Abstract
The pasABC genes of the proteic plasmid addiction system of broad-host-range plasmid pTF-FC2 were autoregulated. The PasA antidote was able to repress the operon 25-fold on its own, and repression was increased to 100-fold when the PasB toxin was also present. Autoregulation appears to be an essential requirement for pas-mediated plasmid stabilization because when the pas genes were placed behind the isopropyl-β-d-thiogalactopyranoside (IPTG)-regulated tac promoter, they were unable to stabilize a heterologous test plasmid.
Plasmid pTF-FC2 is a 12.2-kb, mobilizable, broad-host-range plasmid that was originally isolated from the biomining bacterium Thiobacillus ferrooxidans (9). The plasmid contains a proteic poison-antidote plasmid addiction system (pas) located between the repB and repA genes (Fig. 1) of its IncQ-like replicon (4). This stability system is unusual in that it consists of three genes rather than the two-gene systems identified in other plasmids (7). The pasA gene encodes an antidote, pasB encodes a toxin, and pasC encodes a protein that appears to enhance the neutralizing effect of the antidote (12). Autoregulation is a general property of proteic stabilization systems in which regulation has been studied. For example, the ccd system of plasmid F is autoregulated by a 69-kDa complex of CcdA and CcdB (14), and neither CcdA nor CcdB alone is capable of autorepression. In contrast, the parDE system of plasmid RK2 is autoregulated solely by the ParD antidote protein (10). The parD locus of plasmid R1 is repressed only 30 to 40% by Kis on its own, and the complete Kis-Kid complex is required for maximal repression (11).
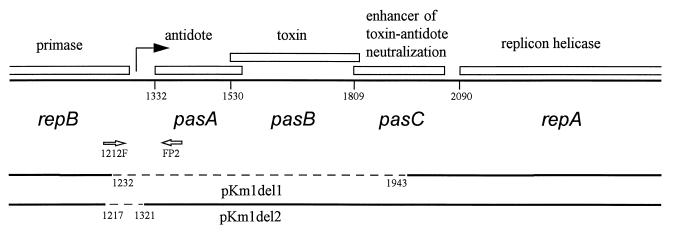
FIG. 1.
Layout of the pTF-FC2 pas showing its location within the plasmid replicon. Numbers below the thick line indicate the positions of the genes relative to that of the ClaI site of pTF-FC2 (5). The positions of the PCR primers used to amplify the pas promoter region and the regions missing from the spontaneous deletions following PasA antidote inactivation (12) (broken lines) are shown below the layout.
We investigated whether the pas of pTF-FC2 is autoregulated and whether the third component of the pas, PasC, plays a role in regulation. Furthermore, we investigated whether autoregulation is a necessary requirement for pas stabilization. It is conceivable that differences in the half-lives of the antidote and toxin proteins together with differences in the levels of toxin and antidote translation may by themselves be sufficient to increase plasmid stability. The bacteria, plasmids, and constructs we used in this study are given in Table 1.
TABLE 1.
Bacteria, plasmid vectors, and pas-containing constructs used in this study
| Strain, plasmid, or construct | Genotype or descriptiona | Reference |
|---|---|---|
| E. coli strains | ||
| JM105 | thi rpsL endA sbcB15 hspR4 Δ(lac-proAB) [F′ traD36 proAB lacIqΔM15] | 15 |
| CSH50-Iq | rpsL Δ(lac-pro) [F′ traD36 proAB lacIqΔM15] | 13 |
| Plasmids | ||
| pMC1403 | ColE1 replicon, AprlacZYA* | 2 |
| pACYC184 | p15a replicon, Cmr Tcr | 3 |
| pKK223-3 | ColE1 replicon, Aprtac | 1 |
| pOU82 | R1 replicon, AprlacZYA | 6 |
| Constructs containing pas genes | ||
| pP2H | pMC1403 vector, Apr, pas region 1240–1362b | This work |
| pTac-pasA-pACYC | pACYC184 vector, Cmr, pas region 1316–1559 | This work |
| pTac-pasB-pACYC | pACYC184 vector, Cmr, pas region 1518–1816 | This work |
| pTac-pasC-pACYC | pACYC184 vector, Cmr, pas region 1789–2028 | This work |
| pTac-pasAB-pACYC | pACYC184 vector, Cmr, pas region 1316–1816 | This work |
| pTac-pasABC-pACYC | pACYC184 vector, Cmr, pas region 1316–2028 | This work |
| pKmM0 | pTF-FC2 replicon, Kmr, 1–4910c | 12 |
| pKmM1 | pTF-FC2 replicon, Kmr, 1–4911 pasA | 12 |
| pKmM2 | pTF-FC2 replicon, Kmr, 1–4911 pasB | 12 |
| pKmM3 | pTF-FC2 replicon, Kmr, 1–4911 pasC | 12 |
| pKmM1del1d | pTF-FC2 replicon, Kmr, 1–4911, Δ1217–1321e | 12 |
| pKmM1del2d | pTF-FC2 replicon, Kmr, 1–4911, Δ1232–1943e | 12 |
| pOU-pasABC | pOU82 R1 replicon, Apr, pas region 1158–2027 | 12 |
| pOU-tac-pasABC | pOU82 R1 replicon, Apr, tac, pas region 1158–2027 | This work |
Apr, ampicillin resistance; Kmr, kanamycin resistance; Cmr, chloramphenicol resistance; Tcr, tetracycline resistance; lacZYA*, lacZYA genes with deletion of lacZ promoter and ATG; tac, trp-lac hybrid promoter.
The pas region numbers are the nucleotide positions relative to those of the ClaI-PstI fragment of pTV100 (5).
Entire pTF-FC2 replicon from the ClaI site to the PstI site of pTV100 including the pas region (5).
Spontaneous deletions of the toxic plasmid pKmM1 (12).
Regions missing from the spontaneous deletions after PasA antidote inactivation (12).
Autoregulation of pasABC.
Regulation of the pasABC genes was investigated by the construction of an in-frame translational fusion of pasA to a lacZ reporter gene. A translational fusion would indicate the cumulative effect of transcriptional and translational regulation. An in-frame translational fusion of pasA to a lacZ reporter gene was constructed by cloning a PCR amplification product which extended for 124 bp upstream of the pasA start into the vector pMC1403. The primers 1212F (5′-CGCCAGGGTTTTCCCAGTCACGAC-3′) and FP2 (5′-AGTAGGGATCCACTTCGGCGGGCAGTCGG-3′) (shown in Fig. 1), were used to amplify the pasA promoter from pTV400 (4). The PCR was carried out by using DynazymeII (Finnenzymes Oy) in a JDI2500 thermocycler (denaturation step of 2 min at 95°C and then 30 cycles, with 1 cycle consisting of 30 s at 95°C, 30 s at 52°C, and 60 s at 72°C). Primer FP2 introduced a BamHI site which allowed in-frame cloning of the fragment into vector pMC1403 to create construct pP2H. DNA sequencing with a Pharmacia ALF express automated DNA sequencer was used to confirm the integrity of the construct. β-Galactosidase assays were performed by the method of Miller (8) on log-phase cultures grown with the appropriate antibiotic selection. The pas-lacZ fusion, pP2H, when placed in Escherichia coli CSH50-Iq gave moderate levels of β-galactosidase activity (252 Miller units) (Table 2). When plasmid pKmM0, which has the PasABC system situated within its natural, broad-host-range, pTF-FC2 replicon, was placed in trans to pP2H, expression of β-galactosidase activity was reduced to 12 Miller units. To identify the repressor of gene expression, β-galactosidase activity was measured in strains in which pKmM0 was replaced by the pKmM0-based pas mutant plasmids pKmM1 (pasA), pKmM2 (pasB), and pKmM3 (pasC) (12). However, the pKmM1 pasA mutant was lethal to Escherichia coli CSH50-Iq, and inactivation of pasB or pasC relieved the repression of lacZ reporter gene expression to a small extent (from 12 to 31 and 14 Miller units, respectively). When two spontaneous pKmM0 pas deletion mutants in which the pasA promoter region (pKmM1del1) or most of pasABC (pKmM1del2) had been deleted (Fig. 1) (12) were placed in trans to pP2H, reporter gene expression was restored to near unrepressed levels. This indicated that PasA was the primary repressor.
TABLE 2.
Regulation of the pasABC genes in E. coli CSH50-Iq containing the pas-lacZ reporter construct, pP2H
| Coresident plasmid | Avg β-galactosidase activitya (Miller units) ± SD | % Activity |
|---|---|---|
| pACYC184 (control) | 252 ± 6 | 100 |
| pKmM0 | 12 ± 1 | 5 |
| pKmM2 | 31 ± 4 | 12 |
| pKmM3 | 14 ± 1 | 6 |
| pKmM1del1 | 185 ± 70 | 73 |
| pKmM1del2 | 206 ± 9 | 82 |
| pTac-pasA-pACYC | 10 ± 1 | 4 |
| pTac-pasB-pACYC | NAb | NA |
| pTac-pasC-pACYC | 226 ± 37 | 90 |
| pTac-pasAB-pACYC | 2 ± 1 | 1 |
| pTac-pasABC-pACYC | 3 ± 2 | 1 |
β-Galactosidase activity was measured three times on each of three independently selected colonies.
No assay possible because of the lethality of the PasB toxin in CSH50-Iq.
To confirm regulation by the pas gene products, constructs of each of the pas genes cloned individually and in combination behind the non-pas-regulated tac promoter of vector pKK223-3 were used. Since both the pMC1403 reporter gene vector and pKK223-3 use ColE1 origins of replication and both are ampicillin resistant, the tac-regulated pas genes were subcloned into the pACYC184 vector. tac-pas fusions were excised from their respective pKK223-3 constructs as PvuI (blunted)-BamHI fragments and cloned into pACYC184 which had been cut with BamHI and ClaI (blunted). These constructs, pTac-pasA-pACYC, pTac-pasB-pACYC, pTac-pasC-pACYC, pTac-pasAB-pACYC, and pTac-pasABC-pACYC were transformed into E. coli CSH50-Iq cells containing pP2H. The level of β-galactosidase expression decreased from 252 to 10 Miller units when only pasA was provided in trans (Table 2). This repression was enhanced when pasAB (2 Miller units) or pasABC (3 Miller units) was present. The pasABC promoter therefore appears to be autorepressed (25-fold) by PasA, and this repression increased when both PasA and PasB were present (100-fold). Regulation by pasB or pasBC could not be tested due to the lethal effects of PasB in the absence of PasA. PasC alone had little effect on the expression of the pasA promoter.
Levels of reporter gene activity varied substantially between strains and experiments. Only the data for E. coli CSH50-Iq, in which reporter gene activity was less variable than in some of the other strains, are presented. Similar experiments with E. coli JM105 showed the same trends, although these data were unreliable because of the greater variability in reporter gene expression (data not shown). PasA was clearly the primary repressor, and this negative regulation was enhanced in the presence of PasB. The pTF-FC2 pas is therefore similar to the parD locus of R1 in that parD is only partially repressed by Kis (30 to 40%) and the complete Kis-Kid complex is required for maximal repression (11).
Effect of expression from a heterologous promoter on pas function.
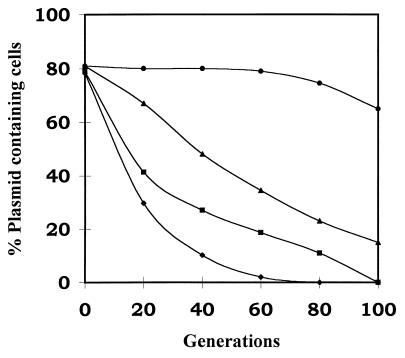
To investigate whether autoregulation is important for the proper functioning of the pas proteic plasmid stability system, we examined the stability of the pOU82 test plasmid containing the pasABC genes under the control of a tac promoter (pOU-tac-pasABC) in E. coli CSH50-Iq. The tac-pas fusions in pOU82 were constructed by excising the pasABC genes linked to the tac promoter from the construct pTac-pasABC on a BamHI-PvuI (blunted) fragment and ligating them into pOU82 which had been cut with EcoRI (blunted) and BamHI. Stability of the pOU-tac-pasABC construct was tested in the presence and absence of pas induction by 2 mM isopropyl-β-d-thiogalactopyranoside (IPTG) (Fig. 2). This was compared with the stability of the pOU82 control and pOU82-pasABC containing the pas genes under the control of the natural promoter. The pOU82-tac-pasABC construct was less stable than the pOU82 control even without induction of the pas genes from the tac promoter. On IPTG induction of the pas genes, the pOU82-tac-pasABC construct was even less stable than without induction. Lower levels of IPTG (0.5 and 1.0 mM) were also used, but the result was similar to that for 2 mM (data not shown). Autoregulatory feedback by PasA-PasB would therefore appear to be an essential feature of the proteic poison-antidote pas for it to stabilize a heterologous test plasmid in E. coli.
FIG. 2.
Stability of plasmids in E. coli CSH50-Iq host cells. Cells containing pOU82 (▴), pOU82-pasABC (•), pOU-tac-pasABC without IPTG induction (■), or pOU-tac-pasABC with 2 mM IPTG (⧫) are shown.
Acknowledgments
We are most grateful to Kenn Gerdes for the gift of plasmid pOU82.
This work was supported by grants from Gencor (now Billiton) Process Research (Randburg, South Africa), the Foundation for Research Development (Pretoria, South Africa) and the THRIP programme of the Department of Trade and Industry.
REFERENCES
- 1.Brosius J, Holy A. Regulation of ribosomal RNA promoters with a synthetic lac operator. Proc Natl Acad Sci USA. 1984;81:6929–6933. doi: 10.1073/pnas.81.22.6929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Casadaban M J, Chou J, Cohen S N. In vitro gene fusions that join an enzymatically active β-galactosidase segment to amino-terminal fragments of exogenous proteins: Escherichia coli plasmid vectors for the detection and cloning of translational initiation signals. J Bacteriol. 1980;142:971–980. doi: 10.1128/jb.143.2.971-980.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chang A C Y, Cohen S N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the p15A cryptic miniplasmid. J Bacteriol. 1978;134:1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dorrington R A, Rawlings D E. Characterization of the minimum replicon of the broad-host-range plasmid pTF-FC2 and similarity between pTF-FC2 and the IncQ plasmids. J Bacteriol. 1990;172:5697–5705. doi: 10.1128/jb.172.10.5697-5705.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dorrington R A, Bardien S, Rawlings D E. The broad-host-range plasmid pTF-FC2 requires a primase-like protein for autonomous replication in Escherichia coli. Gene. 1991;108:7–14. doi: 10.1016/0378-1119(91)90481-p. [DOI] [PubMed] [Google Scholar]
- 6.Gerdes K, Larsen J E L, Molin S. Stable inheritance of plasmid R1 requires two different loci. J Bacteriol. 1985;161:292–298. doi: 10.1128/jb.161.1.292-298.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jensen R B, Gerdes K. Programmed cell death in bacteria: proteic plasmid stabilization systems. Mol Microbiol. 1995;17:205–210. doi: 10.1111/j.1365-2958.1995.mmi_17020205.x. [DOI] [PubMed] [Google Scholar]
- 8.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1983. [Google Scholar]
- 9.Rawlings D E, Kusano T. Molecular genetics of Thiobacillus ferrooxidans. Microbiol Rev. 1994;58:39–55. doi: 10.1128/mr.58.1.39-55.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roberts R C, Spangler C, Helinski D R. Characteristics and significance of the DNA binding activity of plasmid stabilization system protein ParD from the broad-host-range plasmid RK2. J Biol Chem. 1993;268:27109–27117. [PubMed] [Google Scholar]
- 11.Ruiz-Echevarrìa M J, Berzal-Herranz A, Gerdes K, Díaz-Orejas R. The kis and kid genes of the parD maintenance system of plasmid R1 form an operon that is autoregulated at the level of transcription by the co-ordinated action of the Kis and Kid proteins. Mol Microbiol. 1991;5:2685–2693. doi: 10.1111/j.1365-2958.1991.tb01977.x. [DOI] [PubMed] [Google Scholar]
- 12.Smith A S G, Rawlings D E. The poison antidote stability system of the broad-host range Thiobacillus ferrooxidans plasmid pTF-FC2. Mol Microbiol. 1997;26:261–270. doi: 10.1046/j.1365-2958.1997.6332000.x. [DOI] [PubMed] [Google Scholar]
- 13.Smith A S G, Rawlings D E. Efficiency of the pTF-FC2 pas poison-antidote stability system in Escherichia coli is affected by the host strain, and antidote degradation requires the Lon protease. J Bacteriol. 1998;180:187–98. doi: 10.1128/jb.180.20.5458-5462.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tam J E, Kline B C. The F plasmid ccd autorepressor is a complex of CcdA and CcdB proteins. Mol Gen Genet. 1989;219:26–32. doi: 10.1007/BF00261153. [DOI] [PubMed] [Google Scholar]
- 15.Yannisch-Perron C, Vieira J, Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequence of the M13mp18 and pUC19 vectors. Gene. 1985;33:103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]