Abstract
Cerebellar ataxia with neuropathy and vestibular areflexia syndrome (CANVAS) is a late onset, recessively inherited neurodegenerative disorder caused by biallelic, non-reference pentameric AAGGG(CCCTT) repeat expansions within the second intron of replication factor complex subunit 1 (RFC1). To investigate how these repeats cause disease, we generated CANVAS patient induced pluripotent stem cell (iPSC) derived neurons (iNeurons) and utilized calcium imaging and transcriptomic analysis to define repeat-elicited gain-of-function and loss-of-function contributions to neuronal toxicity. AAGGG repeat expansions do not alter neuronal RFC1 splicing, expression, or DNA repair pathway functions. In reporter assays, AAGGG repeats are translated into pentapeptide repeat proteins that selectively accumulate in CANVAS patient brains. However, neither these proteins nor repeat RNA foci were detected in iNeurons, and overexpression of these repeats in isolation did not induce neuronal toxicity. CANVAS iNeurons exhibit defects in neuronal development and diminished synaptic connectivity that is rescued by CRISPR deletion of a single expanded allele. These phenotypic deficits were not replicated by knockdown of RFC1 in control neurons and were not rescued by ectopic expression of RFC1. These findings support a repeat-dependent but RFC1-independent cause of neuronal dysfunction in CANVAS, with important implications for therapeutic development in this currently untreatable condition.
Keywords: Nucleotide Repeat expansion disorders, Short Tandem Repeats, RAN translation, RNA foci, Ataxia, neuropathy, C9orf72 FTD/ALS, Fragile X-associated Tremor/Ataxia Syndrome, Friedreich Ataxia, Neurodegeneration
Summary
Human CANVAS neurons exhibit transcriptional and functional synaptic defects that are corrected by heterozygous repeat deletion but are independent of the gene within which they reside—RFC1.
Introduction
Cerebellar ataxia with neuropathy and vestibular areflexia syndrome (CANVAS) is a recessively inherited, progressive, and debilitating disorder characterized by vestibular, cerebellar, and somatosensory impairments1,2. CANVAS typically presents in late-middle-age, with most patients exhibiting progressive motor imbalance, oscillopsia, dysphagia, dysarthria, and peripheral sensory neuropathy. Post-mortem analyses of CANVAS patients indicate cerebellar and basal ganglia atrophy with diffuse Purkinje cell loss, as well as peripheral sensory neuronopathy including the vestibular, facial, and trigeminal nerves1,3–7 (Figure 1A).
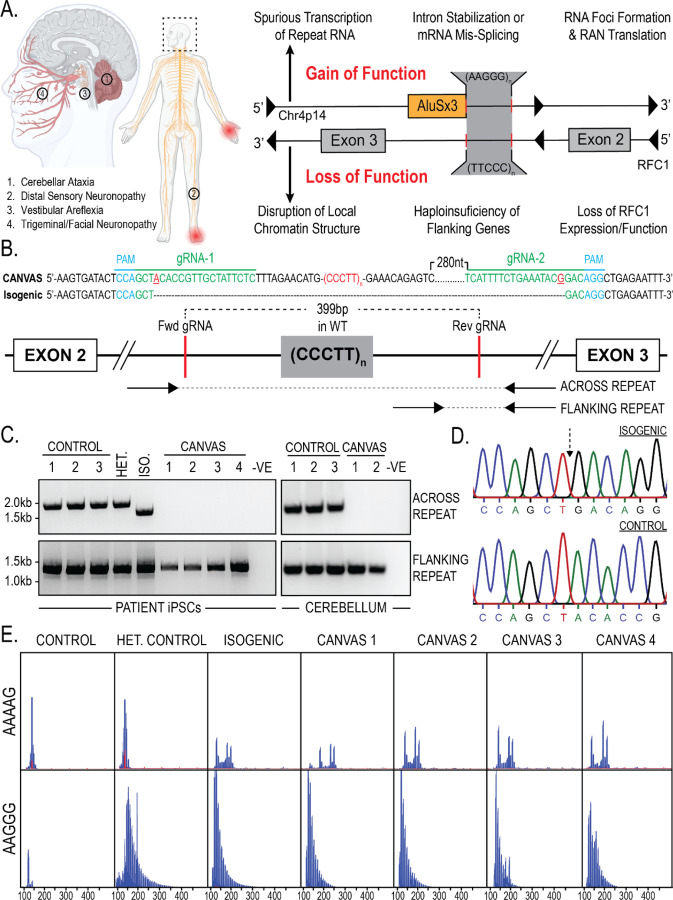
Figure 1 – Repeat characterization and heterozygous correction of CANVAS patient derived iPSC lines.
A) Schematic of brain, CNS, and PNS regions affected in CANVAS (left), and potential mechanisms of repeat toxicity in CANVAS (right). B) Repeat architecture of the expanded locus and CRISPR gRNA design to remove the AAGGG/CCCTT repeat expansion by NHEJ. C) Endpoint PCR of gDNA extracted from CANVAS patient and control derived iPSC lines and CANVAS and control cerebellum tissue utilizing the primer pairs outlines in (1B) to screen for the presence of WT repeat, mutant repeat expansion, or deletion of expanded repeat. D) Chromatogram of Sanger Sequencing identifying AAGGG/CCCTT allele deletion in heterozygous isogenic line indicating the expected NHEJ join point compared to control iPSC line. E) Repeat Primed PCR of the expanded locus in CANVAS patient and control derived iPSC lines utilizing anti AAAAG and AAGGG probes.
CANVAS results from a biallelic, non-reference, pentameric CCCTT(AAGGG) repeat expansion in the second intron of replication factor complex subunit 1 (RFC1) and this same expansion also causes late-onset idiopathic ataxia and sensory neuropathy in isolation8–10. Normally, this locus harbors a short (AAAAG)~11 repeat, and individuals harboring a heterozygous (AAGGG)exp allele with the wild type (AAAAG)~11 allele do not develop CANVAS spectrum phenotypes, consistent with a recessive pattern of inheritence8,9. Since the initial description of AAGGG expansions at this locus, several further pathogenic repeat expansion conformations have been described: ACAGG expansions have been identified in CANVAS patients of Oceania and East Asian descent11, and more recently the 100,000 genomes project database has identified AAGGC, AGGGC, and AGAGG expansions, either in the homozygous or compound heterozygous state with the AAGGG expansion12. Furthermore, AAAGG repeat expansions previously reported to be non-pathogenic in the homozygous state and compound heterozygous state with AAGGG expansions have since been found to be pathogenic when present at significantly expanded states12. RFC1-associated repeat expansions are common, with a minor allelic frequency of ~0.7–6.8% and an expected homozygous pathogenic repeat population frequency of 1 in 625 individuals worldwide—making it one of the most common causes of inherited ataxia and sensory neuropathy8,13,14
Despite clinical interest in CANVAS, the mechanisms by which this repeat expansion causes pathogenesis and neuronal death are unknown. Initial studies suggested that RFC1 mRNA and protein expression are unaltered in the context of the repeat expansion, which is inconsistent with the classical mechanism by which recessively inherited repeat expansions elicit a loss of function for the genes in which they reside8,10,15,16. However, identification of rare compound heterozygotic CANVAS patients harboring RFC1 truncating and splicing loss-of-function mutations with single allele repeat-expansions suggests a role for RFC1 function in CANVAS17–19. Alternatively, the repeats could potentially elicit toxicity through dose-dependent gain of function mechanisms (such as repeat associated non-AUG initiated (RAN) translation or repeat RNA-protein complex formation) that only manifest in homozygosity or in combination with RFC1 haploinsufficiency20,21 (Figure 1A). Limited postmortem and cell-based analyses performed to date have not demonstrated classic pathological hallmarks seen in other repeat expansion disorders, such as repeat RNA foci or ubiquitinated inclusions8.
Understanding the pathogenic nature of this repeat expansion is further complicated by its manifestation within two distinct genetic elements on opposing strands. The AAGGG repeat sits at the 3’ end of an AluSx3 transposable element on the sense strand, while the complementary CCCTT repeat is embedded deep within the large intron 2 region of RFC1, potentially allowing for two distinct and context-dependent mechanisms of toxicity. Alu elements are ancient retro-transposition artifacts that make up roughly 10% of the human genome (~1 million copies)22 and serve as reservoirs of potential regulatory functions that have actively driven primate evolution, with evidence to suggest roles for intronic Alu elements in mRNA splicing23,24 (constitutive and alternative), RNA editing25, and protein translation26. Moreover, the AAGGG repeat sequence found on the opposing strand to RFC1 at this AluSx3 3’ end is predicted to form G-quadruplex secondary structures implicated in repeat expansion disease pathogenesis in both transcribed RNA and DNA during transcription and DNA replication fork formation27.
Given the paucity of empiric data on these repeats and the multiple potential mechanisms underpinning CANVAS, we investigated how these repeats cause disease, including independent evaluation of previously tested ideas and direct measures of the ability of these repeats to elicit neurotoxicity. We generated multiple CANVAS patient-derived and CRISPR-corrected isogenic induced pluripotent stem cell (iPSC) lines and differentiated them into neurons. Through functional neuronal assays, transcriptomic analyses, and in vitro toxicity investigations, we identify deficits in key neuronal cellular pathways in CANVAS neurons that are rescued upon CRISPR correction of a single expanded repeat allele. In contrast, loss of RFC1 expression fails to recapitulate these cellular and molecular phenotypes and reprovision of RFC1 into CANVAS iNeurons is insufficient to reverse pathologic cascades. Together, these studies support a repeat-dependent mechanism of toxicity that operates outside of the canonical functions of RFC1.
Results
CANVAS patient-derived and CRISPR-corrected heterozygous isogenic iPSC lines
Homozygous non-reference repeat expansions in RFC1 could potentially elicit neurodegeneration through multiple gain-of-function or loss-of-function mechanisms (Figure 1A). To assess these possibilities systematically, we generated a series of CANVAS patient derived-derived iPSC lines from 4 patients and controls, as well as one family member with a heterozygous RFC1 expansion (Supplementary Figure 1A–B). As an additional experimental control, we generated an isogenic line through deletion of the expanded allele in patient one iPSC line through CRISPR-Cas9 genome editing (Figure 1B–D). Successful deletion of a single expanded allele was achieved in CANVAS patient 1 iPSCs (Figure 1C), and the specificity of this deletion was confirmed by Sanger sequencing of PCR products (Figure 1D). However, we were unable to generate biallelic homozygous deletions of this AluSx3 element and expanded repeat despite multiple rounds of editing and re-editing in both CANVAS and control cell lines, consistent with prior reports28. As such, the heterozygous deletion line was taken forward for experimental analysis. Cell lines were confirmed to possess AAGGG/CCCTT repeat expansion alleles by repeat-primed PCR (Figure 1E) in combination with the results obtained by screening PCR (Figure 1C).
Translated AAGGG repeat products are detected in CANVAS patient brains
To investigate the potential of repeat-dependent gain-of-function mechanisms in CANVAS pathogenesis (Figure 1A), we generated repeat-containing reporter constructs encompassing various repeat motifs within intronic sequence contexts (Figure 2A). Wild-type AAAAG/CTTTT and mutant CANVAS AAGGG/CCCTT repeat expansions were generated by recursive directional ligation29 and were ligated into plasmid backbones containing 150bp of upstream intronic sequence deriving from the genomic strand within which the repeat motif would be found; AAAAG/AAGGG repeats for the sense strand AluSx3 containing sequence and CTTTT/CCCTT repeats for the antisense RFC1 intron 2 containing sequence.
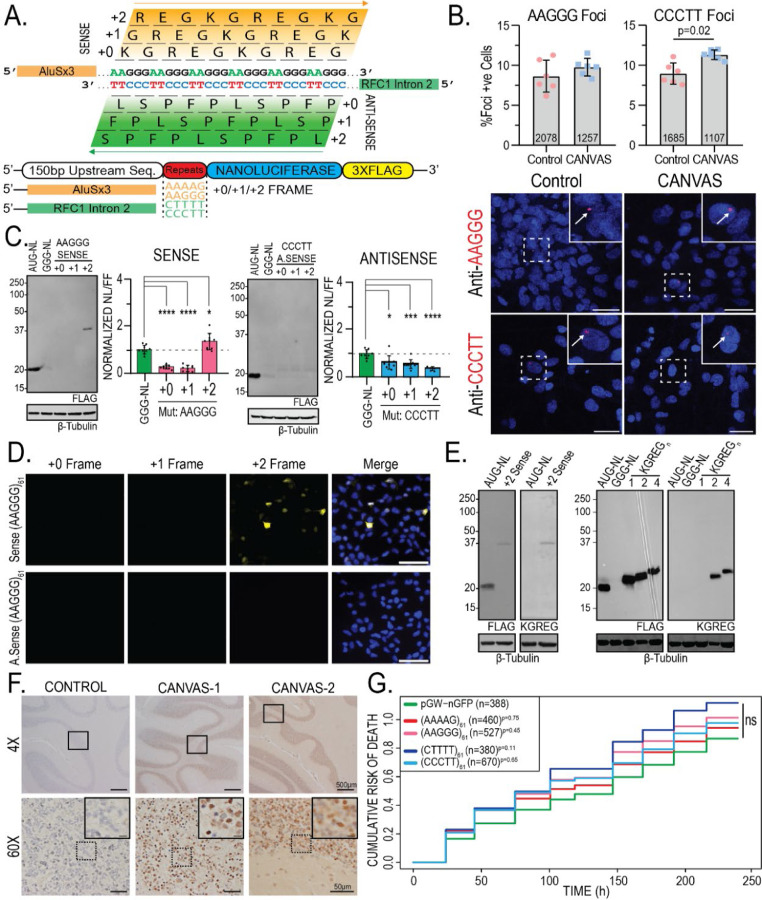
Figure 2 – Translated AAGGG repeat products are detected in CANVAS patient brains.
A) Schematic of reading frames and peptide products from the sense and antisense strands of the repeat expansion locus. B) Confocal Images of representative CANVAS patient and control neurons after RNA HCR with anti-AAGGG or anti-CCCTT fluorescent probes, scale = 10 µm, and quantification of foci positive neurons for control (n=3) and CANVAS (n=3) patient neurons with total n-numbers of neuronal cells analyzed indicated. AAGGG (F(5, 12) = 3.619, P=0.074), CCCTT (F(5, 12) = 8.293, P=0.011). n = 2 biological replicates from 3 independent patient derived cell lines. Data were analyzed by one-way ANOVA with Sidak’s post-hoc multiple comparison tests. C) Expression analysis of lysates from HEK293 cells transfected with control plasmid or plasmids encoding intronic sense or antisense AAGGG/CCCTT repeat reporters in the +0/+1/+2 reading frames (left) and Nano-luciferase expression assay quantification (right). N = 3 technical replicates from a total of 7 biological replicates. Data were analyzed by one-way ANOVA with Sidak’s post-hoc multiple comparison tests. D) ICC of HEK293 cells transfected with plasmids encoding intronic sense or antisense AAGGG/CCCTT repeat reporters with C-terminal triple-tags in the +0/+1/+2 reading frames. E) Expression analysis of lysates from HEK293 cells transfected with control plasmid +2 Sense (AAGGG)61 plasmid using anti-FLAG M2 (1:1000) and anti-KGREG (1:100) antibodies (left). F) IHC of control (n=1) and RFC1 expansion CANVAS (n=2) patient post-mortem cerebellar vermis tissue stained with sense anti-KGREG antibody (1:100, acid AR). Scale = 500 µm (4x), 50 µm (60x) and 20 µm (inset). Full IHC results of 3x control tissues are shown in supplementary figure 2C. G) Cumulative Hazard Plot for primary rat cortical neurons transfected with CANVAS intronic expression plasmids containing 61 repeats of the indicated type over a 10-day period, n = 8 technical replicates, 3 biological replicates, n numbers of cells shown per condition. Error = SD.
We first assessed whether expression of these repeat containing constructs elicit RNA foci formation by using repeat-targeting fluorescent probes and RNA HCR-FISH (Supplementary Figure 2A). In HEK293 cells, sense (AAGGG) repeat-directed probes detected nuclear and cytoplasmic RNA foci that co-localized with anti-nanoluciferase probes in transfected cells and generated patterns of expression that were different from nanoluciferase expression alone. Most of the nuclear and all the cytoplasmic signal was ablated by RNase treatment (Supplementary Figure 2A). A similar pattern was seen for antisense (CCCTT) repeat-directed probes, suggesting that both the sense and antisense pentanucleotide repeats can form RNA condensates within cells. To assess if such foci are detectable in patient cells, we performed RNA HCR-FISH with these same probes in control (n=3) and CANVAS (n=3) patient iPSC-derived neurons (Figure 2B, Supplementary Figure 2B–C). No significant difference was observed between CANVAS and control neurons for sense AAGGG repeat-RNA foci detection (F(5, 12) = 3.619, P=0.074). In contrast, antisense CCCTT repeat-RNA foci were detected in CANVAS neurons at greater rates compared to control cells (F(5, 12) = 8.293, P=0.011). However, the overall abundance of foci was low, and the specificity was imperfect, with 8.94% of control neurons and 11.3% of CANVAS neurons showing antisense CCCTT RNA foci in blinded assays (Figure 2B, Supplementary Figure 2D).
The native sequences surrounding the repeats are predicted to contain both AUG and non-AUG near-cognate codons upstream of the repeats without any intervening stop codons—potentially placing the repeats within open reading frames. To assess whether these repeats might be translated in patients, we generated intronic sense and antisense reporter constructs with Nanoluciferase-3xFlag (NL-3F) C-terminal tags in all 3 potential reading frames.
Protein expression and nanoluciferase activity analysis in transfected HEK293 cells showed selective translation of repeat-derived peptides within the sense (AAGGG) strand +2 reading frame (Figure 2C, left). In contrast, no products were detected from the antisense RFC1 intron in all 3 reading frames (Figure 2C, right), despite the presence of an AUG codon in the +0 reading frame. This was also observed by ICC in HEK293 cells, whereby only cells transfected with the +2 sense AAGGG reporter showed translation of any repeat-containing peptides (Figure 2D).
If the AAGGG repeat were to be translated into a protein, it would generate the same pentapeptide repeat protein, polyKGREG, in all 3 potential reading frames. We therefore generated antibodies against a polyKGREG epitope. This antibody showed a high degree of specificity for two or more KGREG repeats, which are otherwise not predicted to occur in the human proteome, in transfected HEK293 cells by immunoblot staining (Supplementary Figure 3A) and strong colocalization with FLAG antibodies by immunocytochemistry (Supplementary Figure 3B). Using this antibody, we confirmed that the +2 sense AAGGG reporter product contained KGREG peptides (Figure 2E). To assess whether sense-strand derived KGREG peptide products accumulate in CANVAS patient brains, we performed immunohistochemistry on control (n=3) and CANVAS (n=2) post-mortem brain samples obtained from the University of Michigan Brain Bank, Harvard University Brain Bank, and the University of Amsterdam Brain Bank (Figure 2F, Supplementary Figure 3C). KGREG staining was selectively detected within cerebellar granule cells in CANVAS patients, with no signal detected in controls. While CANVAS patient brains showed marked Purkinje cell loss, no KGREG staining was observed in the few Purkinje cells that remained (Figure 2F, Supplementary Figure 3C). These data suggest that pentapeptide KGREG repeat proteins may be produced from AAGGG repeats in CANVAS patients in a cell type-specific manner.
Expression of either CGG or GGGGCC repeats in neurons is sufficient to elicit toxicity, which can serve as a proxy for gain-of-function associated neurodegeneration30–34. We therefore tested whether ectopic AAGGG or CCCTT repeat expression might elicit neurotoxicity. Using automated longitudinal fluorescence microscopy35,36, expression of either the intronic WT or mutant sense or antisense reporter constructs in primary rat cortical neurons showed no toxicity over a 10-day period compared to expression of GFP alone (AAAAG61: P=0.75, AAGGG61: P=0.45, CTTTT61: P=0.11, CCCTT61: P=0.65) (Figure 2G). These data suggest that while AAGGG and CCCTT repeats are capable of eliciting RNA foci and being translated into pentapeptide repeat proteins, they do not exhibit intrinsic toxicity and do not accumulate into pathologic aggregates in patient tissues.
Splicing of RFC1 is normal in multiple CANVAS patient derived cell types
Intronic repeat expansions can interfere with pre-mRNA splicing by inducing intron retention within the mature transcript 37,38, stabilizing circular intronic lariat species39, or inducing differential exon usage during splicing40–42. Intronic Alu elements also influence alternative splicing through inclusion of Alu-containing exons, with 85% of Alu containing exons deriving from antisense Alu elements43,44. To assess whether AAGGG/CCCTT repeat expansions induced intron retention, we performed end-point RT-PCR with primers that amplify the exon2-exon3 junction or the exon2-intron2 junction (Figure 3A). There were no differences between cases and controls in terms of intron retention or aberrant intron 2 splicing of RFC1 in patient fibroblasts, iPSC-derived neurons or cortical and cerebellar regions of post-mortem brain (Figure 3A).
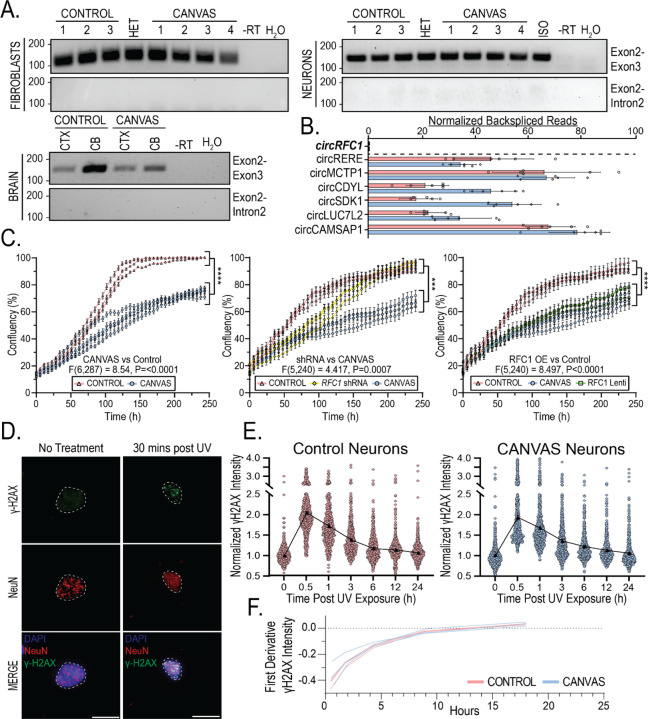
Figure 3 – Canonical functions and expression of RFC1 are normal in CANVAS patient-derived cells.
A) Endpoint RT-PCR utilizing primer sets spanning RFC1 exon2-exon3 or exon2-intron 2 in CANVAS fibroblasts (Top, left), iPSC-derived neurons (Top, right), and CANVAS post-mortem brain (Bottom, left). B) Quantification of normalized circular backspliced read counts for RFC1 and other known circRNA species in CANVAS patient iPSC-derived neurons by paired-end RNASeq analysis. C) 10-day time-course analysis of the rate of cellular division and proliferation in CANVAS (n=4) and control (n=3) fibroblast lines (left, F(6,287) = 8.54, P<0.0001), control fibroblast lines (n=3) mock-treated or treated with RFC1 shRNA lentivirus (center, F(5,240) = 1.314, P=0.131), and CANVAS fibroblast lines (n=3) mock-treated or treated with RFC1 overexpression lentivirus (right, F(5,240) = 2.358, P=0.245). N = 3 biological replicates from 3–4 independent patient cell lines. Data were analyzed by one-way ANOVA with Sidak’s post-hoc multiple comparison tests. D-E) Analysis of recovery after discrete UV exposure and DNA damage in CANVAS patient iPSC-derived neurons. D) Representative images of γ-H2AX staining of iPSC-derived neurons pre- and post-60 mJ/cm2 UV exposure (Scale = 10µm). E) Quantification of mean γ-H2AX staining in CANVAS patient (n=3) and control (n=3) iPSC-derived NeuN+ neuronal nuclei over a 24h period after 60 mJ/cm2 UV exposure (n=16,569 NeuN+ nuclei total). Data were analyzed by one-way ANOVA with post-hoc multiple comparison tests. F) First derivative DNA damage recovery rate curves for CANVAS (n=3) and control (n=3) patient iPSC-derived neurons. Error = SD.
To investigate whether AAGGG/CCCTT repeat expansions trigger RFC1 intron 2 stabilization as a circular lariat species, as may occur in C9orf72 FTD/ALS39, we utilized whole-transcriptome RNA short read sequencing to map RFC1 mRNA splicing isoforms and identify the presence of intronic backspliced reads indicative of circular mRNA species (circRNAs) arising from circular intronic lariats45. Total rRNA-depleted RNA was extracted from 10-week-aged CANVAS patient and control iPSC-derived neurons and paired-end reads were processed and analyzed using packages described in methods. A known list of circRNA species46–50 were detected at comparable levels in CANVAS and control samples (Figure 3B), however, no backspliced reads were identified to map to RFC1 intron 2 region or across the RFC1 transcript, indicating a lack of detectable circular RNA species deriving from the RFC1 locus (Figure 3B).
To assess whether AAGGG/CCCTT repeat expansions affect RFC1 mRNA isoforms, we used DEXSeq51 to assess the normalized differential exon usage of mature spliced RFC1 transcripts in CANVAS patient and control iPSC-derived neurons (Supplementary Figure 4A). No changes in RFC1 exon usage were observed within the N-terminal regions flanking the repeat expansion, or across the entire RFC1 transcript. Similarly, Sashimi plots of RFC1 splicing demonstrate similar splicing and alternative exon usage in CANVAS patient iPSC-derived neurons compared to controls without evidence for alternative non-canonical exon usage for RFC1 in normal or disease states (Supplementary Figure 4B).
Canonical functions and expression of RFC1 are normal in CANVAS patient-derived cells
Prior studies in CANVAS patient fibroblasts, lymphoblasts, frontal cortex and cerebellar vermis of CANVAS patients demonstrated no differences in RFC1 mRNA when compared to controls8. Consistent with prior studies, we observed no changes in RFC1 steady-state mRNA abundance or RFC1 protein expression between control (n=3), CANVAS (n=4), and heterozygous carrier patient fibroblasts (n=1) (Supplementary Figure 5A). Similarly, we observed no changes in RFC1 mRNA or RFC1 protein levels between 6-week-old control (n=3) and CANVAS (n=3) patient iPSC-derived neurons (Supplementary Figure 5B). Analysis in limited post-mortem frontal cortex and cerebellar brain samples showed no change in RFC1 mRNA, with a modest but significant increase in RFC1 protein (Supplementary Figure 5C).
RFC1 plays critical roles in both DNA replication and DNA damage repair pathways as a subunit of the DNA clamp-loader complex52–54. To assess whether AAGGG/CCCTT repeat expansions might interfere with RFC1’s DNA replication functions, we first investigated the rate of cellular proliferation in age- and passage-matched CANVAS (n=4) and control (n=3) patient-derived fibroblasts (Figure 3C, left). We observed a highly significant decrease in cellular proliferation rate for CANVAS fibroblasts when compared to controls (F(6,287) = 8.54, P<0.0001). To determine if this CANVAS fibroblast proliferation phenotype was dependent on RFC1 expression or function, control-derived fibroblasts (n=3/group) were transduced with lentiviral particles encoding shRNAs targeting RFC1 exon 4 and exon 15, or a control shRNA, and were assessed for proliferation rate over a 10-day period as before (Figure 3C, Center). RFC1 knockdown reduced RFC1 protein to nearly undetectable levels (Supplementary Figure 7). RFC1 knockdown in control fibroblasts resulted in an initial lag in cellular proliferation compared to control shRNA-treated fibroblasts (120h, P= 0.001) which recovered to control levels after 200h (Figure 3C, Center, F(5,240) = 1.314, P=0.131), while untreated CANVAS patient fibroblasts continued to show a slower proliferation rate throughout the analysis (F(5,240) = 4.417, P=0.0007). To assess if reprovision of full-length wild-type RFC1 was sufficient to overcome the CANVAS phenotype, we overexpressed full-length RFC1 in CANVAS patient-derived fibroblasts (Figure 3C, Right). CANVAS patient-derived fibroblasts overexpressing RFC1 retained a markedly slowed proliferation rate compared to control fibroblasts (F(5,240) = 8.497, P<0.0001), with no significant improvement over the proliferation rate of CANVAS patient-derived fibroblasts treated with a control lentivirus (F(5,240) = 2.358, P=0.245). These results suggest that AAGGG/CCCTT repeat expansions affect cell proliferation independent of RFC1 protein levels.
CANVAS is predominantly a neuronopathy, and as neurons are post-mitotic, we investigated whether DNA damage repair was dysfunctional in CANVAS patient iPSC-derived neurons. To do this, we analyzed expression of the DNA Damage marker γ-H2AX in 8-week-old control and CANVAS iPSC-derived neurons where basal levels of DNA damage accumulation was comparable between control and CANVAS iPSC-derived neurons (Supplementary Figure 6A). To test for an altered response to DNA damage, CANVAS patient and control iPSC-derived neurons were exposed to UV irradiation (Figure 3D–E). To test for an altered response to DNA damage, CANVAS patient and control iPSC-derived neurons were exposed to UV irradiation (Figure 3D–E). As expected, γ-H2AX levels increased after exposure to 0–120 mJ/cm2 UV irradiation in control iPSC-derived neurons (Supplementary Figure 6B). Control (n=3) and CANVAS patient (n=3) iPSC-derived neurons showed a consistent ~2-fold increase in γ-H2AX reactivity within 30 mins of 60 mJ/cm2 UV irradiation, followed by a slow but consistent decline in γ-H2AX reactivity in all experimental conditions, with return to baseline γ-H2AX reactivity within 6–12h post-irradiation. First-derivative analysis of normalized γ-H2AX indicated no differences in the rate of γ-H2AX recovery after UV induction between CANVAS and control neurons (Figure 3E–F, Supplementary Figure 6C–D), suggesting that DNA damage repair is not affected in CANVAS neurons.
Synaptic genes are downregulated in CANVAS patient-derived neurons
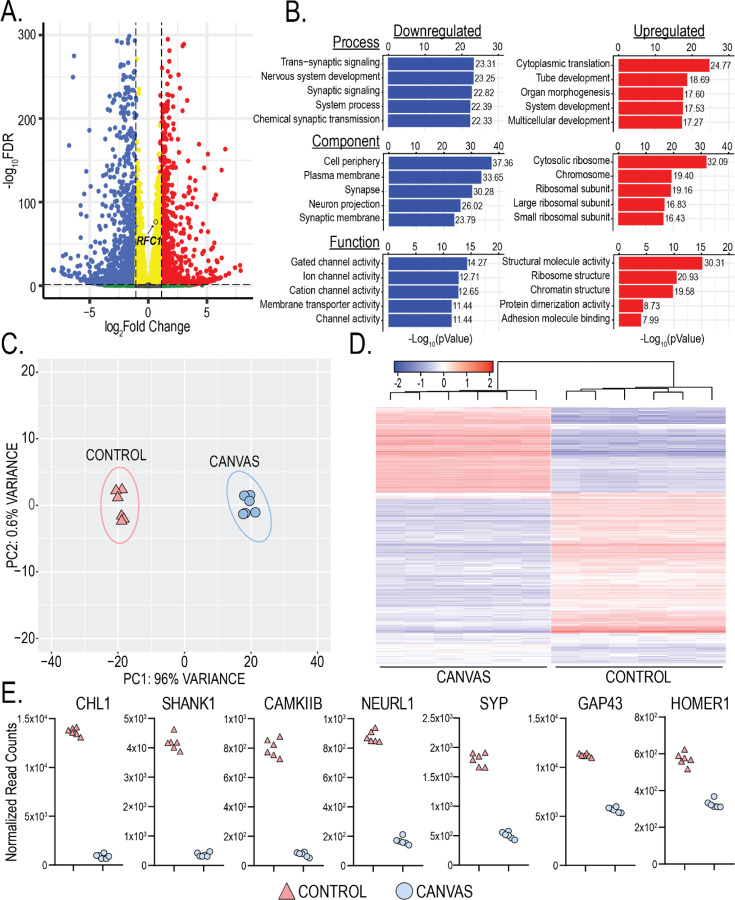
To assess for differences between CANVAS and control iPSC-derived neurons that might provide some insights into disease pathogenesis, we next conducted paired-end sequencing of total RNA extracted from 10-week-old CANVAS patient (n=6) and control (n=6) iPSC-derived neurons to identify potential dysregulated pathways or genes in CANVAS. An average of ~20–30M reads were obtained per sample. After trimming, genome alignment, and filtering for low transcript reads (see methods), we observed that 5.9% (1313) of detected genes were upregulated and 11.7% (2630) were downregulated in CANVAS patient iPSC-derived neurons compared to control neurons (Figure 4A). RFC1 transcripts showed a modest but significant increase in CANVAS iNeurons that was below the prespecified fold-change threshold implemented (Figure 4A), with no RFC1-associated GO-terms identified within overrepresented cellular pathways of these dysregulated transcripts in CANVAS (Figure 4B). Principal component analysis (PCA) clustering of neuronal samples based on gene expression patterns demonstrated discrete and distinct clustering of CANVAS neuronal samples as separate from controls (Figure 4C) with 96% of observable variance seen across principal component 1 (PC1), indicating high variability between control and CANVAS gene expression profiles and a dominant source of variation between conditions. Heatmap analysis of the top dysregulated transcripts detected in CANVAS patient iPSC-derived neurons show a strong preference for reduced expression of transcripts (Figure 4D). Gene ontology (GO) analysis indicated a significant overrepresentation of neuronal signaling processes including synaptic signaling and processes that regulate synaptic signaling within the transcripts downregulated in CANVAS vs control (Figure 4B, Supplementary Figure 8), with many of these transcripts localized to neuronal processes and involved in signaling, channel, or transporter activity. Normalized gene counts for select dysregulated synaptic genes indicates that this reduction in synaptic gene expression is highly significant and encompasses both pre- and post-synaptic genes, as well as genes involved in synaptic organization and signal transduction (Figure 4E). A full analysis of dysregulated pathways is shown in Supplementary Figure 8.
Figure 4 -. Synaptic genes are downregulated in CANVAS neurons.
A) Volcano plot of differentially expressed genes in CANVS patient vs control iPSC-derived neurons, blue = significantly downregulated, red = significantly upregulated, RFC1 labelled. B) Gene Ontology (GO) pathway analysis of the top 5 up/downregulated Biological Process, Cellular Component, and Molecular Function in CANVAS patient vs control iPSC-derived neurons. N = 6 biological replicates from 3 individual CANVAS and control patients. C) Principal Component Analysis (PCA) of CANVAS (n=6) vs control (n=6) patient iPSC-derived neurons. D) Heatmap of normalized expression for the top 1000 genes differentially expressed in CANVAS patient vs control iPSC-derived neurons. E) Normalized gene counts for the top 7 downregulated synaptic-associated genes in CANVAS patient vs control iPSC-derived neurons.
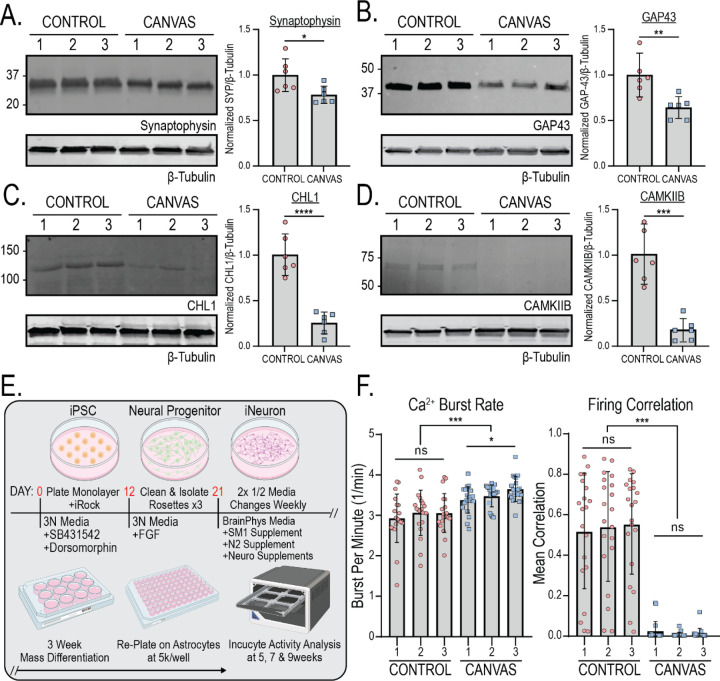
Protein expression analyses of select synaptic genes significantly downregulated in transcriptomic analyses in Figure 4E found similar downregulation at the protein level. Synaptophysin, CHL1, GAP43, and CAMKIIB all showed highly significant reductions in expression at both the transcript and protein level in CANVAS patient iPSC-derived neurons compared to control (Figure 4E, Figure 5A–D).
Figure 5 – CANVAS patient derived neurons exhibit synaptic dysfunction and reduced connectivity.
A-D) Protein expression (left) and normalized quantification (right) of selected synaptic genes identified as downregulated in CANVAS patient iPSC-derived neurons by transcriptomic analysis - (A) Synaptophysin, (B) GAP43, (C) CHL1, (D) CAMKIIB. N = 2 biological replicates from 3 independent patient derived cell lines. Data were analyzed by one-way ANOVA with Sidak’s post-hoc multiple comparison tests. E) Schematic outlining experimental workflow for generating patient iPSC-derived neurons for calcium imaging analysis. F) Analysis of Ca2+ imaging metrics for control (n=3) and CANVAS (n=3) patient iPSC-derived neurons at 9-weeks post-differentiation. Burst Rate (F(5, 114) = 8.268, P<0.0001), Firing Correlation (F(5, 114) = 45.62, P<0.0001). Each data point represents the mean of ~1000–3000 active cells per well (Supplementary Figure 5). Data were analyzed by one-way ANOVA with Sidak’s post-hoc multiple comparison tests. Error = SD.
Spontaneous synaptic activity is impaired in CANVAS patient-derived neurons
Given the dysregulation of synapse-associated transcripts and proteins we observed in CANVAS patient iPSC-derived neurons, we investigated if these alterations in gene expression correlated with observable phenotypic differences in synaptic activity. To accomplish this, we measured spontaneous synaptic activity in control (n=3) and CANVAS (n=3) glutamatergic forebrain neurons with the Incucyte® Neuroburst Orange fluorescent calcium indicator and Incucyte® S3 automated imaging system. Patient-derived neural progenitor cells were differentiated and re-plated at uniform density of 5,000 cells per well on an astrocyte feeder layer within PLO/Laminin coated 96-well plates described in methods (Figure 5E). Neurons were analyzed for active cell number, basal calcium intensity, burst rate, burst strength, burst duration, and network correlation between 3–11 weeks post-differentiation (Figure 5F, Supplementary Figure 9A), and wells with at least 500 active cells were included in the analysis. Both control and CANVAS iPSC-derived neurons consistently had between 1000–2000 active cells per well with minor but statistically significant reductions observed for basal calcium intensity, burst duration, and burst strength in CANVAS neurons (Supplementary Figure 9A). Notably, while control iPSC-derived neurons formed synchronized networks with 50–70% network correlation by 5 weeks post-differentiation (Figures 5F and 6E), CANVAS patient iPSC-derived neurons remained devoid of detectable synchronous firing even 7–11 weeks post-differentiation, with single cells showing independent activity at a 17% increase in firing rate relative to control (Figure 5F, Supplementary Movies).
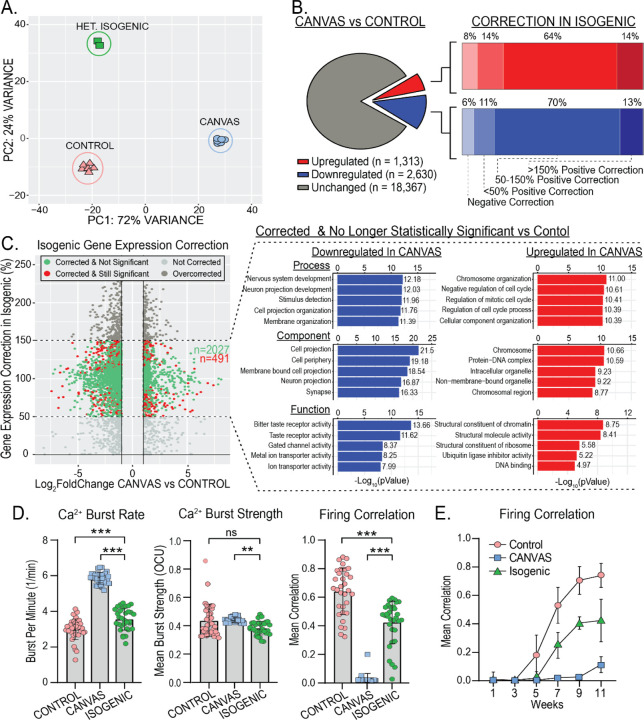
Figure 6 – Heterozygous isogenic correction of CANVAS neurons corrects transcriptomic and synaptic functional deficits.
A) Principal Component Analysis (PCA) of CANVAS (n=6), control (n=6), and CANVAS Heterozygous Isogenic (n=2) patient iPSC-derived neurons. B) Schematic illustrating the total number of genes dysregulated (up & down) in CANVAS vs control (left), with the percentage of these up- or down-dysregulated genes that show negative correction, partial correction, or full correction of expression upon heterozygous isogenic correction of CANVAS patient iPSC-derived neuron line. C) Scatter plot of Log2FoldChange (CANVAS vs control) vs Gene expression correction per gene in the heterozygous isogenic patient iPSC-derived neurons (left), and Gene Ontology (GO) pathway analysis of the top 5 up/downregulated Biological Process, Cellular Component, and Molecular Function for the genes that show 50–150% gene expression in Isogenic Correction vs CANVAS and are non-statistically significant in Isogenic vs control conditions. D) Analysis of Ca2+ imaging metrics for control (n=3), CANVAS (n=3), and Heterozygous Isogenic (n=1) patient iPSC-derived neurons. Burst Rate (F(2, 165) = 279.4, P<0.0001), Burst Strength (F(2, 165) = 4.034, P=0.019), Firing Correlation (F(2, 165) = 185.9, P<0.0001). Each data point represents the mean of ~1000–3000 active cells per well (Supplementary Figure 8). Data were analyzed by one-way ANOVA with Sidak’s post-hoc multiple comparison tests. E) Mean firing correlation of control (n=3), CANVAS (n=3), and heterozygous isogenic (n=1) patient iPSC-derived neurons across 10 weeks of differentiation from week 1 to week 11. Error = SD.
Synaptic gene expression is corrected upon heterozygous correction of RFC1 repeat expansion
To determine the impact of the AAGGG/CCCTT repeat expansion in CANVAS patient iPSC-derived neurons, we generated an isogenic line with a monoallelic deletion of the repeat and compared its gene expression to both CANVAS and control iPSC-derived neuronal lines. Surprisingly, the heterozygous isogenic line almost completely corrected across PC1 that separates CANVAS from control neuronal samples, while variance along PC2 was increased substantially, indicating partial correction of some additional CANVAS-associated variance to control and the emergence of a new source of variance after heterozygous deletion of the AAGGG/CCCTT repeat expansion (Figure 6A, Supplementary Figure 10A–B). This large-scale correction of the CANVAS associated transcriptomic signature is also visible by heatmap analysis illustrating that the heterozygous isogenic patient iPSC-derived neurons exhibit a global gene expression pattern more similar to that of control than CANVAS (Supplementary Figure 10C).
In total, 64% of the CANVAS upregulated genes and 70% of the CANVAS down-regulated genes exhibited at least a 50% correction in expression upon monoallelic deletion of the AAGGG/CCCTT repeat expansion in the CANVAS patient 1 line (Figure 6B), and 80.5% of these genes (2027:491) were no longer significantly different compared to controls (Figure 6C, Green). Importantly, the GO-terms of these gene expression corrected genes correlate with many of the top dysregulated pathways in CANVAS, including multiple synapse-associated processes and functions (Figure 6C, right, Supplementary Figure 10D). Within these pathways, the key synaptic genes found to be most downregulated in CANVAS (Figure 4E) show significant or almost complete restoration in the isogenic neurons compared to control (Supplementary Figure 10E), with CAMKIIB, GAP43, HOMER1, NEURL1, and SYP showing the greatest restoration in the isogenic line.
Dysregulated synaptic activity is rescued by heterozygous correction of RFC1 repeat expansion
We next analyzed spontaneous synaptic activity upon heterozygous isogenic correction in comparison to both control and CANVAS patient iPSC-derived neurons (Figure 6D). Heterozygous isogenic neurons exhibited modest but significant improvements in basal calcium intensity and burst duration compared to CANVAS neurons (Supplementary Figure 9B), as well as large and significant improvements in neuronal burst rate synchronized firing (Figure 6D–E), with an average network correlation of 42% compared to 70% in controls. While these metrics don’t indicate complete recovery of deficits observed in CANVAS versus control, the significant trend towards recovery in all metrics in conjunction with the observed correction in gene expression upon deletion of a single expanded allele in CANVAS neurons suggests a repeat-dependent mechanism of CANVAS pathogenesis.
RFC1 reduction does not mimic CANVAS-associated transcriptomic or functional deficits
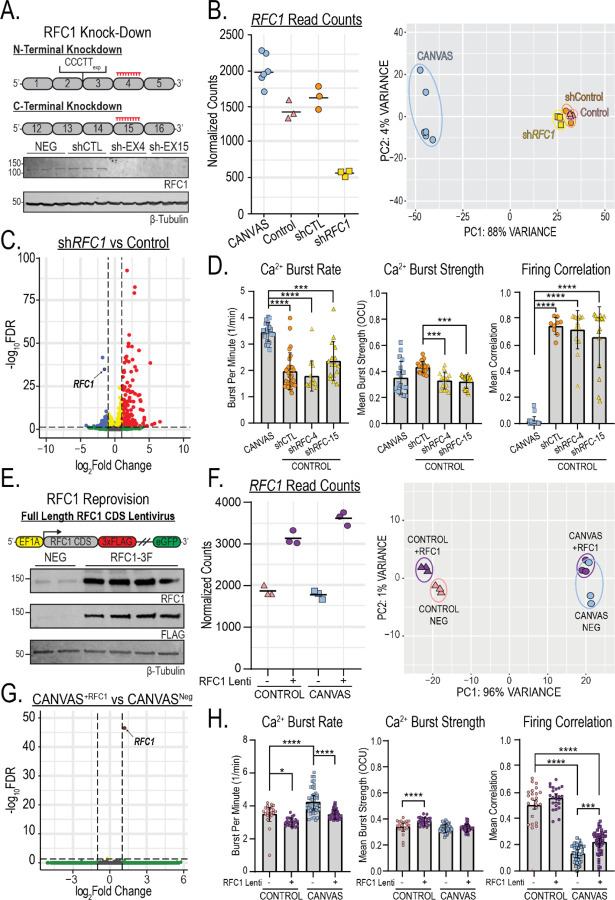
If loss of RFC1 function is central to CANVAS pathogenesis, then targeted loss of RFC1 should recapitulate defects observed in CANVAS patient neurons. To test this hypothesis, we conducted transcriptomic- and functional neuronal analyses of control neurons after sustained (~10 weeks) knockdown of RFC1 in comparison to CANVAS neurons. Treatment with RFC1 shRNA lentivirus led to undetectable levels of RFC1 protein (Figure 7A), and normalized RFC1 gene counts across experimental conditions showed efficient knockdown of RFC1 transcripts with ~63% reduction in comparison to control neurons and ~75% reduction in comparison to CANVAS neurons (Figure 7B, left). Compared to control neurons expressing a non-targeting shRNA, RFC1 knockdown led to statistically upregulated expression of only 0.5% (235) of detected genes and statistically downregulated expression of only 0.23% (103) of detected genes in iPSC-derived neurons. PCA clustering showed no overlap between RFC1 shRNA treated iPSC-derived neurons and CANVAS neurons and instead showed tight clustering with non-targeting shRNA expressing controls (Figure 7B, right, Supplementary Figure 11A–B), indicating that RFC1 knockdown in control neurons does not induce CANVAS-like transcriptomic alterations. Further, volcano plot of RFC1 knockdown vs control neurons (Figure 7C) indicates less dysregulation in gene expression when compared to CANVAS vs control (Figure 4A), with a preference for increased expression for a small number of transcripts and a reduction in RFC1 (circled). Similarly, none of the key synaptic genes found to be highly downregulated in CANVAS (Figure 4E) showed significant dysregulation upon RFC1 knockdown (Supplementary Figure 11C).
Figure 7 – RFC1 knockdown or reprovision fail to recapitulate or correct transcriptomic and synaptic deficits in CANVAS patient-iPSC derived neurons.
A) Schematic outlining approach to knockdown of either the N- or C-terminus of RFC1 by shRNA lentiviral transduction (Top), and analysis of RFC1 expression from control iPSC-derived neurons (GTX129291, 1:1000) indicating successful knockdown of RFC1 (Bottom). B) Normalized read counts for RFC1 transcripts in CANVAS, control iPSC-derived neurons as well as control iPSC-derived neurons treated with either control or anti-RFC1 shRNA lentivirus (left), and Principal Component Analysis (PCA) of CANVAS (n=6), control (n=3), control mock-treated (n=3), and control shRFC1 treated (n=3) patient iPSC-derived neurons. C) Volcano plot of −Log10FDR vs Log2(Fold Change) for RFC1 knockdown vs control, RFC1 labelled. D) Analysis of Ca2+ imaging metrics for CANVAS (n=3) and control (n=3) patient iPSC-derived neurons treated with shControl or shRFC1 exon4/exon15 lentiviruses. Burst Rate (F(3, 78) = 29.6, P<0.0001), Burst Strength (F(3, 78) = 8.265, P<0.0001), Firing Correlation (F(3, 78) = 100.6, P<0.0001). E) Schematic outlining the approach of RFC1 overexpression in CANVAS patient iPSC-derived neurons by lentiviral transduction (Top), and analysis of RFC1 expression in patient iPSC-derived neurons upon lentiviral transduction (Bottom). F) Normalized read counts for RFC1 transcripts in CANVAS and control iPSC-derived neurons transduced with either full-length RFC1 CDS lentivirus or control lentivirus (n=3/group) (left), and Principal Component Analysis (PCA) of CANVAS and control iPSC-derived neurons transduced either full-length RFC1 CDS lentivirus or control lentivirus (n=3/group) (right). G) Volcano plot of −Log10FDR vs Log2(Fold Change) for CANVAS patient-derived neurons transduced with either full-length RFC1 CDS lentivirus or control lentivirus (n=3/group), RFC1 labelled. H) Analysis of Ca2+ imaging metrics for control (n=3) and CANVAS (n=3) patient iPSC-derived neurons treated with control or RFC1-overexpression lentivirus. Burst Rate (F(3, 135) = 31.01, P<0.0001), Burst Strength (F(3, 135) = 16.74, P<0.0001), Firing Correlation (F(3, 135) = 147.3, P<0.0001). Firing Correlation two-way ANOVA treatment vs genotype: F(1,135) = 41.25, P<0.0001, F(1,135) = 36.64, P<0.0001 respectively. Each data point represents the mean of ~1000–3000 active cells per well (Supplementary Figure 5). Data were analyzed by one-way ANOVA with Sidak’s post-hoc multiple comparison tests. Error = SD.
Gene set enrichment analysis (GSEA) of ranked genes from RFC1 knockdown showed an enrichment for annotated RFC1-associated functions (Supplementary Figure 12). Gene ontology (GO) analysis for the most overrepresented cellular pathways within the transcripts dysregulated upon RFC1 knockdown, meanwhile, found that immune and developmental cellular processes were most significantly dysregulated (Figure 7C). There was no enrichment in synaptic-associated processes, in contrast to the significantly dysregulated pathways identified in CANVAS neurons vs control (Figure 4B, Supplementary Figures 8 & 11D).
To assess the impact of RFC1 knockdown on neuronal function, we again used calcium imaging. Compared to control neurons, RFC1 knockdown elicited no differences in basal calcium intensity, burst duration (Supplementary Figure 9C), neuronal burst rate or network firing correlation (Figure 7D) and the RFC1 knockdown neurons remained highly significantly different from CANVAS neurons in these four metrics. The only metric that showed any similarities with the findings in CANVAS neurons was burst strength, where knockdown of RFC1 induced a 24% reduction (Figure 7D, center, P<0.0001), comparable to the levels seen in CANVAS neurons (CANVAS – shRNA1: P=0.915, CANVAS – shRNA2: P=0.786).
Reprovision of RFC1 does not correct CANVAS-associated transcriptomic or functional deficits
To determine if reprovision of RFC1 can correct phenotypic deficits observed in CANVAS patient neurons, we conducted transcriptomic- and functional neuronal analyses of control and CANVAS iPSC-derived neurons after sustained (~10 weeks) expression of either GFP lentivirus or a full-length RFC1 lentivirus construct with a C-terminal 3x FLAG tag and non-fused GFP (Figure 7E). Transduction of this lentivirus significantly boosted RFC1 expression in patient iPSC-derived neurons (Figure 7E), with a ~50–75% increase in detected RFC1 transcripts compared to neurons treated with the GFP control lentivirus only (Figure 7F, left). PCA clustering showed no overlap of RFC1 overexpression CANVAS neurons with control neurons and instead showed tight clustering between genotypes independent of treatment condition (Figure 7F, right), indicating that RFC1 overexpression fails to correct CANVAS neuronal transcriptomic alterations. Further, volcano plot of differential gene expression in CANVAS neurons overexpressing RFC1 compared to those expressing a control GFP lentivirus show that sustained reprovision of RFC1 to CANVAS neurons induces effectively no transcriptomic changes, with only RFC1 found to be differentially expressed between these conditions (Figure 7G). Similarly, comparison of CANVAS neurons overexpressing RFC1 with control neurons (Supplementary Figure 13A) exhibited identical differential gene expression patterns as was observed in Figure 4A. Overexpression of RFC1 in control neurons also triggered minimal changes in global gene expression (Supplementary Figure 13B), and heatmap profiles of global gene expression across all conditions indicate that overexpression of RFC1 does not correct the transcriptomic deficits observed in CANVAS, and elicits negligible effects within control neurons (Supplementary Figure 13C).
When analyzing the functional phenotypes observed for CANVAS neurons, overexpression of RFC1 elicited no differences for metrics that exhibited only minor or no dysregulation in CANVAS compared to controls (Supplementary Figure 7D). Reprovision of RFC1 did reduce the abnormal firing burst rate in CANVAS neurons by 17.6%, which effectively corrected the difference between CANVAS and control neurons. However, RFC1 overexpression also induced a 13.9% reduction in burst rate within control neurons, suggesting that this effect is not specific to CANVAS neurons (Figure 7H, left). 2-way ANOVA analysis indicates that the effect sizes are comparable and that the CANVAS vs control genotype is not a driver of the effect (Treatment: F(1,135)=41.25, P<0.0001, Genotype: F(1,135)=36.64, P<0.0001). Similarly, RFC1 overexpression increased the firing correlation observed for CANVAS neurons (0.13 to 0.22, P=0.001). However, this correction was modest, and these CANVAS neurons remained significantly less correlated than control neurons (P<0.0001, Figure 7H, right).
Discussion
With an estimated carrier frequency upwards of ~6%8,14, non-reference AAGGG/CCCTT repeat expansions in RFC1 are potentially significant contributors to neurologic disease, including both ataxia and sensory neuronopathy. Here we used patient-derived neurons, patient cells and tissues to directly assess how these repeats elicit toxicity. Our findings suggest a specific role for the repeat element in neuronal development and synaptic function that is largely independent of RFC1 expression and its canonical functions. These findings have important implications for therapy development in this currently untreatable disorder.
CANVAS arises from a polymorphic set of biallelic RFC1 repeat expansion motifs comprising AAGGG, AAAGG, ACAGG, AAGGC, AGGGC, and AGAGG motifs in isolation or as heterozygous combinations8,9,11,12. Further, rare CANVAS patients harbor compound heterozygous monoallelic RFC1 expansions combined with loss-of-function mutations that result in RFC1 haploinsufficiency from the non-expanded allele17–19. This, in combination with data indicating a recessive mode of inheritance, suggested to us and others that RFC1 loss-of-function is a pathogenic driver of CANVAS pathogenesis. However, we find that RFC1 expression is normal at both the mRNA and protein level in the context of biallelic expansions in differentiated human iPSC-derived neurons, with no changes in RFC1 mRNA splicing, intron degradation, transcript isoform, or exon usage in CANVAS neurons compared to controls (Figure 3A–B, Supplementary Figure 4). Consistent with this, we observe no deficits in canonical RFC1 functions in either mitotic- and post-mitotic CANVAS patient-derived cell types. RFC1 plays a key role in both DNA replication and DNA damage repair52, but CANVAS patient-derived neurons do not accumulate DNA damage compared to controls, and they exhibit normal rates of recovery after UV-induced DNA damage (Figure 3, Supplementary Figure 6). Deficits in the rate compared to controls. CANVAS patient-derived neurons exhibit significant dysregulation in genes associated with pathways regulating synaptic of cell division and proliferation were observed in CANVAS patient fibroblasts (Figure 3C). However, these deficits were independent of RFC1 function, as they were neither replicated by RFC1 knockdown in control fibroblasts nor corrected by reprovision of RFC1 to CANVAS patient fibroblasts. Despite the clinical genetic data strongly suggesting an RFC1 loss-of-function in CANVAS and our own data showing gene alterations and signaling dysfunction in CANVAS patient iPSC-derived neurons, we find no evidence for a loss of canonical RFC1 function in multiple cell types, pointing towards a more nuanced etiology driving CANVAS pathogenesis at the RFC1 locus.
Repeat associated gain-of-function mechanisms, such as the formation of RNA foci and the presence of ubiquitinated inclusions of RAN translated repeat polypeptides, occur in many repeat expansion neurologic diseases31,37–39,55. Yet, postmortem and cell-based analyses performed to date have not demonstrated any of these pathological features in CANVAS7,8. We detected sense strand AAGGG repeat-derived pentapeptide repeat peptides in cerebellar granule cells in CANVAS patient, but not control, brains (Figure 2, Supplementary Figure 3). We did not detect these proteins or repeat RNA foci in patient iPSC-derived neurons and overexpression of these AAGGG repeats in isolation did not induce neuronal toxicity (Figure 2, Supplementary Figure 2). It is therefore unclear what role – if any-these translated pentapeptide repeats play in the neuropathogenic cascades that precede CANVAS symptom onset. CANVAS patient iPSC-derived neurons showed significant deficits in synaptic gene expression at both the mRNA and protein level structure and organization, regulation of chemical synaptic transmission, and expression of ion channels localized to both the pre- and post-synaptic membranes (Figures 4–5, Supplementary Figure 8). This deficit correlates with phenotypic deficits in synaptic activity (Figures 5–7). While synaptic dysfunction and loss of synaptic connections occurs in numerous neurodegenerative diseases, such as Huntington Disease (HD)56,57, C9orf72 FTD/ALS58,59, and Alzheimer Disease (AD)60,61, the molecular steps preceding this deficit in synaptic gene expression and associated synaptic dysfunction in CANVAS patient neurons is unclear. Intriguingly, monoallelic deletion of the AAGGG expansion significantly corrected both synaptic gene expression defects and synaptic signaling dysfunction in CANVAS neurons (Figure 6). As this isogenic deletion of a single AAGGG expansion does not alter RFC1 expression or neuronal response and recovery after UV induced DNA damage (Supplementary Figure 6), this rescue in synaptic gene expression and neuronal connectivity instead appears to be dependent on the repeat element itself.
If dysfunction in RFC1 protein expression or activity was the cause of CANVAS phenotypes, then we would predict that synaptic dysfunction and gene expression alterations in CANVAS patient-derived neurons would be replicated by knockdown of RFC1 in control neurons. However, sustained (~10 weeks) knockdown of RFC1 with two independent shRNAs in control iPSC-derived neurons elicits no deficits in synaptic function or gene expression changes akin to those seen in CANVAS neurons (Figure 7, Supplementary Figures 9, 11, 12). Similarly, if RFC1 loss impacts CANVAS disease relevant phenotypes, then prolonged reprovision of RFC1 protein into CANVAS patient iPSC-derived neurons should rescue the functional synaptic phenotypes observed. Yet, we observed no correction of CANVAS neuronal transcriptomic signatures after RFC1 reprovision. Moreover, while minor changes were observed in CANVAS neuronal activity with exogenous RFC1 expression, these improvements were both modest and non-specific, with similar effects in control and CANVAS neurons. CANVAS neurons remained highly dysregulated even when expressing RFC1 (Figure 7, Supplementary Figures 9, 11).
In summary, our studies provide strong support for a repeat-dependent mechanism of neuronal dysfunction in CANVAS that operates outside of the canonical functions of RFC1. These findings stand in contrast to clinical genetic studies pointing towards an RFC1 loss-of-function mechanism as a central contributor to the molecular etiology of CANVAS and suggest that replacing or boosting canonical RFC1 function would be ineffective as a therapeutic approach in this condition. Moreover, the mechanism by which these repeats act does not fit into known repeat-associated gain-of-function and loss-of-function boxes previously defined for other repeat expansion disorders. Instead, our findings suggest that these non-reference repeats act through an as-yet undefined novel molecular mechanism that will likely have relevance beyond this condition.
Methods
Dermal fibroblast isolation and iPSC derivation from canvas patient samples
Dermal fibroblasts were obtained from consenting patients clinically diagnosed with CANVAS spectrum disorder and genetically confirmed to possess biallelic RFC1 expansions. Dermal biopsy samples were cultured in Fibroblast Medium (DMEM, 10% (v/v) FBS, 1% (v/v) 100x NEAA, 1% (v/v) Pen/Strep, 1.5% (v/v) 1M HEPES) at 37 ºC and 5% CO2 until fibroblasts emerged from the tissue and were maintained at low passage number and grown to 80–90% confluence prior to episomal reprogramming. On day 0, fibroblasts were detached with Trypsin-EDTA, washed with −/− Phosphate Buffered Saline (PBS), and 3x105 cells were resuspended in 120 µl of R-buffer (Invitrogen) supplemented with 1 µg GFP plasmid, 1µg pCXLE-hUL (Addgene #27080), 1 µg pCXLE-hSK (Addgene #27078), and 1 µg pCXLE-hOCT3/4-shP53 (Addgene #27077) and electroporated using the Neon System (Invitrogen, Condition: 1450 V, 10 ms, 3-pulses). Electroporated fibroblasts were subsequently plated across 3 wells of a Geltrex™ (Thermo Scientific) coated 6-well plate with daily media changes of fresh fibroblast media until day 3. On day 3 cells were changed into a 50/50 ratio of fibroblast media and TeSR™ E-7™ Reprogramming Media (STEMCELL Technologies), and on day 5 were changed into 100% E-7™ Reprogramming Media. Daily media changes of E7™ Reprogramming Media followed until the emergence of iPSC colonies between day 21–28 when iPSC colonies were picked and transferred to Geltrex™ coated 12-well plates containing 1 mL TeSR™-E8™ (STEMCELL Technologies) supplemented with 10 µM ROCK Inhibitor (Y-27632, Cayman Chemical) for 24 h. iPSCs were maintained in TeSR™-E8™ at 37 ºC and 5% CO2 and passaged with 0.5 mM EDTA as needed. Multiple iPSC colonies were picked, expanded, and characterized per patient line before use. iPSC colonies were stained via ICC for pluripotency markers using antibodies against SOX2 (ab5603), OCT4 (ab181557) & Nanog (ab21624), and mRNA expression of pluripotency transcription factors was confirmed by RT-PCR (Supplementary Figure 1A–B). iPSCs were confirmed to have no chromosomal abnormalities by G-band karyotyping (Supplementary Figure 1B, WiCell Research Institute).
Generation of heterozygous isogenic iPSC lines by CRISPR-Cas9
CRISPR gRNAs were designed to remove the expanded AAGGG repeat by non-homologous end joining (NHEJ) utilizing the closest unique PAM sites to the repeat region and obtained from IDT (Figure 1B). iPSCs were detached to a single cell suspension using Accutase™ (ThermoFisher), and 1x106 cells were resuspended in 120 µL R-buffer containing pre-formed Cas9 RNP complexes of (20 µM HiFi Cas9 (IDT), tracR-ATTO550 (IDT), and gRNAs: F: GAGAATAGCAACGGTGTAGCTGG, R: TCATTTTCTGAAATACGGACAGG). The iPSC:RNP mix was electroporated using the Neon system (Condition: 1450 V, 10 ms, 3-pulses), before plating across 3 wells of a Geltrex™ coated 6-well plate with TeSR™-E8™ media supplemented with 10uM ROCK inhibitor (Y-27632) for 24 h. Electroporation efficiency was assessed by nuclear positive fluorescence of TracR ATTO550, and iPSCs were cultured until healthy colonies emerged. Clonal populations were achieved through single-cell isolation and expansion62. Emerging clonal colonies were screened by PCR utilizing primer sets that spanned the repeat, priming either inside or outside the deletion region (Figure 1C, Supplementary Table 1). Utilizing these primer sets, lack of amplification using primer set 1 in conjunction with amplification using primer set 2 indicates a biallelic expansion at this locus, amplification with both primer sets indicates either a biallelic wild-type locus, or the presence of a heterozygous monoallelic expansion when combined with a positive saw-tooth like pattern by repeat-primed PCR. Finally, reduced molecular weight amplification utilizing primer set 1 and full-length amplification with primer set 2 in combination with a positive saw-tooth like pattern by repeat-primed PCR indicates a monoallelic deletion of this locus by CRISPR-Cas9, and reduced molecular weight amplification with primer set 1 and absence of amplification with primer set 2 indicates a biallelic deletion of this locus byCRISPR-Cas9. Successful deletion of a single allele was achieved in CANVAS patient 1 iPSCs (Figure 1C), and the specificity of this deletion was investigated by sanger sequencing of long-range PCR products (Figure 1D).
RFC1 repeat screening and repeat-primed PCR
Cell pellets were obtained from patient-derived cell lines by Accutase™ detachment and centrifugation, or from patient post-mortem brain tissue through gentle neutral protease digestion of brain tissue chunks for 45 mins at 37 °C, followed by mechanical dissociation to single cell slurry by trituration. Genomic DNA was subsequently isolated from cell pellets using gDNA mini-prep kit (Zymo). Screening for repeat expansions in RFC1 was achieved by a combination of short-range repeat-spanning end-point PCR (Supplementary Table 1) with 2X Faststart PCR mastermix (Roche) and by Repeat-Primed PCR using 2X Phusion Flash High-Fidelity PCR Mastermix (Thermo Fisher). End-point PCR was achieved by amplifying across the repeat region where the presence of a single band at the expected size indicated a non-expanded Wild-Type locus, whereas the absence of a band in the presence of a control band utilizing primers that amplified the intronic region adjacent to the repeat locus indicated the presence of a large, expanded region (Figure 1C). Repeat primed PCR of the repeat locus was conducted as previously described8. 5’-FAM labelled PCR products were analyzed through capillary electrophoresis by Laragen Inc. and files were analyzed by Peak Scanner Fragment Analysis Software (Figure 1E). Primers and cycling conditions for all PCR/RP-PCR experiments are outlined in Supplementary Table 1.
Differentiation and maintenance of patient iPSC-derived neurons
iPSCs were differentiated to neural precursor cells (NPCs) and glutamatergic forebrain neurons using a modified dual-SMAD inhibition protocol63 (Supplementary Figure 1C–E). Briefly, iPSCs were detached and plated as a monolayer at 100% confluence in TeSR™-E8™ media containing 10 µM ROCK Inhibitor (Y-27632) for 24 h. On day 2, media was changed to 3N+A Media (241 mL DMEM/F12 with HEPES + L-Glutamine, 241 mL Neurobasal Medium, 5 mL NEAA, 2.5 mL N2 supplement, 5 mL B27 (- Vitamin A), 2.5 mL Glutamax™, 2.5 mL Pen/Strep, 125 µl Insulin, and 3.5 µl BME) supplemented with two SMAD inhibitors: 10 µM SB431542 (Cayman Chemical) and 1 µM dorsomorphin (Cayman Chemical). At day 10–12, neuroepithelial sheets were combed into large clumps, passaged, and maintained on Geltrex™ coated plates in 3N+A media supplemented with 20 ng/mL Fibroblast Growth Factor (FGF), with daily media changes until neuronal rosettes appeared (Supplementary Figure 1D). Rosettes were manually picked and dissociated into single cell NPCs using Accutase™ and plated on to Geltrex™ coated plates in neural expansion medium (3N+A containing 20 ng/mL FGF and 20 ng/mL epidermal growth factor (EGF)) with media changes every other day and passaged as needed using Accutase™. Neuronal differentiation was achieved by plating high-density NPCs and switching to Neuronal Induction Media (490 mL Brainphys, 10 mL SM1, 5 mL N2, 20 ng/mL Brain Derived Neurotrophic Factor (BDNF), 20 ng/mL Glial Derived Neurotrophic Factor (GDNF), 200 nM L-ascorbic acid, and 1 mM bucladesine (dbcAMP)). Neurons were maintained with half medium changes twice per week, with Neuronal Induction Media supplemented with 1 µg/mL laminin once per week. After 3 weeks of neuronal induction, post-mitotic neurons were detached using Accutase™ and replated at a uniform density on PEI & laminin coated plates before experimentation.
Lentivirus production and transduction of iPSC-derived neurons
pLV-eF1-RFC1-eGFP full-length RFC1 expression plasmid was designed and synthesized by VectorBuilder, and pSMART-eF1-RFC1shRNA-tGFP expression plasmids were designed and produced by Horizon Discovery (Table 1). Plasmids were prepped for lentiviral production by bacterial transformation followed by maxi-prep. 80 µg of plasmid DNA was used for lentiviral particle generation at University of Michigan Viral Vector Core. Lentivirus particles were delivered resuspended in DMEM media at 100x concentration (1x108 TU/mL). Lentivirus transduction of patient iPSC-derived neurons occurred on DIV7 post-differentiation: neurons were first treated with 1 µg/mL polybrene for 30 minutes, before being washed with fresh complete Brainphys media and subsequently changed to conditioned media containing lentiviral particles diluted to their working concentration. Neurons were changed to fresh media 24 h after transduction, and fluorescently positive cells appeared ~72 h post-transduction. Efficiency of lentiviral transduction is assessed in supplementary figure 7.
Generation of repeat-containing CANVAS DNA reporter plasmids
Plasmids containing expanded AAGGG/AAAAG or CCCTT/CTTTT repeats were generated using Recursive Directional Ligation (RDL)29. Briefly, gene blocks containing 16 repeats flanked by type IIS restriction sites were produced by Genewiz (Azenta), and these were ligated into pcDNA3.1 plasmids mutated to remove restriction sites of interest. The repeat size was recursively doubled through a process of plasmid digestion to remove the repeat unit, followed by re-ligation back into the donor plasmid that had been nicked open at the 3’ end of the repeat unit. When the repeats were built to the maximal length before significant repeat shrinkage was observed, the repeats were removed by restriction endonuclease digestion, and ligated into pcDNA3.1 reporter plasmids containing 100 nt of sense or antisense intronic sequence (Figure 2A).
Longitudinal survival assays of primary rodent and iPSC-derived neurons
Mixed cortical neurons were dissected from E20 Long–Evans rat pups of both sexes, as previously described35,36. Cortical neurons were cultured at 0.6 × 106 cells mL−1 on 96-well plates. Cultures were maintained at 37 °C in Neuronal Growth Media (NGM - Neurobasal-A supplemented with 2% (v/v) B-27 and 1% (v/v) Glutamax™ (Fisher)). On DIV 4, neurons were co-transfected with 0.1 μg of pGW1-mApple and either 0.1 μg of pGW1-GFP or 0.1 μg of experimental DNA per well of a 96-well culture plate, using Lipofectamine 2000 (Invitrogen). Neurons were imaged at regular 24 h intervals starting 24 h post-transfection using an automated fluorescence microscopy platform detailed in earlier studies35,36. Image processing for each time point and survival analysis for automated fluorescence images were achieved by custom code written in Python or the ImageJ macro language, and cumulative hazard plots were generated using the survival package in R.
Post-mortem brain IHC and antibody generation
Rabbit polyclonal antibodies were generated by Abclonal (Cambridge, MA) against peptides containing (KGREG)7 or (PFPSL)7, corresponding to (AAGGG)21 and (CCCTT)21 respectively. Antibodies were affinity purified from anti-sera. Pre-bleed sera and peptides were used to characterize the antibodies and protein concentrations of the pre-bleed sera were determined. The dilution of pre-bleed sera used had an equal protein concentration as the dilution of antibody used. A 100x excess of peptide was incubated with the antibody prior to use to block the antibodies’ ability to bind to antigen. CANVAS and control brain regions were obtained from the University of Michigan Brain Bank, Harvard University Brain Bank, and the University of Amsterdam Brain Bank with informed consent of the patients or their relatives and the approval of the local institutional review boards (IRB). For immunohistochemistry, paraffin embedded sections were de-paraffinized with a series of xylene washes and decreasing concentrations of ethanol. Antigen retrieval (AR) was achieved with citrate buffer, if necessary. Endogenous peroxidase was quenched with 1% hydrogen peroxide for 30 min. Sections were blocked in 5% normal goat serum (NGS) in Tris, pH 7.6. Primary Antibodies, KGREG7 (Abclonal, 1:100, Acid AR), PFPSL7 (Abclonal, 1:100, Acid AR) were diluted in 5% NGS Tris B (Tris pH 7.6, 0.1% Triton-X 100, 0.5% bovine serum albumin) and were incubated with sections overnight at 4 °C. The following day, sections were washed in Tris A (Tris pH 7.6, 0.1% Triton) and Tris B. Antibody detection was determined using VECTASTAIN Elite ABC HRP kit (Vector Laboratories) following the manufacturer’s protocol. Sections were counterstained with hematoxylin (Vector Laboratories). After dehydrating the slides through a series of increasing concentration of ethanol and then xylenes, coverslips were mounted to the slides using DPX mounting media. Images were taken on an Olympus BX51 microscope.
RNA hybridization chain reaction (HCR) of RNA foci
CANVAS patient and control iPSC-derived neurons were differentiated and maintained to 8 weeks of age as previously described. Cells were washed with (−/−) PBS and subsequently fixed with 4% paraformaldehyde (PFA) and incubated overnight in 70% ethanol. After overnight incubation with 70% ethanol, cells were rehydrated in PBS for 1 h, permeabilized with 0.1% Triton X-100 for 5 min, and blocked with 2% bovine serum albumin (BSA) for 20 min at room temperature. Repeat-containing RNA foci were probed in patient iPSC-derived neurons using DNA probes with additional sequence complementary to Cy5-labeled, self-hybridizing hairpins64–67. Probes against the AAAAG, AAGGG, CTTTT & CCCTT sequences (Supplementary Table 2) were purchased from molecularinstruments.org and applied according to the manufacturer’s protocol. Coverslips were then applied to slides with ProLong Gold Antifade Mounting Medium with DAPI. Then, 10–20 fields per condition were imaged blinded using an inverted, Olympus FV1000, laser-scanning confocal microscope. Channels were imaged sequentially and optimized to eliminate bleed-through. Neurons were imaged in a series of Z-planes to resolve the entire soma and dendritic arbor. Images were analyzed blinded in ImageJ. Average intensity composite images were derived from raw image files. For quantification of individual soma, cell casts were made using threshold images as a guide.
Analysis of DNA damage accumulation and recovery in iPSC-derived neurons
The accumulation of basal DNA damage was analyzed in 8-week-old patient iPSC-derived neurons by Western Blot for the DNA damage marker γ-H2AX (P-S139, Abcam ab11174). The ability to detect DNA damage was tested in patient iPSC-derived neurons by exposure to 0, 15, 30, and 120 mJ/cm2 ultra-violet (UV) irradiation to induce differing degrees of DNA damage, followed by Western Blot analysis for γ-H2AX. Recovery after DNA damage induction in CANVAS patient iPSC-derived neurons was conducted as described: CANVAS patient and control iPSC-derived neurons were plated on PEI/laminin coated glass chamber slides and maintained for 8 weeks as previously described. At 8 weeks, neurons were either fixed with 4% PFA for no-exposure control or irradiated with 60 mJ/cm2 UV. UV Irradiated cells were fixed in 4% PFA at time intervals of: 0.5, 1, 3, 6, 12, and 24 h post-irradiation, and were stained for the neuronal nuclei marker NeuN, DNA marker DAPI and DNA damage marker γ-H2AX. Images were captured in all 3 fluorescent channels at the same exposure for each cell line and time point at 40x magnification with an Olympus IX71 fluorescent microscope and Slidebook 5.5 software, and γ-H2AX reactivity analyzed using FIJI. High throughput quantification of cells was achieved by using a FIJI script to mask NeuN positive nuclei using the parameters of size: 200–1500 pixels2 and circularity: 0.40–1.00. Masked NeuN positive nuclei were subsequently analyzed for γ-H2AX intensity and was quantified and visualized using GraphPad Prism v.9.
Cell proliferation assays
CANVAS patient and control fibroblasts were analyzed for cell proliferation rates through automated longitudinal brightfield microscopy using the Incucyte S3 system. Fibroblasts were seeded into TPP™ 96-well plates at a density of 2,500 cells per well and whole wells were imaged by tiling of 5x images per well using S3 Neuro O/NIR Optical Module 10x magnification with 6 h intervals for a period of 10 days. Cell confluency was automatically analyzed using the Incucyte® S3 Live Cell Analysis Software using the basic analyzer cell confluence settings to mask and quantify individual cell number and absolute confluence with the parameters: segmentation adjustment – 2, hole fill – 0 µm2, adjust size – 4 pixels. 3 control and 4 CANVAS patient fibroblast lines were used, with 12 wells averaged per fibroblast line for technical replicates within each biological replicate, and a total of 3 biological replicates presented. For lentiviral experiments, fibroblasts were transduced with lentiviral particles in 10 cm dish mass culture 7 days prior to re-seeding at experimental density in 96-well plates. Lentiviral particles were diluted within culture medium to a final concentration of 1x.
Calcium imaging and analysis of iPSC-derived neurons
TPP™ tissue-culture treated 96-well plates were prepared by coating with 0.1% polyethyleneimine (PEI) diluted in borate buffer for 24 h, followed by 3x washes in H2O and coating with 1 µg/mL laminin diluted into neuronal medium overnight before use. Rat primary cortical astrocytes (Fisher) were plated at high density and maintained until confluence in Astrocyte Media (DMEM/F12, 10% (v/v) Horse Serum, 10% (v/v) FBS, 3% (v/v) 20% D-glucose solution, 2% (v/v) B27 supplement, 1% (v/v) Glutamax™, 1% (v/v) Pen/Strep antibiotic). CANVAS and control iPSC-derived neurons were differentiated from NPCs in Geltrex™-coated 10 cm plates as previously described for 21 days. 5 days post-differentiation, neurons were transduced with lentiviral particles as previously described based on experimental setup. After 3-weeks neuronal differentiation, neurons were detached using 25% Accutase™ and incubating at 37 ºC for 10 mins. Neurons were diluted in pre-warmed Brainphys neuronal media and gently triturated to detach. Neurons were pelleted by centrifugation and resuspended in 1 mL pre-warmed Brainphys neuronal media before passing through a 45 µm cell strainer to obtain a single cell suspension of neuronal soma. Cells were counted using a hemocytometer with trypan blue as a live cell marker, and neurons were diluted to a concentration of 5,000 live cells in 200 µl complete neuronal media per well of a 96-well plate. 10 days post-replating, neurons were transduced with Incucyte® Neuroburst Orange Lentivirus particles by diluting 5 µl lentivirus into complete Brainphys neuronal media per well, and neurons were maintained as previously described until analysis using the Incucyte® S3 Live Cell Analysis Instrument. Image acquisition was conducted using S3 Neuro O/NIR Optical Module at 4x magnification for 180 s per well in phase and orange fluorescence channels, and data were automatically analyzed using the Incucyte® S3 Live Cell Analysis Software with the parameters: object size − 30 µm, min cell width − 9 µm, sensitivity − 1, min burst intensity − 0.2. Neurons were analyzed for active cell number, basal calcium intensity, burst rate, burst strength, durst duration, and network correlation. Data for different neuronal signaling metrics were compiled for each scan time point and analyzed using GraphPad Prism v.9.
RNA extraction and RT-PCR/qPCR
Total RNA was extracted using TRIzol and chloroform, followed by isopropanol precipitation. RNA pellets were washed in 75%+ ethanol and resuspended in nuclease free H2O. Contaminating gDNA was removed by DNase I digestion (Fisher Scientific) and cleaned up using an RNA clean & concentrator kit (Zymo). Total RNA was quantified by NanoDrop UV/Vis spectrophotometry, and 1 µg of RNA was reverse-transcribed (RT) to cDNA using iScript cDNA synthesis kit (Bio-Rad) with oligo-dT primers for poly-A mRNA selection. RT-PCR was achieved using 2X Faststart PCR mastermix (Roche) using primer sets and cycling conditions described in Supplementary Table 1. RT-qPCR was achieved using Taqman Real-Time PCR Assay(s) (Thermo Fisher) using primer probe sets and cycling conditions described in Supplementary Table 1.
RNA-seq sample preparation and analysis
For RNA-seq experiments, patient iPSC-derived neurons were plated and aged to ~10 weeks as previously described. Total RNA was extracted using TRIzol and chloroform, followed by isopropanol precipitation. RNA pellets were washed in 75%+ ethanol and resuspended in nuclease free H2O. Contaminating gDNA was removed by DNase I digestion (Fisher Scientific) and cleaned up using an RNA clean & concentrator kit (Zymo). Total RNA was quantified by NanoDrop UV/Vis spectrophotometry, analyzed for integrity by Bioanalyzer, and 1.5–2 µg total RNA was sent to Genewiz (Azenta) for rRNA-depletion library preparation and paired-end (PE) sequencing, where between 20–30 M paired-end reads were achieved per sample. Raw FASTA read files were delivered by sFTP transfer and analyzed in Command Line and R-Studio. Read files were quality controlled using FastQC(v0.12.0)68, pre-processed and trimmed using fastp(v0.23.4)69, and aligned to GRCh38 (NCBI GCF_000001405.40) genome using STAR(v2.7.11a) two-pass alignment70. Transcript and gene count matrices were produced using featureCounts(Subread v2.0.6)71. Read count matrices were imported into R-Studio (vRtidyverse/4.2.0) where count normalization and differential gene expression analysis was conducted using DESeq2(v1.40.2)72. Gene Ontology (GO) Pathway Overrepresentation analysis was conducted using GOstats(v2.66.0)73, and Gene Set Enrichment Analysis (GSEA) conducted using clusterProfiler(v4.8.3)74 and gseGO(v3.0.4)74. Plots were generated and visualized using DESeq2(v1.40.2)23, EnhancedVolcano(v1.18.0)75, ggPlot2(v3.4.3)76, and ggRidges(v0.5.4)76. Differential exon usage was analyzed and plotted using DEXSeq(v1.46.0)51, circular RNA analysis was conducted using DCC(v0.5.0) in Python77. Sashimi plots of splice junction analysis were produced using Integrative Genomics Viewer (IGV) Genome Browser78 and exported for modification in Illustrator (Adobe). All harvesting and sequencing of RNA was conducted at the same time within each experimental condition to avoid batch effects. Genes and pathways that showed significant correction in the heterozygous isogenic corrected line from CANVAS to control were identified by binning normalized gene expression values into categories of those showing reduced expression upon heterozygous deletion, 0–50% positive correction in expression compared to control, 50–150% positive correction in expression compared to control, and those genes that showed over 150% correction in comparison to control. These genes were further binned into their statistical significance in difference of expression when compared to control and plotted using ggPlot2(v3.4.3) ref and Gene Ontology (GO) Pathway Overrepresentation analysis was conducted using GOstats(v2.66.0)ref.
Supplementary Material
| Reagent or Resource | Source | Details |
|---|---|---|
| Cell Lines | ||
|
| ||
| Control 1 (2E) | Laboratory of Jack Parent, University of Michigan | Tidball et al.79, Mojica-Perez at al.62 |
| Control 2 (NL-Xa 1071) | Laboratory of Paul Hagerman, UC Davis | Liu et al.80 |
| Control 3 (UM4-6) | MStem Cell Laboratory of Gary Smith, University of Michigan | NIH (hESC-12-0147). Haenfler et al.81 |
| CANVAS 1P | Michigan Medicine (Dermal fibroblast patient sample) | In-house episomal hiPSC reprogramming |
| CANVAS 2P | Michigan Medicine (Dermal fibroblast patient sample) | In-house episomal hiPSC reprogramming |
| CANVAS 3P | Michigan Medicine (Dermal fibroblast patient sample) | In-house episomal hiPSC reprogramming |
| CANVAS 4P | Laboratory of Vikram Khurana, Harvard Medicine, (Fibroblasts) | In-house episomal hiPSC reprogramming |
| CANVAS 1P Isogenic | CRISPR-edited CANVAS 1P line | In-house episomal hiPSC reprogramming |
| CANVAS 2C Heterozygous | Michigan Medicine (Dermal fibroblast patient sample) | In-house episomal hiPSC reprogramming |
|
| ||
| Patient Brains | ||
|
| ||
| Control 1 | University of Michigan Brain Bank | T-346 |
| Control 2 | University of Michigan Brain Bank | T-357 |
| Control 3 | University of Michigan Brain Bank | 02-AA-185 |
| CANVAS 1 | Harvard University Brain Bank | BN-16-198-77 |
| CANVAS 2 | University of Amsterdam Brain Bank | 2008-030 |
|
| ||
| iPSC Reprogramming | ||
|
| ||
| pCXLE-hUL | Addgene | plasmid #27080 |
| pCXLE-hSK | Addgene | plasmid #27078 |
| pCXLE-hOCT3/4-shP53 | Addgene | plasmid #27077 |
| TeSR™E7™ Reprogramming Media | STEMCELL Technologies | #05914 |
|
| ||
| iPSC Maintenance | ||
|
| ||
| TeSR™E8™ Maintenance Media | STEMCELL Technologies | #05990 |
| ROCK Inhibitor (Y-27632) | Cayman Chemicals | #10005583 |
| EDTA 0.5M | Lonza | #BMA51201 |
|
| ||
| Lentiviral Constructs | ||
|
| ||
| Lenti-EV-GFP-VSVG | University of Michigan Vector Core | 100x (1x108 TU/mL) |
| Lenti-pGipZ Scramble Control | University of Michigan Vector Core | 100x (1x108 TU/mL) |
| Lenti-eF1a-RFC1-3xF-GFP | VectorBuilder | 100x (1x108 TU/mL) |
| Lenti-shRFC1-Exon4-GFP | Horizon Discovery | 100x (1x108 TU/mL) |
| Lenti-shRFC1-Exon15-GFP | Horizon Discovery | 100x (1x108 TU/mL) |
|
| ||
| SMAD-Neuron Differentiation | ||
|
| ||
| Brainphys Neuronal SM1 Kit | STEMCELL Technologies | #05792 |
| N-2 Supplement | ThermoFisher Scientific | #17502001 |
| Laminin | Millipore Sigma | #L2020 |
| Recombinant Human BDNF | PeproTech | #450-02 |
| Recombinant Human GDNF | PeproTech | #450-10 |
| Bucladesine (dbcAMP) | Cayman Chemicals | #14408 |
| L-Ascorbic Acid | Sigma | #A4403 |
| Accutase™ | ThermoFisher | #00-4555-56 |
|
| ||
| Antibodies | ||
|
| ||
| FLAG M2 | Sigma | #F1804 (1:1000 WB, 1:100 ICC) |
| KGREG | Abclonal | (1:500 WB, 1:100 ICC/IHC) |
| PFPSL | Abclonal | (1:500 WB, 1:100 ICC/IHC) |
| γ-H2AX | Abcam | #ab11174 (1:2000 WB, 1:500 ICC) |
| RFC1 | GeneTex | #GTX129291 (1:2000 WB) |
| β-Tubulin | DSHB | #E7 (1:1000 WB) |
| Oct4 | Abcam | #ab181557 (1:250 ICC) |
| Nanog | Biolegend | #674202 (1:250 ICC) |
| NeuN | MilliporeSigma | #MAB377 (1:500 ICC) |
| CHL1 | Abcam | #ab106269 (1:500 WB) |
| CAMKIIB | Fisher | #13-9800 (1:1000 WB) |
| Synaptophysin | Abcam | #ab32127 (1:1000 WB) |
| SHANK1 | Novus | #NB300-167 (1:1000 WB) |
| GAP43 | Fisher | #33-5000 (1:2000 WB) |
| AlexaFluor Rabbit-488 | ThermoFisher | #A-11008 (1:1000 ICC 2°) |
| AlexaFluor Rabbit-555 | ThermoFisher | #A-21428 (1:1000 ICC 2°) |
| AlexaFluor Mouse-488 | ThermoFisher | #A-11001 (1:1000 ICC 2°) |
| AlexaFluor Mouse-555 | ThermoFisher | #A-21422 (1:1000 ICC 2°) |
| IRDye Rabbit-680rd | LI-COR | #926-68071 (1:5000 WB 2°) |
| IRDye Rabbit-800cw | LI-COR | #926-32211 (1:5000 WB 2°) |
| IRDye Mouse-680rd | LI-COR | #926-68070 (1:5000 WB 2°) |
| IRDye Mouse-800cw | LI-COR | #926-32210 (1:5000 WB 2°) |
Acknowledgements
This work was supported by funding from the National Institute of Neurological Disorders and Stroke (R21NS129096-01, R01NS086810-09 to P.K.T; R01NS097542, R01NS113943, and R56NS128110 to S.J.B), National Institute of Child Health and Human Development (P50HD104463-04). P.K.T was also supported by Veterans Health Administration (BLRD BX004842) and philanthropic donations. Y.P and A.S were supported by NIH Postbaccalaureate Research Education Program (PREP, PAR-22-220 R25). J.M was supported by the UM Medical Scientist Training Program (MSTP). We wish to thank the patients and their families for the donation of fibroblast and post-mortem tissue samples for this study. We also thank the University of Michigan Human Stem Cell and Genome Editing Core, Dr Jack Parent, and Sandra Mojica-Perez for their continuous support in generating CRISPR-corrected iPSC clones and for the use of their Incucyte S3 instrument.
Footnotes
Conflicts of interest
The authors declare no direct conflicts of interest related to the content of this manuscript. No commercial forces had editorial or supervisory input on the content of the manuscript or its figures.
P.K.T. holds a shared patent on ASOs with Ionis Pharmaceuticals. He has served as a consultant with Denali Therapeutics, and he has licensed technology and antibodies to Denali and Abcam. V.K. is a co-founder of and senior advisor to DaCapo Brainscience and Yumanity Therapeutics, companies focused on CNS diseases.
Data and materials availability
RNASeq data will be publicly available upon publication of this manuscript. Code for analyses and any materials used for this study are available upon request.
References
- 1.Cortese Andrea, Curro’ Riccardo, Vegezzi Elisa, Yau Wai Yan, Houlden Henry, and Reilly Mary M (2022). Cerebellar ataxia, neuropathy and vestibular areflexia syndrome (CANVAS): genetic and clinical aspects. Pract Neurol 22, 14. 10.1136/practneurol-2020-002822. [DOI] [PubMed] [Google Scholar]
- 2.Szmulewicz David J., Roberts Leslie, McLean Catriona A., MacDougall Hamish G., Halmagyi G. Michael, and Storey Elsdon (2016). Proposed diagnostic criteria for cerebellar ataxia with neuropathy and vestibular areflexia syndrome (CANVAS). Neurology: Clinical Practice 6, 61. 10.1212/CPJ.0000000000000215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paisán-Ruiz C., and Jen J.C. (2020). CANVAS with cerebellar/sensory/vestibular dysfunction from RFC1 intronic pentanucleotide expansion. Brain 143, 386–390. 10.1093/brain/awaa015. [DOI] [PubMed] [Google Scholar]
- 4.Ishai R., Seyyedi M., Chancellor A.M., McLean C.A., Rodriguez M.L., Halmagyi G.M., Nadol J.B. Jr., Szmulewicz D.J., and Quesnel A.M. (2021). The Pathology of the Vestibular System in CANVAS. Otology & Neurotology 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Szmulewicz D.J., Roberts L., McLean C.A., MacDougall H.G., Chancellor A.M., Mossman S., Lamont D., Storey E., and Halmagyi G.M. (2013). Clinico-pathological correlates in cerebellar ataxia with neuronopathy and vestibular areflexia syndrome (CANVAS). Journal of the Neurological Sciences 333, e632. 10.1016/j.jns.2013.07.2196. [DOI] [Google Scholar]
- 6.Szmulewicz David J., McLean Catriona A., Rodriguez Michael L., Chancellor Andrew M., Mossman Stuart, Lamont Duncan, Roberts Leslie, Storey Elsdon, and Halmagyi G. Michael (2014). Dorsal root ganglionopathy is responsible for the sensory impairment in CANVAS. Neurology 82, 1410. 10.1212/WNL.0000000000000352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khurana V., de Gusmao C.M., Glover M., and Helgager J. (2021). Case 20–2021: A 69-Year-Old Man with Ataxia. N Engl J Med 385, 165–175. 10.1056/NEJMcpc2004992. [DOI] [PubMed] [Google Scholar]
- 8.Cortese A., Simone R., Sullivan R., Vandrovcova J., Tariq H., Yan Y.W., Humphrey J., Jaunmuktane Z., Sivakumar P., Polke J., et al. (2019). Biallelic expansion of an intronic repeat in RFC1 is a common cause of late-onset ataxia. Nature Genetics 51, 649–658. 10.1038/s41588-019-0372-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rafehi H., Szmulewicz D.J., Bennett M.F., Sobreira N.L.M., Pope K., Smith K.R., Gillies G., Diakumis P., Dolzhenko E., Eberle M.A., et al. (2019). Bioinformatics-Based Identification of Expanded Repeats: A Non-reference Intronic Pentamer Expansion in RFC1 Causes CANVAS. The American Journal of Human Genetics 105, 151–165. 10.1016/j.ajhg.2019.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cortese A., Tozza S., Yau W.Y., Rossi S., Beecroft S.J., Jaunmuktane Z., Dyer Z., Ravenscroft G., Lamont P.J., Mossman S., et al. (2020). Cerebellar ataxia, neuropathy, vestibular areflexia syndrome due to RFC1 repeat expansion. Brain 143, 480–490. 10.1093/brain/awz418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scriba C.K., Beecroft S.J., Clayton J.S., Cortese A., Sullivan R., Yau W.Y., Dominik N., Rodrigues M., Walker E., Dyer Z., et al. (2020). A novel RFC1 repeat motif (ACAGG) in two Asia-Pacific CANVAS families. Brain 143, 2904–2910. 10.1093/brain/awaa263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dominik N., Magri S., Currò R., Abati E., Facchini S., Corbetta M., MacPherson H., Bella D.D., Sarto E., Stevanovski I., et al. (2023). Normal and pathogenic variation of RFC1 repeat expansions: implications for clinical diagnosis. Brain, awad240. 10.1093/brain/awad240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cortese A., Reilly M.M., and Houlden H. (2020). RFC1 CANVAS / Spectrum Disorder GeneReviews. Pagon M. P. A. Ghayda M Mirzaa Roberta A., Wallace Stephanie E., Bean Lora JH, Gripp Karen W., Amemiya Anne, ed. (University of Washington; ). [PubMed] [Google Scholar]
- 14.Traschütz Andreas, Cortese Andrea, Reich Selina, Dominik Natalia, Faber Jennifer, Jacobi Heike, Hartmann Annette M., Rujescu Dan, Montaut Solveig, Echaniz-Laguna Andoni, et al. (2021). Natural History, Phenotypic Spectrum, and Discriminative Features of Multisystemic RFC1 Disease. Neurology 96, e1369. 10.1212/WNL.0000000000011528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gisatulin M., Dobricic V., Zühlke C., Hellenbroich Y., Tadic V., Münchau A., Isenhardt K., Bürk K., Bahlo M., Lockhart P.J., et al. (2020). Clinical spectrum of the pentanucleotide repeat expansion in the RFC1 gene in ataxia syndromes. Neurology 95, e2912. 10.1212/WNL.0000000000010744. [DOI] [PubMed] [Google Scholar]
- 16.Martelli A., Napierala M., and Puccio H. (2012). Understanding the genetic and molecular pathogenesis of Friedreich’s ataxia through animal and cellular models. Disease Models & Mechanisms 5, 165–176. 10.1242/dmm.008706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ronco R., Perini C., Currò R., Dominik N., Facchini S., Gennari A., Simone R., Stuart S., Nagy S., Vegezzi E., et al. (2023). Truncating Variants in RFC1 in Cerebellar Ataxia, Neuropathy, and Vestibular Areflexia Syndrome. Neurology 100, e543. 10.1212/WNL.0000000000201486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arteche-López A., Avila-Fernandez A., Damian A., Soengas-Gonda E., Fuente R.P. de la, Gómez P.R., Merlo J.G., Burgos L.H., Fernández C.C., Rosales J.M.L., et al. (2023). New Cerebellar Ataxia, Neuropathy, Vestibular Areflexia Syndrome cases are caused by the presence of a nonsense variant in compound heterozygosity with the pathogenic repeat expansion in the RFC1 gene. Clinical Genetics 103, 236–241. 10.1111/cge.14249. [DOI] [PubMed] [Google Scholar]
- 19.Weber S., Coarelli G., Heinzmann A., Monin M.-L., Richard N., Gerard M., Durr A., and Huin V. (2023). Two RFC1 splicing variants in CANVAS. Brain 146, e14–e16. 10.1093/brain/awac466. [DOI] [PubMed] [Google Scholar]
- 20.Cleary J.D., Pattamatta A., and Ranum L.P.W. (2018). Repeat-associated non-ATG (RAN) translation. Journal of Biological Chemistry 293, 16127–16141. 10.1074/jbc.R118.003237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bajc Česnik A., Darovic S., Prpar Mihevc S., Štalekar M., Malnar M., Motaln H., Lee Y.-B., Mazej J., Pohleven J., Grosch M., et al. (2019). Nuclear RNA foci from C9ORF72 expansion mutation form paraspeckle-like bodies. Journal of Cell Science 132, jcs224303. 10.1242/jcs.224303. [DOI] [PubMed] [Google Scholar]
- 22.Lander E.S., Linton L.M., Birren B., Nusbaum C., Zody M.C., Baldwin J., Devon K., Dewar K., Doyle M., FitzHugh W., et al. (2001). Initial sequencing and analysis of the human genome. Nature 409, 860–921. 10.1038/35057062. [DOI] [PubMed] [Google Scholar]
- 23.Payer L.M., Steranka J.P., Ardeljan D., Walker J., Fitzgerald K.C., Calabresi P.A., Cooper T.A., and Burns K.H. (2019). Alu insertion variants alter mRNA splicing. Nucleic Acids Research 47, 421–431. 10.1093/nar/gky1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sorek R., Ast G., and Graur D. (2002). Alu-Containing Exons are Alternatively Spliced. Genome Research 12, 1060–1067. 10.1101/gr.229302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim D.D.Y., Kim T.T.Y., Walsh T., Kobayashi Y., Matise T.C., Buyske S., and Gabriel A. (2004). Widespread RNA Editing of Embedded Alu Elements in the Human Transcriptome. Genome Research 14, 1719–1725. 10.1101/gr.2855504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen L.-L., and Yang L. (2017). ALUternative Regulation for Gene Expression. Trends in Cell Biology 27, 480–490. 10.1016/j.tcb.2017.01.002. [DOI] [PubMed] [Google Scholar]
- 27.Robinson J., Raguseo F., Nuccio S.P., Liano D., and Di Antonio M. (2021). DNA G-quadruplex structures: more than simple roadblocks to transcription? Nucleic Acids Research 49, 8419–8431. 10.1093/nar/gkab609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Davies K.C., Bozaoglu K., and Lockhart P.J. (2023). Generation and heterozygous repair of human iPSC lines from three individuals with cerebellar ataxia, neuropathy and vestibular areflexia syndrome (CANVAS) carrying biallelic AAGGG expansions in RFC1. Stem Cell Research 68, 103047. 10.1016/j.scr.2023.103047. [DOI] [PubMed] [Google Scholar]
- 29.Rohilla K.J., Ovington K.N., Pater A.A., Barton M., Henke A.J., and Gagnon K.T. (2020). Systematic microsatellite repeat expansion cloning and validation. Human Genetics 139, 1233–1246. 10.1007/s00439-020-02165-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wright S.E., Rodriguez C.M., Monroe J., Xing J., Krans A., Flores B.N., Barsur V., Ivanova M.I., Koutmou K.S., Barmada S.J., et al. (2022). CGG repeats trigger translational frameshifts that generate aggregation-prone chimeric proteins. Nucleic Acids Res 50, 8674–8689. 10.1093/nar/gkac626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rodriguez C.M., Wright S.E., Kearse M.G., Haenfler J.M., Flores B.N., Liu Y., Ifrim M.F., Glineburg M.R., Krans A., Jafar-Nejad P., et al. (2020). A native function for RAN translation and CGG repeats in regulating fragile X protein synthesis. Nature Neuroscience 23, 386–397. 10.1038/s41593-020-0590-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.He F., Flores B.N., Krans A., Frazer M., Natla S., Niraula S., Adefioye O., Barmada S.J., and Todd P.K. (2020). The carboxyl termini of RAN translated GGGGCC nucleotide repeat expansions modulate toxicity in models of ALS/FTD. Acta Neuropathologica Communications 8, 122. 10.1186/s40478-020-01002-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Malik I., Tseng Y.-J., Wright S.E., Zheng K., Ramaiyer P., Green K.M., and Todd P.K. (2021). SRSF protein kinase 1 modulates RAN translation and suppresses CGG repeat toxicity. EMBO Mol Med 13, e14163. 10.15252/emmm.202114163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Flores B.N., Dulchavsky M.E., Krans A., Sawaya M.R., Paulson H.L., Todd P.K., Barmada S.J., and Ivanova M.I. (2016). Distinct C9orf72-Associated Dipeptide Repeat Structures Correlate with Neuronal Toxicity. PLOS ONE 11, e0165084. 10.1371/journal.pone.0165084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barmada S.J., Ju S., Arjun A., Batarse A., Archbold H.C., Peisach D., Li X., Zhang Y., Tank E.M.H., Qiu H., et al. (2015). Amelioration of toxicity in neuronal models of amyotrophic lateral sclerosis by hUPF1. Proceedings of the National Academy of Sciences of the United States of America 112, 7821–7826. 10.1073/pnas.1509744112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Arrasate M., Mitra S., Schweitzer E.S., Segal M.R., and Finkbeiner S. (2004). Inclusion body formation reduces levels of mutant huntingtin and the risk of neuronal death. Nature 431, 805–810. [DOI] [PubMed] [Google Scholar]
- 37.Niblock M., Smith B.N., Lee Y.-B., Sardone V., Topp S., Troakes C., Al-Sarraj S., Leblond C.S., Dion P.A., Rouleau G.A., et al. (2016). Retention of hexanucleotide repeat-containing intron in C9orf72 mRNA: implications for the pathogenesis of ALS/FTD. Acta Neuropathologica Communications 4, 18. 10.1186/s40478-016-0289-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sznajder Ł.J., Thomas J.D., Carrell E.M., Reid T., McFarland K.N., Cleary J.D., Oliveira R., Nutter C.A., Bhatt K., Sobczak K., et al. (2018). Intron retention induced by microsatellite expansions as a disease biomarker. Proceedings of the National Academy of Sciences 115, 4234–4239. 10.1073/pnas.1716617115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang S., Latallo M.J., Zhang Z., Huang B., Bobrovnikov D.G., Dong D., Livingston N.M., Tjoeng W., Hayes L.R., Rothstein J.D., et al. (2021). Nuclear export and translation of circular repeat-containing intronic RNA in C9ORF72-ALS/FTD. Nature Communications 12. 10.1038/s41467-021-25082-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bidichandani S.I., Ashizawa T., and Patel P.I. (1998). The GAA Triplet-Repeat Expansion in Friedreich Ataxia Interferes with Transcription and May Be Associated with an Unusual DNA Structure. The American Journal of Human Genetics 62, 111–121. 10.1086/301680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wieben E.D., Aleff R.A., Tang X., Butz M.L., Kalari K.R., Highsmith E.W., Jen J., Vasmatzis G., Patel S.V., Maguire L.J., et al. (2017). Trinucleotide Repeat Expansion in the Transcription Factor 4 (TCF4) Gene Leads to Widespread mRNA Splicing Changes in Fuchs’ Endothelial Corneal Dystrophy. Investigative Ophthalmology & Visual Science 58, 343–352. 10.1167/iovs.16-20900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang N., and Ashizawa T. (2017). RNA toxicity and foci formation in microsatellite expansion diseases. Current Opinion in Genetics & Development 44, 17–29. 10.1016/j.gde.2017.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nakama M., Otsuka H., Ago Y., Sasai H., Abdelkreem E., Aoyama Y., and Fukao T. (2018). Intronic antisense Alu elements have a negative splicing effect on the inclusion of adjacent downstream exons. Gene 664, 84–89. 10.1016/j.gene.2018.04.064. [DOI] [PubMed] [Google Scholar]
- 44.Lev-Maor G., Ram O., Kim E., Sela N., Goren A., Levanon E.Y., and Ast G. (2008). Intronic Alus Influence Alternative Splicing. PLOS Genetics 4, e1000204. 10.1371/journal.pgen.1000204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang M., Hou J., Müller-McNicoll M., Chen W., and Schuman E.M. (2019). Long and Repeat-Rich Intronic Sequences Favor Circular RNA Formation under Conditions of Reduced Spliceosome Activity. iScience 20, 237–247. 10.1016/j.isci.2019.08.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang R., Zhou X., Luo G., Zhang J., Yang M., and Song C. (2021). CircRNA RERE Promotes the Oxidative Stress-Induced Apoptosis and Autophagy of Nucleus Pulposus Cells through the miR-299–5p/Galectin-3 Axis. Journal of Healthcare Engineering 2021, 2771712. 10.1155/2021/2771712. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 47.Dal Molin A., Hofmans M., Gaffo E., Buratin A., Cavé H., Flotho C., de Haas V., Niemeyer C.M., Stary J., Van Vlierberghe P., et al. (2021). CircRNAs Dysregulated in Juvenile Myelomonocytic Leukemia: CircMCTP1 Stands Out. Frontiers in Cell and Developmental Biology 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wei Y., Chen X., Liang C., Ling Y., Yang X., Ye X., Zhang H., Yang P., Cui X., Ren Y., et al. (2020). A Noncoding Regulatory RNAs Network Driven by Circ-CDYL Acts Specifically in the Early Stages Hepatocellular Carcinoma. Hepatology 71. [DOI] [PubMed] [Google Scholar]
- 49.Chen Y., Li X., Meng S., Huang S., Chang S., and Shi J. (2022). Identification of Functional CircRNA–miRNA–mRNA Regulatory Network in Dorsolateral Prefrontal Cortex Neurons of Patients With Cocaine Use Disorder. Frontiers in Molecular Neuroscience 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Luo Z., Lu L., Tang Q., Wei W., Chen P., Chen Y., Pu J., and Wang J. (2021). CircCAMSAP1 promotes hepatocellular carcinoma progression through miR-1294/GRAMD1A pathway. Journal of Cellular and Molecular Medicine 25, 3793–3802. 10.1111/jcmm.16254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Anders S., Reyes A., and Huber W. (2012). Detecting differential usage of exons from RNA-seq data. Genome Research 22, 2008–2017. 10.1101/gr.133744.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Majka J., and Burgers P.M.J. (2004). The PCNA–RFC Families of DNA Clamps and Clamp Loaders. In Progress in Nucleic Acid Research and Molecular Biology (Academic Press; ), pp. 227–260. 10.1016/S0079-6603(04)78006-X. [DOI] [PubMed] [Google Scholar]
- 53.Tomida J., Masuda Y., Hiroaki H., Ishikawa T., Song I., Tsurimoto T., Tateishi S., Shiomi T., Kamei Y., Kim J., et al. (2008). DNA Damage-induced Ubiquitylation of RFC2 Subunit of Replication Factor C Complex *. Journal of Biological Chemistry 283, 9071–9079. 10.1074/jbc.M709835200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Overmeer R.M., Gourdin A.M., Giglia-Mari A., Kool H., Houtsmuller A.B., Siegal G., Fousteri M.I., Mullenders L.H.F., and Vermeulen W. (2010). Replication Factor C Recruits DNA Polymerase δ to Sites of Nucleotide Excision Repair but Is Not Required for PCNA Recruitment. Molecular and Cellular Biology 30, 4828–4839. 10.1128/MCB.00285-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Boivin M., Deng J., Pfister V., Grandgirard E., Oulad-Abdelghani M., Morlet B., Ruffenach F., Negroni L., Koebel P., Jacob H., et al. (2021). Translation of GGC repeat expansions into a toxic polyglycine protein in NIID defines a novel class of human genetic disorders: The polyG diseases. Neuron 109, 1825–1835.e5. 10.1016/j.neuron.2021.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Milnerwood A.J., and Raymond L.A. (2010). Early synaptic pathophysiology in neurodegeneration: insights from Huntington’s disease. Trends in Neurosciences 33, 513–523. 10.1016/j.tins.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 57.Ravalia A.S., Lau J., Barron J.C., Purchase S.L.M., Southwell A.L., Hayden M.R., Nafar F., and Parsons M.P. (2021). Super-resolution imaging reveals extrastriatal synaptic dysfunction in presymptomatic Huntington disease mice. Neurobiology of Disease 152, 105293. 10.1016/j.nbd.2021.105293. [DOI] [PubMed] [Google Scholar]
- 58.Ghaffari L.T., Trotti D., Haeusler A.R., and Jensen B.K. (2022). Breakdown of the central synapses in C9orf72-linked ALS/FTD. Frontiers in Molecular Neuroscience 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gelon P.A., Dutchak P.A., and Sephton C.F. (2022). Synaptic dysfunction in ALS and FTD: anatomical and molecular changes provide insights into mechanisms of disease. Frontiers in Molecular Neuroscience 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Meftah S., and Gan J. (2023). Alzheimer’s disease as a synaptopathy: Evidence for dysfunction of synapses during disease progression. Frontiers in Synaptic Neuroscience 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Martínez-Serra R., Alonso-Nanclares L., Cho K., and Giese K.P. (2022). Emerging insights into synapse dysregulation in Alzheimer’s disease. Brain Communications 4, fcac083. 10.1093/braincomms/fcac083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mojica-Perez S.P., Stokes K., Jaklic D.C., Jahagirdar S., Uhler M., Parent J.M., and Niu W. (2023). Protocol for selecting single human pluripotent stem cells using a modified micropipetter. STAR Protocols 4, 102629. 10.1016/j.xpro.2023.102629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shi Y., Kirwan P., and Livesey F.J. (2012). Directed differentiation of human pluripotent stem cells to cerebral cortex neurons and neural networks. Nature Protocols 7, 1836–1846. 10.1038/nprot.2012.116. [DOI] [PubMed] [Google Scholar]
- 64.Choi H.M.T., Calvert C.R., Husain N., Huss D., Barsi J.C., Deverman B.E., Hunter R.C., Kato M., Lee S.M., Abelin A.C.T., et al. (2016). Mapping a multiplexed zoo of mRNA expression. Development (Cambridge) 143, 3632–3637. 10.1242/dev.140137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Choi H.M.T., Chang J.Y., Trinh L.A., Padilla J.E., Fraser S.E., and Pierce N.A. (2010). Programmable in situ amplification for multiplexed imaging of mRNA expression. Nature Biotechnology 28, 1208–1212. 10.1038/nbt.1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Huss D., Choi H.M.T., Readhead C., Fraser S.E., Pierce N.A., and Lansford R. (2015). Combinatorial analysis of mRNA expression patterns in mouse embryos using hybridization chain reaction. Cold Spring Harbor Protocols 2015, 259–268. 10.1101/pdb.prot083832. [DOI] [PubMed] [Google Scholar]
- 67.Glineburg M.R., Zhang Y., Krans A., Tank E.M., Barmada S.J., and Todd P.K. (2021). Enhanced detection of expanded repeat mRNA foci with hybridization chain reaction. Acta Neuropathologica Communications 9, 73. 10.1186/s40478-021-01169-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Andrews S. (2010). FastQC: A Quality Control Tool for High Throughput Sequence Data. [Online].
- 69.Chen S., Zhou Y., Chen Y., and Gu J. (2018). Fastp: An ultra-fast all-in-one FASTQ preprocessor. In Bioinformatics (Oxford University Press; ), pp. i884–i890. 10.1093/bioinformatics/bty560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dobin A., Davis C.A., Schlesinger F., Drenkow J., Zaleski C., Jha S., Batut P., Chaisson M., and Gingeras T.R. (2013). STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21. 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liao Y., Smyth G.K., and Shi W. (2014). FeatureCounts: An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 30, 923–930. 10.1093/bioinformatics/btt656. [DOI] [PubMed] [Google Scholar]
- 72.Love M.I., Huber W., and Anders S. (2014). Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biology 15. 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Falcon S., and Gentleman R. (2007). Using GOstats to test gene lists for GO term association. Bioinformatics 23, 257–258. 10.1093/bioinformatics/btl567. [DOI] [PubMed] [Google Scholar]
- 74.Wu T., Hu E., Xu S., Chen M., Guo P., Dai Z., Feng T., Zhou L., Tang W., Zhan L., et al. (2021). clusterProfiler 4.0: A universal enrichment tool for interpreting omics data. Innovation 2. 10.1016/j.xinn.2021.100141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Blighe K., Rana S., and Lewis M. (2023). EnhancedVolcano: Publication-ready volcano plots with enhanced colouring and labeling.
- 76.Wilke C.O. (2022). ggridges: Ridgeline Plots in “ggplot2.”
- 77.Cheng J., Metge F., and Dieterich C. (2016). Specific identification and quantification of circular RNAs from sequencing data. Bioinformatics 32, 1094–1096. 10.1093/bioinformatics/btv656. [DOI] [PubMed] [Google Scholar]
- 78.Robinson J.T., Thorvaldsdóttir H., Winckler W., Guttman M., Lander E.S., Getz G., and Mesirov J.P. (2011). Integrative genomics viewer. Nature Biotechnology 29, 24–26. 10.1038/nbt0111-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tidball A.M., Dang L.T., Glenn T.W., Kilbane E.G., Klarr D.J., Margolis J.L., Uhler M.D., and Parent J.M. (2017). Rapid Generation of Human Genetic Loss-of-Function iPSC Lines by Simultaneous Reprogramming and Gene Editing. Stem Cell Reports 9, 725–731. 10.1016/j.stemcr.2017.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Liu J., Kościelska K.A., Cao Z., Hulsizer S., Grace N., Mitchell G., Nacey C., Githinji J., McGee J., Garcia-Arocena D., et al. (2012). Signaling defects in iPSC-derived fragile X premutation neurons. Human Molecular Genetics 21, 3795–3805. 10.1093/hmg/dds207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Haenfler J.M., Skariah G., Rodriguez C.M., Monteiro da Rocha A., Parent J.M., Smith G.D., and Todd P.K. (2018). Targeted Reactivation of FMR1 Transcription in Fragile X Syndrome Embryonic Stem Cells. Frontiers in Molecular Neuroscience 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
RNASeq data will be publicly available upon publication of this manuscript. Code for analyses and any materials used for this study are available upon request.