Abstract
In the era of cancer immunotherapy, there is a high interest in combining conventional cancer therapies such as radiotherapy with drugs that stimulate the immune system. The observation that ionizing radiation applied to mouse tumors could delay the growth of distant lesions (“abscopal effect”) and this was potentiated by immunostimulatory drugs, led to clinical trials in which often only one lesion was irradiated. The results of these first clinical trials combining radio and immunotherapy are now becoming available. These results show that, while immunotherapy potentiates the local effects of radiotherapy, the abscopal effect is still infrequent. Transcriptomic analysis of resected colorectal cancer (CRC) metastases allows to distinguish three molecular subtypes with distinct potential to benefit from localized therapies and/or immunotherapy; the subtype with characteristics consistent with the existence of preexisting immunity is the most likely to respond to radiation. Recent preclinical data suggests these preexistent T cells can survive radiation and contribute to its therapeutic effect. In this review, we discuss possible reasons for the preclinical/clinical discrepancies regarding the abscopal effect, and we propose irradiation of multiple or all tumors combined with systemic immunotherapy, patient selection based on tumor subtype, and rational therapeutic combinations that take into account preexisting immunity, as possible avenues to increase the efficacy of radio-immunotherapy.
Introduction
The discovery of the important role of the immune system in the therapeutic effect of ionizing radiation (IR) and the development of cancer immunotherapy has led to increased interest in combining the two modalities. The first clinical trials of radio-immunotherapy were initiated based on encouraging case reports and preclinical results but lacked a robust framework establishing the optimal radiotherapy dose, fractionation rate, target selection, and timing. Nonetheless, many clinical trials testing this combination are currently ongoing. Accumulating data will guide future trial design in order to optimize the therapeutic ratio of radio-immunotherapy in the treatment of metastatic cancer. Concomitantly, the correlation of patient outcomes with peripheral blood analyses and tissue biopsies from these studies and further preclinical investigation may enable new mechanistic insights regarding the interaction between IR and cancer immunity that could potentially have far-reaching clinical applications. Here, we review some clinical and preclinical data that are re-shaping our understanding of the interaction between radiotherapy and immunotherapy.
Some lessons from the clinic
1. Local therapy improves cancer-specific outcomes in oligometastatic disease and this benefit may be potentiated by systemic immunotherapy
In 1995, Hellman and Weichselbaum proposed the oligometastatic state as an intermediate phenotype between locoregionally confined malignancy and widespread metastatic disease, largely characterized by clinical features, including a numerically limited number of lesions and a slow pace of progression (1). The implication of this hypothesis involves the possibility of significantly benefitting and potentially curing a subset of metastatic patients with localized therapies (e.g., surgery or radiotherapy). In several recent phase II clinical trials, patients with oligometastatic disease received standard of care treatment including surveillance with or without metastasis-directed ablative radiotherapy to all visible sites of disease (2–5). While the primary site, number of lesions treated, and radiation dose and fractionation differed across these studies, they all showed an improvement in meaningful endpoints, including but not limited to progression-free survival (PFS) and overall survival (OS).
Expanding these results to the setting of radio-immunotherapy, two recently published prospective studies in metastatic non-small cell lung cancer (NSCLC) treated patients with Pembrolizumab with or without locally ablative therapy including stereotactic body radiotherapy (SBRT) (6, 7). The first, which treated only one metastatic site with SBRT, did not significantly improve PFS or OS, though there was a trend towards improved response rates. The second, which treated all metastatic sites as in the above oligometastatic studies, did demonstrate a significant increase in PFS of approximately 12 months as compared with historic controls; of note, this improvement was numerically greater than those seen in the studies investigating locally ablative therapy alone. We will discuss the implications of these differing conclusions within the context of our abscopal and preclinical discussions below.
2. Immunotherapy works best when tumor burden is smallest
It is becoming increasingly clear that cancer immunotherapy is more effective when treating patients with limited disease burden. Analysis of the KEYNOTE-001 trial, in which patients with advanced melanoma were treated with Pembrolizumab, revealed that the baseline sum of lesion(s) size below the median (10.2 cm) was independently associated with OS (8), and most complete responses occurred in patients with tumors that were smaller yet (< 5 cm) (9). Translational data provides a mechanistic underpinning for these findings: in stage IV melanoma patients treated with Pembrolizumab, it is the ratio of T-cell reinvigoration (fold change in %PD1+ki67+ CD8+ T cells post vs. pre-treatment) to baseline tumor burden that best predicts clinical outcomes, rather than either factor alone (10).
The smallest tumor burden is one that cannot be detected radiographically. Indeed, patients treated with immunotherapy in the adjuvant setting for malignancies with a high metastatic propensity often see the greatest clinical benefits. In stage III-IV completely resected melanoma, adjuvant treatment with anti-PD1 agents markedly improved recurrence-free survival in several studies (11, 12). Similarly, patients with locally advanced NSCLC treated with definitive chemoradiation experienced significantly improved PFS and OS with the addition of adjuvant Durvalumab versus placebo in the PACIFIC trial (13).
Studies other than the PACIFIC trial in the setting of combined immunotherapy and radiotherapy support the assertion that less disease (fewer lesions) leads to better outcomes. In a trial in which patients with metastatic solid tumors were treated with radiotherapy to one metastatic site concurrently with daily injections of GMCSF, systemic responses were observed only in patients with less than six lesions (14). In another trial of metastatic prostate cancer patients treated with one 8 Gy fraction to a single bone lesion with or without systemic ipilimumab, patients were more likely to benefit from the addition of ipilimumab if they had one compared to two or more bone metastases (15).
3. The abscopal effect is rare
The abscopal effect (from the Latin ab- for “away from” and scopus for “target”) is the regression of unirradiated tumor lesions in a patient treated with radiotherapy and was first described in 1953 (16). In the intervening decades, the abscopal effect had largely been viewed by radiation oncologists as an interesting but exceedingly unusual phenomenon with little hope for application in the clinic. In the era of cancer immunotherapy, a new appreciation of the role played by the immune system in governing the therapeutic effect of ionizing radiation has caused a major spike in interest in this phenomenon. Underlying this enthusiasm is the hope that immunotherapy would amplify the rare systemic effects of radiotherapy, while radiotherapy would serve as an “in situ vaccine” (17), rendering immune-excluded tumors suddenly responsive to immunotherapy. Indeed, a systematic analysis of the literature on the abscopal effect (18) found 46 case reports described in 31 studies that spanned 50 years (1964–2014). Of those 31 studies, 17 (55%) were published in the last nine years, with several of these case reports having an outsized impact on the field (19, 20).
It is currently unclear, however, that the addition of radiotherapy, at least when treating only one or a few sites with abscopal intent, significantly adds to the systemic efficacy of immunotherapy. Most available data has been presented in the form of the aforementioned case reports or single-arm, early phase clinical trials (21, 22) testing different radioimmunotherapy combinations with conflicting results. In the abscopal study by Theelen and colleagues (6), the addition of single-site SBRT to pembrolizumab in metastatic NSCLC demonstrated a trend towards improved response rates but did not meet its other efficacy endpoints; however, the PD-L1-negative patient subgroup did derive significant benefit from SBRT. Similarly, in the study by Kwon and colleagues in metastatic prostate cancer (15), there was no difference in OS between those treated with single-site radiotherapy plus ipilimumab versus those who received single-site radiotherapy alone. Again, a small subgroup of patients—in this case those with small tumor burden as outlined above—did demonstrate a benefit.
Contrast these results with the radioimmunotherapy trials using radiotherapy to treat all sites of known disease, e.g., eschewing the abscopal approach for a cytoreductive one. The PACIFIC trial in locally advanced NSCLC demonstrated a previously unimaginable 12-month prolongation in time to distant metastasis and death (13), while the study by Bauml and colleagues in oligometastatic NSCLC demonstrated a 12-month improvement in PFS over historical controls (7).
Taken together, the clinical observations to date strongly suggest that the most effective way to apply radioimmunotherapy is to treat as many sites with local therapy as possible (23), with the goal of increasing the potentially synergistic local and systemic effects of both modalities. Whereas immunotherapy can increase the local effect of radiotherapy in all treated sites as will be discussed below, the radiotherapeutically debulked tumor burden would allow immunotherapy to better eliminate micro-metastatic disease.
Preclinical evidence for the abscopal effect
Although the abscopal effect was defined in 1953, it remained largely limited to case reports for most of the following five decades. In 1999, Chakravarty et al. reported that in a metastatic mouse lung cancer model, co-administration of high-dose local IR (60 Gy) in the primary tumor and daily injections of Flt3L to expand dendritic cells in vivo dramatically increased survival due to a reduction in the number of spontaneous lung metastases (24). Also in 1999, the Weichselbaum and Schreiber labs showed that intratumoral injection of an adenoviral vector with interleukin-12 enhanced local anti-tumor effects of irradiation and suppressed microscopic tumor growth at a distant site (25). Demaria et al. expanded the early observations with IR+Flt3L to breast cancer models (26) and also pioneered the use of immune checkpoint inhibitors (specifically, anti-CTLA-4) in combination with IR (27). These early studies showed that the addition of immunotherapy was critical for increasing the abscopal effect of IR, and that host T cells were required for this effect. Also more effective local tumor control, achieved by 2 (compared with 1) consecutive doses of 12 Gy in combination with anti-CTLA4 translated into higher survival rates (27). A widely cited preclinical study by Dewan et al. in 2009 reported that fractionated IR, specifically 3 doses of 8 Gy each, was more efficacious in controlling abscopal tumors when combined with anti-CTLA-4 therapy than single high dose of 20 Gy in 2-flank TSA mammary and MCA38 colon carcinoma models (28). However, this might not be applicable to all tumor types and immunotherapies combined with IR. Doses as little as 2 Gy (26) and as large as 60 Gy (24) effectively mediated abscopal responses in 67NR mammary carcinoma and LLC lung cancer models, respectively, and a single 12 Gy dose in combination with anti-PD-L1 blockade was sufficient to cause an abscopal response in the TUBO mammary carcinoma model (29). PD-1/PD-L1 blockade combined with IR showed good results in part due to the IR-induced up-regulation of PD-L1 in the tumor microenvironment (29, 30). In recent years, attempts to potentiate the abscopal response have focused on the combination of IR with multiple therapies simultaneously. These therapies include T cell checkpoint blockade and costimulatory antibodies, e.g., anti-41BB (31) or anti-CD40 (32), TGFβ blockade (33, 34), chemotherapy (35), and immunotherapeutics that modify macrophage function (36). In this context, the combination of IR with approaches that allow for localized delivery of immunotherapeutic drugs (37, 38) are of special interest, since they might promote efficacy while minimizing adverse effects of the multiple drugs used. As an example, a clever study by Schrand et al. used IR to induce intratumoral VEGF expression, which caused accumulation of VEGF-41BB aptamer conjugates in the tumor, leading to increased antitumor efficacy and decreased toxicity (38).
Why the abscopal effect in patients is less frequent than expected from preclinical research
As discussed above, preclinical investigation led to high expectations to induce systemic anti-tumor effects through the combination of immunotherapy and single site-directed radiotherapy. However, clinically available data so far shows somewhat disappointing results when using this approach. One obvious reason behind these different outcomes is the limited ability of the mouse models used to faithfully recapitulate metastatic cancer in patients. Preclinical studies of the abscopal effect used most frequently “two-flank” mouse tumor models (26, 28, 29, 31, 32, 35) in which the same transplantable cell line is injected s.c in two distant locations in the mouse, and only one tumor is irradiated; the abscopal effects are followed in the untreated lesion. In this model, the genetic and environmental factors defining these “abscopal” tumors are almost identical to those of the “primary” tumor, and therefore, immune responses directed to antigens present in one of the tumors can sometimes also recognize the abscopal tumor. Some transplantable cell lines exist that give rise to spontaneously metastatic tumors (e.g., used in (24); however, it is likely that even these relatively quickly established metastases (6 weeks) fail to recapitulate the complexity of human metastatic cancer. Two recent studies using whole-exome sequencing and immunohistochemistry-derived data highlight the existence of high inter-metastases heterogeneity within the same patient (39, 40), to the point that each metastasis can be approached as a different disease based on different mutational and clonal composition and potential for dissemination (40). Indeed, this study of 31 metastases collected over 11 years from two cases of stage IV CRC found that most (76%) coding mutations were unique to 1 metastasis, and only a few mutations were shared by all metastases in one of the patients, while the other patient had no mutations that were shared among all lesions. Not surprisingly, TCR diversity (alpha and beta V-J recombinations) was also high among metastases of the same patient. In each patient, a single synchronous metastasis expanded the metastatic lineage, proving a different dissemination potential. Interestingly, low recurrence risk among individual tumor clones was associated with high immunoscore (reflective of CD8+ T cell density), and low tumor burden, confirming the above discussed conclusions from both clinical cohorts and at the individual patient (inter-metastasis) level, that immunotherapy works best with limited tumor burden. Another important observation from these studies is that mutational load by itself does not determine T-cell reactivity against tumors. Indeed, immunoscore was not associated with mutational load in the two CRC patients study (40). In another study where metastatic lesions from a high-grade serous ovarian cancer patient that were regressing after chemotherapy were compared with other lesions from the same patient that were progressing (39), it was found that regressor vs. progressor behavior was not explained by the presence of specific mutations or neoepitopes. However, regressor lesions showed higher TCR clonotype diversity and expansion, implicating the individual metastasis microenvironments as possible determinants of the generation of effective T-cell responses. In this regard, it is interesting that irradiation of liver metastases resulted in higher T-cell activation (observed in the peripheral blood) when compared with irradiation of lung metastases in a study using SBRT and ipilimumab (41). The patients studied in the Jimenez-Sanchez and Angelova et al. reports had an exceptionally long survival; this could raise questions about the universality of the findings discussed, in that perhaps the unusual duration of the disease allowed for more heterogeneity to develop, and therefore these three patients could represent an extreme of the spectrum. Nevertheless, mixed responses (simultaneous growth and shrinkage of metastatic lesions) within the same patient are relatively common, accounting for 8.6–33.8% of patients with CRC (42, 43), and are associated with significantly worse OS (42). Overall, the existence of mixed responses and in general inter-metastasis tumor heterogeneity could account for the low rates of abscopal responses observed in the clinic.
How to make radio-immunotherapy combinations more effective
Lessons learned from the clinical trials performed to date, and preclinical investigations into the interactions among IR, the tumor microenvironment, and intratumoral T cells should guide the design of more effective radio-immunotherapy combinations. Here we propose some specific ways this could be achieved:
1. Irradiate all sites
Based on the observations discussed above and other evidence, we have recently proposed that potential synergies between immunotherapy and radiotherapy require treatment of all or most sites of metastatic disease (44). This conclusion is shared by others in the field (23) and is based on recent technical advances that allow for delivery of high doses of IR with high precision and lower toxicities. Also, this approach is supported by observations from a exploratory subset analysis in our recent clinical trial combining Pembrolizumab and SBRT indicating that tumors that had to be partially irradiated (due to technical constraints) showed similar tumor control compared with those that were completely irradiated. This suggests that, even in cases where irradiation to all sites might not be possible, partial irradiation of all lesions could potentially suffice. One argument against this approach is that T cells might be radiosensitive and radiotherapy in this context might be immunosuppressive, which we discuss below.
2. Apply knowledge on the biology of tumors
The Pitroda and Weichselbaum labs recently performed an integrated transcriptomic analysis (mRNA+miRNA) of CRC liver metastases after resection (45), with the goal of distinguishing different molecular subtypes of CRC metastases and their relation to clinical outcomes. We found that molecular features associated with distinct clinical outcomes in primary CRC failed to do so in patients with resected liver metastases. Integrated transcriptomic analysis, however, defined 3 molecular subtypes of CRC metastases associated with different overall survival rates. Subtype 1, named “canonical” for its increased expression of cell proliferation and altered cell cycle and DNA repair pathways, was present in 33% of the samples and associated with poor survival. This subtype showed low expression of immune markers. In contrast, subtype 2, or “immune” (28%), had a high expression of T-cell activation, antigen presentation and IFN signaling genes, and was associated with the most favorable overall survival. Finally, subtype 3, or “stromal” (39%), was characterized by the expression of some immune markers together with strong activation of epithelial-mesenchymal transition, angiogenesis and extracellular matrix remodeling pathways. The stromal subtype had low survival rates. These three subtypes are reminiscent of the “immune desert/inflamed/excluded” phenotypes described for patients treated with checkpoint inhibitors (46), (47). When a cohort of metastatic urothelial cancer patients treated with anti-PD-L1 with the “excluded” phenotype was investigated (47), it was found that lack of response to treatment in that cohort correlated with a signature of TGFβ signaling in fibroblasts. Previous studies had shown “sequestering” of T cells by activated fibroblasts producing CXCL12 in dense extracellular matrix deposits surrounding human lung and pancreatic ductal adenocarcinoma tumors (48, 49). Consistent with the notion that fibroblastic activation by TGFβ contributes to T-cell exclusion from tumor beds, by keeping T cells in the peritumoral stromal region, treatment of murine tumors with anti-TGFβ improved the therapeutic efficacy of anti-PD-L1 blockade presumably by counteracting the fibroblastic “barrier,” which resulted in increased intratumoral T-cell infiltration (47). The genetic/environmental factors that determine the “stromal”/“excluded” tumor type have yet to be fully elucidated; however, since tumor fibroblasts derive predominantly from locally available (50) normal fibroblasts (51), one possibility is that seeding of metastatic cells into anatomic locations that are rich in mesenchymal/stromal cells, as part of the normal histological composition of that organ, or even as small deposits present within organs to mediate repair and wound healing, could preferentially give rise to stromal/T cell excluded tumor lesions. In any case, these preclinical and clinical observations suggest that strategies directed to block TGFβ, potentially combined with immunotherapy and radiation, would be most effective in patients with molecular “stromal” subtypes. Patients with “immune” molecular tumor subtypes who benefit the most from localized therapies (surgery, radiotherapy) (45) might well overlap with the “inflamed” tumor patients who benefit the most from immunotherapy (46), and therefore either therapeutic approach, or even a combination of the two, would be the most logical choice for these patients. The “canonical” subtype and/or immune-excluded patients are the least likely to benefit from immunotherapy; however, the DNA repair abnormalities present in tumors of that molecular subtype might make them more susceptible to treatment with pharmacological agents that amplify the DNA damage induced by radiotherapy such as PARP inhibitors (45).
3. Consider intratumoral T cells during individual tumor radiotherapy
For tumors with the “excluded” phenotype, it has been frequently proposed that irradiation could attract T cells to the tumor, turning “cold” tumors that cannot respond to immunotherapy into “hot” tumors that can be treated with immunotherapy (52), but this has not been conclusively proven. Preclinical studies have convincingly shown that irradiation can increase T-cell infiltration of tumors (53–57). Based on the lack of clinical evidence, it is possible that irradiation could attract more T cells in tumors that have low but detectable levels of T cells, whereas it may not have the same effect in tumors that are completely devoid of T cells. For murine tumors that have a detectable T cell population at baseline (comparable to the “inflamed” phenotype), we have recently reported that many T cells present in the tumor before irradiation survive even after high doses of IR (58). In addition, we found that blockade of T-cell infiltration by the S1P1 inhibitor FTY720 did not affect the radiation response if the tumor was established before administration of the drug, but if the drug was started at the time of implantation, irradiation was ineffective. This suggests that preexisting T cells are in certain conditions sufficient to mediate the local cytotoxic effects of radiotherapy, unlike some immunotherapeutics, which required newly infiltrating T cells (59). This is relevant because some investigators have proposed that intensity modulated radiotherapy, which “spreads the dose,” might be immunosuppressive by decreasing circulating T cells. The high radio-resistance of T cells in tumors is not the only exception to the rule that T lymphocytes are one of the most radiosensitive cells in the organism, since we find that also tissue-resident T cells from certain solid tissues (e.g., intraepithelial lymphocytes in the gut) are partially radio-resistant. It is likely that solid tissue/tumor residence imparts some common characteristics to the T cells that reside within them, and indeed, intratumoral and tissue-resident T cells seem to have more similar transcriptomes than intratumoral and lymphoid tissue-derived T cells (58). Behind those similar characteristics must lie common molecular factors present both in transplantable murine tumors and the epithelium of the gut. TGFβ was tested as a possible candidate that would explain both the general similarities and the radio-resistant phenotype, based on the transcriptional analysis of intratumoral T cells, which implicated TGFβ as a master regulator of their phenotype. Together with IL15, TGFβ present in tissues is required for the formation and maintenance of TRM (60). Furthermore, exposure to TGFβ has been shown to increase the radioresistance of non-malignant and malignant cells (61–64). Accordingly, we found that treatment with anti-TGFβ antibodies resulted in higher T-cell densities in the tumors; however, these T cells were more sensitive to irradiation than intratumoral T cells in IgG-treated mice (58). Not all T cells were destroyed after IR in anti-TGFβ treated mice, suggesting that other mechanisms might contribute to an increase radio-resistance in intratumoral T cells.
Examples of potential alternative mechanisms that have been implicated in increasing radio-resistance in the context of cancer are hypoxia and integrin-signaling. Hypoxia has long been known to be an obstacle to effective radiotherapy (65), and many approaches to radiosensitize tumors by targeting hypoxia have been developed over the years (66). In addition, cellular contact with extracellular matrix proteins can increase radio-resistance by promoting DNA damage repair and activation of Akt/MAPK signaling pathways (67). It will be important to discern which of these mechanisms favor radioresistance of both cancer and T cells or are specific for one or the other. This might be very relevant for combinatorial strategies where drugs with radio-sensitizing effect are used, such as anti-TGFβ agents. In strategies featuring TGFβ blockade, treatment has usually been initiated before radiation treatment, with the goal of radiosensitizing cancer cells (61). To what extent the possible radiosensitization of preexisting intratumoral T cells using this approach might limit the potential of combinatorial strategies involving radiation and TGFβ-targeting drugs remains to be elucidated. It will be also helpful to determine whether carefully timed treatments would overcome any potentially harmful interactions. Use of strategies that radiosensitize cancer but not T cells, if existent, would be preferable.
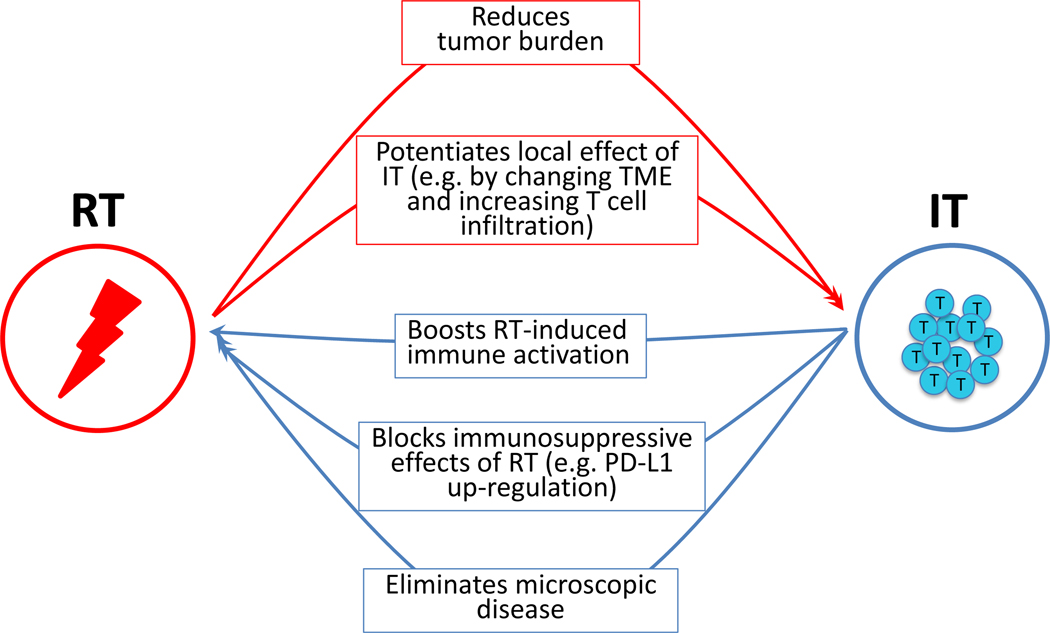
In summary, we propose here a model in which irradiation and immunotherapy synergize (Figure 1) to exert more potent local effects in the irradiated tumors, rather than to elicit a systemic immune response, and suggest avenues to adequately tailor clinical therapies based on the molecular type of the cancer being treated and lessons learned from preclinical investigation on the effects of irradiation on preexisting immunity.
Figure 1.
Mechanisms of cooperation between radiation therapy (RT) and immunotherapy (IT). Red arrows indicate ways RT can help IT achieve greater overall tumor control; blue arrows show ways IT can help RT with the same goal. TME, tumor microenvironment.
Acknowledgements
The authors would like to thank Sean P. Pitroda for helpful suggestions and Amy K. Huser for editorial help. This work was supported by funds from the Ludwig Foundation for Cancer Research and Regeneron Pharmaceuticals (to RW) and NIH R21 CA226582 (to RW and AA).
RW has received commercial grants from Regeneron and has ownership interests in Boost.
Abbreviations:
- IR
ionizing radiation
- PFS
progression-free survival
- OS
overall survival
- NSCLC
non-small cell lung cancer
- SBRT
stereotactic body radiotherapy
Footnotes
AA and SG have no conflict of interest to disclose.
References
- 1.Hellman S, Weichselbaum RR. Oligometastases. J Clin Oncol 1995; 13:8–10. [DOI] [PubMed] [Google Scholar]
- 2.Gomez DR, Tang C, Zhang J, Blumenschein GR Jr., Hernandez M, Lee JJ, et al. Local Consolidative Therapy Vs. Maintenance Therapy or Observation for Patients With Oligometastatic Non-Small-Cell Lung Cancer: Long-Term Results of a Multi-Institutional, Phase II, Randomized Study. J Clin Oncol 2019; 37:1558–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iyengar P, Wardak Z, Gerber DE, Tumati V, Ahn C, Hughes RS, et al. Consolidative Radiotherapy for Limited Metastatic Non-Small-Cell Lung Cancer: A Phase 2 Randomized Clinical Trial. JAMA Oncol 2018; 4:e173501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ost P, Reynders D, Decaestecker K, Fonteyne V, Lumen N, De Bruycker A, et al. Surveillance or Metastasis-Directed Therapy for Oligometastatic Prostate Cancer Recurrence: A Prospective, Randomized, Multicenter Phase II Trial. J Clin Oncol 2018; 36:446–53. [DOI] [PubMed] [Google Scholar]
- 5.Palma DA, Olson R, Harrow S, Gaede S, Louie AV, Haasbeek C, et al. Stereotactic ablative radiotherapy versus standard of care palliative treatment in patients with oligometastatic cancers (SABR-COMET): a randomised, phase 2, open-label trial. Lancet 2019; 393:2051–8. [DOI] [PubMed] [Google Scholar]
- 6.Theelen W, Peulen HMU, Lalezari F, van der Noort V, de Vries JF, Aerts J, et al. Effect of Pembrolizumab After Stereotactic Body Radiotherapy vs Pembrolizumab Alone on Tumor Response in Patients With Advanced Non-Small Cell Lung Cancer: Results of the PEMBRO-RT Phase 2 Randomized Clinical Trial. JAMA Oncol 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bauml JM, Mick R, Ciunci C, Aggarwal C, Davis C, Evans T, et al. Pembrolizumab After Completion of Locally Ablative Therapy for Oligometastatic Non-Small Cell Lung Cancer: A Phase 2 Trial. JAMA Oncol 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Joseph RW, Elassaiss-Schaap J, Kefford R, Hwu WJ, Wolchok JD, Joshua AM, et al. Baseline Tumor Size Is an Independent Prognostic Factor for Overall Survival in Patients with Melanoma Treated with Pembrolizumab. Clin Cancer Res 2018; 24:4960–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Robert C, Ribas A, Hamid O, Daud A, Wolchok JD, Joshua AM, et al. Durable Complete Response After Discontinuation of Pembrolizumab in Patients With Metastatic Melanoma. J Clin Oncol 2018; 36:1668–74. [DOI] [PubMed] [Google Scholar]
- 10.Huang AC, Postow MA, Orlowski RJ, Mick R, Bengsch B, Manne S, et al. T-cell invigoration to tumour burden ratio associated with anti-PD-1 response. Nature 2017; 545:60–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eggermont AMM, Blank CU, Mandala M, Long GV, Atkinson V, Dalle S, et al. Adjuvant Pembrolizumab versus Placebo in Resected Stage III Melanoma. N Engl J Med 2018; 378:1789–801. [DOI] [PubMed] [Google Scholar]
- 12.Weber J, Mandala M, Del Vecchio M, Gogas HJ, Arance AM, Cowey CL, et al. Adjuvant Nivolumab versus Ipilimumab in Resected Stage III or IV Melanoma. N Engl J Med 2017; 377:1824–35. [DOI] [PubMed] [Google Scholar]
- 13.Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, et al. Overall Survival with Durvalumab after Chemoradiotherapy in Stage III NSCLC. N Engl J Med 2018; 379:2342–50. [DOI] [PubMed] [Google Scholar]
- 14.Golden EB, Chhabra A, Chachoua A, Adams S, Donach M, Fenton-Kerimian M, et al. Local radiotherapy and granulocyte-macrophage colony-stimulating factor to generate abscopal responses in patients with metastatic solid tumours: a proof-of-principle trial. Lancet Oncol 2015; 16:795–803. [DOI] [PubMed] [Google Scholar]
- 15.Kwon ED, Drake CG, Scher HI, Fizazi K, Bossi A, van den Eertwegh AJ, et al. Ipilimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184–043): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol 2014; 15:700–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mole RH. Whole body irradiation; radiobiology or medicine? Br J Radiol 1953; 26:234–41. [DOI] [PubMed] [Google Scholar]
- 17.Formenti SC, Demaria S. Radiation therapy to convert the tumor into an in situ vaccine. Int J Radiat Oncol Biol Phys 2012; 84:879–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abuodeh Y, Venkat P, Kim S. Systematic review of case reports on the abscopal effect. Curr Probl Cancer 2016; 40:25–37. [DOI] [PubMed] [Google Scholar]
- 19.Golden EB, Demaria S, Schiff PB, Chachoua A, Formenti SC. An abscopal response to radiation and ipilimumab in a patient with metastatic non-small cell lung cancer. Cancer Immunol Res 2013; 1:365–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Postow MA, Callahan MK, Barker CA, Yamada Y, Yuan J, Kitano S, et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med 2012; 366:925–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brody JD, Ai WZ, Czerwinski DK, Torchia JA, Levy M, Advani RH, et al. In situ vaccination with a TLR9 agonist induces systemic lymphoma regression: a phase I/II study. J Clin Oncol 2010; 28:4324–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luke JJ, Lemons JM, Karrison TG, Pitroda SP, Melotek JM, Zha Y, et al. Safety and Clinical Activity of Pembrolizumab and Multisite Stereotactic Body Radiotherapy in Patients With Advanced Solid Tumors. J Clin Oncol 2018; 36:1611–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brooks ED, Chang JY. Time to abandon single-site irradiation for inducing abscopal effects. Nat Rev Clin Oncol 2019; 16:123–35. [DOI] [PubMed] [Google Scholar]
- 24.Chakravarty PK, Alfieri A, Thomas EK, Beri V, Tanaka KE, Vikram B, et al. Flt3-ligand administration after radiation therapy prolongs survival in a murine model of metastatic lung cancer. Cancer Res 1999; 59:6028–32. [PubMed] [Google Scholar]
- 25.Seetharam S, Staba MJ, Schumm LP, Schreiber K, Schreiber H, Kufe DW, et al. Enhanced eradication of local and distant tumors by genetically produced interleukin-12 and radiation. Int J Oncol 1999; 15:769–73. [DOI] [PubMed] [Google Scholar]
- 26.Demaria S, Ng B, Devitt ML, Babb JS, Kawashima N, Liebes L, et al. Ionizing radiation inhibition of distant untreated tumors (abscopal effect) is immune mediated. Int J Radiat Oncol Biol Phys 2004; 58:862–70. [DOI] [PubMed] [Google Scholar]
- 27.Demaria S, Kawashima N, Yang AM, Devitt ML, Babb JS, Allison JP, et al. Immune-mediated inhibition of metastases after treatment with local radiation and CTLA-4 blockade in a mouse model of breast cancer. Clin Cancer Res 2005; 11:728–34. [PubMed] [Google Scholar]
- 28.Dewan MZ, Galloway AE, Kawashima N, Dewyngaert JK, Babb JS, Formenti SC, et al. Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody. Clin Cancer Res 2009; 15:5379–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Deng L, Liang H, Burnette B, Beckett M, Darga T, Weichselbaum RR, et al. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest 2014; 124:687–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dovedi SJ, Adlard AL, Lipowska-Bhalla G, McKenna C, Jones S, Cheadle EJ, et al. Acquired resistance to fractionated radiotherapy can be overcome by concurrent PD-L1 blockade. Cancer Res 2014; 74:5458–68. [DOI] [PubMed] [Google Scholar]
- 31.Rodriguez-Ruiz ME, Rodriguez I, Garasa S, Barbes B, Solorzano JL, Perez-Gracia JL, et al. Abscopal Effects of Radiotherapy Are Enhanced by Combined Immunostimulatory mAbs and Are Dependent on CD8 T Cells and Crosspriming. Cancer Res 2016; 76:5994–6005. [DOI] [PubMed] [Google Scholar]
- 32.Rech AJ, Dada H, Kotzin JJ, Henao-Mejia J, Minn AJ, Twyman-Saint Victor C, et al. Radiotherapy and CD40 Activation Separately Augment Immunity to Checkpoint Blockade in Cancer. Cancer Res 2018; 78:4282–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vanpouille-Box C, Diamond JM, Pilones KA, Zavadil J, Babb JS, Formenti SC, et al. TGFbeta Is a Master Regulator of Radiation Therapy-Induced Antitumor Immunity. Cancer Res 2015; 75:2232–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rodriguez-Ruiz ME, Rodriguez I, Mayorga L, Labiano T, Barbes B, Etxeberria I, et al. TGFbeta Blockade Enhances Radiotherapy Abscopal Efficacy Effects in Combination with Anti-PD1 and Anti-CD137 Immunostimulatory Monoclonal Antibodies. Mol Cancer Ther 2019; 18:621–31. [DOI] [PubMed] [Google Scholar]
- 35.Luo R, Firat E, Gaedicke S, Guffart E, Watanabe T, Niedermann G. Cisplatin Facilitates Radiation-Induced Abscopal Effects in Conjunction with PD-1 Checkpoint Blockade Through CXCR3/CXCL10-Mediated T-cell Recruitment. Clin Cancer Res 2019; 25:7243–55. [DOI] [PubMed] [Google Scholar]
- 36.Caetano MS, Younes AI, Barsoumian HB, Quigley M, Menon H, Gao C, et al. Triple Therapy with MerTK and PD1 Inhibition Plus Radiotherapy Promotes Abscopal Antitumor Immune Responses. Clin Cancer Res 2019; 25:7576–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mills BN, Connolly KA, Ye J, Murphy JD, Uccello TP, Han BJ, et al. Stereotactic Body Radiation and Interleukin-12 Combination Therapy Eradicates Pancreatic Tumors by Repolarizing the Immune Microenvironment. Cell Rep 2019; 29:406–21 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schrand B, Verma B, Levay A, Patel S, Castro I, Benaduce AP, et al. Radiation-Induced Enhancement of Antitumor T-cell Immunity by VEGF-Targeted 4–1BB Costimulation. Cancer Res 2017; 77:1310–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jimenez-Sanchez A, Memon D, Pourpe S, Veeraraghavan H, Li Y, Vargas HA, et al. Heterogeneous Tumor-Immune Microenvironments among Differentially Growing Metastases in an Ovarian Cancer Patient. Cell 2017; 170:927–38 e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Angelova M, Mlecnik B, Vasaturo A, Bindea G, Fredriksen T, Lafontaine L, et al. Evolution of Metastases in Space and Time under Immune Selection. Cell 2018; 175:751–65 e16. [DOI] [PubMed] [Google Scholar]
- 41.Tang C, Welsh JW, de Groot P, Massarelli E, Chang JY, Hess KR, et al. Ipilimumab with Stereotactic Ablative Radiation Therapy: Phase I Results and Immunologic Correlates from Peripheral T Cells. Clin Cancer Res 2017; 23:1388–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van Kessel CS, Samim M, Koopman M, van den Bosch MA, Borel Rinkes IH, Punt CJ, et al. Radiological heterogeneity in response to chemotherapy is associated with poor survival in patients with colorectal liver metastases. Eur J Cancer 2013; 49:2486–93. [DOI] [PubMed] [Google Scholar]
- 43.Allison KH, Sledge GW. Heterogeneity and cancer. Oncology (Williston Park) 2014; 28:772–8. [PubMed] [Google Scholar]
- 44.Weichselbaum RR. The 46th David A. Karnofsky Memorial Award Lecture: Oligometastasis-From Conception to Treatment. J Clin Oncol 2018:JCO1800847. [DOI] [PubMed] [Google Scholar]
- 45.Pitroda SP, Khodarev NN, Huang L, Uppal A, Wightman SC, Ganai S, et al. Integrated molecular subtyping defines a curable oligometastatic state in colorectal liver metastasis. Nat Commun 2018; 9:1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hegde PS, Karanikas V, Evers S. The Where, the When, and the How of Immune Monitoring for Cancer Immunotherapies in the Era of Checkpoint Inhibition. Clin Cancer Res 2016; 22:1865–74. [DOI] [PubMed] [Google Scholar]
- 47.Mariathasan S, Turley SJ, Nickles D, Castiglioni A, Yuen K, Wang Y, et al. TGFbeta attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature 2018; 554:544–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Salmon H, Franciszkiewicz K, Damotte D, Dieu-Nosjean MC, Validire P, Trautmann A, et al. Matrix architecture defines the preferential localization and migration of T cells into the stroma of human lung tumors. J Clin Invest 2012; 122:899–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ene-Obong A, Clear AJ, Watt J, Wang J, Fatah R, Riches JC, et al. Activated pancreatic stellate cells sequester CD8+ T cells to reduce their infiltration of the juxtatumoral compartment of pancreatic ductal adenocarcinoma. Gastroenterology 2013; 145:1121–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Arina A, Idel C, Hyjek EM, Alegre ML, Wang Y, Bindokas VP, et al. Tumor-associated fibroblasts predominantly come from local and not circulating precursors. Proc Natl Acad Sci U S A 2016; 113:7551–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dominguez CX, Muller S, Keerthivasan S, Koeppen H, Hung J, Gierke S, et al. Single-cell RNA sequencing reveals stromal evolution into LRRC15+ myofibroblasts as a determinant of patient response to cancer immunotherapy. Cancer Discov 2019. [DOI] [PubMed] [Google Scholar]
- 52.Turning Sevenich L. “Cold” Into “Hot” Tumors-Opportunities and Challenges for Radio-Immunotherapy Against Primary and Metastatic Brain Cancers. Front Oncol 2019; 9:163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Filatenkov A, Baker J, Mueller AM, Kenkel J, Ahn GO, Dutt S, et al. Ablative Tumor Radiation Can Change the Tumor Immune Cell Microenvironment to Induce Durable Complete Remissions. Clin Cancer Res 2015; 21:3727–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gupta A, Probst HC, Vuong V, Landshammer A, Muth S, Yagita H, et al. Radiotherapy promotes tumor-specific effector CD8+ T cells via dendritic cell activation. J Immunol 2012; 189:558–66. [DOI] [PubMed] [Google Scholar]
- 55.Lugade AA, Moran JP, Gerber SA, Rose RC, Frelinger JG, Lord EM. Local radiation therapy of B16 melanoma tumors increases the generation of tumor antigen-specific effector cells that traffic to the tumor. J Immunol 2005; 174:7516–23. [DOI] [PubMed] [Google Scholar]
- 56.Matsumura S, Wang B, Kawashima N, Braunstein S, Badura M, Cameron TO, et al. Radiation-induced CXCL16 release by breast cancer cells attracts effector T cells. J Immunol 2008; 181:3099–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zheng W, Skowron KB, Namm JP, Burnette B, Fernandez C, Arina A, et al. Combination of radiotherapy and vaccination overcomes checkpoint blockade resistance. Oncotarget 2016; 7:43039–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Arina A, Beckett M, Fernandez C, Zheng W, Pitroda S, Chmura SJ, et al. Tumor-reprogrammed resident T cells resist radiation to control tumors. Nat Commun 2019; 10:3959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yost KE, Satpathy AT, Wells DK, Qi Y, Wang C, Kageyama R, et al. Clonal replacement of tumor-specific T cells following PD-1 blockade. Nat Med 2019; 25:1251–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mackay LK, Rahimpour A, Ma JZ, Collins N, Stock AT, Hafon ML, et al. The developmental pathway for CD103(+)CD8+ tissue-resident memory T cells of skin. Nat Immunol 2013; 14:1294–301. [DOI] [PubMed] [Google Scholar]
- 61.Bouquet F, Pal A, Pilones KA, Demaria S, Hann B, Akhurst RJ, et al. TGFbeta1 inhibition increases the radiosensitivity of breast cancer cells in vitro and promotes tumor control by radiation in vivo. Clin Cancer Res 2011; 17:6754–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ewan KB, Henshall-Powell RL, Ravani SA, Pajares MJ, Arteaga C, Warters R, et al. Transforming growth factor-beta1 mediates cellular response to DNA damage in situ. Cancer Res 2002; 62:5627–31. [PubMed] [Google Scholar]
- 63.Kirshner J, Jobling MF, Pajares MJ, Ravani SA, Glick AB, Lavin MJ, et al. Inhibition of transforming growth factor-beta1 signaling attenuates ataxia telangiectasia mutated activity in response to genotoxic stress. Cancer Res 2006; 66:10861–9. [DOI] [PubMed] [Google Scholar]
- 64.Konge J, Leteurtre F, Goislard M, Biard D, Morel-Altmeyer S, Vaurijoux A, et al. Breast cancer stem cell-like cells generated during TGFbeta-induced EMT are radioresistant. Oncotarget 2018; 9:23519–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Thomlinson RH, Gray LH. The histological structure of some human lung cancers and the possible implications for radiotherapy. Br J Cancer 1955; 9:539–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dewhirst MW. A potential solution for eliminating hypoxia as a cause for radioresistance. Proc Natl Acad Sci U S A 2018; 115:10548–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sandfort V, Koch U, Cordes N. Cell adhesion-mediated radioresistance revisited. Int J Radiat Biol 2007; 83:727–32. [DOI] [PubMed] [Google Scholar]