not specified Double‐blinded: no Withdrawals documented: partial Intention‐to‐treat analysis (ITT): modified | |
| Participants | Geographic setting: Europe Number enrolled: 589 Number randomized, control: 284 Number analyzed, control: 284 Number randomized, treatment: 287 Number analyzed, treatment: 287 Median/mean age control group: 73 Median/mean age treatment group: 72 | |
| Interventions | Control: goserelin 3.6 mg sc Treatment: goserelin 3.6 mg sc plus flutamide 750 mg Median time to follow‐up: 56.2 months Lost to follow‐up: NA | |
| Outcomes | Overall survival Cancer‐specific survival Progression‐related outcomes Time to treatment failure Adverse events | |
| Notes | Percentage M1 disease, control: 58% Percentage M1 disease, treatment: 56% Percentage poorly differentiated tumor, control: 16% Percentage poorly differentiated tumor, treatment: 12% | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Zalcberg 1996.
| Methods | Multicenter Randomization: Not specified Double‐blinded: yes Withdrawals documented: partial Intention‐to‐treat analysis (ITT): modified | |
| Participants | Geographic setting: Australia Number enrolled: 222 Number randomized, control: 110 Number analyzed, control: 110 Number randomized, treatment: 112 Number analyzed, treatment: 112 Median/mean age control group: 71 Median/mean age treatment group: 72 | |
| Interventions | Control: bilateral orchiectomy plus placebo Treatment: bilateral orchiectomy plus flutamide 750 mg Median time to follow‐up: NA Lost to follow‐up: NA | |
| Outcomes | Overall survival Cancer‐specific survival Progression‐related outcomes Time to treatment failure Adverse events | |
| Notes | Percentage M1 disease, control: 98% Percentage M1 disease, treatment: 100% Percentage poorly differentiated tumor, control: NS Percentage poorly differentiated tumor, treatment: NS | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| de Voogt 1990 | Treatment using steroidal compound cyproterone (CPA). |
| Di Silverio 1990 | Treatment using steroidal compound cyproterone (CPA). |
| Jorgensen 1993 | Treatment using steroidal compound cyproterone (CPA). |
| Klosterhalfen 1987 | Treatment using steroidal compound cyproterone (CPA). |
| Robinson 1995 | Treatment using steroidal compound cyproterone (CPA). |
| Thorpe 1996 | Treatment using steroidal compound cyproterone (CPA). |
| Williams 1990 | Treatment using steroidal compound cyproterone (CPA). |
Sources of support
Internal sources
Dept. of Veterans Affairs Health Services Research and Development Program, USA.
External sources
American Cancer Society, USA.
Declarations of interest
B Schmitt has co‐authored a draft manuscript with the Blue Cross/Blue Shield Technology Evaluation Center project on hormonal therapies for prostate cancer.
CL Bennett is (1) on the speaker's bureau for Schering‐Plough and ALZA; (2) has received unrestricted grant support from Schering‐Plough, AstraZeneca, and Cell Pathways; and (3) was a consultant to the Blue Cross/Blue Shield Technology Evaluation Center project on hormonal therapies for prostate cancer.
TJ Wilt was a consultant to the Blue Cross/Blue Shield Technology Evaluation Center project on hormonal therapies for prostate cancer.
Edited (no change to conclusions)
References
References to studies included in this review
Beland 1990 {published data only}
- *Beland G, Elhilali M, Fradet Y, Laroche B, Ramsey EW, Trachtenberg J, Venner PM, Tewari HD. A controlled trial of castration with and without nilutamide in metastatic prostatic carcinoma. Cancer 1990;66(5 Suppl):1074‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Beland G. Combination of Anandron with orchiectomy in treatment of metastatic prostate cancer. Results of a double‐blind study. Urology 1991;37(2 Suppl):25‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Beland G, Elhilali M, Fradet Y, Laroche B, Ramsey EW, Trachtenberg J, Venner PM. Total androgen blockade for metastatic cancer of the prostate. Am J Clin Oncol 1988;11(Suppl 2):S187‐90. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Beland G, Elhilali M, Fradet Y, Laroche B, Ramsey EW, Trachtenberg J, Venner PM, Tewari HD. A controlled trial of castration with and without nilutamide in metastatic prostatic carcinoma. Cancer 1990b;66(5 Suppl):1074‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Beland G, Elhilali M, Fradet Y, Laroche B, Ramsey EW, Trachtenberg J, Venner PM, Tewari HD. Total androgen ablation: Canadian experience. Urol Clin North Am 1991;18(1):75‐82. [MEDLINE: ] [PubMed] [Google Scholar]
- Beland G, Elhilali M, Fradet Y, Laroche B, Ramsey EW, Venner PM, Tewari HD. Total androgen blockade vs orchiectomy in stage D2 prostate cancer. Prog Clin Biol Res 1987;243A:391‐400. [MEDLINE: ] [PubMed] [Google Scholar]
- The Canadian Anandron Study Group. Total androgen ablation in the treatment of metastatic prostatic cancer. The Canadian Anandron Study Group. Semin Urol 1990;8(3):159‐65. [MEDLINE: ] [PubMed] [Google Scholar]
Boccardo 1993 {published data only}
- Boccardo F, Pace M, Rubagotti A, Guarneri D, Decensi A, Oneto F, Martorana G, Giuliani L, Selvaggi F, Battaglia M, et al. Goserelin acetate with or without flutamide in the treatment of patients with locally advanced or metastatic prostate cancer. The Italian Prostatic Cancer Project (PONCAP) Study Group. Eur J Cancer 1993;29A(8):1088‐93. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bono 1998 {published data only}
- Bono AV, DiSilverio F, Robustelli della Cuna G, Benvenuti C, Brausi M, Ferrari P, Gibba A, Galli L. Complete androgen blockade versus chemical castration in advanced prostatic cancer: analysis of an Italian multicentre study. Italian Leuprorelin Group. Urol Int 1998;60(Suppl 1):18‐24. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Brisset 1987 {published data only}
- Brisset JM, Boccon‐Gibod L, Botto H, Camey M, Cariou G, Duclos JM, Duval F, Gonties D, Jorest R, Lamy L, et al. Anandron (RU 23908) associated to surgical castration in previously untreated stage D prostate cancer: a multicenter comparative study of two doses of the drug and of a placebo. Prog Clin Biol Res 1987;243A:411‐22. [MEDLINE: ] [PubMed] [Google Scholar]
Crawford 1989 {published data only}
- *Crawford ED, Eisenberger MA, McLeod DG, Spaulding JT, Benson R, Dorr FA, Blumenstein BA, Davis MA, Goodman PJ. A controlled trial of leuprolide with and without flutamide in prostatic carcinoma. N Engl J Med 1989;321(7):419‐24. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Benson RC. Total androgen blockade: the United States experience. Eur Urol 1993;24(Suppl 2):72‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Benson RC, Crawford ED, Eisenberger MA, McLeod DG, Spaulding JT, Dorr FA. National Cancer Institute study of luteinizing hormone‐releasing hormone plus flutamide versus luteinizing hormone‐releasing hormone plus placebo. Semin Oncol 1991;5(Suppl 6):9‐12. [MEDLINE: ] [PubMed] [Google Scholar]
- Crawford ED. Changing concepts in the management of advanced prostate cancer. Urology 1995;46(6):899‐901. [DOI] [PubMed] [Google Scholar]
- Crawford ED. Combination studies with leuprolide. Eur Urol 1990;18(Suppl 3):30‐3. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Crawford ED. Total androgen blockade: the NCI study [abstract]. In: International Testicular and Prostatic Cancer Conference. 1990 Oct 4‐6; Toronto, Canada. p. International Testicular and Prostatic Cancer Conference. 1990 Oct 4‐6; Toronto, Canada. p. 91.
- Crawford ED, Allen JA. Treatment of newly diagnosed state D2 prostate cancer with leuprolide and flutamide or leuprolide alone, phase III, intergroup study 0036. J Steroid Biochem Mol Biol 1990;37(6):961‐3. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Crawford ED, Blumenstein BA, Goodman PJ, Davis MA, Eisenberger MA, McLeod DG, Spaulding JT, Benson R, Dorr FA. Leuprolide with and without flutamide in advanced prostate cancer. Cancer 1990;66(5 Suppl):1039‐44. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Crawford ED, Goodman P, Blumenstein B. Combined androgen blockade: leuprolide and flutamide versus leuprolide and placebo. Semin Urol 1990;8(3):154‐8. [MEDLINE: ] [PubMed] [Google Scholar]
- Crawford ED, Nabors WL. Total androgen blockade: American experience. Urol Clin North Am 1991;18(1):55‐63. [PubMed] [Google Scholar]
- Eisenberger MA, Crawford ED, McLeod DG, Benson R, Dorr FA, Blumenstein B. A comparison of leuprolide and flutamide vs leuprolide alone in newly diagnosed stage D2 prostate cancer: prognostic and therapeutic importance of the minimal disease subset [abstract]. Proc Annu Meet Am Soc Clin Oncol 1992;11:A619. [Google Scholar]
- Eisenberger MA, Crawford ED, Wolf M, Blumenstein B, McLeod DG, Benson R, Dorr FA, Benson M, Spaulding JT. Prognostic factors in stage D2 prostate cancer; important implications for future trials: results of a cooperative intergroup study (INT.0036). Semin Oncol 1994;21(5):613‐9. [MEDLINE: ] [PubMed] [Google Scholar]
- McLeod DG, Crawford ED, Blumenstein BA, Eisenberger MA, Dorr FA. Controversies in the treatment of metastatic prostate cancer. Cancer 1992;70(1 Suppl):324‐8. [DOI] [PubMed] [Google Scholar]
Crawford 1990 {published data only}
- Crawford ED, Kasimis BS, Gandara D, Smith JA, Soloway MS, Lange PH, Lynch DF, Al‐Juburi A, Bracken RB, Wise HA, et al. A randomized, controlled clinical trial of leuprolide and anandron (LA) vs leuprolide and placebo (LP) for advanced prostate cancer (D2cap) [abstract]. Proc Annu Meet Am Soc Clin Oncol 1990;9:A523. [Google Scholar]
Denis 1993 {published data only}
- *Denis LJ, Carnelro de Moura JL, Bono A, Sylvester R, Whelan P, Newling D, Depauw M. Goserelin acetate and flutamide versus bilateral orchiectomy: a phase III EORTC trial (30853). EORTC GU Group and EORTC Data Center. Urology 1993;42(2):119‐29. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Carvalho AP, Moura JL, Denis L, Newling D, Smith P, Bono A, Sylvester R, Pauw M, Ongena P. Zoladex and flutamide vs orchidectomy: a phase III EORTC 30853 trial. EORTC Urological Group. Prog Clin Biol Res 1989;303:129‐43. [PubMed] [Google Scholar]
- Denis L, Keuppens F, Newling D, Smith PH, Calais da Silva F, Sylvester R, DePauw M, Onegana P and members of the EORTC‐GU Group Belgium. Orchidectomy versus total androgen blockade: a phase III EORTC 30853 study. GnRH Analogues Cancer Human Reproduction 1990;3:117‐27. [Google Scholar]
- Denis L, Keuppens F, Robinson M, Mahler C, Smith P, Pinto de Carvalho AP, Newling D, Bono A, Sylvester R, Pauw M, et al. Complete androgen blockade: data from an EORTC 30853 trial. Semin Urol 1990;8(3):166‐74. [PubMed] [Google Scholar]
- Denis L, Robinson M, Mahler C, Smith P, Keuppens F, Moura JL, Bono A, Newling D, Sylvester R, Pauw M, et al. Orchidectomy versus Zoladex plus Eulexin in patients with metastatic prostate cancer (EORTC 30853). J Steroid Biochem Mol Biol 1990;37(6):951‐9. [DOI] [PubMed] [Google Scholar]
- Denis L, Smith P, Carneiro de Moura JL, Newling D, Bono A, Keuppens F, Mahler C, Robinson M, Sylvester R, Pauw M, et al. Total androgen ablation: European experience. The EORTC GU Group. Urol Clin North Am 1991;18(1):65‐73. [PubMed] [Google Scholar]
- Denis L, Smith PH, Moura JL, Newling DW, Bono A, Keuppens F, Robinson, M, Mahler C, Sylvester R, Pauw M, et al. Orchidectomy vs. Zoladex plus flutamide in patients with metastatic prostate cancer. The EORTC GU Group. Eur Urol 1990;18(Suppl 3):34‐40. [DOI] [PubMed] [Google Scholar]
- Keuppens F, Denis L, Smith P, Carvalho AP, Newling D, Bond A, Sylvester R, Pauw M, Vermeylen K, Ongena P. Zoladex and flutamide versus bilateral orchiectomy. A randomized phase III EORTC 30853 study. The EORTC GU Group. Cancer 1990;66(5 Suppl):1045‐57. [DOI] [PubMed] [Google Scholar]
- Keuppens F, Whelan P, Carneiro de Moura JL, Newling D, Bono A, Denis L, Robinson M, Mahler C, Sylvester R, Pauw M, et al. Orchiectomy versus goserelin plus flutamide in patients with metastatic prostate cancer (EORTC 30853). European Organization for Research and Treatment Cancer‐ Genitourinary Group. Cancer 1993;72(12 Suppl):3863‐9. [DOI] [PubMed] [Google Scholar]
- Newling DW, Denis L, Vermeylan K. Orchiectomy versus goserelin and flutamide in the treatment of newly diagnosed metastatic prostate cancer. Analysis of the criteria used in the European Organization for Research and Treatment Cancer‐ Genitourinary Group Study 30853. Cancer 1993;72(12 Suppl):3793‐8. [DOI] [PubMed] [Google Scholar]
- Newling DW, Pavone Macaluse M, Smith P, Voogt HJ, Robinson MR, Schroder FH, Denis L, Jones EG, Pauw M, Sylvester R. Update of EORTC clinical trials in prostate cancer. The EORTC Genito‐Urinary Group. Semin Urol 1992;10(1):65‐71. [PubMed] [Google Scholar]
Dijkman 1997 {published data only}
- *Dijkman GA, Janknegt RA, Reijke TM, Debruyne FM. Long‐term efficacy and safety of nilutamide plus castration in advanced prostate cancer, and the significance of early prostate specific antigen normalization. International Anandron Study Group. J Urol 1997;158(1):160‐3. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Dijkman GA, Fernandez del Moral P, Debruyne FM, Janknegt RA. Improved subjective responses to orchiectomy plus nilutamide (Anandron) in comparison to orchiectomy plus placebo in metastatic prostate cancer. International Anandron Study Group. Eur Urol 1995;27(3):196‐201. [DOI] [PubMed] [Google Scholar]
- Janknegt RA. Total androgen blockade with the use of orchiectomy and nilutamide (Anandron) or placebo as treatment of metastatic prostate cancer. International Anandron Study Group. Cancer 1993;72(12 Suppl):3874‐7. [DOI] [PubMed] [Google Scholar]
- Janknegt RA, Abbou CC, Bartoletti R, Bernstein‐Hahn L, Bracken B, Brisset JM, Silva FC, Chisholm G, Crawford ED, Debruyne FM, et al. Orchiectomy and nilutamide or placebo as treatment of metastatic prostate cancer in a multinational double‐blind randomized trial. J Urol 1993;149(1):77‐82. [DOI] [PubMed] [Google Scholar]
Eisenberger 1997 {published and unpublished data}
- *Eisenberger M, Blumenstein B, Crawford ED, Miller G, McLeod D, Loehrer P, Wilding G, Sears K, Culkin DJ, Thompson IM, Bueschen AJ, Lowe BA. Bilateral orchidectomy with or without flutamide for metastatic prostate cancer. N Engl J Med 1998;339:1036‐42. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Eisenberger M, Crawford ED, McLeod D, Loehrer P, Wilding G, Blumenstein B. A comparison of bilateral orchidectomy (orch) with or without flutamide in stage D2 prostate cancer (PC) (NCI INT‐0105 SWOG/ECOG). Proc Annu Meet AM Soc Clin Oncol 1997;16:A3. [Google Scholar]
Ferrari 1993 {published data only}
- Ferrari P, Castagnetti G, Ferrari G, Pollastri CA, Tavoni F, Dotti A. Combination treatment in M1 prostate cancer. Cancer 1993;72(12 Suppl):3880‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ferrari 1996 {published data only}
- Ferrari P, Castagnetti G, Ferrari G, Baisi B, Dotti A. Combination treatment versus LHRH alone in advanced prostatic cancer. Urol Int 1996;56(Suppl 1):13‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Fourcade 1990 {published data only}
- Fourcade RO, Cariou G, Coloby P, Colombel P, Coulange C, Grise P, Mangin P, Soret JY, Poterre M. Total androgen blockade with Zoladex plus flutamide vs. Zoladex alone in advanced prostatic carcinoma: interim report of a multicenter, double‐blind, placebo‐controlled study. Eur Urol 1990;18(Suppl 3):45‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Iversen 1990 {published data only}
- *Iversen P, Christensen MG, Friis E, Hornbol P, Hvidt V, Iversen HG, Klarskov P, Krarup T, Lund F, Mogensen P, et, al. A phase III trial of zoladex and flutamide versus orchiectomy in the treatment of patients with advanced carcinoma of the prostate. Cancer 1990;66(5 Suppl):1058‐66. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Iversen P. Zoladex plus flutamide vs. orchidectomy for advanced prostatic cancer. Danish Prostatic Cancer Group (DAPROCA). Eur Urol 1990;18(Suppl 3):41‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Iversen P, Rasmussen F, Klarskov P, Christensen IJ. Long‐term results of Danish Prostatic Cancer Group trial 86. Goserelin acetate plus flutamide versus orchiectomy in advanced prostate cancer. Cancer 1993;72(12 Suppl):3851‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Knonagel 1989 {published data only}
- Knonagel H, Bolle JF, Hering F, Senn E, Hodel T, Neuenschwander H, Biedermann C. Therapy of metastatic prostatic cancer by orchiectomy plus Anandron versus orchiectomy plus placebo. Initial results of a randomized multicenter study. Helv Chir Acta 1989;56(3):343‐5. [MEDLINE: ] [PubMed] [Google Scholar]
Namer 1990 {published data only}
- *Namer M, Toubol J, Caty A, Couette JE, Douchez J, Kerbrat P, Droz JP. A randomized double‐blind study evaluating Anandron associated with orchiectomy in stage D prostate cancer. J Steroid Biochem Mol Biol 1990;37(6):909‐15. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Namer M, Amiel J, Toubal J. Anandron (RU 23908) associated with orchiectomy in stage D prostate cancer. Preliminary results of a randomized, double‐blind study. Am J Clin Oncol 1988;11(Suppl 2):S191‐6. [DOI] [PubMed] [Google Scholar]
Navratil 1987 {published data only}
- Navratil H. Double‐blind study of Anandron versus placebo in stage D2 prostate cancer patients receiving buserelin. Results on 49 cases from a multicentre study. Prog Clin Biol Res 1987;243A:401‐10. [MEDLINE: ] [PubMed] [Google Scholar]
Periti 1995 {published data only}
- Periti P, Rizzo M, Mazzei T, Mini E. Depot leuprorelin acetate alone or with nilutamide in the treatment of metastatic prostate carcinoma: interim report of a multicenter, double‐blind, placebo‐controlled study [abstract]. Can J Infect 1995;6(Suppl C):292C. [Google Scholar]
Schulze 1988 {published data only}
- Schulze H, Kaldenhoff H, Senge T. Evaluation of total versus partial androgen blockade in the treatment of advanced prostatic cancer. Urol Int 1988;43(4):193‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Tyrrell 1991 {published data only}
- *Tyrrell CJ, Altwein JE, Klippel F, Varenhorst E, Lunglmayr G, Boccardo F, Holdaway IM, Haefliger JM, Jordaan JP. A multicenter randomized trial comparing the luteinizing hormone‐releasing hormone analogue goserelin acetate alone and with flutamide in the treatment of advanced prostate cancer. The International Prostate Cancer Study Group. J Urol 1991;146(5):1321‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Jurincic CD, Horlbeck R, Klippel KF. Combined treatment (goserelin plus flutamide) versus monotherapy (goserelin alone) in advanced prostate cancer: a randomized study. Semin Oncol 1991;18(5 Suppl 6):21‐5. [MEDLINE: ] [PubMed] [Google Scholar]
- Jurincic CD, Winkler C, Horlbeck R, Gasser A, Klippel KF. The treatment of advanced prostatic carcinoma with goserelin acetate (Zoladex (r)) and goserelin acetate plus (Fugerel (r)). Dtsch Z Onkol 1992;24(3):65‐70. [Google Scholar]
- Lunglmayr G. 'Zoladex' versus 'Zoladex' plus flutamide in the treatment of advanced prostate cancer. First interim analysis of an international trial. International Prostate Cancer Study Group. Prog Clin Biol Res 1989;303:145‐51. [MEDLINE: ] [PubMed] [Google Scholar]
- Tyrrell CJ, Altwein JE, Klippel F, Varenhorst E, Lunglmayr G, Boccardo F, Holdaway IM, Haefliger JM, Jordaan JP, Sotarauta M. Multicenter randomized trial comparing Zoladex with Zoladex plus flutamide in the treatment of advanced prostate cancer. Survival update. International Prostate Cancer Study Group. Cancer 1993;72(12 Suppl):3878‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Zalcberg 1996 {published data only}
- Zalcberg JR, Raghaven D, Marshall V, Thompson PJ. Bilateral orchidectomy and flutamide versus orchidectomy alone in newly diagnosed paptients with metastatic carcinoma of the prostate‐an Australian multicentre trial. Br J Urol 1996;77(6):865‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
de Voogt 1990 {published data only}
- Voogt HJ, Klijn JG, Studer U, Schroder F, Sylvester R, Pauw M. Orchidectomy versus Buserelin in combination with cyproterone acetate, for 2 weeks or continuously, in the treatment of metastatic prostatic cancer. Preliminary results of EORTC‐trial 30843. J Steroid Biochem Mol Biol 1990;37(6):965‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Di Silverio 1990 {published data only}
- Silverio F, Serio M. [Zoladex in prostatic carcinoma]. Drugs Exp Clin Res 1990;16(Suppl):19‐29. [MEDLINE: ] [PubMed] [Google Scholar]
- Silverio F, Serio M, D'Eramo G, Sciarra F. Zoladex vs. Zoladex plus cyproterone acetate in the treatment of advanced prostatic cancer: a multicenter Italian study. Eur Urol 1990;18(Suppl 3):54‐61. [DOI] [PubMed] [Google Scholar]
Jorgensen 1993 {published data only}
- Jorgensen T, Tveter KJ, Jorgensen LH. Total androgen suppression: experience from the Scandinavian Prostatic Cancer Group Study No. 2. Eur Urol 1993;24(4):466‐70. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Klosterhalfen 1987 {published data only}
- Klosterhalfen H, Becker H. Results of a 10‐year randomised prospective study in metastasised prostate cancer. Aktuel Urol 1987;18(5):234‐6. [Google Scholar]
Robinson 1995 {published data only}
- Newling DW, Pavone Macaluse M, Smith P, Voogt HJ, Robinson MR, Schroder FH, Denis L, Jones WG, Pauw M, Sylvester R. Update of EORTC clinical trials in prostate cancer. The EORTC Genito‐Urinary Group. Semin Urol 1992;10(1). [PubMed] [Google Scholar]
- Robinson MR. A further analysis of European Organization for Research and Treatment of Cancer protocol 30805. Orchidectomy versus orchidectomy plus cyproterone acetate versus low‐dose diethylstilbestrol. Cancer 1993;72(12 Suppl). [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Robinson MR. Complete androgen blockade: the EORTC experience comparing orchidectomy versus orchidectomy plus cyproterone acetate versus low‐dose stilboestrol in the treatment of metastatic carcinoma of the prostate. Prog Clin Biol Res 1987;243A:383‐90. [PubMed] [Google Scholar]
- Robinson MR. EORTC protocol 30805: a phase III trial comparing orchidectomy versus orchidectomy and cyproterone acetate and low dose stilboestrol in the management of metastatic carcinoma of the prostate. Prog Clin Biol Res 1988;260:101‐10. [PubMed] [Google Scholar]
- Robinson MR, Smith PH, Richards B, Newling DW, Pauw M, Sylvester R. The final analysis of the EORTC Genito‐Urinary Tract Cancer Co‐Operative Group phase III clinical trial (protocol 30805) comparing orchidectomy, orchidectomy plus cyproterone acetate and low dose stilboestrol in the management of metastatic carcinoma of the prostate. Eur Urol 1995;28(4):273‐83. [PubMed] [Google Scholar]
Thorpe 1996 {published data only}
- Thorpe SC, Azmatullah S, Fellows GJ, Gingell JC, O'Boyle PJ. A prospective, randomised study to compare goserelin acetate (Zoladex) versus cyproterone acetate (Cyprostat) versus a combination of the two in the treatment of metastatic prostatic carcinoma. Eur Urol 1996;29(1):47‐54. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Williams 1990 {published data only}
- Williams G, Asopa R, Abel PD, Smith C. Pituitary adrenal and gonadal endocrine suppression for the primary treatment of prostate cancer. Br J Urol 1990;65(5):504‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Additional references
Bennett 1999
- Bennett CL, Tosteson T, Weinberg P, et al. Maximum androgen blockade with flutamide in conjunction with surgical or medical castration therapy for metastatic prostate cancer: a literature‐based meta‐analysis of 9 randomized trials and 4, 128 patients. Prostate cancer and prostatic diseases. Prostate Cancer Prostatic Dis 1999;2:4‐8. [DOI] [PubMed] [Google Scholar]
Conn 1991
- Conn PM, Crowley WF Jr. Gonadotropin‐releasing hormone and its analogue. N Engl J Med 1991;324:93‐103. [DOI] [PubMed] [Google Scholar]
Crawford 1989
- Crawford ED, Eisenberger MA, McLeod DG, Spaulding JT, Benson R, et al. A controlled trial of leuprolide with and without flutamide in prostatic carcinoma. N Engl J Med 1989;321(7):419‐24. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Denis 1993a
- Denis LJ, Carnelro de Moura JL, Bono A, Sylvester R, Whelan P, Newling D, Depauw M. Goserelin acetate and flutamide versus orchiectomy: a phase III EORTC trial (30853). Urology 1993;42(2):119‐30. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Denis 1993b
- Denis L, Murphy GP. Overview of phase III trials on combined androgen treatment in patients with metastatic prostate cancer. Cancer 1993;72(12 Suppl):3888‐95. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hasselblad 1998
- Hasselblad V. Meta‐analysis of multitreatment studies. Med Decision Making 1998;18(1):37‐43. [DOI] [PubMed] [Google Scholar]
Landis 1999
- Landis SH, Murray T, Bolden S, Wingo PA. Cancer Statistics, 1999. A Cancer Journal for Clinicians 1999;49:8‐11. [DOI] [PubMed] [Google Scholar]
Moinpour 1998
- Moinpour CM, Savage M, Troxel A, Lovato L, et al. Quality of life in advanced prostate cancer: results of a randomized therapeutic trial. J Natl Cancer Inst 1998;90:1537‐44. [DOI] [PubMed] [Google Scholar]
PCTOG 1995
- Prostate Cancer Trialists' Overview Group. Maximum androgen blockade in advanced prostate cancer: An overview of 22 randomized trials with 3283 deaths in 5710 patients. Lancet 1995;346:265‐69. [MEDLINE: ] [PubMed] [Google Scholar]
References to other published versions of this review
Aronson 1999
- Aronson N, Seidenfeld J, Samson DJ, et al. Relative Effectiveness and Cost‐Effectiveness of Methods of Androgen Suppression in the Treatment of Advanced Prostate Cancer. Evidence Report/Technology Assessment No. 4. (Prepared by Blue Cross/Blue Shield Association Evidence‐based Practice Center under Contract No. 290‐97‐0015). AHCPR Publication No. 99‐E0012. Rockville, MD: Agency for Health Care Policy and Research, May 1999. [PMC free article] [PubMed] [Google Scholar]


