Abstract
Consolidation of initially encoded hippocampal representations in the neocortex through reactivation is crucial for long-term memory formation, and is facilitated by the coordination of hippocampal sharp-wave ripples (SWRs) with cortical oscillations during non-REM sleep. However, the contribution of high-frequency cortical ripples to consolidation is still unclear. We used continuous recordings in the hippocampus and prefrontal cortex (PFC) over the course of spatial learning and show that independent PFC ripples, when dissociated from SWRs, predominantly suppress hippocampal activity in non-REM sleep. PFC ripples paradoxically mediate top-down suppression of hippocampal reactivation, which is inversely related to reactivation strength during coordinated CA1-PFC ripples. Further, we show non-canonical, serial coordination of ripples with cortical slow and spindle oscillations. These results establish a role for cortical ripples in regulating consolidation.
Systems memory consolidation is the process through which newly formed memories are transferred to cortical regions for long term storage, and models of memory consolidation such as the standard systems theory of consolidation and multiple trace theory posit a predominantly unidirectional interaction between the hippocampus and cortical regions (1–3). This consolidation process is facilitated through the coordination of patterns of activity that span a range of timescales and oscillatory frequencies and has been extensively studied in the context of the hippocampal-prefrontal cortical circuit. The most prominent of these activity patterns are hippocampal sharp-wave ripples (SWRs), a phenomenon critical for memory consolidation (4). These high frequency bursts during non-REM (NREM) sleep reactivate hippocampal representations of previous experiences, engage the prefrontal cortex (PFC), and are associated with brain wide changes in activity, hypothesized to support integration of mnemonic representations in distributed cortical circuits (5–8). Similarly, high frequency ripple bursts have been observed in the neocortex, including PFC, that synchronize local activity and have been reported to be coupled to hippocampal SWRs (9, 10). However, whether and how PFC ripples reciprocally engage hippocampal activity, and especially hippocampal reactivation, in support of consolidation is unclear.
Independent and coordinated ripple events across CA1 and PFC
Here, we simultaneously recorded populations of CA1 (n = 3,316) and PFC (n = 2,474) neurons (fig. S1) from animals (n = 8 rats) in NREM sleep (mean = 13.24 ± 0.83 min per epoch, fig. S2) throughout the course of learning a spatial alternation task that requires hippocampal-prefrontal interactions (11, 12). Activity was monitored across multiple run and sleep sessions during learning. We detected cortical ripples in PFC as previously described (9, 10), and classified them as either temporally coupled to hippocampal SWRs (i.e., coordinated ripples) or independent (Fig. 1A-D). We thus separated ripples based on the interregional overlap of these events as independent PFC ripples, independent CA1 SWRs (termed independent SWRs), and coordinated CA1-PFC ripples (fig. S3,S4). Independent PFC ripple and independent CA1 SWR events were more prevalent than coupled CA1-PFC ripples events in NREM sleep (fig. S3B; independent PFC ripple rate: 0.46 ± 0.02 Hz; independent CA1 ripple rate: 0.67 ± 0.02 Hz; coordinated ripple rate: 0.16 ± 0.01 Hz). We hypothesized that these two kinds of events, independent and coordinated, may subserve different mnemonic processes within the hippocampal-prefrontal network, and found key differences. Coordinated events were longer in duration and had higher cell participation (fig. S5A,C), consistent with previous reports that long duration events are important for performing behavioral tasks with a high memory demand (13). The rate of independent PFC ripples increased over time and learning as compared to CA1 SWRs, and was correlated with task performance in the subsequent behavioral session (Fig. 1E, fig. S3C). Conversely, within each individual sleep session, independent SWR rate decreased over time, shifting the balance between independent and coordinated SWRs over the course of a sleep session (fig. S3D,E). The rate of coordinated CA1-PFC ripples also increased with learning and was correlated with the increase in overall PFC ripple rate, suggesting a role for PFC in driving the increase in coupling during later sleep epochs (Fig. 1F, fig. S3F). Finally, coordinated CA1-PFC ripples were also associated with higher spindle power (Fig. 1G), indicating enhanced cross-frequency coupling between the hippocampus and PFC during coordination, which is critical for memory consolidation (14–16).
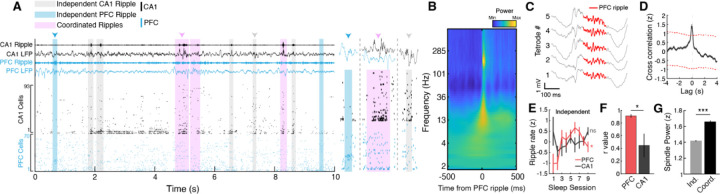
Fig. 1. Independent and coordinated ripple events in CA1 and PFC.
(A) Example CA1 and PFC raster plot illustrating the occurrence of independent CA1 and PFC ripples and coordinated events. Events indicated by the arrowheads are expanded on right to show LFP and single unit activity. (B) PFC ripple triggered spectrogram combined across all animals (n = 8 rats) and epochs (n = 72 epochs; n = 37,335 ripples). Note the associated spindle band activity (12–16 Hz) during these events. (C) Example PFC ripple event shown across 5 tetrodes. Detected ripple event is highlighted in red. (D) Cross-correlation between all ripple events in CA1 and PFC. Red dashed line indicate the 95% confidence intervals for jittered data. (E) Independent ripple rate over the 9 sleep epochs (left, PFC: F = 2.44, *p = 0.03; CA1: F = 1.18, p = 0.34, main effect of time, repeated measures ANOVA). (F) The correlations between overall and coordinated ripple rate over time for CA1 and PFC events suggest PFC driven coupling (PFC, r = 0.91 ± 0.03; CA1, r = 0.45 ± 0.18, *p = 0.01). (G) Peak spindle power during independent vs coordinated PFC ripple events (Independent = 1.42 ± 0.01, Coordinated = 1.66 ± 0.01, ***p = 2.11×10−66).
Bidirectional modulation of CA1 neurons during PFC ripples
Previous reports have shown that PFC neurons are bidirectionally modulated by hippocampal SWRs (17–19), so here we examined the effect of independent PFC ripples on CA1 neuronal activity. Interestingly, a large majority of CA1 cells, both putative pyramidal cells and interneurons (classification shown in fig. S6), were suppressed during independent PFC ripples, while PFC activity was enhanced by the local ripples (Fig. 2A,B; interneuron modulation shown in figs. S7A). In addition, suppression in CA1 during PFC ripples peaked prior to PFC excitation (Fig. 2C), indicating attenuation of CA1 activity through a cortical-hippocampal-cortical loop that may enhance information transfer within the neocortex (20, 21). The neuronal activity modulation in CA1 and PFC was dependent on the ripples examined (Fig. 2A, fig. S8A-C), either local ripples in CA1/ PFC or coordinated ripples in CA1-PFC, especially with coordinated ripples predominantly driving excitation in both regions in contrast to independent cortical ripples, highlighting that these distinct events may differ in their contribution to mnemonic processes.
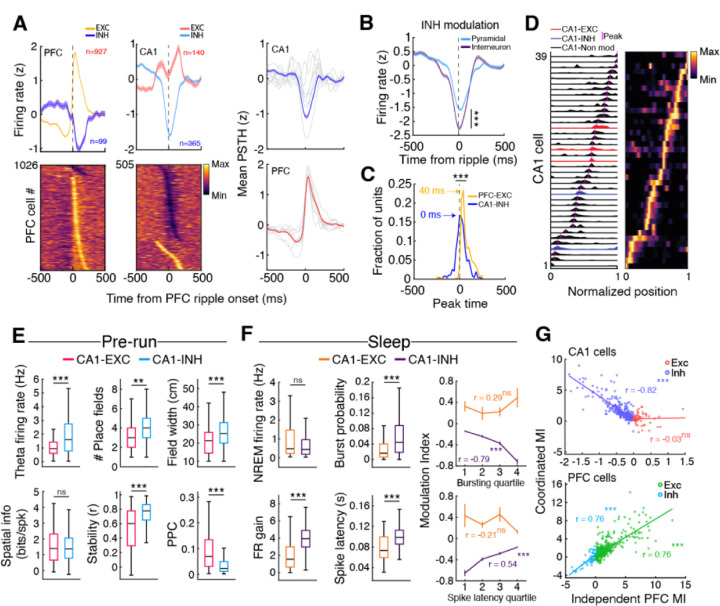
Fig. 2. Predominant suppression of CA1 activity during independent PFC ripples.
(A) Independent PFC ripple triggered modulation in PFC (left) and CA1 (middle). (Right) Overall modulation in each area showing neurons excited or suppressed during ripples, with predominantly excitation in PFC and predominantly suppression in CA1. Gray lines indicate individual animals. (B) The degree of suppression was higher in INH interneurons as compared to pyramidal cells (±200 around ripple onset; ***p = 1.37×10−9). (C) Timing of peak suppression for CA1 INH cells (interneurons and pyramidal cells) and PFC EXC cells (Comparison of peak timing distributions, ***p = 4.90×10−46). (D) Example firing maps for CA1 EXC, INH, and non-modulated cells during one trajectory. (E) Differences in spatial properties for CA1 EXC and INH pyramidal cells during the run session preceding the sleep epoch in which the cells were modulated (Theta firing rate, EXC = 1.20 ± 0.10 Hz, INH = 2.05 ± 0.10 Hz, ***p = 2.08×10−6; Number of fields, EXC = 3.3 ± 0.17, INH = 3.99 ± 0.11, **p = 0.002; Field width, EXC = 21.67 ± 0.91 cm, INH = 26.56 ± 0.61 cm, ***p = 1.1×10−4; Spatial information (bits/spike), EXC = 1.52 ± 0.12 bits, INH = 1.49 ± 0.06 bits, p = 0.83; Stability, EXC = 0.54 ± 0.03, INH = 0.72 ± 0.01, ***p = 9.01×10−7; Pairwise phase consistency, EXC = 0.10 ± 0.01, INH = 0.05 ± 0.004, ***p = 3.31×10−10). (F) (Left ) Mean NREM firing rate and intra-SWR firing dynamics for CA1 EXC and INH cells during coordinated ripples (NREM firing rate, EXC = 1.07 ± 0.11 Hz, INH = 0.81 ± 0.05 Hz, p = 0.18; FR gain, EXC = 2.02 ± 0.16, INH = 4.07 ± 0.10, ***p = 2.88×10−27; Burst probability, EXC = 0.031 ± 0.006, INH = 0.075 ± 0.005, ***p = 6.38×10−5; Spike latency, EXC = 0.080 ± 0.006, INH = 0.099 ± 0.001, ***p = 1.61×10−5). (Right) Relationship between SWR bursting incidence (top, EXC: r = 0.29, p = 0.08, INH: r = −0.79, ***p = 5.20×10−69) or spike latency for CA1 EXC and INH cells (bottom, EXC: r = −0.21, p = 0.21, INH: r = 0.54, *** p = 9.51×10−25) with modulation index (MI) during independent PFC ripple events. (G) Relationship between MI during coordinated ripples and independent PFC ripples (top, CA1, EXC: r = 0.03, p = 0.82; INH: r = −0.82, ***p = 6.84×10−80; bottom, PFC, EXC: r = 0.76, ***p = 1.34×10−92; INH: r = 0.76, ***p = 1.51×10−10). Note the negative relationship for CA1 INH cells, indicating that stronger the recruitment during coordinated ripples, the more inhibited the cells are during independent PFC ripples.
Further examination of the inhibited (INH, n = 365) and excited (EXC, n = 140) CA1 pyramidal neurons during run sessions revealed representational differences (Fig. 2D,E). INH cells had higher theta firing rates, had wider and more numerous fields, and had greater stability between sessions, whereas EXC cells were more strongly locked to the hippocampal theta oscillation (Fig. 2E, fig. S8D). During NREM sleep, EXC and INH cells had similar baseline firing rates but had distinct intra-SWR firing dynamics. EXC cells were generally active earlier in SWR events and INH cells were more strongly excited (Fig. 2F). The degree of CA1 modulation by PFC ripples was also predictive of intra-CA1-SWR dynamics (Fig. 2F, right) and was negatively correlated with modulation during coordinated CA1-PFC ripples (Fig. 2E), indicating a role for PFC ripples in influencing the content of hippocampal activity during coordinated ripples. In particular, the suppression of CA1 INH cells by PFC ripples that are strongly recruited during coordinated CA1-PFC ripples suggests that independent PFC ripples suppress hippocampal reactivation during spatial learning. Similar relationships were also found in the population of putative CA1 interneurons, demonstrating a broad top-down influence of PFC ripples on CA1 activity (fig. S7B-D). Thus, PFC ripples delineate transient intervals where hippocampal activity is largely suppressed, potentially modifying circuit dynamics for effective information processing or transfer.
Top-down suppression of CA1 reactivation
We then examined population activity in CA1 and PFC during these ripple events using assembly analysis to explicitly look at reactivation during ripples (Fig. 3A,C). Corroborating the single unit results, assemblies within a region (CA1 n = 585, PFC n = 320) were robustly reactivated during their respective local ripple events (Fig. 3B,D, fig. S9B,C) and were strongly co-active during coordinated CA1-PFC ripple events as expected for a memory consolidation process (fig. S9D,G). CA1 EXC cells had higher weights and contribution to reactivation strength (Fig. 3D, fig. S9I), possibly owing to the stronger theta phase locking within this population during run sessions (Fig. 2E, fig. S8D). Assemblies in CA1 and PFC exhibited gradients of reactivation strengths across events (fig. S10), and the degree of reactivation strength in CA1 was negatively correlated across independent PFC ripples and coordinated CA1 SWRs (Fig. 3E), confirming that assemblies that are strongly reactivated during coordinated CA1-PFC ripples are highly suppressed by independent PFC ripples. This CA1 suppression was also strongest during the later sleep sessions when PFC ripple rate peaked (Fig. 1E, fig. S9E). Interestingly, the degree of suppression of CA1 assemblies during independent PFC ripples was related to assembly reinstatement in subsequent behavioral sessions (fig. S9H), suggesting a role for this top-down suppression in modifying subsequent CA1 coactivity patterns. In addition, both reactivation strength and within-area coactivity in PFC and CA1 differed across ripple events, and population activity in PFC and CA1 was able to distinguish between independent and coordinated ripples in each respective area (Fig. 3F,G, fig. S9F). We observed similar relationships for joint CA1-PFC reactivation strength. Joint CA1-PFC coactivity differed between independent and coordinated SWRs, and showed a similar negative relationship between reactivation strength during coordinated ripples vs. independent PFC ripples (fig. S11). These results indicate that independent and coordinated ripples underlie different modes of information exchange between CA1 and PFC, and further that stronger the reactivation in CA1 during coordinated CA1-PFC ripples, the stronger the suppression during PFC ripples.
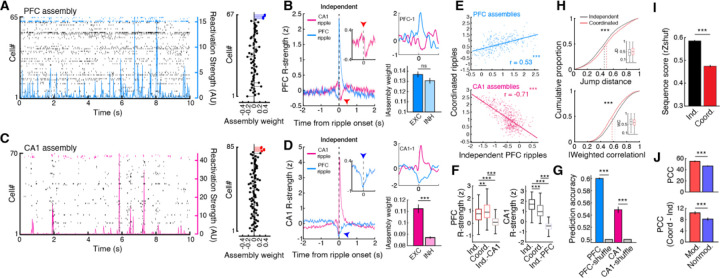
Fig. 3. Assembly reactivation during independent and coordinated ripple events.
(A) (Left) Example PFC raster plot during NREM sleep with the reactivation strength of an assembly overlaid. (Right) Neuron weights for the plotted assembly on the left, with member cells highlighted in blue. (B) (Left) Independent ripple triggered PFC reactivation strength. (Right) Example PFC assembly aligned to ripple events (top) and the absolute weights of EXC and INH PFC cells (modulation during independent CA1 SWRs, EXC = 0.137 ± 0.003, INH = 0.131 ± 0.003, p = 0.37). (C) and (D) Same as in (A) and (B) but for CA1 (modulation during independent PFC ripples, EXC = 0.112 ± 0.004, INH = 0.087 ± 0.001, ***p = 8.04×10−7). (E) Relationship between Z-scored assembly reactivation strength during independent PFC ripples and coordinated ripples (PFC: r = 0.53, ***p = 1.28×10−23; CA1: r = −0.71, ***p = 4.25×10−87). Note that PFC reactivation strength is correlated across events, whereas CA1 assemblies that are highly suppressed during independent PFC ripples are strongly reactivated during coordinated ripples. (F) Reactivation strength during within-area independent and coordinated ripples and during opposing area independent events (PFC: Independent-PFC = 0.71 ± 0.03, Coordinated-PFC = 0.96 ± 0.04, Independent-CA1 = 0.13 ± 0.02, **p = 0.0023, ***p = 3.21×10−33, ***p = 2.12×10−53 for independent-PFC vs coordinated-PFC, independent-PFC vs independent-CA1, and coordinated-PFC vs independent-CA1, respectively; CA1: Independent-CA1 = 1.67 ± 0.03, Coordinated-CA1 = 1.11 ± 0.03, Independent-PFC = −0.51 ± 0.02, ***p = 2.44×10−18, ***p = 7.45×10−239, ***p = 1.14×10−128 for independent-CA1 vs coordinated-CA1, independent-CA1 vs independent-PFC, and coordinated-CA1 vs independent-PFC, respectively; Kruskal-Wallis test, Bonferroni corrected for multiple comparison). (G) Prediction of intra-area ripple type (independent or coordinated) using spike count data (PFC: data = 0.6 ± 0.001, shuffle = 0.5 ± 4.64×10−5, ***p = 3.39×10−50; CA1: data = 0.55 ± 0.005, shuffle = 0.50 ± 1.37×10−4, ***p = 1.28×10−26). (H) Jump distance and absolute weighted correlation for significant CA1 replay events decoded during independent CA1 SWRs and coordinated ripples (Jump distance: Independent = 0.468 ± 0.003, Coordinated = 0.505 ± 0.005, ***p = 2.73×10−11; Weighted correlation: Independent = 0.620 ± 0.002, Coordinated = 0.585 ± 0.003, ***p = 1.10×10−17). (I) Sequence degradation (rZshuf) of independent and coordinated replay events after shuffling individual cells’ linearized firing fields (Independent = 0.585 ± 0.004, Coordinated = 0.475 ± 0.005, ***p = 5.46×10−76). (J) Per-cell contribution (PCC) to all significant replay for CA1 modulated vs non-modulated cells (top, modulated = 55.04 ± 1.28, non-modulated = 46.65 ± 1.02, ***p = 2.39×10−7) and the difference in PCC (coordinated minus independent) to independent and coordinated events (bottom, modulated = 10.44 ± 0.57, non-modulated = 8.17 ± 0.55, ***p = 9.88×10−4).
Since it has been previously demonstrated that hippocampal reactivation and replay are separable components of the dynamic hippocampal code (22), we examined how association with PFC ripples may impact CA1 replay. We found that replay events associated with coordinated SWRs were less sequential in structure, having higher normalized jump distances and lower weighted correlations than independent-SWR associated replay (Fig. 3H, fig. S12). Sequential unit spiking was also more consistent, as measured by rank-order correlation (23), and had higher dimensionality during independent SWRs, further highlighting the influence of PFC on SWR content (fig. S12D,E). Coordinated-SWR replay exhibited a larger degree of sequence degradation when place cell templates are shuffled as compared to independent-SWR replay, possibly due to a decrease in overall reactivation strength and coactivity during these events (Fig. 3F,I, fig. S9F). Furthermore, PFC ripple modulated CA1 cells had higher per-cell contribution (PCC) to replay than non-modulated cells (Fig. 3J, top). Although both modulated and non-modulated cells contributed more strongly to coordinated-SWR replay, which is expected due to the greater sequence degradation observed in these events, modulated cells had higher PCC differences (Fig. 3J, bottom). This overall higher contribution of modulated cells to coordinated-SWR replay and the differences observed between independent and coordinated events further suggest a role for PFC in shaping the content of hippocampal reactivation and replay during sleep.
Sequential coupling of sleep oscillations across CA1 and PFC
In addition to the recent observation of temporal coupling of hippocampal-cortical ripples contributing to memory consolidation, the coordination of hippocampal SWRs with cortical slow oscillations (SOs) and spindles is known to be important for consolidation (14, 24, 25). To this end, we investigated the incidence of ripple events in the context of these oscillations that span multiple timescales. Independent and coordinated CA1 SWRs exhibited differential coupling with spindles and SOs, with only coordinated CA1-PFC ripples associated with cortical spindles and not independent CA1 SWRs (Fig. 4A, fig. S13). Population activity in CA1 and PFC also had unique responses surrounding independent vs. coordinated events (fig. S14). Additionally, CA1 replay events, specifically during coordinated CA1-PFC ripples, were enriched during up states of the SO (Fig. 4B,C, fig. S15B). These results, along with the finding that coordinated CA1-PFC ripples were associated with a higher cortical spindle power (Fig. 1G), led us to hypothesize distinct temporal organization of independent and coordinated ripple events with slow oscillations and spindles.
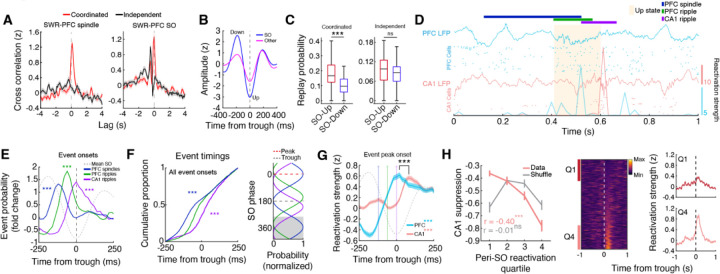
Fig. 4. Serially coupled spindles, ripples, and slow oscillations.
(A) Cross-correlation between independent or coordinated CA1 SWRs with PFC spindles and SOs (trough time). Note that only coordinated CA1-PFC ripples tend to be associated with spindle oscillations. Furthermore, the timing of SWRs relative to SOs is different between these events, with coordinated ripples occurring primarily at the trough and independent SWRs preceding SO troughs. (B) Average waveforms for SOs and other, lower amplitude slow waves extracted from PFC LFPs. (C) Probability of coordinated and independent ripple associated CA1 replay during up and down states (Coordinated: up = 0.19 ± 0.02, down = 0.11 ± 0.01, ***p = 2.30×10−4; Independent: up = 0.09 ± 0.01, down = 0.08 ± 0.01, p = 0.18). (D) Raster showing the reactivation strengths of example CA1 and PFC assemblies in relation to LFP events. Note the sequential organization of events with PFC leading CA1. (E) Event probability fold change of spindles and ripples aligned to SO troughs (Spindles, ***p = 2.84×10−14; PFC ripples, ***p = 1.66×10−7; CA1 ripples, ***p = 3.86×10−5, ttest vs 0), showing the spindle-PFC ripple-CA1 ripple timing. (F) (Left) Cumulative proportion of event timings within ±250 ms of SO troughs (Spindles vs PFC ripples, ***p = 2.41×10−16, PFC ripples vs CA1 ripples, ***p = 4.62×10−13), and (Right) preferred SO phase for each event type (Spindles vs PFC ripples, U2 = 2.63, ***p = 5.73×10−23, PFC ripples vs CA1 ripples, U2 = 2.54, ***p = 3.61×10−22, Watson’s U2 test). Gray shaded area indicates repeated data for visualization. (G) SO trough aligned CA1 and PFC reactivation strength during SO associated coordinated events where PFC ripples precede CA1 SWRs (PFC vs shuffle, ***p = 4.91×10−19; CA1 vs shuffle, ***p = 2.12×10−39; PFC vs CA1 timing, ***p = 7.96×10−5). (H) (Left) Relationship between independent PFC ripple associated CA1 suppression and SO upstate CA1 reactivation strength (data: r = −0.40, ***p = 3.01×10−21, shuffle: r = −0.01, p = 0.86), again showing a negative relationship. (Middle) SO trough aligned reactivation strength for all CA1 assemblies. (Right) Z-scored SO trough aligned CA1 reactivation strength plotted for the first and fourth quartiles.
We observed an overall higher prevalence of coordinated events where PFC ripples precede CA1 SWRs (fig. S16A), which is consistent with PFC ripples potentially driving ripple coordination (Fig. 1F), and therefore focused on these events for the subsequent analyses to investigate sequential coupling of events in CA1 and PFC. We quantified the onset of PFC spindles, ripples, and CA1 SWRs relative to the trough (up state) of SOs and calculated the fold change probability of each event around SOs. We found a sequential organization of these oscillatory events that suggests a thalamocortical-hippocampal directionality, with spindles preceding coordinated PFC-CA1 ripple events (Fig. 4D,E) as well as independent PFC ripples (fig. S15D). This temporal relationship with spindles was not present for independent CA1 SWRs (fig. S15D) and differed for coordinated events where CA1 SWRs preceded PFC ripples (fig. S15E). The embedding of PFC ripple events between spindles and CA1 SWRs (Fig. 4E,F) would allow for the coordination of PFC and CA1 assembly reactivation. Indeed, PFC and CA1 reactivation was temporally organized surrounding SO troughs in a manner that is consistent with cortico-hippocampal directionality (Fig. 4G). In addition, the strength of up state aligned reactivation in CA1 assemblies was related to the degree of suppression during independent PFC ripples (Fig. 4H), suggesting a role of PFC ripples in prioritizing certain CA1 memory traces for long-term storage or modification. Additionally, differences in coordinated events (PFC leading vs lagging), such as the timing and content of CA1 and PFC reactivation (fig. S16), suggests a bidirectional coordinated ripple-mediated interaction with potentially distinct roles in consolidation.
Memory consolidation is a dynamic process that is dependent on the interaction between multiple brain systems, and previous studies have shown that enhanced cross-frequency coupling between the hippocampus and PFC enhances performance on memory related tasks (14, 26). Our finding that independent and coordinated events across the hippocampal-prefrontal circuit elicit differential responses suggests separate roles in systems memory consolidation. Notably, the suppression of CA1 reactivation by independent PFC ripples is unexpected based on current models of systems consolidation (1–3). While coordinated ripple events across hippocampal-neocortical circuits are thought to support memory consolidation through the coordinated reactivation of behaviorally relevant representations (9, 19), the roles of independent events in CA1 and PFC are unclear. One possibility is that independent CA1 SWRs (independent meaning uncoupled from PFC ripple activity, specifically) preferentially engage other brain areas to support different aspects of consolidation depending on the nature of the experience. Indeed, there are widespread, bidirectional changes in activity surrounding SWRs (5, 27), which could reflect distinct processes if examined on an event-by-event basis. Future studies investigating multiple regions of interest simultaneously will allow for the discretization of events spanning a wide range of brain areas and may reveal a multiplex hierarchy, within which specific components can be selectively engaged depending on mnemonic demands (28).
The broad suppression that spans multiple cell types in CA1 during PFC ripples indicates that these cortical ripples have a unique contribution to the consolidation of recently acquired memories. The robust and predictive nature of this suppression suggests that PFC may have a role in modulating or biasing the content of hippocampal reactivation to influence what information to consolidate within the hippocampus, and in turn, the neocortex. Furthermore, modulation during PFC ripples may indicate a mechanism through which the E-I balance within the CA1 circuit is modified to maintain a specific level of criticality (29) or to control the excitatory drive in the population of CA1 pyramidal cells, thus increasing the signal to noise ratio to allow for more robust information transfer (30). Concurrently, CA1 suppression may also be a mechanism through which memory interference in PFC is attenuated to allow for restricted ripple mediated information transfer and plasticity throughout the neocortex to support memory integration through synchrony for semantic memories (20, 21, 31). We hypothesize that PFC ripples may be associated with consolidation within cortical networks while suppressing hippocampal reactivation, and elucidation of the brain-wide processes associated with this suppression will require monitoring multiple cortical areas in future studies. However, it is important to note—given that the suppression of CA1 initiates prior to PFC reactivation during independent PFC ripple events, there are likely other brain regions contributing to this process. Future studies investigating this bidirectional modulation of CA1 in the context of the laminar distribution of cells (deep vs superficial), their projection profiles to different cortical targets, such as PFC and entorhinal cortex, and the state of plasticity (plastic vs rigid) will yield a clearer picture of the multimodal dynamics present in the hippocampus (32–34).
The finding that highly suppressed CA1 assemblies are strongly reactivated during coordinated events, which are coupled with spindles and SOs, a salient signature of consolidation, also suggests a mechanism through which PFC prioritizes specific CA1 assemblies for consolidation based on future utility or generalizability (35). In the context of memory reconsolidation, suppression may be involved in preventing destabilization of certain salient CA1 memory traces (36). Many studies of reconsolidation utilize experimental paradigms that are performed over the course of multiple days. It has been shown that specific changes in hippocampal dynamics potentially linked to consolidation, namely representational drift (37), are a function of experience (38, 39); however, our longitudinal study design wherein animals are exposed to multiple behavioral sessions in a day can maintain a specific level of lability in CA1 that is consistent with circuit dynamics underlying reconsolidation. Given the persistent reactivation that occurs in CA1 subsequent to novel experience (40), PFC mediated suppression may be a mechanism through which CA1 activity is normalized to a level that is optimal for the induction of transcriptional processes that underlie memory maintenance or integration (36).
Previous human fMRI studies have reported hippocampal suppression primarily during retrieval stopping, which is typically investigated in contexts where intrusive memories are proactively suppressed (41, 42). Our finding that coordinated SWR associated replay events have lower dimensionality and sequential structure raises the alternative possibility that PFC may be involved in a process that prevents certain neural patterns from being consolidated through disruption of hippocampal sequential activity. SWRs are thought to facilitate retrieval through the reactivation of hippocampal ensembles, but the mechanisms through which these activity patterns are distinguished based on utility for consolidation are unclear. There must be a way for the brain to separate activity that is elicited by actual experience from novel, unrelated activity patterns that are a result of internally generated mechanisms to maintain a faithful rendering of reality (8, 43). Although these coordinated SWR associated replay events quantitatively represent sequences of behaviorally relevant activity, the up and downstream brain areas conveying and receiving information respectively may play roles in determining the nature of the top-down influence of PFC on hippocampal sequential activity (28). This type of reality monitoring is crucial for the purported role of SWRs in imagination (8), and further elucidation of the mechanisms underlying this process may lead to a deeper understanding of how it goes awry in neurological disorders such as schizophrenia (43).
Our results highlight the dynamic and multidimensional nature of memory consolidation and uncover a previously unknown PFC ripple mediated phenomenon with a distinct suppressive reactivation role that is involved in modifying hippocampal representations and biasing CA1 reactivation in sleep for effective future behavior. Notably, the ability to distinguish SWR events based on long-range interregional coordination affords the opportunity to study this widespread hippocampal phenomenon in greater detail and may be able to reconcile how SWR diversity may play a role in different aspects of cognition (33, 44–46).
Supplementary Material
Funding Statement
National Institutes of Mental Health grant R01MH112661 (SPJ).
National Institutes of Health grant 2T32NS007292-36 (JDS).
Footnotes
Competing interests: Authors declare that they have no competing interests.
Data and materials availability: Data underlying these results will be uploaded in the NWB (Neurodata Without Borders) format to DANDI (ID#TBD) upon acceptance. Code to replicate these results will be available on our lab GitHub (http://github.com/JadhavLab/PrefrontalRipples) upon acceptance.
References
- 1.Buzsaki G., Two-stage model of memory trace formation: a role for "noisy" brain states. Neuroscience 31, 551–570 (1989). [DOI] [PubMed] [Google Scholar]
- 2.Squire L. R., Alvarez P., Retrograde amnesia and memory consolidation: a neurobiological perspective. Curr Opin Neurobiol 5, 169–177 (1995). [DOI] [PubMed] [Google Scholar]
- 3.Nadel L., Moscovitch M., Memory consolidation, retrograde amnesia and the hippocampal complex. Curr Opin Neurobiol 7, 217–227 (1997). [DOI] [PubMed] [Google Scholar]
- 4.Girardeau G., Zugaro M., Hippocampal ripples and memory consolidation. Curr Opin Neurobiol 21, 452–459 (2011). [DOI] [PubMed] [Google Scholar]
- 5.Logothetis N. K. et al. , Hippocampal-cortical interaction during periods of subcortical silence. Nature 491, 547–553 (2012). [DOI] [PubMed] [Google Scholar]
- 6.Buzsaki G., Hippocampal sharp wave-ripple: A cognitive biomarker for episodic memory and planning. Hippocampus 25, 1073–1188 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shin J. D., Jadhav S. P., Multiple modes of hippocampal-prefrontal interactions in memory-guided behavior. Curr Opin Neurobiol 40, 161–169 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Joo H. R., Frank L. M., The hippocampal sharp wave-ripple in memory retrieval for immediate use and consolidation. Nat Rev Neurosci 19, 744–757 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khodagholy D., Gelinas J. N., Buzsaki G., Learning-enhanced coupling between ripple oscillations in association cortices and hippocampus. Science 358, 369–372 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vaz A. P., Inati S. K., Brunel N., Zaghloul K. A., Coupled ripple oscillations between the medial temporal lobe and neocortex retrieve human memory. Science 363, 975–978 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maharjan D. M., Dai Y. Y., Glantz E. H., Jadhav S. P., Disruption of dorsal hippocampal - prefrontal interactions using chemogenetic inactivation impairs spatial learning. Neurobiol Learn Mem 155, 351–360 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shin J. D., Tang W., Jadhav S. P., Dynamics of Awake Hippocampal-Prefrontal Replay for Spatial Learning and Memory-Guided Decision Making. Neuron 104, 1110–1125 e1117 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fernandez-Ruiz A. et al. , Long-duration hippocampal sharp wave ripples improve memory. Science 364, 1082–1086 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maingret N., Girardeau G., Todorova R., Goutierre M., Zugaro M., Hippocampo-cortical coupling mediates memory consolidation during sleep. Nat Neurosci 19, 959–964 (2016). [DOI] [PubMed] [Google Scholar]
- 15.Latchoumane C. V., Ngo H. V., Born J., Shin H. S., Thalamic Spindles Promote Memory Formation during Sleep through Triple Phase-Locking of Cortical, Thalamic, and Hippocampal Rhythms. Neuron 95, 424–435 e426 (2017). [DOI] [PubMed] [Google Scholar]
- 16.Staresina B. P., Niediek J., Borger V., Surges R., Mormann F., How coupled slow oscillations, spindles and ripples coordinate neuronal processing and communication during human sleep. Nat Neurosci 26, 1429–1437 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jadhav S. P., Rothschild G., Roumis D. K., Frank L. M., Coordinated Excitation and Inhibition of Prefrontal Ensembles during Awake Hippocampal Sharp-Wave Ripple Events. Neuron 90, 113–127 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang D. V., Ikemoto S., Coordinated Interaction between Hippocampal Sharp-Wave Ripples and Anterior Cingulate Unit Activity. J Neurosci 36, 10663–10672 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tang W., Shin J. D., Frank L. M., Jadhav S. P., Hippocampal-Prefrontal Reactivation during Learning Is Stronger in Awake Compared with Sleep States. J Neurosci 37, 11789–11805 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arnulfo G. et al. , Long-range phase synchronization of high-frequency oscillations in human cortex. Nat Commun 11, 5363 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dickey C. W. et al. , Widespread ripples synchronize human cortical activity during sleep, waking, and memory recall. Proc Natl Acad Sci U S A 119, e2107797119 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu C., Todorova R., Tang W., Oliva A., Fernandez-Ruiz A., Associative and predictive hippocampal codes support memory-guided behaviors. Science 382, eadi8237 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stark E., Roux L., Eichler R., Buzsaki G., Local generation of multineuronal spike sequences in the hippocampal CA1 region. Proc Natl Acad Sci U S A 112, 10521–10526 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klinzing J. G., Niethard N., Born J., Mechanisms of systems memory consolidation during sleep. Nat Neurosci 22, 1598–1610 (2019). [DOI] [PubMed] [Google Scholar]
- 25.Todorova R., Zugaro M., Hippocampal ripples as a mode of communication with cortical and subcortical areas. Hippocampus 30, 39–49 (2020). [DOI] [PubMed] [Google Scholar]
- 26.Geva-Sagiv M. et al. , Augmenting hippocampal-prefrontal neuronal synchrony during sleep enhances memory consolidation in humans. Nat Neurosci 26, 1100–1110 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Karimi Abadchi J. et al. , Spatiotemporal patterns of neocortical activity around hippocampal sharp-wave ripples. Elife 9, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Buzsaki G., Tingley D., Space and Time: The Hippocampus as a Sequence Generator. Trends Cogn Sci 22, 853–869 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O'Byrne J., Jerbi K., How critical is brain criticality? Trends Neurosci 45, 820–837 (2022). [DOI] [PubMed] [Google Scholar]
- 30.Malik R., Li Y., Schamiloglu S., Sohal V. S., Top-down control of hippocampal signal-to-noise by prefrontal long-range inhibition. Cell 185, 1602–1617 e1617 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Navarro Lobato I. et al. , Increased cortical plasticity leads to memory interference and enhanced hippocampal-cortical interactions. Elife 12, (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grosmark A. D., Buzsaki G., Diversity in neural firing dynamics supports both rigid and learned hippocampal sequences. Science 351, 1440–1443 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de la Prida L. M., Potential factors influencing replay across CA1 during sharp-wave ripples. Philos Trans R Soc Lond B Biol Sci 375, 20190236 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sharif F., Tayebi B., Buzsaki G., Royer S., Fernandez-Ruiz A., Subcircuits of Deep and Superficial CA1 Place Cells Support Efficient Spatial Coding across Heterogeneous Environments. Neuron 109, 363–376 e366 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sun W., Advani M., Spruston N., Saxe A., Fitzgerald J. E., Organizing memories for generalization in complementary learning systems. Nat Neurosci 26, 1438–1448 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alberini C. M., The role of reconsolidation and the dynamic process of long-term memory formation and storage. Front Behav Neurosci 5, 12 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Driscoll L. N., Duncker L., Harvey C. D., Representational drift: Emerging theories for continual learning and experimental future directions. Curr Opin Neurobiol 76, 102609 (2022). [DOI] [PubMed] [Google Scholar]
- 38.Geva N., Deitch D., Rubin A., Ziv Y., Time and experience differentially affect distinct aspects of hippocampal representational drift. Neuron 111, 2357–2366 e2355 (2023). [DOI] [PubMed] [Google Scholar]
- 39.Khatib D. et al. , Active experience, not time, determines within-day representational drift in dorsal CA1. Neuron 111, 2348–2356 e2345 (2023). [DOI] [PubMed] [Google Scholar]
- 40.Giri B., Miyawaki H., Mizuseki K., Cheng S., Diba K., Hippocampal Reactivation Extends for Several Hours Following Novel Experience. J Neurosci 39, 866–875 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Anderson M. C., Hanslmayr S., Neural mechanisms of motivated forgetting. Trends Cogn Sci 18, 279–292 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Meyer A. K., Benoit R. G., Suppression weakens unwanted memories via a sustained reduction of neural reactivation. Elife 11, (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Simons J. S., Garrison J. R., Johnson M. K., Brain Mechanisms of Reality Monitoring. Trends Cogn Sci 21, 462–473 (2017). [DOI] [PubMed] [Google Scholar]
- 44.Reichinnek S., Kunsting T., Draguhn A., Both M., Field potential signature of distinct multicellular activity patterns in the mouse hippocampus. J Neurosci 30, 15441–15449 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ramirez-Villegas J. F., Logothetis N. K., Besserve M., Diversity of sharp-wave-ripple LFP signatures reveals differentiated brain-wide dynamical events. Proc Natl Acad Sci U S A 112, E6379–6387 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nitzan N., Swanson R., Schmitz D., Buzsaki G., Brain-wide interactions during hippocampal sharp wave ripples. Proc Natl Acad Sci U S A 119, e2200931119 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shin J. D., Tang W., Jadhav S. P., Protocol for geometric transformation of cognitive maps for generalization across hippocampal-prefrontal circuits. STAR Protoc 4, 102513 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schmitzer-Torbert N., Jackson J., Henze D., Harris K., Redish A. D., Quantitative measures of cluster quality for use in extracellular recordings. Neuroscience 131, 1–11 (2005). [DOI] [PubMed] [Google Scholar]
- 49.Harris K. D., Hirase H., Leinekugel X., Henze D. A., Buzsaki G., Temporal interaction between single spikes and complex spike bursts in hippocampal pyramidal cells. Neuron 32, 141–149 (2001). [DOI] [PubMed] [Google Scholar]
- 50.Mizuseki K., Diba K., Pastalkova E., Buzsaki G., Hippocampal CA1 pyramidal cells form functionally distinct sublayers. Nat Neurosci 14, 1174–1181 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sullivan D. et al. , Relationships between hippocampal sharp waves, ripples, and fast gamma oscillation: influence of dentate and entorhinal cortical activity. J Neurosci 31, 8605–8616 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kim J., Gulati T., Ganguly K., Competing Roles of Slow Oscillations and Delta Waves in Memory Consolidation versus Forgetting. Cell 179, 514–526 e513 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Siapas A. G., Lubenov E. V., Wilson M. A., Prefrontal phase locking to hippocampal theta oscillations. Neuron 46, 141–151 (2005). [DOI] [PubMed] [Google Scholar]
- 54.Lubenov E. V., Siapas A. G., Hippocampal theta oscillations are travelling waves. Nature 459, 534–539 (2009). [DOI] [PubMed] [Google Scholar]
- 55.Vinck M., van Wingerden M., Womelsdorf T., Fries P., Pennartz C. M., The pairwise phase consistency: a bias-free measure of rhythmic neuronal synchronization. Neuroimage 51, 112–122 (2010). [DOI] [PubMed] [Google Scholar]
- 56.Cheng S., Frank L. M., New experiences enhance coordinated neural activity in the hippocampus. Neuron 57, 303–313 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Peyrache A., Khamassi M., Benchenane K., Wiener S. I., Battaglia F. P., Replay of rule-learning related neural patterns in the prefrontal cortex during sleep. Nat Neurosci 12, 919–926 (2009). [DOI] [PubMed] [Google Scholar]
- 58.van de Ven G. M., Trouche S., McNamara C. G., Allen K., Dupret D., Hippocampal Offline Reactivation Consolidates Recently Formed Cell Assembly Patterns during Sharp Wave-Ripples. Neuron 92, 968–974 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zarzoso V., Comon P., Robust independent component analysis by iterative maximization of the kurtosis contrast with algebraic optimal step size. IEEE Trans Neural Netw 21, 248–261 (2010). [DOI] [PubMed] [Google Scholar]
- 60.Davidson T. J., Kloosterman F., Wilson M. A., Hippocampal replay of extended experience. Neuron 63, 497–507 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Karlsson M. P., Frank L. M., Awake replay of remote experiences in the hippocampus. Nat Neurosci 12, 913–918 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Farooq U., Dragoi G., Emergence of preconfigured and plastic time-compressed sequences in early postnatal development. Science 363, 168–173 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Valero M. et al. , Sleep down state-active ID2/Nkx2.1 interneurons in the neocortex. Nat Neurosci 24, 401–411 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.