Abstract
Objective:
There is no clear consensus regarding the contour of prostatic glandular intraluminal concretions. This study enlightens the rational approach toward deciphering the true nature of these concretions and evaluates their role in normal routine histology of the prostate gland.
Methods:
Fifty hematoxylin and eosin slides that were prepared from procured transrectal biopsy specimens of normal prostate glands from asymptomatic patients suspected of having a prostatic disease but later found to be normal were retrospectively observed for the staining, contour, and positioning of the aggregated masses or concretions within their prostatic lumina and were then compared with the blood prostate specific antigen (PSA) levels.
Results:
Although significant associations highlighting the utility of these masses in presumed pathological states of the gland were drawn by comparing their staining parameters and contours to those of their respective PSA levels, their interluminal contour variations and vivid staining appearances did not necessarily rule out the possibility of some of them being artefacts, provided they were assessed in totality with the surrounding acini. Intensely eosinophilic concretions were found in patients with a high mean age and those with high PSA levels.
Conclusions:
Prostatic intraluminal masses that were rounded tended to indicate pathological shifts within the gland; however, the possibility of them changing to artefacts during slide preparations could not be ruled out.
Keywords: Lumen, complexes, ageing, masses
Abstract
Amaç:
Prostatik glandüler intraluminal konkresyonların konturuna ilişkin net bir fikir birliği yoktur. Bu çalışma, bu oluşumların gerçek doğasını çözmeye yönelik rasyonel yaklaşımı aydınlatmakta ve bunların prostat bezinin normal rutin histolojisindeki rolünü değerlendirmektedir.
Yöntemler:
Prostatik hastalığı olduğundan şüphelenilen ancak daha sonra normal olduğu tespit edilen asemptomatik hastaların normal prostat bezlerinden elde edilen transrektal biyopsi örneklerinden hazırlanan 50 hematoksilen ve eozin slaytı, prostat lümenlerinde toplanmış kitleler veya konkresyonların lekelenme, kontur ve konumlanması açısından geriye dönük olarak incelendi ve daha sonra kan prostat spesifik antijen (PSA) seviyeleri ile karşılaştırıldı.
Bulgular:
Her ne kadar bu kitlelerin bezin varsayılan patolojik durumlarındaki yararlığını vurgulayan anlamlı ilişkiler, boyama parametreleri ve konturları ilgili PSA düzeyleriyle karşılaştırılarak ortaya konmuş olsa da, bir kısmı çevredeki asinüslerle bir bütün olarak değerlendirilmek kaydıyla, interlüminal kontur varyasyonları ve canlı boyama görünümleri ile bunların bazılarının artefakt olma olasılığı dışlanmadı. Yüksek yaş ortalaması ve PSA düzeyi olan hastalarda yoğun eozinofilik konkresyonlar saptandı.
Sonuçlar:
Yuvarlak prostatik lümen içi kitleler, bez içindeki patolojik şiftleri gösterme eğilimindeydi; ancak slayt hazırlıkları sırasında bunların artefaktlara dönüşme olasılığı göz ardı edilemez.
Keywords: Lümen, kompleksler, yaşlanma, kütleler
INTRODUCTION
Although the prostate gland is considered to be a fibromuscular cum glandular organ, its glandular component is yet to be properly explored, because the origins as well as the roles played by the so-called prostatic concretions have been the subject of differing opinions of late1,2. These lamellated, homogeneous concretional masses that are found within the prostatic acini are believed to have been formed either because of precipitated epithelial shedding due to wear and tear of the glandular acini, secretions that are formed because of seepage of exudates combined with calcium that press upon each other within the confines of the acinar boundary, or a mix of both1,3. Nevertheless, there still seems to remain an element of uncertainty in the discernment of their exact staining nature by routine hematoxylin and eosin (H&E) stain methods that contradict their fundamental composition and formation as observed by various workers2,4. This in turn has paved the way for a differing consensus on their role during the aging process of the gland that has formed the basis of this study2,5. Clinical evidence negating the usefulness of prostatic concretions as a measuring tool for prostatic glandular assessment also puts them at risk of being a characteristic feature of this gland6,7. Hence, this study was conducted with the sole aim of enlightening the readers regarding the true nature of these concretions concerning their contrasting staining characteristics and positioning within the lumen of their prostatic glandular acini, apart from studying their vivid contours, which would help the readers arrive at a consensus in differentiating these concretional masses from those of the actual artefacts per se. This study has also attempted to compare the prostatic intraluminal masses observed in the biopsied slides of patients with asymptomatic prostatic conditions to the levels of prostate specific antigen (PSA) in their blood thereby helping us understand whether or not these prostatic masses were found to have a significant focused pathological shift from their original normal benign aggregational state. The novelty of this study lies in the fact that this is the first study to decipher the usefulness of the various contours and staining patterns of each of these prostatic intraluminal masses in routine histology, as they were for a long time considered to be the key features of the prostate as a whole, without considering the possibility of some of these patterns being diffused from each other with frequent scattering, thereby overcoming the tendency to label them as just secretions. This would also provide an apt platform for justifying their role as either mere artefacts during the preparation of slides or as specific hallmark features of the gland per se during routine histology slide demonstrations.
MATERIALS and METHODS
This observational study involved a retrospective cross-sectional examination of routinely stained histopathological slides of normal prostate glands from patients who were biopsied through the transrectal route under ultrasound guidance after a suspicious per rectal examination without any symptomatic prostatic pathology and were later diagnosed as normal. The images of 72 individual prostatic H&E slides from 72 different patients that were stored in the bio-lab computer for the period between November 1, 2022, and April 30, 2023 were initially screened. Later, 22 of those slides were excluded because they were either old or improperly stained or lacked clinical data about the patient’s condition that warranted a biopsy. Therefore, after exclusion from the total slide population lot of 72, 50 slides were finally selected and observed. Each of the 50 slides was sectioned from the glandular submucous zone of the prostate glands of individual patients concerned, the chosen slides were prepared and taken in such a way that one complete section of the glandular submucous zone was visible under a bright field microscope. Only those slides with a numerical PSA value in their accompanying diagnostic report were chosen for the study. Slides that were stained with non-routine stains other than H&E were excluded from the study because the prime purpose of this study was to help histologists gain insight into the normal contour and nature of prostatic concretions to justify their role in routine histological teaching. This study was conducted at a tertiary care medical national institute in collaboration with a private diagnostic lab, which provided technical assistance involving the procurement of reports related to routine histological biopsies. This study received approval from both the institute research review board and AIIMS BBN Institute Ethics Committee (IEC Ref No: AIIMS/BBN/IEC/MAR/2023/244, date: 01.03.2023). The sample size for this study was calculated using the software “calculator.net”, and the effective sample size of 50 slides was arrived after calculating the effect size whose coefficient value was found to be 3.1 by the graph pad prism software. For a slide sample population size of 50, keeping in mind that the number of patients who came to the lab with indications of prostate biopsies per year was 50 (as per the available turn-over records from the concerned lab), the expected proportion of slides was kept at 20% (obtained from a previous related reference study4 on the prostate gland). Assuming an absolute margin of error of 5% for a confidence interval of 95%, the sample size was estimated to be 48. However, 50 slides from the initially screened lot of 72 were finally taken into consideration because they were available, freshly prepared, and healthy.
The 50 selected slides were observed at 10x and 40x magnifications using a Zeiss Axio-Cam 208 binocular microscope attached to a Dell Inspiron 24 computer with an in-built DSL camera. A thorough zig-zag method of observation of the slides was performed from left to right, covering all the fields of a slide, respectively, for each of the slides, and the images of the intraluminal masses within the prostatic acini that were assumed to be the concretions were captured and observed for their contour and staining. The features of those masses were noted as having been observed in at least 70% of the acini per slide for each of the 50 slides, and an attempt was made to determine if separate associations existed between the staining characteristics of the luminal masses and the mean PSA levels of the patients, as well as between the contour patterns of the masses and the mean PSA levels of the patients. The observations of the slides and photographs were done by three independent researchers to avoid bias, and each of the 50 slides were independently evaluated by the three researchers. It was agreed in unison, conforming to the norms of the institute research board, that in the case of differences in opinion between the researchers regarding the contour of prostatic concretions, a higher expert opinion from outside the institute may be acquired. However, the evaluation, opinions, and findings expressed by the researchers throughout the study were in complete agreement with each other. In addition, the anonymity of patients with regard to their clinical histories and PSA levels was maintained.
Statistical Analysis
The independent variables between the two groups were compared using the students unpaired t-test. Only two groups were compared at a time, and a p-value of 0.05 was considered significant for a type 1 alpha error of 0.5 with a 95% confidence interval. The statistical interpretations, mean values, and standard deviations were performed using the graph pad prism software belonging to the second version (2.0), and the baseline calculations were performed using the Microsoft Excel worksheet 2010 version. The contrast, clarity, and transfer of the biopsied images stained with H&E from the microscope to the digital gadgets were performed using the ZEN 2.0 software inbuilt and incorporated into the computer attached to the Zeiss microscope.
RESULTS
Of the 50 examined slides that were taken from the glandular regions of the prostate gland from patients with asymptomatic conditions of the prostate, 23 of them were found to have wholly basophilic and homogeneous masses in at least 70% of their acini, whereas 11 of those slides were found to have wholly eosinophilic masses in at least 70% of their acini with a p-value of 0.0444 that would indicate a significant strength (Table 1). This is in contrast to the views of a typical concretion as evidenced in literature1,3,5 wherein the concretional masses were supposed to be predominantly eosinophilic with a basophilic outer rim. This deviation may indicate a shift in these masses as artefacts during slide preparations rather than true concretions. Only 8 of the slides were found to have the typical staining characteristics of a concretion, as described in the literature, in which those masses possessed an eosinophilic core surrounded by an outer basophilic rim, which denoted the outer calcium cover. The remaining 8 slides did not show the presence of any aggregated mass or bodies within their acinar lumina, further raising the possibility that these masses were artefacts arising from slide preparation, as evidenced by their lack of consistency in some of the slides. The mean PSA values of the patients from whom the slides were biopsied were found to be significantly associated with the concretional staining, and the p-value was 0.0444 (Table 1). As observed from Table 1, slides containing typical aggregated masses with an eosinophilic core and outer basophilic rim were associated with normal PSA levels, in contrast to the relatively high PSA levels observed in those slides with wholly eosinophilic masses. This could possibly suggest the role of inflammation or inflammatory triggers within the gland for the evaporation of calcified deposits over the core, leading to a lack of basophilic staining of concretions. However, as wholly basophilic concretions do not exist by themselves, as evidenced by literature4,6,7, the possibility of them being artefacts cannot be ruled out either.
Table 1. Association between the staining characteristics of acinar masses and blood PSA levels of patients.

The contours of the intraluminal masses within the acinar lumina were also compared independently to that of the blood PSA levels, irrespective of their staining characteristics, and a significant association was found between both of them, as depicted in Table 2. It was seen that higher PSA levels were associated with compact and circumscribed masses in contrast to the scattered masses that were associated with significantly low PSA levels. This could signify a contributive role played using the inflammatory processes within the gland in the compaction of a prostatic concretion.
Table 2. Association between the contour of acinar masses and blood PSA levels of patients irrespective of their staining features.

The aforementioned various contours of these masses that were observed within the acinar luminaare shown below in the merged photographs (Figure 1) of H&E-stained biopsied slides from patients. It was noted by the authors that there were round masses and uncircumscribed masses within the prostatic acinar lumina. The uncircumscribed masses could be either compact or diffuse, apart from the possibility of scattered masses (Figure 1). Irrespective of whether they were localised or scattered, these masses were found to either co-exist within the particular lumen of an acinus either alone or combined (Figure 1), or they existed solitarily in separately distinct acini (Figure 1), giving us the impression that some of these masses could possibly have become detached artefacts during the course of slide preparations, while many could still have been true concretional masses, wherein the representativeness of the aged processes of the gland per se could have been retained in them. Certain smaller acini, however, were not found to contain any of these masses (Figure 1), raising the possibility that these masses may have a predilection toward larger, spacious acini, wherein the pathological influences over the aging processes of the gland might be reflected upon much better with fewer boundary constraints. However, the inconsistency in the appearance of these concretions in some of the acini also does not rule out the possibility of them being artefacts. An attempt was made to determine if there existed an association between the number of stained masses in 70% of the acini and the mean age of the patients from whom the biopsied slides were obtained (Table 3). A significant association (p-value =0.0005) between them was found.
Figure 1.

Various contours of prostatic intraluminal masses. Compact, diffuse and scattered concretions are noticed in various acini.
H&E: Hematoxylin and eosin
Table 3. Age distribution of prostatic masses according to their staining.

In our study, we observed that as the age of the patients increased, there was a predominance of eosinophilic staining in the masses, or else the acini did not contain any mass at all. Conversely, as the mean age of the patients decreased, there was a change in the staining pattern of the masses, and they were no longer eosinophilic; instead, they were basophilic, with the staining first limited to the outer core and then gradually occupying the entire mass as the patients’ mean age thoroughly decreased, indicated by the significant p-value of 0.0005 (Table 3).
DISCUSSION
Most medical schools employ routine histological demonstrations of prostate gland slides to teach first-year medical students during their practical hours1,2. A histologist observing such slides at random while also teaching medical students for the same could be at times puzzled, looking at the prostatic concretions that frequent the internal lumina of the acini that are found within the glandular regions of the prostate, because these concretions, being commonly noticed in routinely stained prostatic histological slides, are considered to be an amorphous mixture of desquamated epithelial cellular debris and worn out collagenous fibres, which had been lining the prostatic acini for long periods of time, but which peeled off and/or mixed with secretions due to wear and tear along with combined mucins, giving them a homogeneous nature because of which they were frequently seen to be brightly stained with eosin and appeared bright pink1,3. However, there are certain others who have hypothesized the presumed role of ‘calcium-mixed-cellular exudates’, with uncertainty, in the formation of these structures rather than attributing them to desquamated epithelial cells per se4,5. Hence, there is a discrepancy of opinion about whether or not calcium does accumulate around its core, leading to the question of prostatic age determination3,5. Considering the classical school of thought that there tends to be an encirclement of calcium deposits around these concretions owing to the heavily dense and repelling positive charges of the ions of the former from the negative interior of the latter, these concretions ought to appear blue on their outer rim when compared to their dense, eosinophilic central core6,7,8 (Figure 2). However, such a phenomenon could not be uniformly observed or appreciated in all of the slides of the biopsied specimens in this study (Table 1), thereby raising concerns over their interpretation in routine histological slide preparations. Moreover, it would be highly immature to broadly comment upon the staining of these intraluminal masses without considering the possibilities of their association with inflammatory tendencies, because such comments without proper reasoning would make it a hard target for controversies related to the former being labeled as mere artefacts (Figure 3) rather than definitive prostatic masses per se. Hence, in this study, an attempt was made to compare the mean PSA values of the patients from whom biopsies were performed to the staining patterns of their prostatic concretions (Table 1), and it was thus revealed that eosinophilic concretions occurred at high mean PSA levels in contrast to the basophilic masses observed at relatively low PSA levels. In addition, the ideally described concretions (in literature) with a blue rim and pink inner core occurred at a mediocre mean PSA level of 2.7, which was somewhere intermediate between the high and low levels, respectively (Table 1). These findings suggest the possibility of heavy calcium efflux from the core due to the massive influx of inflammatory exudates with desquamated cellular debris into the concretions when tendencies for inflammatory triggers are more within the gland (as denoted by the relatively high PSA values in patients with eosinophilic masses in Table 1). This could be the reason why most of the fresh specimens of the prostate gland obtained through transrectal biopsies using ultrasound guidance and surgical interventions from patients with warranted indications of a biopsy suggesting an inflammatory assault on the gland generally showed a diffuse pink to normal pink colour on staining their concretions with H&E near their core, within their acini, unless supersaturated with amyloid, as evidenced in literature9,10. Although one may justify the reason behind it to be either defects in proper staining or slide ageing, there simply could not be a random coincidence in this study where most of the acinar lumina exhibited either a wholly basophilic or a wholly eosinophilic staining of their concretions, wherein, the majority of them being diversely sectioned from the glandular regions of the prostate, exhibited the same staining pattern throughout the acini of a particular slide altogether (Table 1, 3). This raises concerns over their utility in estimating the wear and tear of the gland as well as with regard to their aging, contrary to the conventionally held school of thought, where rimmed basophilic concretions with an inner pink core tended to be exaggerated in aged glands1,2,3,5,7. In addition, as evidenced by the findings that were revealed in this study, which showed that no masses were found at a mean age of 67.5 years, whereas the typical concretional masses having a pink core with an outer blue rim were found at a mean age of 52.5 years (Table 3), it would be imperative to conclude that the classical school of thought no longer holds good or might have its own pitfalls. Moreover, wholly basophilic masses were found at a mean age of 44.5 years and wholly eosinophilic masses were found at a mean age of 66.5 years, suggesting that the deposition of calcium into the lumina of the gland gets replaced and/or overlaid by the superfluous deposits of eroded cells with the advancement of glandular age7,10. This also brings us to the question of whether or not latent amyloid forms bear a causative role in the development of these concretions, as most of the specimens observed in the literature failed to reveal the beta-pleated arrangement of their amyloidogenic collagenous twirls in the presence of calcium and epithelial flakes11,12,13,14. Here, it would be imperative for us to understand that amyloid accumulations within the viscera may be associated with an increase in the number of concretions, as also seen with regard to the aging of the gland, and a causal role between them for the same is yet to be established15,16.
Figure 2.

An ideal prostatic concretion. Round and centered position of the concretion in the acinus is noticeable.
H&E: Hematoxylin and eosin
Figure 3.

Factors tending to suggest the possibility of concretions being artefacts. Certain concretions due to their eccentric positioning may suggest the possibility of artefacts.
H&E: Hematoxylin and eosin
Moreover, clinical evidence suggests that, apart from calcium, other minerals such as magnesium and phosphorus in their apatite forms might also play a role in the attributes of prostatic calculi and other crystals that might be found to have an indirect link with the formation of concretions15,17. Hence, the utility of knowing the precise nature of the staining characteristics of all mineral accumulations, including that of calcium17,18, could play a pivotal role in determining whether or not the concretions found in routine histological slides are worth mentioning to be considered as hallmark features of prostate glands rather than being considered as mere staining artefacts arising out of fixation that happened to be observed by chance (Figure 3) or a combination of both in some or all of the acini. Also, a definitive size limit of the concretions is yet to be analyzed or has not yet been calculated in proportion to the age-related changes of the gland13,19. Moreover, the exact number of prostatic concretions that could accumulate within a particular lumen of an acinus is also an element of concern because ageing as a whole does not reflect the singularity of accumulated concretions as conventionally thought, but rather a degenerative process that could lead to the possibility of more than one concretion within an acinus14,20. This again tends to shift the nature of these concretions toward the possibility of them being artefacts (Figure 3). However, the findings in this study (Table 3) show that although there is a significant association between the age of the gland and its staining pattern, it does not adequately reflect the role played by calcium in prostatic ageing, or rather, its role could have been possibly masked by the predominant eosinophilic concretional staining pattern observed in patients with a heightened mean age (Table 3).
The authors of this article would prefer to refer to these concretions as just “intraluminal acinar masses” when observed randomly or retrospectively without trying to comment upon the composition of those masses, as it would be immature to comment on them without clinical correlations. However, based on their experiences teaching histology to undergraduates, the authors of this article have agreed in unison that multiple forms of these intraluminal masses do exist with regard to their staining and contour (Table 4), (Figure 1), and have also arrived at the conclusion that these masses just need to be demonstrated without further indulging into their causative role.
Table 4. Various features of the prostatic intraluminal masses observed by the authors in this study.
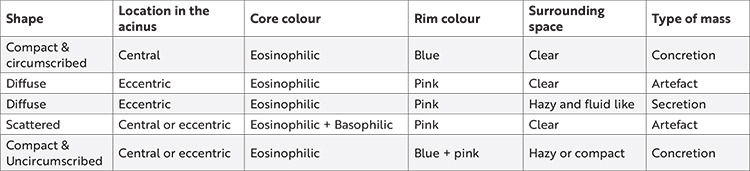
Table 4 along with Figures 1 and 3 would also serve to enlighten the readers regarding the differentiation of prostatic concretions from diffuse acinar secretions during slide examinations of the prostate, apart from focusing only on the artefacts per se5,18. Some clinicians attribute the prostatic calculi and crystal formations within the lobes of the prostate gland as precursor units of prostatic secretions that eventually give rise to concretions eventually11,16. As per the literature, it was hypothesized that the tendencies toward inflammation or inflammatory triggers within the prostate, aided by those aforementioned precursor crystals and/or calculi, could serve as a nidus to emancipate the formation of compacted secretions, which in turn may lead to the formation of concretions, much before the pathological processes of the gland begin to set in12,17,19. Hence, in this study, the PSA was used to compare the contour and positioning of the concretions, as the former would aptly indicate any silent, ongoing insults within the glandular elements of the latter, thereby helping us attribute a plausible pathological association between both of them. In this study (Table 2), it was revealed that most patients whose PSA levels were low had fragmented prostatic masses compared with the localized masses in those with high mean PSA levels, thereby alluding to the previous hypothesis12,19 that the roundness and compaction of these masses with their apt localization within their acinar lumina might indicate a silent inflammatory trend within the internal milieu of the prostate, possibly due to calculi or crystals. Hence, it would be apt to consider the fact that sonologists and surgeons usually prefer to measure the seeded nests of the prostatic calculi or crystals directly by ultrasound and analyze them rapidly through biopsies rather than waiting for the slow concretions to be formed per se10,14,15. Hence, the quest to explore the compositional nature and staining patterns of these prostatic concretions has become obsolete in the clinical arena, owing to the fact that these prostatic secretions and concretions not only act as a nidus for malignant growth but also serve as natural culture media for the growth of several bacteria, as evidenced by the growth of biofilms over the same that could eventually lead to urinary tract infections17,19,20,21. Hence, some clinicians preferred the term “wasteosomes” over prostatic concretions because they were essentially wasted remnants without proper use, as evidenced by transrectal prostate biopsies after ultrasound examination3,18,22. Since the very purpose of histological studies is not only to correlate with the clinical findings but also to motivate the students toward the clinical and surgical setting, the authors rightfully feel that the utility of these concretions in being the characteristics of a normal prostate gland as a teaching aid for beginners of normal histology is in doubt, particularly because only pathological specimens of the prostate gland may reveal the true nature of these concretions after correlation with biopsies and ultrasound7,11,15,18,19. Moreover, the true core of the concretions may be lost during routine histological tissue processing, giving rise to the possibility of partial artefacts in some acini and true concretions in certain others21,22.
Because this is a retrospective observational study, the results of this study do not necessarily reflect the changes that are bound to occur with the evolving morphologies of the prostatic concretions over the course of time with the shifting changes of the gland. Thus, to supplement this problem, prospective cohorts of benign asymptomatic prostatic conditions may be chosen and followed up by taking serial biopsy sections from those patients at different periods of time until the changes in the morphology of their concretions may be compared, which may provide more discrete evidence regarding the same. However, there would always be the problem of patient compliance, especially with regard to the indications that would warrant a normal histological biopsy for study purposes, as patients would be reluctant to give their consent for the same.
CONCLUSION
Compact intraluminal prostatic masses and intensely eosinophilic masses may indicate an ongoing occult pathological shift in the gland from a benign state. Purely basophilic intraluminal masses as well as those masses that are immensely scattered cum fragmented, haphazardly pink, and/or eccentrically placed within the prostatic acinar lumina supplemented with ill-defined margins may suggest the possibility of them being artefacts.
Acknowledgments
The authors of this research study would like to thank the Review board, Institute Research Cell and Ethics Committee of All India Institute of Medical Sciences, Bibinagar, Hyderabad Metropolitan Region, Telangana, India, for granting them the permission to carry out this study by collaborating with a private diagnostic lab specialized in prostate biopsies. The authors would also like to acknowledge the technical assistants/lab personnel from Fusion diagnostic labs, New Delhi, India who were helpful in providing the researchers with the PSA values and photographs of the concerned slides in this study by maintaining the anonymity of the patients concerned.
Footnotes
Ethics
Ethics Committee Approval: This study received approval from both the institute research review board and AIIMS BBN Institute Ethics Committee (IEC Ref No: AIIMS/BBN/IEC/MAR/2023/244, date: 01.03.2023).
Informed Consent: Retrospective study.
Peer-review: Externally and internally peer-reviewed.
Author Contributions
Surgical and Medical Practices: S.S., Concept: S.S., R.M., M.C., Design: S.S., R.M., Data Collection and/or Processing: S.S., Analysis and/or Interpretation: S.S., R.M., M.C., Literature Search: S.S., R.M., M.C., Writing: S.S., R.M., M.C.
Conflict of Interest: The authors have no conflict of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.McNeal JE. Normal histology of the prostate. Am J Surg Pathol. 1988;12:619–33. doi: 10.1097/00000478-198808000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Ittmann M. Anatomy and Histology of the Human and Murine Prostate. Cold Spring Harb Perspect Med. 2018;8:a030346. doi: 10.1101/cshperspect.a030346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Riba M, Del Valle J, Augé E, Vilaplana J, Pelegrí C. From corpora amylacea to wasteosomes: History and perspectives. Ageing Res Rev. 2021;72:101484. doi: 10.1016/j.arr.2021.101484. [DOI] [PubMed] [Google Scholar]
- 4.Sfanos KS, Wilson BA, De Marzo AM, Isaacs WB. Acute inflammatory proteins constitute the organic matrix of prostatic corpora amylacea and calculi in men with prostate cancer. Proc Natl Acad Sci U S A. 2009;106:3443–8. doi: 10.1073/pnas.0810473106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chatterjee S. Artefacts in histopathology. J Oral Maxillofac Pathol. 2014;18(Suppl 1):S111–6. doi: 10.4103/0973-029X.141346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rifkin MD, Dähnert W, Kurtz AB. State of the art: endorectal sonography of the prostate gland. AJR Am J Roentgenol. 1990;154:691–700. doi: 10.2214/ajr.154.4.1690499. [DOI] [PubMed] [Google Scholar]
- 7.Awojobi OA, Junaid TA, Nkposong EO. Transrectal biopsy of the prostate: a review of 186 biopsies. Afr J Med Med Sci. 1983;12:117–9. [PubMed] [Google Scholar]
- 8.Antico E, Busilacchi P, Candelari R, Cavalli ML, Maggi G. Confronto tra citologia e microisotologia nel prelievo ecoguidato della prostata con aghi sottili. Radiol Med. 1990;79:83–6. [PubMed] [Google Scholar]
- 9.Zajicek J. Cytology of infradiaphragmatic organs. 5. Prostatic gland and seminal vesicles. Monogr Clin Cytol. 1979;7:129–65. [PubMed] [Google Scholar]
- 10.Torres Ramírez C, Aguilar Ruíz J, Zuluaga Gómez A, del Río Samper S, Issa Khozouz N. Estructura de los calculos prostáticos primarios o endógenos [Structure of primary prostatic endogenous calculi] Arch Esp Urol. 1979;32:581–90. [PubMed] [Google Scholar]
- 11.Kim WB, Doo SW, Yang WJ, Song YS. Influence of prostatic calculi on lower urinary tract symptoms in middle-aged men. Urology. 2011;78:447–9. doi: 10.1016/j.urology.2010.12.056. [DOI] [PubMed] [Google Scholar]
- 12.Kwon YK, Choe MS, Seo KW, et al. The effect of intraprostatic chronic inflammation on benign prostatic hyperplasia treatment. Korean J Urol. 2010;51:266–70. doi: 10.4111/kju.2010.51.4.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meares EM Jr. Infection stones of prostate gland. Laboratory diagnosis and clinical management. Urology. 1974;4:560–6. doi: 10.1016/0090-4295(74)90490-7. [DOI] [PubMed] [Google Scholar]
- 14.Eykyn S, Bultitude MI, Mayo ME, Lloyd-Davies RW. Prostatic calculi as a source of recurrent bacteriuria in the male. Br J Urol. 1974;46:527–32. doi: 10.1111/j.1464-410x.1974.tb03851.x. [DOI] [PubMed] [Google Scholar]
- 15.Thomas BA, Robert JT. Prostatic calculi. J Urol. 1927;18:470–93. [Google Scholar]
- 16.Bartoletti R, Cai T, Nesi G, et al. The impact of biofilm-producing bacteria on chronic bacterial prostatitis treatment: results from a longitudinal cohort study. World J Urol. 2014;32:737–42. doi: 10.1007/s00345-013-1145-9. [DOI] [PubMed] [Google Scholar]
- 17.Shoskes DA, Lee CT, Murphy D, Kefer J, Wood HM. Incidence and significance of prostatic stones in men with chronic prostatitis/chronic pelvic pain syndrome. Urology. 2007;70:235–8. doi: 10.1016/j.urology.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 18.Suh JH, Gardner JM, Kee KH, Shen S, Ayala AG, Ro JY. Calcifications in prostate and ejaculatory system: a study on 298 consecutive whole mount sections of prostate from radical prostatectomy or cystoprostatectomy specimens. Ann Diagn Pathol. 2008;12:165–70. doi: 10.1016/j.anndiagpath.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 19.Donlan RM, Costerton JW. Biofilms: survival mechanisms of clinically relevant microorganisms. Clin Microbiol Rev. 2002;15:167–93. doi: 10.1128/CMR.15.2.167-193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dessombz A, Méria P, Bazin D, Daudon M. Prostatic stones: evidence of a specific chemistry related to infection and presence of bacterial imprints. PLoS One. 2012;7:e51691. doi: 10.1371/journal.pone.0051691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cai T, Tessarolo F, Caola I, et al. Prostate calcifications: A case series supporting the microbial biofilm theory. Investig Clin Urol. 2018;59:187–93. doi: 10.4111/icu.2018.59.3.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Soto SM, Smithson A, Martinez JA, Horcajada JP, Mensa J, Vila J. Biofilm formation in uropathogenic Escherichia coli strains: relationship with prostatitis, urovirulence factors and antimicrobial resistance. J Urol. 2007;177:365–8. doi: 10.1016/j.juro.2006.08.081. [DOI] [PubMed] [Google Scholar]


