Abstract
Microglia, as the immune cells of the central nervous system (CNS), play dynamic roles in both health and diseased conditions. The ability to genetically target microglia using viruses is crucial for understanding their functions and advancing microglia-based treatments. We here show that resident microglia can be simply and specifically targeted using adeno-associated virus (AAV) vectors containing a 466-bp DNA fragment from the human IBA1 (hIBA1) promoter. This targeting approach is applicable to both resting and reactive microglia. When combining the short hIBA1 promoter with the target sequence of miR124, up to 95% of transduced cells are identified as microglia. Such a simple and highly specific microglia-targeting strategy may be further optimized for research and therapeutics.
Keywords: microglia, virus transduction, adeno-associated virus, AAV, lentivirus, human IBA1
INTRODUCTION
Microglia serve as the immune cells of the central nervous system (CNS), maintaining homeostasis under normal conditions. They become activated and play dynamic roles during neuroinflammation, neural damage, or under degenerative conditions (1–6). Depending on the pathological processes, microglia can serve as phagocytes and secrete proinflammatory or anti-inflammatory mediators to regulate degeneration, repair and regeneration in the CNS (7). These behaviors of microglia are tightly regulated by extra- and intra-cellular signaling pathways and transcriptional programs (8, 9). Genetic manipulation of microglia will help understand their function as well as hold promising potential for developing new treatments for CNS diseases.
Viral vectors such as lentivirus and adeno-associated virus (AAV) are clinically relevant tools for genetic manipulation of different cell types (10–13). Even though lentivirus and AAV can be used to transduce most brain cells, microglia seem to be resistant to the transduction using those vectors (13–17). The first analysis using AAV2 showed that microglia could be transduced but gene expression was not detectable, suggesting that microglia could have an intrinsic mechanism to prevent transgene expression or virus degradation (18). The inclusion of microglial specific promoters like F4/80 and CD68 in AAV improved microglia transduction in culture; however, transduction efficiency remained very low in mice (19). Attempts to improve AAV capsids also failed to induce strong and stable microglia transgene expression (20).
In this study, we initially aimed to reprogram microglia for neural regeneration, as we have previously done with resident astrocytes and NG2 glia (21–26). However, multiple attempts failed to efficiently transduce microglia with lentivirus under several routinely used promoters. We therefore conducted in vivo screens of additional promoters that could drive gene expression through lentivirus in resident microglia. We mainly focused on human gene promoters with the expectation that such evolutionarily conserved promoters could eventually be used for therapeutics. Based on the results of lentiviruses, we further revealed that a 466-bp fragment of the human IBA1 (hIBA1) promoter, when employed in AAV vectors, was sufficient to drive microglia-specific gene expression. During the process of this study and supporting our results, it was recently reported that a 1.7-kb mouse Iba1 promoter was also capable of driving microglia-specific expression through AAVs (27). Notwithstanding, our remarkably short hIBA1 promoter will allow AAVs to package much larger gene inserts. Although clinically relevant AAV capsids, such as AAV5 and AAV8, can be used to target microglia, our hIBA1 promoter should be compatible with the recently developed capsid variants for microglia (28, 29).
RESULTS
Specific targeting of microglia with lentivirus under the hIBA1 promoter
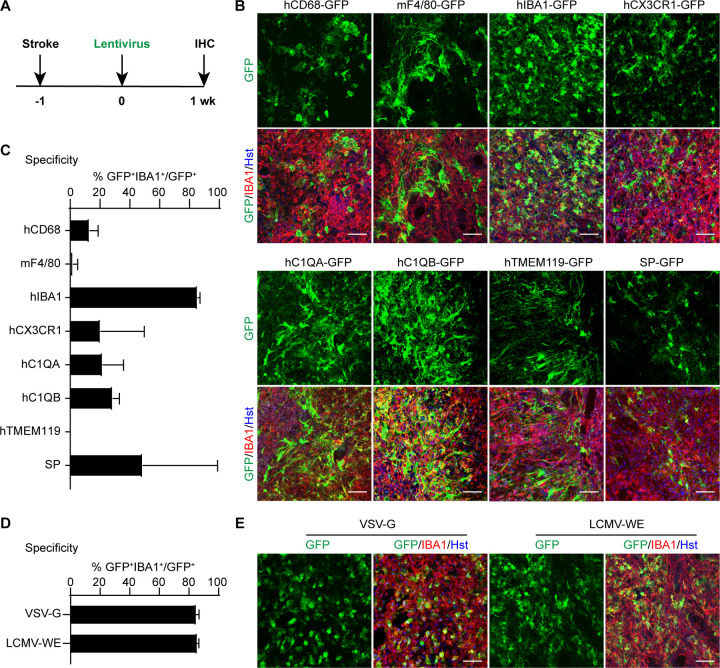
We first examined the lentiviral delivery system to target microglia in the adult mouse brain. We screened eight different promoters associated with specific gene expression in microglia. Except for the mouse mF4/80 promoter, the remaining seven promoters are human origin with an aim of targeting human microglia for clinical therapy. They include the hCD68, hIBA1, hCX3CR1, hC1QA, hC1QB, hTMEM119, and SP promoters. The reporter GFP was put under the control of these promoters, and the lentivirus was packaged with the VSV-G envelope. To include reactive microglia which are critically involved in neurological diseases, we induced ischemic stroke through the MCAO procedure one week before virus injections (Fig. 1A). One week post virus (wpv) delivery, we analyzed the expression of the reporter GFP expression and the microglia-specific marker IBA1 in the injected mouse striatum. GFP+IBA1+ cells were detected in the striatum with all the promoters tested; however, the highest microglia-specific GFP expression was driven by the hIBA1 promoter (85.64 ± 0.72 %; Fig. 1B, C). GFP driven by the other promoters was detected in both microglia and cells that morphologically appeared to be neurons and astrocytes. Because the best transduction efficiency for microglia was driven by the hIBA1 promoter, we decided to focus our subsequent studies on this promoter.
Figure 1. In vivo screens for microglia-targeting lentivirus.
(A) Schematic diagram of the experimental procedure. One week post MCAO-induced stroke, lentiviruses with various promoter-driven GFP were injected into the striata and examined after another week (wk).
(B) Representative confocal images showing marker expression for the indicated lentiviruses. Scale bars, 50 μm.
(C) Quantifications showing high microglia-specificity of GFP expression in mice injected with the lenti-hIBA1-GFP (mean ± SEM; n=3 mice per group).
(D) Quantifications showing comparable specificity of lentivirus packaged with either VSV-G or LCMV-WE envelope (mean ± SEM; n=3 mice per group).
(E) Representative confocal images showing marker expression for the indicated lentiviruses. Scale bars, 50 μm.
Microglia-specific expression is not affected by the lentiviral envelopes
Cell type-specificity of lentiviruses was reported to be influenced by the viral envelopes (30). To test this possibility, we compared the hIBA1-GFP lentivirus packaged with either the VSV-G or the LCMV-WE envelope. When GFP+IBA1+ cells were examined at 1 wpv, both enveloped lentiviruses showed high targeting specificity for microglia in the injected striatum (84.96 ± 1.04% for VSV-G and 85.64 ± 0.75 % for LCMV-WE; Fig. 1D, E).
Specific targeting of microglia with scAAVs under the hIBA1 promoter
Lentiviral and AAV vectors are widely applied for gene expression in vivo. Even though both systems allow stable transgene expression, AAVs are more broadly employed for in vivo gene therapy due to their non-integration nature and minimal inflammatory response (11, 12, 31, 32). We first examined the self-complementary AAV (scAAV) since it was reported to drive stronger gene expression than the single strand AAV (ssAAV) (33). scAAV-hIBA1-GFP was then packaged with 8 different serotypes, including AAV1, AAV2, AAV5, AAV6, AAV6m, AAV8, AAV9 and PHP.eB. They were injected into the striatum one week post L-NIO-induced stroke (which produces more constant and confined infarcts when compared to the MCAO procedure) and examined 7 days later (Fig. 2A). All these scAAVs produced a robust expression of GFP in IBA1+ cells but with varying degrees of specificity and efficiency (Fig. 2B–D). Quantification of GFP+IBA1+ cells showed that both scAAV5 and scAAV8 had the highest degrees of specificity (93.68 ± 1.14% and 77.42 ± 4.97%, respectively; Fig. 2C) and efficiency (69.7 ± 7.23% and 82.11 ± 0.8%, respectively; Fig. 2D) for microglia.
Figure 2. In vivo screens for microglia-targeting AAVs.
(A) Schematic diagram of the experimental procedure. One week post L-NIO-induced stroke, scAAVs with various serotypes were injected into the striata and examined after another week (wk).
(B) Representative confocal images showing marker expression for the indicated scAAVs. Scale bars, 50 μm.
(C) Quantifications showing microglia-specificity of GFP expression for the indicated scAAVs (mean ± SEM; n=3 mice per group).
(D) Quantifications showing microglia transduction efficiency for the indicated scAAVs (mean ± SEM; n=3 mice per group).
(E) Schematic diagram of the experimental procedure. One week post L-NIO-induced stroke, scAAV5 or scAAV8 was injected into the striata and examined after another 4 week (wk).
(F) Representative confocal images showing marker expression for the indicated scAAVs. Scale bars, 50 μm.
(G) Quantifications showing the specificity and transduction efficiency for microglia (mean ± SEM; n=3 mice per group).
To determine whether transgene expression could be maintained for longer time in vivo, we injected the virus and conducted analysis at 4 wpv (Fig. 2E). Both scAAV5 and scAAV8 could mediate robust GFP expression in microglia at this longer delay after virus injection (Fig. 2F–G). Cell type-specificity and transduction efficiency were comparable to what were observed at 1 wpv.
Identification of the minimal hIBA1 promoter
The AAV vector has a limited packaging capacity, with about ~4.5 kb for ssAAVs and an even smaller size for scAAV (34, 35). A minimal promoter will help increase the size of the transgene that could be packaged. Our original hIBA1 promoter is about 760 bp in length, which is already relatively small. To determine whether it could be further shortened, we made a series of truncations starting from the 5’ end and subcloned them into the ssAAV vector. They were then packaged with serotype 5 and injected into the striatum of adult mouse with or without L-NIO-induced stroke (Fig. 3A–B). When examined at 1 wpv, the 466-bp hIBA1a promoter showed a similar expression pattern as the original hIBA1 promoter, with high microglia specificity and transduction efficiency (70.51 ± 5.12% and 82.26 ± 2.62%, respectively; Fig. 3C–E). Of note, the microglia-specificity was comparable in brains with or without the stroke (70.51 ± 5.15% for sham and 91.93 ± 1.98% for stroke; Fig. 3D), although the transduction efficiency tended to be slightly lower than the non-stroke condition (Fig. 3E). The other shorter promoters, especially hIBA1c and hIBA1d, performed worse. These results suggest that the 466-bp hIBA1a promoter could be used for subsequent experiments. When examined at 4 wpv, however, all these viruses showed a lower microglia-specificity and transduction efficiency (Fig. S1), indicating a time-dependent effect for the ssAAV virus.
Figure 3. In vivo screens for the minimal microglia-targeting promoter.
(A) Schematic diagram of the experimental procedure. ssAAV5 viruses with different promoters were injected into sham mice or mice with L-NIO-induced stroke. Brains were analyzed one week later.
(B) Diagram of the examined ssAAVs with different lengths of hIBA1 promoter.
(C) Representative confocal images showing marker expression for the indicated ssAAV5s. Scale bars, 50 μm.
(D) Quantifications showing microglia-specificity of GFP expression for the indicated ssAAV5s (mean ± SEM; n=3 mice per group).
(E) Quantifications showing microglia transduction efficiency for the indicated ssAAV5s (mean ± SEM; n=3 mice per group).
High microglia-specificity of scAAV under the hIBA1a promoter
Since the above ssAAVs had dramatically reduced microglia specificity and transduction efficiency at 4 wpv, we then evaluated whether packaging the virus as scAAV would improve the outcome. For this purpose, we prepared the scAAV5-hIBA1a-GFP virus, injected it into the striatum of adult mouse with or without L-NIO-induced stroke, and examined gene expression at both 1 and 4 wpv (Fig. 4A). We found strong GFP expression in microglia at 1 wpv, in both non-injured and injured brains (Fig. 4B). The efficiency and specificity for microglia transduction was not only maintained but also slightly increased at 4 wpv, reaching nearly 80% under the stroke condition (87.62 ± 1.16% for specificity and 88.74 ± 1.12% for efficiency; Fig. 4B–D). These results suggest that the hIBA1a promoter could drive highly microglia-specific expression when packaged into scAAV.
Figure 4. Increased long-term specificity and efficiency for the minimal hIBA1a promoter when packaged in scAAV.
(A) Schematic diagram of the experimental procedure. scAAV5 virus with the hIBA1a promoter was injected into sham mice or mice with L-NIO-induced stroke. Brains were analyzed 1 week or 4 weeks later.
(B) Representative confocal images showing marker expression for the indicated conditions. Scale bars, 50 μm.
(C) Quantifications showing microglia-specificity of GFP expression for the indicated conditions (mean ± SEM; n=3 mice per group).
(E) Quantifications showing microglia transduction efficiency for the indicated conditions (mean ± SEM; n=3 mice per group).
miR124T confers high specificity of ssAAV under the hIBA1a promoter
Although scAAV can improve the stability for microglia transduction, its limited packaging size could be a problem for larger genes. To maintain microglia specificity but also keep higher packaging capacity, we redesigned a new ssAAV vector under the hIBA1a promoter. Since the vast majority of GFP+ non-microglia cells were neurons, we inserted after the transgene a synthetic sequence containing 4 copies of the targeting sequence of miR124 (Fig. 5A, miR124T). miR124 is a microRNA that is highly enriched in neurons (36). The insertion of miR124T after the transgene is expected to cause silencing of the transgene in neurons but not in other cells (37). We packaged the ssAAV5-hIBA1a-GFP-miR124T virus and injected it into the striatum of mouse without prior injury (Fig. 5A). When examined at 4 wpv, we observed robust and highly specific GFP expression in IBA1+ microglia (Fig. 5B). Quantification showed that 94.78 ± 0.59% of GFP+ cells were also IBA1+ (Fig. 5C), indicating a remarkably high microglia-specificity. The remaining few GFP+ but IBA1− cells mainly exhibited the morphology of astrocytes or neurons. Transduction efficiency surrounding the injected brain area was also high, reaching 85.83 ± 1.79% (Fig. 5D).
Figure 5. miR124T confers high specificity of ssAAV under the hIBA1a promoter.
(A) Schematic diagram of the experimental procedure. The miR124T sequence was inserted into the 3’ end of the GFP gene. ssAAV5 virus was then injected into the striatum of adult wildtype mouse and analyzed 4 weeks later.
(B) Representative confocal images showing marker expression in the injection area and an area away from the injection site. Neurons are marked by the NeuN staining. Scale bars, 50 μm.
(C) Quantifications showing high microglia-specificity of GFP expression (mean ± SEM; n=3 mice per group).
(D) Quantifications showing high microglia transduction efficiency (mean ± SEM; n=3 mice per group).
DISCUSSION
Our results show that brain microglia can be specifically targeted with AAVs containing a 466-bp hIBA1 promoter (hIBA1a). The microglia specificity is further improved when the target sequence of miR124 (miR124T) is included in the AAV vector to inhibit transgene expression in neurons. The hIBA1a promoter and miR124T could be similarly integrated into lentiviral vectors for lentivirus-mediated targeting of resident microglia.
Although it is well-known that viral tropism is determined by surface proteins in the viral envelope or capsid, cell type-specificity of transgene expression is also controlled by the gene promoters (12, 13, 38, 39). In lentiviral vectors, our in vivo screens showed that the GFP reporter driven by the hIBA1 promoter exhibited the highest microglia-specificity when compared to any other tested promoters, including the synthetic promoter (SP), hCD68, mF4/80, hCX3CR1, hC1QA, and hC1QB. Future experiments may be needed to tease out the regulatory genomic elements that drive microglia-restricted expression by these latter genes. Of note, microglia-specificity of lentiviral vectors with the hIBA1 promoter was not majorly affected by the viral envelope, as we found a comparable specificity for lentivirus packaged with either the VSV-G or the LCMV-WE envelope. Such a result further highlights the importance of the specific promoter for gene expression in microglia.
Because of its low toxicity and minimal induction of host immune responses, AAVs are broadly employed for gene expression in vivo (11, 32). Among the many natural capsids of AAVs, AAV1, AAV2, AAV5, AAV6, AAV8, and AAV9 are the most frequently used for research and therapeutics. Our results showed that AAV vector containing the hIBA1 promoter, when packaged with any of these above capsids, could drive transgene expression in microglia. Nonetheless, AAV5 and AAV8 gave rise to a comparably high transduction specificity and efficiency for microglia, while the previously reported AAV6 mutant capsid (AAV6m) (20) performed the worst. The non-microglia cells for these above tested capsids are mainly neurons and astrocytes, which seem to be the default cell types for majority AAVs even with glia-specific promoter (15, 40). To reduce non-specific transgene expression in neurons, we inserted into the AAV vector with 4 copies of the target sequence of miR124, a microRNA highly enriched in neurons (36). Such a strategy greatly improved targeting specificity and long-term expression through AAVs in brain microglia; and it could be similarly employed in the lentiviral vectors.
A major limitation of AAVs is their relatively small packaging capacity for foreign DNAs, with roughly 4.5 kb for ssAAVs (34) and an even smaller size for scAAVs (35). Our systematic truncation analysis showed that a 466-bp genomic fragment of the hIBA1 promoter is still sufficient to drive microglia-specific gene expression, therefore permitting insertion of larger genes in both ssAAV and scAAV vectors. Our result is in sharp contrast to a recent report, showing a 1.7-kb fragment of the mouse Iba1 promoter is needed for expression in microglia (27). Additionally, our short hIBA1 promoter is expected to work in human microglia; therefore, it should have broader applications than the much longer mouse Iba1 promoter.
In conclusion, our study identified a simple method to specifically target resident microglia with AAVs or lentiviruses. It could be further facilitated with mutant AAV capsids for microglia (28, 29). Our method should be a valuable contribution to microglia-based research and therapeutics.
MATERIALS AND METHODS
Animals.
Wildtype C57/BL6J mice were purchased from the Jackson Laboratory. Adult male and female mice at 2–3 months of age were used unless otherwise stated. All mice were housed under a 12 h light/dark cycle and had ad libitum access to food and water in the animal facility. Experimental animals were randomized, and the experimenters were not blinded to the allocation of animals during experiments and outcome assessment. All experimental procedures and protocols were approved by the Institutional Animal Care and Use Committee at University of Texas Southwestern.
Stroke.
Strokes were induced by either middle cerebral artery occlusion (MCAO) or L-NIO injection. For MCAO, mice were anesthetized with isoflurane. The left common and external carotid arteries were exposed and ligated. Occlusion of the middle cerebral artery (MCA) was accomplished through the insertion of a filament from the basal part of the external carotid artery and advancing it in the internal carotid artery toward the location of MCA branching from the circle of Willis. The occlusion lasted 20 min and reperfusion was initiated by removing the suture from the internal carotid artery. Once the suture was removed, the internal carotid artery was ligated. L-NIO induced ischemia was selected for the following experiments because it produced constant and confined infarcts in the striatum. For L-NIO induced ischemia, a craniotomy was performed overlying the injection sites of the cortex. A Hamilton syringe was filled with L-NIO (27 μg/μL in sterile physiological saline; Calbiochem), secured onto the stereotaxic arm, and connected to a pressure pump. Three injections (each of 0.3 μL of L-NIO solution) were made in the following coordinates: anterior-posterior (AP) +1 mm, medio-lateral (ML) +2 mm, dorsoventral from the skull (DV) −2 mm; AP +1 mm, ML +2 mm, DV −2.5 mm; and AP +1 mm, ML +2 mm, DV −3 mm. Injections were made at a rate of 3 μL/min, targeting the striatum. Localized vasoconstriction leads to focal ischemia in the striatum. In Sham experiments, the needle was placed at the same coordinates as the stroke experiments, but L-NIO was not injected in the striatum.
Lentiviral vectors and virus preparation.
The lentiviral vector SP–GFP was generated by sub-cloning the macrophage synthetic promoter (SP, ~400 bp, which harbors multiple myeloid/macrophage cis elements) into the CS–CDF–CG–PRE vector (41). This SP-GFP vector was used to construct other lentiviral vectors. The human CD68 promoter and enhancer (hCD68, ~800 bp) was subcloned from pcDNA3-hCD68prm (Addgene #34837). The mouse F4/80 promoter (mF4/80, ~670 bp) was PCR-amplified from mouse genomic DNA. The human IBA1 promoter (hIBA1, ~760 bp), human TMEM119 promoter (hTMEM119, ~570 bp), human CX3CR1 promoter (hCX3CR1, ~700 bp), human C1QA promoter (hC1QA, ~560 bp), and human C1QB promoter (hC1QB, ~550 bp) were PCR-amplified from human genomic DNA. All vectors were verified through restriction enzyme digestions and DNA sequencing. Replication-deficient virus was produced in HEK293T cells by transient transfection with lentiviral vectors and packaging plasmids (pMDL, pREV, and VSV-G or pHCMV-LCMV-WE envelopes). Lentivirus was collected by PEG precipitation and tittered as described previously (21).
AAV vectors and virus production.
The single strand AAV (ssAAV) vectors were based on the AAV-hGFAP-GFP vector (40) by replacing the hGFAP promoter with the hIBA1 promoter. The self-complementary AAV (scAAV) vectors were based on the scAAV-CAG-GFP vector (Addgene #83279) by replacing the CAG promoter with the hIBA1 promoter. All vectors were verified through restriction enzyme digestions and DNA sequencing. AAV viruses were packaged with pAd-deltaF6 (Addgene #112867) and one of the following helper plasmids: pAAV2/1 (Addgene #112862), pAAV2/2 (Addgene #104963), pAAV2/5 (Addgene #104964), pAAV2/6 (Addgene #110660), pAAV2/6m (20), pAAV2/8 (Addgene #112864), pAAV2/9 (Addgene #112865), or pUCmini-iCAP-PHP.eB (Addgene #103005). Briefly, HEK293T cells were transfected with the packaging plasmids and a vector plasmid. Three days later, virus was collected from the cell lysates and culture media. Virus was purified through iodixanol gradient ultracentrifugation, washed with PBS, and concentrated with 100K PES concentrator (Pierce™, Thermo Scientific). Viral titers were determined by quantitative PCR with ITR primers (forward: 5-GGAACCCCTAGTGATGGAGTT-3; reverse: 5-CGGCCTCAGTGAGCGA-3).
Stereotactic brain injections.
Viruses were stereotactically injected into the striata of adult mice as described previously (24). Briefly, mice were placed on a stereotactic frame (Kopf, USA) under isoflurane anesthesia. A vertical skin incision exposed the Bregma on the skull used for guiding the location of the Burr hole. Injection coordinates were as follows: AP +1.0 mm, ML −2.0 mm, and DV −3.0 mm. 2 μL of lentivirus with an original titer of ~2e9 cfu/mL or 2 μL of AAV with an original titer of 0.5–8e13 GC/mL was injected using a 10-μL Hamilton syringe and a 33-gauge beveled tip metal needle. Virus was injected at a rate of 0.5 μL/min until the total volume was delivered. The needle was left in place for 5 minutes before it was slowly removed from the brain.
Immunohistochemistry and quantification.
Mice were sacrificed and fixed by intracardial perfusion with 4% paraformaldehyde in PBS. Brains were dissected out, post-fixed overnight and cryoprotected with 30% sucrose at 4°C for 48 hrs. Coronal brain sections were sectioned on a sliding microtome set at 40 μm thickness. The following primary antibodies were used: GFP (GFP-1020, chicken, 1:1000, Aves Labs), GFAP (ab4674, chiken, 1:500, Abcam), IBA1 (019-19741, rabbit, 1:1000, Waco), and NeuN (266 004, guinea pig, 1:500, Cedarlane-synaptic system). Alexa Fluor 488-, 594- or 647-conjugated corresponding secondary antibodies from Jackson ImmunoResearch were used for indirect fluorescence. Nuclei were counterstained with Hoechst 33342 (Hst). Images were captured and examined by a Zeiss LSM 700 confocal microscope. Three to five random confocal images with marker-positive cells around the injection side were analyzed for each animal. Microglia-specificity was calculated as % of GFP+IBA1+/IBA1+ cells, whereas viral transduction efficiency was calculated as % of GFP+IBA1+/IBA1+ cells.
Supplementary Material
Significance Statement.
Brain microglia play critical roles in human health and diseases. Genetic manipulation of these cells will offer numerous therapeutic opportunities. However, there is a lack of relevant strategies to target these cells with high specificity since they are traditionally considered to be refractory to virus transduction. Through in vivo screening of many promoters, this study identified a short promoter from the human IBA1 gene. When incorporated into lentivirus or adeno-associated virus vectors, this promoter proves effective in driving gene expression with high specificity for brain microglia. Such a simple strategy will facilitate specific approaches for microglia-based research.
Acknowledgments
We thank members of the C.-L.Z. laboratory for helpful discussions, reagents and technical assistance, Yuhua Zou for maintaining mouse colonies, and Paramita Chakrabarty for providing AAV6 mutant capsid.
Funding Statement
C.-L.Z. is a W.W. Caruth, Jr. Scholar in Biomedical Research and supported by the TARCC, the Decherd Foundation, the Pape Adams Foundation, and NIH grants NS092616, NS127375, NS117065, and NS111776.
Footnotes
DECLARATIONS
Conflict of interests
A patent application was filed on using viruses to target microglia/macrophages.
Availability of data and material
All data generated or analyzed during this study are included in the article or its supplementary information files.
REFERENCES
- 1.Colonna M., Butovsky O., Microglia Function in the Central Nervous System During Health and Neurodegeneration. Annu Rev Immunol 35, 441–468 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kanmogne M., Klein R. S., Neuroprotective versus Neuroinflammatory Roles of Complement: From Development to Disease. Trends Neurosci 44, 97–109 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Navarro V. et al. , Microglia in Alzheimer’s Disease: Activated, Dysfunctional or Degenerative. Front Aging Neurosci 10, 140 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu Y., Dissing-Olesen L., MacVicar B. A., Stevens B., Microglia: Dynamic Mediators of Synapse Development and Plasticity. Trends Immunol 36, 605–613 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leng F., Edison P., Neuroinflammation and microglial activation in Alzheimer disease: where do we go from here? Nat Rev Neurol 17, 157–172 (2021). [DOI] [PubMed] [Google Scholar]
- 6.Bachiller S. et al. , Microglia in Neurological Diseases: A Road Map to Brain-Disease Dependent-Inflammatory Response. Front Cell Neurosci 12, 488 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen Y., Colonna M., Two-faced behavior of microglia in Alzheimer’s disease. Nat Neurosci 10.1038/s41593-021-00963-w (2021). [DOI] [PubMed] [Google Scholar]
- 8.Holtman I. R., Skola D., Glass C. K., Transcriptional control of microglia phenotypes in health and disease. J Clin Invest 127, 3220–3229 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Troutman T. D., Kofman E., Glass C. K., Exploiting dynamic enhancer landscapes to decode macrophage and microglia phenotypes in health and disease. Mol Cell 81, 3888–3903 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haery L. et al. , Adeno-Associated Virus Technologies and Methods for Targeted Neuronal Manipulation. Front Neuroanat 13, 93 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haggerty D. L., Grecco G. G., Reeves K. C., Atwood B., Adeno-Associated Viral Vectors in Neuroscience Research. Mol Ther Methods Clin Dev 17, 69–82 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bedbrook C. N., Deverman B. E., Gradinaru V., Viral Strategies for Targeting the Central and Peripheral Nervous Systems. Annu Rev Neurosci 41, 323–348 (2018). [DOI] [PubMed] [Google Scholar]
- 13.O’Carroll S. J., Cook W. H., Young D., AAV Targeting of Glial Cell Types in the Central and Peripheral Nervous System and Relevance to Human Gene Therapy. Front Mol Neurosci 13, 618020 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maes M. E., Colombo G., Schulz R., Siegert S., Targeting microglia with lentivirus and AAV: Recent advances and remaining challenges. Neurosci Lett 707, 134310 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aschauer D. F., Kreuz S., Rumpel S., Analysis of transduction efficiency, tropism and axonal transport of AAV serotypes 1, 2, 5, 6, 8 and 9 in the mouse brain. PLoS One 8, e76310 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Balcaitis S., Weinstein J. R., Li S., Chamberlain J. S., Moller T., Lentiviral transduction of microglial cells. Glia 50, 48–55 (2005). [DOI] [PubMed] [Google Scholar]
- 17.Wang S. K., Lapan S. W., Hong C. M., Krause T. B., Cepko C. L., In Situ Detection of Adeno-associated Viral Vector Genomes with SABER-FISH. Mol Ther Methods Clin Dev 19, 376–386 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Su W. et al. , Recombinant adeno-associated viral (rAAV) vectors mediate efficient gene transduction in cultured neonatal and adult microglia. J Neurochem 136 Suppl 1, 49–62 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cucchiarini M., Ren X. L., Perides G., Terwilliger E. F., Selective gene expression in brain microglia mediated via adeno-associated virus type 2 and type 5 vectors. Gene Ther 10, 657–667 (2003). [DOI] [PubMed] [Google Scholar]
- 20.Rosario A. M. et al. , Microglia-specific targeting by novel capsid-modified AAV6 vectors. Mol Ther Methods Clin Dev 3, 16026 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Niu W. et al. , In vivo reprogramming of astrocytes to neuroblasts in the adult brain. Nat Cell Biol 15, 1164–1175 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Su Z., Niu W., Liu M. L., Zou Y., Zhang C. L., In vivo conversion of astrocytes to neurons in the injured adult spinal cord. Nat Commun 5, 3338 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tai W. et al. , In vivo reprogramming of NG2 glia enables adult neurogenesis and functional recovery following spinal cord injury. Cell Stem Cell 28, 923–937.e924 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang Y. et al. , A single factor elicits multilineage reprogramming of astrocytes in the adult mouse striatum. Proc Natl Acad Sci U S A 119, e2107339119 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tai W., Zhang C. L., In vivo cell fate reprogramming for spinal cord repair. Curr Opin Genet Dev 82, 102090 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tai W., Xu X. M., Zhang C. L., Regeneration Through in vivo Cell Fate Reprogramming for Neural Repair. Front Cell Neurosci 14, 107 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Okada Y. et al. , Development of microglia-targeting adeno-associated viral vectors as tools to study microglial behavior in vivo. Commun Biol 5, 1224 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Young A. et al. , Targeted evolution of adeno-associated virus capsids for systemic transgene delivery to microglia and tissue-resident macrophages. Proceedings of the National Academy of Sciences 120, e2302997120 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin R. et al. , Directed evolution of adeno-associated virus for efficient gene delivery to microglia. Nat Methods 19, 976–985 (2022). [DOI] [PubMed] [Google Scholar]
- 30.Z. X. Cronin J, Reiser J., Altering the tropism of lentiviral vectors through pseudotyping. Curr Gene Ther 5, 387–398 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li C., Samulski R. J., Engineering adeno-associated virus vectors for gene therapy. Nat Rev Genet 21, 255–272 (2020). [DOI] [PubMed] [Google Scholar]
- 32.Naso M. F., Tomkowicz B., Perry W. L. 3rd, Strohl W. R., Adeno-Associated Virus (AAV) as a Vector for Gene Therapy. BioDrugs 31, 317–334 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McCarty D. M., Self-complementary AAV vectors; advances and applications. Mol Ther 16, 1648–1656 (2008). [DOI] [PubMed] [Google Scholar]
- 34.Dong J. Y., Fan P. D., Frizzell R. A., Quantitative analysis of the packaging capacity of recombinant adeno-associated virus. Hum Gene Ther 7, 2101–2112 (1996). [DOI] [PubMed] [Google Scholar]
- 35.Wu J. et al. , Self-complementary recombinant adeno-associated viral vectors: packaging capacity and the role of rep proteins in vector purity. Hum Gene Ther 18, 171–182 (2007). [DOI] [PubMed] [Google Scholar]
- 36.Smirnova L. et al. , Regulation of miRNA expression during neural cell specification. Eur J Neurosci 21, 1469–1477 (2005). [DOI] [PubMed] [Google Scholar]
- 37.Colin A. et al. , Engineered lentiviral vector targeting astrocytes in vivo. Glia 57, 667–679 (2009). [DOI] [PubMed] [Google Scholar]
- 38.Gray S. J. et al. , Optimizing promoters for recombinant adeno-associated virus-mediated gene expression in the peripheral and central nervous system using self-complementary vectors. Hum Gene Ther 22, 1143–1153 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Merienne N., Le Douce J., Faivre E., Deglon N., Bonvento G., Efficient gene delivery and selective transduction of astrocytes in the mammalian brain using viral vectors. Front Cell Neurosci 7, 106 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang L. L. et al. , Revisiting astrocyte to neuron conversion with lineage tracing in vivo. Cell 184, 5465–5481 e5416 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.He W. et al. , Development of a synthetic promoter for macrophage gene therapy. Hum Gene Ther 17, 949–959 (2006). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in the article or its supplementary information files.