Abstract
Recent advances have expanded the role of lipid droplets (LDs) beyond passive lipid storage, implicating their involvement in various metabolic processes across mammalian tissues. Neuronal LDs, long debated in existence, have been identified in several neural structures, raising questions about their contribution to neurodegenerative disorders. Elucidating the specific chemical makeup of these organelles within neurons is critical for understanding their implication in neural pathologies. This study outlines an improved methodology to stimulate and isolate mature LDs from cultured primary neurons, offering insights into their unique lipid-protein composition. Integrating this method with high-throughput techniques may unveil disease-specific alterations in lipid metabolism, providing avenues for potential therapeutic interventions.
Keywords: Neurodegenerative disorders, Neurons, Lipid droplets, Lipidomic
INTRODUCTION
Lipid droplets (LDs) have been traditionally viewed as a passive reservoir of the esterified lipids in adipocytes. Recent advancements in imaging and biochemical techniques have, however, detected the presence of LDs in most mammalian tissues and confirmed their contribution to various metabolic processes ranging from energy production, and membrane synthesis to signaling pathways (see Farese et. al.7 and Olzmann et. al.4 for a comprehensive review). Neurons, with their complex polarized morphology and high energy demand, are not exempt from the importance of lipid metabolism, and lipids are recently gaining attention as a potential key player in the functioning and malfunctioning of neurons. Contrary to the prevalent belief that LDs are non-existent in neurons, it has been detected in motor neurons of drosophila8, axons of Aplysia9, cerebral cortex of mice brain10,11, and neuroblastoma-derived cell lines12.
Neurodegenerative diseases, like Alzheimer’s disease, Parkinson’s disease, and Hereditary spastic paraplegia are multifactorial disorder that shares a common theme of progressive degeneration of neurons, but the precise etiology of these disorders remains elusive. Interestingly, abnormal accumulation of LDs in neurodegenerative disorders10,13,14 has raised the question of whether the disability of triglyceride mobilization from LDs contributes to the cellular dysfunction and deterioration of neurons in specific brain regions of patients. A large body of evidence suggests that neuronal LDs might serve as the site for accumulating both neuroprotective and toxic lipid, and protein species15,16. Moreover, we recently discovered that electrical activity triggers DDHD2 to facilitate transfer of fatty acids (FAs) from the triglyceride-rich LDs to mitochondria for ATP synthesis in dissociated hippocampal neurons. Consequently, comprehending the exact contributions of the lipids and proteins associated with LDs in neuronal function demands their isolation without any contamination of other cellular organelles. Here, we present a refined and highly reproducible method to facilitate the formation of mature LDs in cultured primary neurons and their purification using the sucrose density-gradient method.
Advantages
To the best of our knowledge, this is the first study where we successfully induced LD formation in dissociated neurons and purified them using a density-based floatation method. We carried out lipidomic studies on purified neuronal LDs to identify previously unknown lipids associated with them. The present study provides a snapshot of lipid-protein milieu linked to neuronal LDs. A successful integration of the method with high-throughput omics techniques provides an excellent opportunity to identify targets of disease-specific alterations in lipid and protein composition of neuronal LDs.
Applications
Neurons are polarized cells, whose architecture can be divided into soma, dendrites, and axons. Axons extend further away from the soma and support the release of neurotransmitter, that in turn requires a constant supply of ATP at nerve terminals. We recently discovered that the triglyceride lipase DDHD2 is highly enriched at the synaptic terminals and blocking its activity leads to local LD accumulation. These data suggests that neurons constantly use TGs to maintain synaptic functions. Pharmacological inhibition of the TG lipase results in the formation of near micron size LDs in dissociated cortical neurons, which in turn allow the purification of organelle suitable for analysis of lipid and protein composition.
Limitations
Lipids on the monolayer membrane of LDs are synthesized at the ER leaflets or on-site of maturing LDs. Proteins, on the other hand are trafficked from the ER membrane via membrane bridge or from the cytosol by amphipathic α-helices. Certain lipids, and cytosolic proteins in particular, are susceptible to degradation or dissociation during the physical purification process and might not reflect in the downstream omics studies. Furthermore, the detailed composition of LDs depends on their size, and therefore analytical approaches that report population averages, such as typical mass spectrometry, necessarily miss size-based variation in LD content. Therefore, further biochemical and immunocytochemical methods applied on either intact neurons or isolated LDs are warranted to discern the native and size dependent composition and functions of neuronal LDs.
MATERIALS
-
Biological
Animals: Wild-type rats and mice used for the experiments were of the Sprague-Dawley strain (Charles River code 400, RRID: RGD_734476), and CD1 backgrounds respectively. !CAUTION All animal-related experiments were performed in accordance with protocols approved by the Weill Cornell Medicine and Yale University School of Medicine IACUC.
-
Chemical
1X PBS: (ThermoFisher, Cat. # 10010031)
KLH45: Sigma, Cat. # SML1998
PIPES: Sigma, Cat. # P6757
EGTA: Sigma, Cat. # E3889
MgSO4: Sigma, Cat. # M3409
KOH: Sigma, Cat. # 221473
Complete Protease Inhibitor Cocktail: Roche, Cat. # 45-11697498001 ΔCritical Prepare fresh before use.
PMSF: Sigma, Cat. # P7626 !CAUTION PMSF is acutely toxic. Use disposable gloves while handling.
Sucrose: Sigma, Cat. # S0389
Glyceryl trioleate: Sigma, Cat. # T7140
CellMask™ plasma membrane stain: Invitrogen, Cat. # C10045
BODIPY: Thermo Scientific, Cat. # D3922
TLC plates: Millipore, Cat. # 1.05721.0001
CuSO4: Supelco, Cat. # 102791
H3PO4: Sigma, Cat. # 345245
Organic solvents: Chloroform (Sigma, Cat. # 288306); Methanol (Sigma, Cat. # 34860); n-Hexane (Sigma, Cat. # 34859); Diethyl ether (Sigma, Cat. # 309966); Acetic acid (Sigma, Cat. # 320099) !CAUTION The organic solvents are highly toxic and unstable explosive. Avoid contact with flames and always handle them in the fume hood. Dispose them in accordance with the institutional and local guidelines.
Lipids: Glyceryl trioleate (Sigma, Cat. # T7140); SPLASH® LIPIDOMIX Mass Spec standard (Avanti, Cat. # 330707-1EA) ΔCritical Lipids are unstable and prone to oxidation under atmospheric conditions. So parge the lipids with N2 gas before the storage. Avoid using plastic or polymer tubes for storage of the lipids. Use glass vials for the storage of lipids dissolved in chloroform or any other organic solvent.
-
Equipments
Cell disruption nitrogen chamber: Parr Instrument, Cat. # 4639
Ultracentrifuge: Beckman Coulter, Optima L-100 XP ultracentrifuge
SW40 Ti rotor: Beckman Coulter, Cat. # 331302)
SW40 14-ml ultracentrifuge tube: Beckman Coulter, Cat. # 331374
Needles and plastic syringes
Hamilton syringes: 1-10 μl 10-100 μl and 0.1-1 ml
Microcapillary pipets: Kimble Glass, Cat. # 71900-25
Particle analyzer: Anton Paar Litesizer 500 equipped with Kalliope 2.8.0 software
Microscope: Zeiss LSM880 laser scanning confocal microscope
ImageJ software: https://imagej.nih.gov/ij/
Agilent Poroshell 120 (EC-C18, 2.1 x 100 mm, 2.7 μm, 1000 bar) HPLC column
Agilent 1290 Infiniti II LC system
Agilent 6546 LC/Q-TOF mass spectrometer
PROCEDURE
- Primary culture of Cortical neurons
-
1Coat 10 nos. culture dishes of 150 mm size with 50 μg/ml poly-d-lysine for 16 hours before starting the procedure of cortical culture.
-
2Dissect out both the cortices of brain from postnatal day 0 (P0) mouse or rat pups and place in ice cold HBSS.Δ Critical step: Dissect out and remove the parts of the brain other than cortex to ensure the purity of the cortical neurons. Other regions of the brain such as hippocampus, cerebellum etc. can also be cultured separately using specific protocols for a comparative study.
-
3Slice the cortices into small pieces (~1 mm) using a dissection knife and allow digestion in HBSS solution containing papain (20 u/ml) and DNase (20 μg/ml) for 15-20 minutes at 37 °C.
-
4Triturate the digested tissue slices by pipetting back and forth with 1 ml tip and filter the debris out using a 40 μm cell strainer.Δ Critical step: Cell strainer with wider mesh size may allow cell debris to pass through, while ones with narrow mesh size will restrict the passage of dissociated cortical cells. Wet the strainer with 5 ml HBSS before pouring cell suspension to prevent clogging.
-
5Wash the dissociated cortical cells with HBSS and plate in the coated petri plates at 12,000 - 20,000 cells/cm2 density.
-
6Exchange the plating media after 3 hours with neurobasal media containing 2% B-27 and 0.5 mM L-glutamine.
-
7Maintain the cortical neurons at 37 °C in humidified incubator containing 5% CO2.
-
8Replenish 30% of the total volume with prewarmed complete neurobasal media at DIV4, 7 and 14.
-
1
- Induction of Lipid droplets in cortical neurons
-
9Administer KLH45 (5 μM) into the cortical neurons cultured in complete neurobasal media on DIV-14 to DIV-21 and return to the incubator for 24 hours.Δ Critical step: To confirm the action of KLH45 and subsequent induction of LDs, stain the neurons parallelly cultured in chamber slide with BODIPY 493/503 (follow step 25) and image with fluorescence or confocal microscope.
-
9
- Disruption of cortical neurons
-
10Rinse the neurons twice with ice cooled 1X PBS.
-
11Pour 400 μl 0.9M MEPS buffer (ice cooled supplemented with 1X protease inhibitor cocktail and 0.2 mM PMSF) to the layer of cortical neurons and scrap using a sterile plastic cell-scrapper.
-
12Transfer the cells to a pre-cooled cell disruption vessel. Dissolve oxygen-free nitrogen gas to the cytosol for 20 minutes under high pressure (750 pounds per square inch, psi).
-
13Connect the release port to a collection chamber on ice by silicon pipe and release the pressure slowly to collect the disrupted neurons.Δ Critical step: Mount 30 μl lysed neurons between two glass slides (thickness: 0.18 mm) and observe onto light microscope at 40X magnification to confirm the lysis of cortical neurons. Higher pressure leads to complete homogenization of the cells as well as membranous organelles, therefore optimize the pressure and time for N2 cavitation is required to achieve near complete lysis of neurons.
-
10
- Separation of intact neurons, ruptured cell membrane and nucleus
-
14Transfer the lysed neurons into a fresh 15 ml falcon tube and centrifuge at 1800 g for 10 minutes at 4 °C.
-
15Collect the supernatant (called post-nuclear supernatant or PNS) in a fresh tube and place on ice until the gradient is set-up. An aliquot of 500 μl PNS can be retained on ice or snap frozen in liquid nitrogen to be use as control for purification efficiency.
-
14
- Purification of LDs by density-based floatation
-
16Transfer 2.5 M MEPS at the bottom of a clear SW-41 centrifuge tube and overlay PNS over it. Gently mix the two layers with cut-tip pipette.Δ Critical step: The ratio of MEPS (2.5 M) and PNS (prepared in 0.9 M MEPS) should be such that the final molarity of the mixture is ≥ 1.5. Split the PNS and set up gradient in two SW-41 tubes that can be easily balanced against each other during ultracentrifugation.
-
17Overlay the bottom layer with 2 ml each of 1.2 M and 0.5 M MEPS from the bottom to top carefully without disturbing the interfaces.Δ Critical step: Use a smooth pipette with cut-tips to layer the sucrose gradients. A messy layering of the sucrose solutions will result in the incomplete isolation of LDs and contamination of other organelles.
-
18Overlay 2-3 ml of 0 M MEPS over the layer of 0.5 M MEPS.Δ Critical step: The volume of the top layer of 0 M MEPS should be up to the maximum permissible height of the SW41 tube. Larger the volume of the top layer, purer will the isolated LDs. Salts like 100 mM sodium carbonate can be added to the top layer to remove the loosely bound non-specific proteins to the LDs35.
-
19Transfer the SW-41 tubes to pre-cooled (at 4°C) SW-41-Ti swinging buckets and centrifuge at 1,74,000 g for 1.5 hours at 4 °C.
-
20Transfer the buckets on the ice and collect the floating top layer of LDs using 25 gauge slanted needle attached to a 1 ml syringe.Δ Critical step: Collect the LD sample with the minimum possible volume of 0 M MEPS buffer without disturbing the underlaying layers containing undesired membranes and cytosolic proteins. If the LDs appear dilute, centrifuge the sample at 14,000 g at 4 °C for 10 minutes and carefully remove the buffer at the bottom using a fine needle without disturbing the floating LD layer on the top.
-
21Snap freeze aliquots of the isolated LDs samples in liquid nitrogen and store at −80 °C to preserve the lipids and LD associated proteins until further use.Δ Critical step: The LDs samples can be used within 6 months after preparation. Always thaw the LD samples on the ice.
-
16
- Dynamic light scattering
-
22Dilute the LD samples in ultrapure water (1:50) and load onto dynamic light scatter particle analyzer (Anton Paar Litesizer 500).
-
23Use the Kalliope 2.8.0 software to acquire the size distribution of LDs following manufacturer’s guidelines.
-
22
- Confocal micrography
-
24Prepare a staining solution containing 0.5X CellMask™ PM Stain (from 1000X stock) and 1X BODIPY 493/503 (from 1 mg/ml, i.e. 1000X stock) in 1X PBS.
-
25Transfer 10 μl of LDs sample or 1:100 diluted membrane samples to two separate tubes containing freshly prepared staining solutions and incubate for 10 minutes at room temperature.
-
26Mount the stained samples on clean glass slides under coverslips and seal the edges with clear nail polish.Δ Critical step: Washing of the unbound CellMask™ and BODIPY493/503 stains are not required. The optimum concentration of the stains may vary between 0.5X to 2X depending on the size and density of the LDs.
-
27Image the samples on confocal microscope equipped with appropriate lasers for CellMask™ and BODIPY493/503 stains and analyze the images using ImageJ software.
-
24
- Thin layer chromatography
-
28Thaw an aliquot (100-150 μl) of purified LD sample on ice, adjust the volume to 500 μl with ultrapure water and transfer it to a clean glass tube.
-
29Add 2 ml methanol and 1 ml chloroform to the tube, vortexed the mixture for 1 minute and place it at 4 °C for 14-16 hours.
-
30Add 1 ml each of chloroform and water to the tube, vortex it again for 1 minute and allow it to sit undisturbed at room temperature for 30 minutes, or until the organic phase (lipid containing lower layer) is distinctly separated from aqueous phase (protein containing upper layer).Δ Critical step: If the protein content of sample is too high, the phases are not separated well under gravity. To separate such samples, prepare the sample in glass centrifuge tube and spin at 100-200 g for 10 minutes at 4 °C.
-
31Transfer the organic phase to another clean glass tube and evaporate the solvent under stream of oxygen-free nitrogen gas.
-
32Resuspended the dried lipids in 30 μl chloroform and spot them onto a silica TLC plate that has been pre-cleaned with chloroform.
-
33Separate the spotted lipids by two-solvent systems A and B, first half in solvent system A [n-hexane/diethyl ether/acetic acid (60:40:4)] followed by solvent system B [n-hexane/diethyl ether (59:1)]. Air-dry the solvent from TLC plate in a chemical hood.
-
34Visualize the lipids by spraying the TLC plate with a solution of 10% CuSO4 and 8% H3PO4 followed by charring the plate for 15-20 minutes in a preheated oven at 180 °C.Δ Critical step: To monitor specific lipids in the sample, spot standard lipids on separate spots on the level of other samples on the same plate. In case of any suspicion of lipid contamination in solvents, include a spot of solvent blank.
-
35Capture the image of the TLC plate using a grayscale scanner and quantify the band intensities along the lipid migration lengths using ImageJ software.
-
28
- Extraction of lipids for LCMS/QToF
-
36Thaw LD samples on ice and transfer to 2 ml click-seal microcentrifuge tube.
-
37Add MTBE (methyl-tert-butyl ether) and spike the samples with Splashmix as the internal standards.
-
38Sonicate the samples for 15 minutes at ice-cooled sonication bath.
-
39Centrifuge the sample at 1000 g for 10 minutes at 4 °C to separate the lipid-containing phase from the precipitated pellet of proteins.
-
40Transfer the lipid-containing organic phase to 1.5 ml microcentrifuge tube and evaporate the solvents using a vacuum concentrator without heating.
-
41Resuspend the extracted lipids in n-butanol.Δ Critical step: This step should be performed in cold room maintained at 4 °C. Any bubble in the LC vials should be removed by gently flickering the tube with your fingers.
-
36
- LCMS/QToF
-
42Load 3μl of this mixture onto an Agilent Poroshell 120 (EC-C18, 2.1 x 100 mm, 2.7 μm, 1000 bar) column using an Agilent 1290 Infiniti II LC.
-
43Use Mobile phase A (60% Acetonitrile, 40% H2O, 7.5 mM Ammonium Acetate) and Mobile phase B (90% IPA, 10% Acetonitrile, 7.5 mM Ammonium Acetate) to separate peaks for detection on an Agilent Quadrupole Time-Of-Flight 6546 mass spectrometer.
-
44The gradient started at 85% (15% B) and decreased to 70% A over 2 minutes and then 52% A over 30 seconds. The gradient then slowly decreased to 18% A over 12.5 minutes and then 1% A in one minute and these concentrations held for 4 minutes.
-
45Allow the gradient to restore at 85% A and wash the column for next 5 minutes.
-
46Identify the lipids using MS-DIAL 4.7 (Ref. 36) and quantify each lipid species using the moles of corresponding class-specific lipid standard present in Splashmix.
-
42
Recipe
KLH45: Add 2.18 ml DMSo to a vial of 5 mg KLH45 to prepare a 5 mM stock solution. Vortex for 5 min and store the aliquots at −20 °C. The solution is stable up to 6 months under strictly maintained temperature.
MEPS buffers: Prepare sucrose solutions of 0.9 M, 2.5 M, 1.2 M, 0.5 M & 0 M (M indicates molarity of sucrose) in ultrapure water. Add PIPES (35 mM, pH 7.2), EGTA (5 mM) and MgSO4 (5 mM) to the sucrose solutions and maintain the pH to 7.2. The filtered stocks solutions can be used up to 6 months after preparation, if stored at 4°C. Add freshly prepared protease inhibitor cocktail (1X) immediately before starting the experiment. Δ Critical PIPES is not readily soluble in water at pH ≤ 7. Prepare a 1 M stock solution of PIPES by dissolving it in water and, while stirring, adjust the pH to 7.2 using KOH pellets. To facilitate the solubilization of 2.5 M sucrose, immerse the tube in a water-bath set at temperature 70 °C.
TLC solvent systems: Prepare 208 ml TLC ‘solvent A’ by mixing 120 ml n-hexane, 80 ml diethyl ether and 8 ml acetic acid (ratio 60:40:4); and 240 ml TLC ‘solvent B’ by mixing 236 ml n-hexane and 4 ml diethyl ether (ratio 59:1). Δ Critical TLC ‘solvent A’ and ‘solvent B’ should be prepared and stored in narrow-mouth glass containers with glass stopper. It can be used within for 6 months unless exposed to air that may change the composition of solvents.
Extraction solvent: Add 5 ml methanol to 15 ml methyl tert-butyl ether (MTBE) to prepare 20 ml extraction solvent. Δ Critical The solvent is highly volatile and therefore should not left open in air.
Mobile phase A: Prepare the required volume of the phase A by mixing the following: 60% LCMS-Grade Acetonitrile, 40% MilliQ Water and 7.5 mM Ammonium Acetate.
Mobile phase B: Prepare the required volume of the phase B by mixing the following: 90% LCMS-Grade IPA, 10% LCMS-Grade Acetonitrile and 7.5 mM Ammonium Acetate.
Troubleshooting
| Problem | Possible reasons | Potential Solutions |
|---|---|---|
| Underdeveloped neuronal processes | 1. Neurons were not healthy 2. Culture used earlier than DIV-14 |
1. Plate neurons at adequate density and ensure the quality of culture media 2. Allow neurons in culture for DIV-14 or more |
| Failure of LD induction | KLH45 was degraded | Reconstitute fresh KLH45, store at appropriate temperature in small aliquots and avoid freeze-thaw cycle |
| Incomplete lysis of neurons | Pressure of nitrogen in cell disruption vessel was low | Increase the pressure in nitrogen vessel |
| Low count of LDs | 1. Incomplete lysis of cells 2. Gradients layers of high volume 3. Loss during LD collection |
1. Ensure complete lysis of neurons 2. Use smaller tube for small samples 3. Tube slicer can be used |
| Aggregation of LDs | Electrolyte in the solution was low | 1. Check the concentration and pH of buffers 2. Additional floatation step in low concentration of glycerol, 0.1-1 M NaCl or 100 mM Na2CO3 (pH 11.5)35 |
| Disrupted LD membrane | Homogenization was too harsh | Reduce the nitrogen pressure |
| Contamination of membrane | 1. Wide diameter tube used 2. Density gradient was messy |
1. Preferably use tubes of narrow diameter for prep. with small number of neurons 2. Use smooth pipette with wide orifice tips on a sturdy platform |
| High phospholipid in TLC | Membrane contamination | Follow the above steps and ensure the quality of LDs before lipid extraction |
| Smearing of protein bands in western blot | 1. Proteins were degraded 2. Incomplete extraction of lipids during protein precipitation |
1. Add protease inhibitors to all MEPS buffers and set-up the gradient on ice 2. Increase the centrifugation speed to separate the lipid phase from proteins |
Discussion
Our knowledge about lipid droplets has evolved substantially in past few decades, transitioning from a passive depot of lipids to the dynamic organelle involved in diverse cellular functions. Recent studies establish dysregulation of lipids and excessive accumulation LDs as hallmark of metabolic disorders such as obesity37, diabetes38, atherosclerosis39 and fatty liver disease40. However, despite the multiple reports of lipid accumulation in neurodegenerative disorders, the proteo-lipid identity of the organelle in neurons remained completely unknown. The establishment of a methodology facilitating the isolation of lipid droplets from neurons stands as a pivotal advancement, allowing focused exploration into the role of these organelles in neural functions. This method offers an opportunity to study lipid dynamics in neuronal metabolism, shedding light on their contributions to energy production and signaling, critical for comprehending neurodegenerative conditions. The successful deployment of the isolation technique also holds the promise in identifying novel biomarkers and disease-specific alterations, potentially elucidating the mechanisms underlying neurodegenerative disorders characterized by aberrant LD accumulation. However, acknowledging potential constrains such as size-dependent variations and alteration in lipid-protein compositions during purification process, complementary approaches like FACS, SILAC, and proximity-labelling may be indispensable in addressing size-dependent variations in LD protein complexes. Overall, this methodology marks a significant stride in neurobiology and lipid research, offering an entry point to unravel the complexities of LDs in neural contexts and their implications in neurological disorders.
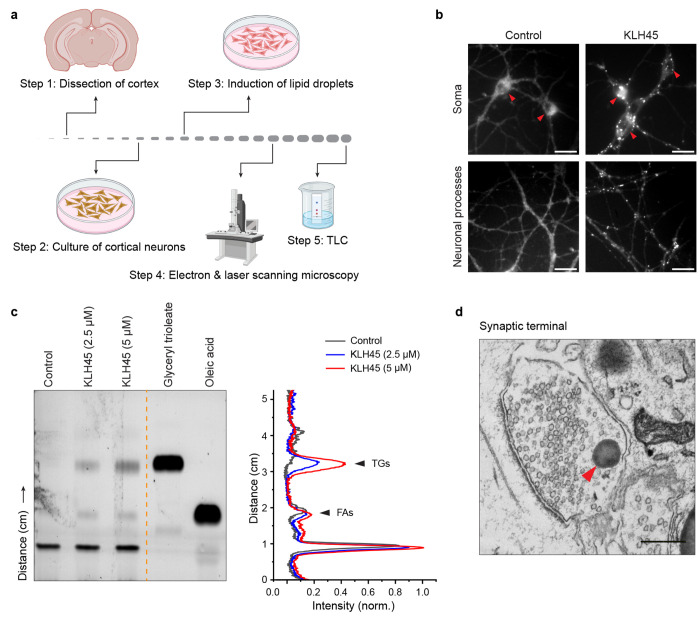
Fig.1|.
Induction of lipid droplets in dissociated cortical or hippocampal neurons. a, Schematic illustration of protocol steps to induce formation of LDs in the cortical neurons and confirmation by microscopy and thin layer chromatography (TLC). The cortical neurons from rodent brain (coronal section shown) cultured on poly-d-lysin coated dishes are treated with KLH45 at DIV 14-21. b, Fluorescence micrographs of control and KLH45 treated cortical neurons after BODIPY staining. Lipids droplets are apparent as globular structures both in the soma (red arrowheads) and neuronal processes after KLH45 induction. scale bar: 20 μm. c, TLC showing the dose dependent accumilation of triglyceride (TGs) in the cortical neurons after inhibition of neuron-specific triglyceride lipase with KLH45. Standard TG (glyceryl trioleate) and free fatty acid (oleic acid) are shown on the right side of yellow line as references. Line intensity profile of control and two KLH45 tretaed (2.5 μM and 5 μM) neurons is shown in the right panel. d, Electron micrograph showing accumulation of LDs (red arrowhead) in the vicinity of synaptic vesicles at a synaptic terminal of a hippocampal neuron. scale bar: 500 nm.
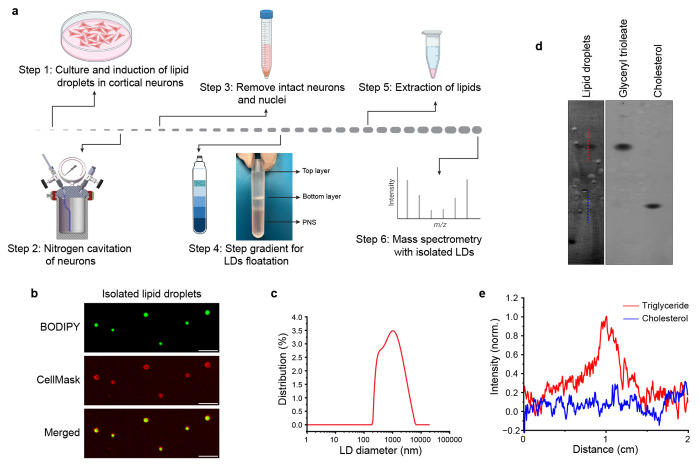
Fig.2|. Isolation of cortical lipid droplets and their physical characterisation.
a, Schematic illustration for the steps for the isolation of lipid droplets from KLH45 induced cortical neurons using sucrose step-density gradient. The LDs are collected from the top layer of sucrose density gradient as shown in step 4. b, Laser scanning confocal micrographs of the purified LDs stained with BODIPY (triglyceride rich neutral core) and CellMask (phospholipid rich monolayer), scale bar: 5 μm. c, Size distribution of the cortical lipid droplets analyzed by dynamic light scattering instrument, Anton Paar Lifesizer 500. The size of the LDs varies from 200 nm to 5 μm. d, Thin layer chromatography with the lipid droplets isolated from cortical neurons. Triglyceride (Glyceryl trioleate) and cholesterol are shown on the right side for reference. d, Line intensity profile plotted for the triglyceride and cholesterol (shown by red and blue lines in d). The intensity profile indicates that the LDs are highly enriched with triglycerides.
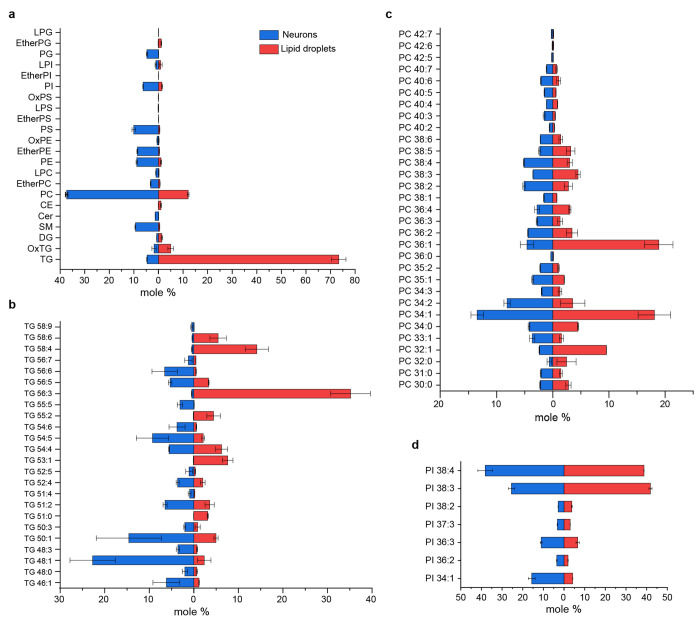
Fig.3|. Lipidomic analysis purified LDs from cortical neurons and comparision with whole neuron lipids.
a, Distribution of lipid classes identified by LCMS performed with the lipids extracted from LDs and whole neuron. The lipid classes include LPG: Lysophosphatidylglycerol, EtherPG: Ether-linked phosphatidylglycerol, PG: Phosphatidylglycerol, LPI: Lysophosphatidylinositol, EtherPI: Ether-linked phosphatidylinositol, PI: Phosphatidylinositol, OxPS: Oxidized phosphatidylserine, LPS: Lysophosphatidylserine, EtherPS: Ether-linked phosphatidylserine, PS: Phosphatidylserine, OxPE: Oxidized phosphatidylethanolamine, EtherPE: Ether-linked phosphatidylethanolamine, PE: Phosphatidylethanolamine, LPC: Lysophosphatidylcholine, EtherPC: Ether-linked phosphatidylcholine, PC: Phosphatidylcholine, CE: Cholesteryl ester, Cer: Ceramide, SM: Sphingomyelin, DG: Diacylglycerol, OxTG: Oxidized triglyceride, TG: Triglyceride. The graph reprensents mole % (of total lipid) of indicated lipid classes in the two samples. b-d, Bar graphs represent the top three enriched lipids species (TG, PC and PI) identified in neuronal lipid droplets compared with whole neuron lipids. TG and PC represents the two major lipids in the LD fraction. Data is represented as mean ± SE. Complete lipid profile is attached as supplementary information with the manuscript.
Table 1:
Methods for lipid droplets purification
| Method | Principle | Biological source | Size of LDs | Ref. |
|---|---|---|---|---|
| Differential Centrifugation | Size and density | CHO K2, Liver, Brown adipose tissue | 0.5-10 μm | 17,18 |
| Density Gradient Centrifugation | Buoyant density | Liver, 3T3-L1 adipocytes | 0.5-5 μm | 19,20 |
| OptiPrep™ Density Gradient Medium | ER kit based | Liver | 0.27-6.37 μm | 21 |
Table 2:
Purification of lipid droplets from animal tissues
| Tissues | Species | Buffers | Size of LDs | Ref. |
|---|---|---|---|---|
| Liver | Rat | KCl; NaCl, Sucrose | 0.5-2 μm | 18,20 |
| Brown adipose tissue | Mouse | Tricine, Sucrose | 0.5-20 μm | 22 |
| White adipose tissue | Mouse | Tris, Na3VO4 NaF, EDTA | NA | 23 |
| Skeletal muscle | Mouse | Buffer A: Tricine, Sucrose Buffer B: HEPES, KCl, MgCl2 | < 1 μm | 24 |
| Cardiac muscle | Mouse | Tris-EDTA-Sucrose | < 1 μm | 25 |
| Mammary tissue | Mouse | KH2PO4, MgCl2, Sucrose | 0.5-6 μm | 26,27 |
| Intestinal mucosa | Mouse | EDTA | 0.8 ± 0.4 μm | 28 |
| Kidney (Renal papillae) | Pig, Rabbit, Dog, Rat | Sucrose | 0.4-0.8 㮼m | 29,30 |
| Kidney (Renal medulla) | Rat, Rabbit | Sucrose | ~0.5 μm | 31,32 |
| Placenta | Human | Tris-EDTA-Sucrose; Buffer A: Tricine, Sucrose Buffer B: HEPES, KCl, MgCl2 | < 1 μm | 33,34 |
NA: not analyzed
Historic overview.
Lipid droplets as an organelle remained ignored for almost a century after its discovery in 19th century by Richard Altmann and E.B Wilson1,2. A series of discoveries in 1990s outlined the importance of their role in cellular functioning and metabolic disorders reignited interest and prompted the development of techniques for LD isolation from live tissues3,4. Irrespective of their source, LDs mainly constitute a core of triglycerides and sterol esters that makes them highly buoyant in aqueous medium enabling their separation from other organelles by floatation. Initial attempts to systematically isolate LDs from plant-based sources date back to 1970s5,6. Subsequent refinement in the methodology (See table 1) extraction of the purest form of LDs from animal sources for lipidomic and proteomic studies devoid of any membrane contamination (See table 2). Isolation of pure LDs requires careful disruption of cell membrane without rupturing internal membranous organelles. Moreover, the buoyancy of the LDs varies with the size of LDs (100 nm to more than 20 μm in some cell types) and their association of organelles (ER, mitochondria, endosomes etc.) prompted modifications in LD purification protocols to avoid co-isolation of undesirable membrane fragments.
Acknowledgements
We thank Yumei Wu and Pietro De Camilli for providing EM image of synaptic terminal; Anoop N. Pillai, Grace Dearden and Anant Menon for providing lab facilities for TLC analysis; Rachel McAllister for critical insights in lipidomic analysis. This research was supported in part by NIH grants (NS036942 & NS11739) to TAR and in part by Aligning Science Across Parkinson’s ASAP-000580 and ASAP-020608 through the Michael J. Fox Foundation for Parkinson’s Research (MJFF). For open access, the authors have applied a CC BY public copyright license to all Author Accepted Manuscripts arising from this submission.
Footnotes
Competing Interest Statement: The authors declare no competing interests.
Data availability
The source data related to the findings of the manuscript are provided as supplementary information with this manuscript and can be used for scientific purposes with permission of corresponding authors.
References
- 1.Altmann R. Die Elementarorganismen und ihre Beziehungen zu den Zellen. (de Gruyter, 1894). [Google Scholar]
- 2.Wilson E. B. The cell in development and inheritance. (Macmillan, 1900). [Google Scholar]
- 3.Ding Y. et al. Isolating lipid droplets from multiple species. Nat Protoc 8, 43–51 (2013). 10.1038/nprot.2012.142 [DOI] [PubMed] [Google Scholar]
- 4.Olzmann J. A. & Carvalho P. Dynamics and functions of lipid droplets. Nat Rev Mol Cell Biol 20, 137–155 (2019). 10.1038/s41580-018-0085-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jacks T. J. Yatsu L. Y. & Altschul A. M. Isolation and characterization of peanut spherosomes. Plant Physiol 42, 585–597 (1967). 10.1104/pp.42.4.585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yatsu L. Y. Jacks T. J. & Hensarling T. P. Isolation of spherosomes (oleosomes) from onion, cabbage, and cottonseed tissues. Plant Physiol 48, 675–682 (1971). 10.1104/pp.48.6.675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Farese R. V. Jr. & Walther T. C. Lipid droplets finally get a little R-E-S-P-E-C-T. Cell 139, 855–860 (2009). 10.1016/j.cell.2009.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Papadopoulos C. et al. Spastin binds to lipid droplets and affects lipid metabolism. PLoS Genet 11, e1005149 (2015). 10.1371/journal.pgen.1005149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Savage M. J. Goldberg D. J. & Schacher S. Absolute specificity for retrograde fast axonal transport displayed by lipid droplets originating in the axon of an identified Aplysia neuron in vitro. Brain Res 406, 215–223 (1987). 10.1016/0006-8993(87)90785-2 [DOI] [PubMed] [Google Scholar]
- 10.Inloes J. M. et al. The hereditary spastic paraplegia-related enzyme DDHD2 is a principal brain triglyceride lipase. Proc Natl Acad Sci U S A 111, 14924–14929 (2014). 10.1073/pnas.1413706111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Renvoise B. et al. Reep1 null mice reveal a converging role for hereditary spastic paraplegia proteins in lipid droplet regulation. Hum Mol Genet 25, 5111–5125 (2016). 10.1093/hmg/ddw315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holtta-Vuori M., Salo V. T., Ohsaki Y., Suster M. L. & Ikonen E. Alleviation of seipinopathy-related ER stress by triglyceride storage. Hum Mol Genet 22, 1157–1166 (2013). 10.1093/hmg/dds523 [DOI] [PubMed] [Google Scholar]
- 13.Hamilton L. K. et al. Aberrant Lipid Metabolism in the Forebrain Niche Suppresses Adult Neural Stem Cell Proliferation in an Animal Model of Alzheimer’s Disease. Cell Stem Cell 17, 397–411 (2015). 10.1016/j.stem.2015.08.001 [DOI] [PubMed] [Google Scholar]
- 14.Han X. et al. Plin4-Dependent Lipid Droplets Hamper Neuronal Mitophagy in the MPTP/p-Induced Mouse Model of Parkinson’s Disease. Front Neurosci 12, 397 (2018). 10.3389/fnins.2018.00397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alarcon-Gil J. et al. Neuroprotective and Anti-Inflammatory Effects of Linoleic Acid in Models of Parkinson’s Disease: The Implication of Lipid Droplets and Lipophagy. Cells 11 (2022). 10.3390/cells11152297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cole N. B. et al. Lipid droplet binding and oligomerization properties of the Parkinson’s disease protein alpha-synuclein. J Biol Chem 277, 6344–6352 (2002). 10.1074/jbc.M108414200 [DOI] [PubMed] [Google Scholar]
- 17.Zhang S. et al. Morphologically and Functionally Distinct Lipid Droplet Subpopulations. Sci Rep 6, 29539 (2016). 10.1038/srep29539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DiAugustine R. P. Schaefer J. M. & Fouts J. R. Hepatic lipid droplets. Isolation, morphology and composition. Biochem J 132, 323–327 (1973). 10.1042/bj1320323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brasaemle D. L. Wolins N. E. Isolation of Lipid Droplets from Cells by Density Gradient Centrifugation. Curr Protoc Cell Biol 72, 3 15 11–13 15 13 (2016). 10.1002/cpcb.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ontko J. A. Perrin L. W. & Horne L. S. Isolation of hepatocellular lipid droplets: the separation of distinct subpopulations. J Lipid Res 27, 1097–1103 (1986). [PubMed] [Google Scholar]
- 21.Brettschneider J. et al. Rapid Lipid Droplet Isolation Protocol Using a Well-established Organelle Isolation Kit. J Vis Exp (2019). 10.3791/59290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu J. et al. Lipid droplet remodeling and interaction with mitochondria in mouse brown adipose tissue during cold treatment. Biochim Biophys Acta 1853, 918–928 (2015). 10.1016/j.bbamcr.2015.01.020 [DOI] [PubMed] [Google Scholar]
- 23.Kanshin E. et al. The stoichiometry of protein phosphorylation in adipocyte lipid droplets: analysis by N-terminal isotope tagging and enzymatic dephosphorylation. Proteomics 9, 5067–5077 (2009). 10.1002/pmic.200800861 [DOI] [PubMed] [Google Scholar]
- 24.Zhang H. et al. Proteome of skeletal muscle lipid droplet reveals association with mitochondria and apolipoprotein a-I. J Proteome Res 10, 4757–4768 (2011). 10.1021/pr200553c [DOI] [PubMed] [Google Scholar]
- 25.Wang H., Lei M., Hsia R. C. & Sztalryd C. Analysis of lipid droplets in cardiac muscle. Methods Cell Biol 116, 129–149 (2013). 10.1016/B978-0-12-408051-5.00008-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu C. C., Howell K. E., Neville M. C., Yates J. R. 3rd & McManaman J. L. Proteomics reveal a link between the endoplasmic reticulum and lipid secretory mechanisms in mammary epithelial cells. Electrophoresis 21, 3470–3482 (2000). [DOI] [PubMed] [Google Scholar]
- 27.Hood L. F. & Patton S. Isolation and characterization of intracellular lipid droplets from bovine mammary tissue. J Dairy Sci 56, 858–863 (1973). 10.3168/jds.S0022-0302(73)85267-1 [DOI] [PubMed] [Google Scholar]
- 28.Soayfane Z. et al. Exposure to dietary lipid leads to rapid production of cytosolic lipid droplets near the brush border membrane. Nutr Metab (Lond) 13, 48 (2016). 10.1186/s12986-016-0107-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bojesen I. Quantitative and qualitative analyses of isolated lipid droplets from interstitial cells in renal papillae from various species. Lipids 9, 835–843 (1974). 10.1007/BF02532606 [DOI] [PubMed] [Google Scholar]
- 30.Nissen H. M. & Bojesen I. On lipid droplets in renal interstitial cells. IV. Isolation and identification. Z Zellforsch Mikrosk Anat 97, 274–284 (1969). 10.1007/BF00344762 [DOI] [PubMed] [Google Scholar]
- 31.Bohman S. O. & Maunsbach A. B. Ultrastructure and biochemical properties of subcellular fractions from rat renal medulla. J Ultrastruct Res 38, 225–245 (1972). 10.1016/s0022-5320(72)90001-9 [DOI] [PubMed] [Google Scholar]
- 32.Comai K., Farber S. J. & Paulsrud J. R. Analyses of renal medullary lipid droplets from normal, hydronephrotic, and indomethacin treated rabbits. Lipids 10, 555–561 (1975). 10.1007/BF02532360 [DOI] [PubMed] [Google Scholar]
- 33.Mannik J., Meyers A. & Dalhaimer P. Isolation of cellular lipid droplets: two purification techniques starting from yeast cells and human placentas. J Vis Exp (2014). 10.3791/50981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hernandez-Albaladejo I. et al. A method for lipid droplet isolation from human placenta for further analyses in clinical trials. Acta Obstet Gynecol Scand 93, 1198–1202 (2014). 10.1111/aogs.12461 [DOI] [PubMed] [Google Scholar]
- 35.Fujiki Y., Hubbard A. L., Fowler S. & Lazarow P. B. Isolation of intracellular membranes by means of sodium carbonate treatment: application to endoplasmic reticulum. J Cell Biol 93, 97–102 (1982). 10.1083/jcb.93.1.97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tsugawa H. et al. MS-DIAL: data-independent MS/MS deconvolution for comprehensive metabolome analysis. Nat Methods 12, 523–526 (2015). 10.1038/nmeth.3393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zimmermann R. et al. Fat mobilization in adipose tissue is promoted by adipose triglyceride lipase. Science 306, 1383–1386 (2004). 10.1126/science.1100747 [DOI] [PubMed] [Google Scholar]
- 38.Samuel V. T. & Shulman G. I. Mechanisms for insulin resistance: common threads and missing links. Cell 148, 852–871 (2012). 10.1016/j.cell.2012.02.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Paul A. Chang B. H., Li L., Yechoor V. K. & Chan L. Deficiency of adipose differentiation-related protein impairs foam cell formation and protects against atherosclerosis. Circ Res 102, 1492–1501 (2008). 10.1161/CIRCRESAHA.107.168070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McManaman J. L. et al. Perilipin-2-null mice are protected against diet-induced obesity, adipose inflammation, and fatty liver disease. J Lipid Res 54, 1346–1359 (2013). 10.1194/jlr.M035063 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The source data related to the findings of the manuscript are provided as supplementary information with this manuscript and can be used for scientific purposes with permission of corresponding authors.